Abstract
In the present study, inhibitory effect of the methanol extract of Raphanus sativus root on lipid peroxidation has been carried out in normal rats. Graded doses of methanol extract of root of the plant (40, 80 and 120 mg kg−1 body weight) were administered orally for 15 days to experimental treated rats. Distilled water was administered to experimental control rats. At the end of experiment, rats were killed by decapitation after ether anesthesia. Blood and liver were collected to measure thiobarbituric acid reactive substance, reduced glutathione and activity of catalase. Results indicated that the extract of R. sativus root reduced the levels of thiobarbituric acid reactive substance significantly in all experimental treated groups (P < 0.05) as compared to the experimental control group. It also increased the levels of reduced glutathione and increased the activity of catalase. In vitro experiments with the liver of experimental control and experimental treated rats were also carried out against cumene hydroperoxide induced lipid peroxidation. The extract inhibited in vitro cumene hydroperoxide induced lipid peroxidation. R. sativus inhibits lipid peroxidation in vivo and in vitro. It provides protection by strengthening the antioxidants like glutathione and catalase. Inclusion of this plant in every day diet would be beneficial.
Keywords: catalase, glutathione, lipid peroxidation, Raphanus sativus, thiobarbituric acid reactive substance
Introduction
Free radicals are continuously produced in body of all living organisms mainly due to oxidation processes. Antioxidant system of the body is generally able to combat the oxidative stress produced after normal physiological processes (1). Modern civilization is facing a variety of mental and physical stress, pollutant stress, stress caused by consuming fast food, etc. These stresses culminate into generation of free radicals and the antioxidant system of body fails to combat this situation. Therefore there is a need to supplement our diet in such a way that it could help strengthen the antioxidant system of the body by inhibiting lipid peroxidation and prevent chronic diseases (2).
Raphanus sativus is an annual herb, consumed as vegetable. It belongs to the family Brassicaceae. In Africa, root of the plant is used for cure of many diseases like gall bladder trouble, diabetes, hepatitis and gastrointestinal disorders. Fresh radish has been reported to increase the protein digestibility. Roots, flowers and pods of the plant are active against gram-positive bacteria like Staphylococcus aureus and Bacillus subtillis (3). It decreases blood glucose levels in diabetic rats (4). It improves the histopathology of colon mucosa in the rats fed high fat diet (5). Gastrointestinal and uterine tone modulatory activities of R. sativus are reported by Ghayur and Gilani (6). Its antioxidant effects are reported in alimentary hyperlipidemic rats (7). The present study aims at assessing the inhibitory effect of R. sativus on lipid peroxidation in normal rats.
Methods
Plant Material and Preparation of Extract
Fresh R. sativus roots were obtained from local farmers and its authenticity was confirmed by Botany section of the Biology department, University of Botswana. Methanol extract was prepared after chopping the roots into thin slices and drying. Dried roots were crushed to powder and soaked with 70% methanol for 3 days. After that, extract was filtered and dried in Buchi-type rotary vaporizer. The yield was 6% of the dried weight.
Chemicals
All the chemicals used were of analytical grade and bought from Sigma Chemicals.
Rats
Male albino rats of Wistar strain of ∼200–250 g were used for all the experiment. Animals had free access to water and were fed on commercial diet.
Experimental Design
In Vivo Experiment
Twenty rats were used for this experiment and were divided into four groups of five each. Group C was normal control group administered distilled water; group E1 was the experimental group receiving the plant extract (40 mg kg−1) body weight; group E2 was the experimental group, receiving the plant extract (80 mg kg−1) body weight; group E3 experimental group received the plant extract (120 mg kg−1) body weight for 15 days. Distilled water and extract were administered orally with the help of gastric tube.
At the end of the experiment, rats were killed by decapitation after ether anesthesia. Blood was collected from brachial artery; plasma was separated from it and frozen. The liver was harvested and washed with cold normal salineand frozen. Thiobarbituric acid reactive substance (TBARS), reduced glutathione (GSH) and catalase were measured in the plasma and liver.
In Vitro Experiment
Livers from groups C and E3 rats were also subjected to in vitro analyses. Ten percent liver homogenates were prepared in phosphate buffer saline (pH 7.4) under chilled condition. Supernatant was collected after centrifugation of the homogenate. All in vitro studies were performed with supernatant. To study the effect on TBARS, 1.5 mmol of cumene hydroperoxide (CHP) was used while to study reduced glutathione 0.3 mmol of CHP was used (8).
Supernatant of liver homogenate from experimental control rats of group C of in vivo experiment was divided into two parts NC and NE and incubated as follows: NC 3 ml of liver homogenate + CHP + 300 µl distilled water. NE 3 ml of liver homogenate + CHP + 300 µl extract dissolved in distilled water (0.6%). Liver homogenate from experimental treated rats of group E3 of in vivo experiment was divided into two parts E3C and E3E and treated as follows: E3C 3 ml of liver homogenate + CHP + 300 µl distilled water. E3E 3 ml of liver homogenate + CHP + 300 µl extract dissolved in distilled water (0.6%). Samples from each incubation mixture were collected at 10, 20 and 40 min to measure TBARS and reduced glutathione. TBARS and reduced glutathione were measured before incubation and considered as values at 0 min.
Biochemical Analysis
Estimation of Lipid Peroxidation
Lipid peroxidation in plasma and liver was estimated in terms of TBARS by the method described by Sushmakumari et al. (9) with little modification. Then 0.1 ml of plasma was treated with 2 ml of TCA–TBA–HCL (TBA 0.37%, 0.25 N HCl and 15% TCA) reagent (1: 1: 1) and incubated in boiling water bath for 10 min. After that, the mixture was cooled, mixed with 2 ml of freshly prepared 1 M NaOH. The absorbance was measured at 535 nm. It is expressed as millimoles per deciliter for plasma and as millimoles per gram wet liver tissue.
Estimation of Reduced Glutathione
Reduced glutathione in plasma and liver was measured by the method of Ellman (10). Then 0.25 ml of plasma was mixed with 0.5 ml of precipitating buffer (5% TCA in 1 mM EDTA) and centrifuged. Supernatant was collected and mixed with 2.5 ml of 0.1 M phosphate buffer (pH 8.0). Color was developed by adding 100 µl 5,5-dithiobis(2-nitrobenzoic acid) (0.01%) and read at 412 nm. It is expressed as milligrams per deciliter for plasma and milligrams per gram wet liver tissue.
Assay of Catalase Activity
The activity of catalase was assayed by the method described by Bisswanger (11). To 0.98 ml H2O2 solution (10 mM), 0.2 ml of plasma was added. Decrease in the absorption at 240 nm was followed. The catalase activity was calculated using the millimolar extinction coefficient of H2O2 (0.071 mmol cm−1) and the activity was expressed as micromoles of H2O2 oxidized per minute per milligram protein. Protein was measured by the method of Lowry et al. (12). Data were analyzed using the Student's t-test.
Results
In Vivo Study
Effect of R. sativus on TBARS in Plasma and Liver
Changes in levels of plasma and liver TBARS after daily administration of R. sativus root extract to normal rats over 15 days are presented in Table 1. The extract reduces TBARS levels of liver and plasma in all experimental groups but in a dose-dependent manner. Results of E1 and E2 are similar, but differ significantly from NC group (P < 0.05). Results in E3 group are more significant (P < 0.01) where extract dose was 160 mg kg−1 body weight. An increase of dose from 40 to 160 mg kg−1 shows a 19% reduction in TBARS levels of plasma.
Table 1.
Effect of R. sativus root extract on TBARS, reduced glutathione and catalase activity after 15 days administration in normal rats
| Groups | Treatment | TBARS (mean ± SEM) | GSH (mean ± SEM) | Catalase (mean ± SEM) | |||
|---|---|---|---|---|---|---|---|
| Plasma (mmol dl−1) | Liver (mmol g−1 wet tissue) | Plasma (mg dl−1) | Liver (mg g−1 wet tissue) | Plasma (U dl−1) | Liver (U mg−1 protein) | ||
| C | Control group (administered distilled water everyday) | 0.21 ± 0.02 | 0.31 ± 0.11 | 7.50 ± 1.07 | 13.21 ± 0.98 | 45.30 ± 2.03 | 70.35 ± 2.35 |
| E1 | Experimental group (administered extract, 40 mg kg−1 body weight) | 0.16 ± 0.07* | 0.25 ± 0.03* | 12.36 ± 2.03* | 21.39 ± 1.19* | 47.93 ± 3.25 | 75.15 ± 2.5 |
| E2 | Experimental group (administered extract, 80 mg kg−1 body weight) | 0.14 ± 0.03* | 0.23 ± 0.01* | 15.16 ± 1.97* | 20.42 ± 1.53* | 60.30 ± 2.91* | 99.33 ± 3.01* |
| E3 | Experimental group (administered extract, 160 mg kg−1 body weight) | 0.13 ± 0.07* | 0.19 ± 0.02* | 19.76 ± 2.39* | 23.91 ± 2.01* | 78.61 ± 2.51** | 118.71 ± 3.03** |
Note: N = 5 in all groups.
*P < 0.05 when compared with control.
**P < 0.01 when compared with control.
R. sativus Increases Reduced Glutathione Levels in Plasma and Liver
Changes in levels of plasma and liver reduced glutathione after daily administration of R. sativus root extract to normal rats over 15 days are presented in Table 1. The extract increases reduced glutathione levels in plasma and liver. Changes in liver glutathione and plasma glutathione are significant in all treated groups (P < 0.05) when compared with NC. Increase is more pronounced in E3 for both plasma and liver glutathione. Plasma glutathione increased by 39% upon increase in the dose from 40 to 160 mg kg−1.
Extract of R. sativus increases Activity of Catalase
The extract also increases the activity of catalase. No significant activity of catalase has been exhibited at the dose 40 mg kg−1 body weight. Activity is significant at both doses (80 and 120 mg kg−1) but most significant with the dose 120 mg kg−1 body weight in E3 for both plasma and liver catalase (P < 0.001).
In Vitro Study
Extent of TBARS Formation in the Reaction Mixture After Incubation with CHP and R. sativus Extract
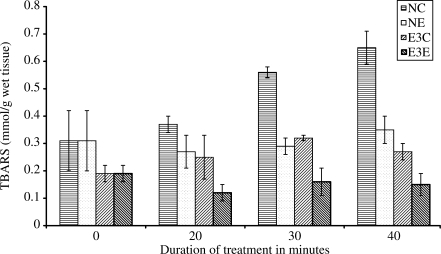
In vitro effect of R. sativus root extract on TBARS in normal and pre-treated rat liver homogenates in CHP-induced peroxidation are presented in Fig. 1. Results show continuous increment in the levels of TBARS in NC from 0 up to 40 min. In NE where extract is included in incubation, a fall in TBARS values is noted up to 20 min. After that it shows an increment. At 40 min, 13% increase is noted when compared with the level at 10 min. In E3C, liver homogenate does not show significant protection against CHP-induced lipid peroxidation and continuous increase in TBARS is observed from 0 min value. Results show an increment of TBARS in both NC and E3C but the increment is larger in NC. Comparison of percent increase at 40 min shows that in NC the increment is 52%, while in E3C it is 29% when compared with 0 min observation. In E3E, where the liver homogenate from pre-treated rats was incubated with extract, a significant difference is noted between the values at 0 and 40 min. Here TBARS registers 21% fall in its level at 40 min.
Figure 1.
In vitro effect of R. sativus root extract on TBARS in experimental control and experimental treated rat liver homogenate in CHP-induced lipid peroxidation. NC: homogenate from experimental control rats + distilled water + CHP, NE: homogenate from experimental control rats + extract + CHP, E3C: homogenate from experimental treated rats (received 120 mg kg−1 body weight)+distilled water+CHP, E3E: homogenate from experimental treated rats (received 120 mg kg−1 body weight) + extract + CHP. N = 5 in all groups, P < 0.05 when NE and E3C compared with NC at 10, 20 and 40 min, P < 0.01 when E3E compared with NC at 10, 20 and 40 min.
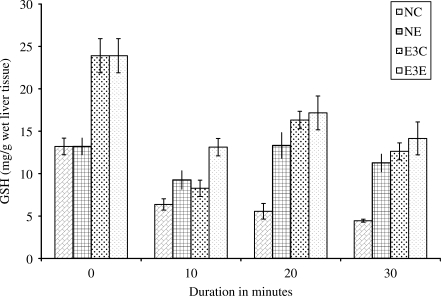
Levels of Reduced Glutathione in the Reaction Mixture After Incubation with CHP and R. sativus Extract
In vitro effect of R. sativus root extract on reduced glutathione in normal and pre-treated rat liver homogenates in CHP-induced peroxidation are presented in Fig. 2. Results show continuous decrease in the levels of reduced glutathione in NC from 0 up to 40 min. In all the three sets of experiment, NE, E3C and E3E, reduced glutathione registers a fall at 10 min, an increase at 20 min and again falls at 40 min. Although the levels of glutathione show increasing and decreasing trends at interval of 10 min in NE, N3C and N3E, their levels show the significant difference from levels in NC at all the observed points and a gradual reduction from 0 min.
Figure 2.
In vitro effect of R. sativus root extract on GSH in experimental control and experimental treated rat liver homogenate in CHP-induced lipid peroxidation. NC: homogenate from experimental control rats + distilled water + CHP, NE: homogenate from experimental control rats + extract + CHP, E3C: homogenate from experimental treated rats (received 120 mg kg−1 body weight) + distilled water + CHP, E3E: homogenate from experimental treated rats (received 120 mg kg−1 body weight) + extract + CHP. N = 5 in all groups, P < 0.05 when NE, E3E and E3C compared with NC at 20 and 40 min.
Discussion
Oxidation of lipid molecules of membrane causes its damage resulting into the development of several physiological and pathological disorders. Inhibition of lipid peroxidation by any means is the best way to avoid these disorders in the body. In the present study, the effect of R. sativus on lipid peroxidation has been analyzed and results clearly show that it has inhibitory effects. Daily administration of R. sativus root extract reduces TBARS levels in all experimental groups in a dose-dependent manner (Table 1). TBARS are diagnostic indices of lipid peroxidation and tissue injury due to oxidative stress (13). Low levels of TBARS in all experimental groups indicated inhibition of lipid peroxidation. Thus the extract potentiates the activity of liver to fight against lipid peroxidation. This is well supported from in vitro results (Fig. 1). Continuous accumulation of TBARS in NC from 0 up to 40 min indicates the lipid peroxidation caused by CHP. Low levels of TBARS in NE up to 20 min and its further increment at 40 min indicates that extract is able to inhibit oxidative stress caused by CHP only up to 20 min. Pre-treatment of rats with extract reduces the rate of in vitro lipid peroxidation. This is evidenced by comparing the percent increase in TBARS level from 0 up to 40 min in NC and E3C. The increase in TBARS concentration is 52% in NC, while in E3E it is 29%. Continuous decrease and low levels of TBARS in E3E set of experiment where rats were pre-treated with extract for 15 days and liver homogenate was incubated with the extract and CHP, indicates that this plant helps in preparing liver to fight against toxin-induced lipid peroxidation. The plant also ensures immediate protection against toxins is very clear from the results of NE set where the rats were not pre-treated with the extract but their liver homogenate was incubated with extract.
From the results it is also evident that the plant inhibits lipid peroxidation by increasing the activity of enzymatic antioxidants like catalase and also by increasing or maintaining the levels of glutathione. The liver is the main organ involved in storage as well as detoxification of xenobiotics (14). Hepatocytes are highly specialized to synthesize GSH from its pre-cursors or to recycle it from oxidized glutathione (GSSG). Administration of extract for 15 days to the experimental rats increases the levels of reduced glutathione in liver and the blood as well. High levels of reduced glutathione protects the cell from oxidative stress and hence from damage (15). This plant also maintains glutathione levels (Table 1) and hence renders protection against oxidative stresses. In vitro experiments with liver homogenate show depletion and recovery response of reduced glutathione against CHP-induced lipid peroxidation in NE, E3C and E3E (Fig. 2). This depletion and recovery response might be due to the recycling process of glutathione (16). In NC, this response is not observed. This could be due to lipid peroxidation occurring at a higher rate than the recycling of glutathione. It appears that this plant is involved in the redox cycle of glutathione. A detailed study is required to confirm this.
Catalase is an enzymatic antioxidant and helps in neutralizing the toxic effect of H2O2. Hydrogen peroxide is not reactive enough to cause a chain of lipid peroxidation reactions, but its combination with super oxide radical produces hydroxyl radical that is highly reactive and thus initiates lipid oxidation reactions (17). Catalase converts hydrogen peroxide to water and non-reactive oxygen species, thus prevents generation of hydroxyl radical and protects the cells from oxidative damage. Results of this study also demonstrate protection through catalase. The activity of catalase in all experimental groups (E1–E3) is higher than in the control group but is most significant in E3 (P < 0.05).
Thus it is clear from this study that R. sativus inhibits lipid peroxidation and provides protection by strengthening the antioxidants like glutathione and catalase. Inclusion of this plant in everyday diet would be beneficial.
Acknowledgments
The author is thankful to Research and Publication Committee, University of Botswana, for providing funds to carry out this work. We are also thankful to Mr Antony, demonstrator, Botany Section, Department of Biological Sciences for providing us with the drawing of the plant.
References
- 1.Yu BP. Cellular defence against damage from reactive oxygen species. Physiol Rev. 1994;74:139–62. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 2.Pierre SH, Georges AA, Simon G, Michel B. Natural health products, modulation of immune functions and prevention of chronic diseases. Evid Based Complement Alternat Med. 2005;4:513–20. doi: 10.1093/ecam/neh125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan KI, Khan FJ, Shahida K. Antimicrobial activity of Alum ceppa and Raphanus sativus. J Pharmacol. 1985;6:59–72. [Google Scholar]
- 4.Chaturvedi P, Akala H. Effect of Raphanus sativus root extracts on glucose level in normal and diabetic rats. J Appl Zool Res. 2001;12:172–7. [Google Scholar]
- 5.Sipos P, Hagymasi K, Lugasi A, Feher E, Blazovics A. Effect of black radish root (R. sativus L var niger) on the colon mucosa in rats fed a fat rich diet. Phytother Res. 2002;16:677–9. doi: 10.1002/ptr.950. [DOI] [PubMed] [Google Scholar]
- 6.Ghayur MN, Gilani AH. Gastrointestinal stimulatory and uterotonic activities of dietary radish leaves extract are mediated through multiple pathways. Phytother Res. 2005;19:750–5. doi: 10.1002/ptr.1753. [DOI] [PubMed] [Google Scholar]
- 7.Lugasi A, Blazovics A, Hagymasik K, Kocsis I, Kery A. Antioxidant effect of squeezed juice from black radish (R. sativus L var niger) in alimentary hyperlipidaemia in rats. Phytother Res. 2005;19:587–91. doi: 10.1002/ptr.1655. [DOI] [PubMed] [Google Scholar]
- 8.Sharma M, Pandey S, Chaturvedi P, Tripathi YB. Protective effect of Rubia cordifolia in isolated rat liver homogenate. Indian J Exp Biol. 1994;32:100–3. [PubMed] [Google Scholar]
- 9.Sushmakumari S, Jaydeep A, Kumar JS, Menon VP. Effect of carnitine on malonyldialdehyde, taurine and glutathione levels in heart of rats subjected to myocardial stress by isoprotenol. Indian J Exp Biol. 1989;27:134–7. [PubMed] [Google Scholar]
- 10.Ellman GC. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 11.Bisswanger H. Practical Enzymology. Wiley-VCH; 2004. p. 79. [Google Scholar]
- 12.Lowry AH, Rosenbrough MJ, Farr AL, Randall RJ. Protein measurement with Folin-Phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 13.Janero DR. Malonyldialdehyde and thiobarbituric acid- reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 14.Kretschmar M, Klinger W. The hepatic glutathione system and influence of xenobiotics. Exp Pathol. 1990;38:145–64. doi: 10.1016/s0232-1513(11)80201-x. [DOI] [PubMed] [Google Scholar]
- 15.Jaya DS, Augustines J, Menon VP. Role of peroxides, glutathione and antiperoxidative enzymes in alcohol and drug toxicity. Ind J Exp Biol. 1993;31:453–9. [PubMed] [Google Scholar]
- 16.Clahsen PC, Moison RM, Holtzer CA, Berger HM. Recycling of glutathione during oxidative stress in erythrocytes of the newborn. Pediatr Res. 1992;32:399–402. doi: 10.1203/00006450-199210000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Lubec B, Hayn M, Denk W, Bauer G. Brain lipid peroxidation and hydroxyl radical attack following the intravenous infusion of hydrogen peroxide in an infant. Free Radic Biol Med. 1996;21:219–23. doi: 10.1016/0891-5849(96)00018-4. [DOI] [PubMed] [Google Scholar]