Abstract
Bromelain, a widely used pineapple extract with cysteine protease activity, has been shown to have immunomodulatory effects in a variety of immune system models. The purpose of the present study was to determine the effects of orally administered bromelain in an ovalbumin (OVA)-induced murine model of acute allergic airway disease (AAD). To establish AAD, female C57BL/6J mice were sensitized with intraperitoneal (i.p.) OVA/alum and then challenged with OVA aerosols for 3 days. Mice were gavaged with either (phosphate buffered saline)PBS or 200 mg/kg bromelain in PBS, twice daily for four consecutive days, beginning 1 day prior to OVA aerosol challenge. Airway reactivity and methacholine sensitivity, bronchoalveolar lavage (BAL) cellular differential, Th2 cytokines IL-5 and IL-13, and lung histology were compared between treatment groups. Oral bromelain-treatment of AAD mice demonstrated therapeutic efficacy as evidenced by decreased methacholine sensitivity (P ≤ 0.01), reduction in BAL eosinophils (P ≤ 0.02) and IL-13 concentrations (P ≤ 0.04) as compared with PBS controls. In addition, oral bromelain significantly reduced BAL CD19+ B cells (P ≤ 0.0001) and CD8+ T cells (P ≤ 0.0001) in AAD mice when compared with controls. These results suggest that oral treatment with bromelain had a beneficial therapeutic effect in this murine model of asthma and bromelain may also be effective in human conditions.
Keywords: airway inflammation, asthma, CD19+ B Cells, CD8+ T Cells, cysteine protease, eosinophils, IL-13, immuno-modulation
Introduction
The prevalence and severity of asthma continues to increase despite new pharmacological advances for both acute treatment and chronic disease management. The benefits of lifestyle changes and alternative asthma treatments are being investigated in the hope that safe and efficacious adjunctive therapies will be added to current treatment formulary (1–8). According to the National Institutes of Health, over 60% of Americans use complementary and alternative medicine with ∼20% using natural products or specific botanical formulations to supplement their health care needs (9). Botanicals such as Boswellia serrata, Petasites hybridus, Astragalus membranaceus, Echinacea angustifolia and Ananas comosus (common pineapple) and specific extracts such as bromelain from pineapple are being investigated as therapeutic agents in inflammatory conditions such as ulcerative colitis, multiple sclerosis and asthma (10–17).
Bromelain is a commonly used botanical extract, and is normally delivered as a powder either encapsulated in gelatin or prepared in an enteric coated tablet. Bromelain is available in combination with other natural products such as in Phlogenzym® (Bromelain, trypsin and rutosid trihydrate), Wobenzym® (Bromelain, trypsin, rutosid trihydrate, pancreatin, papain and phymotrypsin) and BCQ® (Bromelain, Boswellia serrata, Curcuma longa and quercetin) or as a single stand-alone product. Bromelain (enzyme classification 3.4.22.32) is a combination of sulfur-containing cysteine endopeptidases that have a broad specificity for the cleavage of proteins. One mechanism of action proposed to account for bromelain's therapeutic activity is the cleavage of lymphocyte cell surface receptors such as CD4, CD8, CD44 and CD62L. Receptor cleavage can result in altered cell communication, cell trafficking and cell signaling pathways leading to the modulation of pro- and anti-inflammatory cytokines such as IL-2, IL-4, IL-6 and TNF-α (18–22).
Experimental animal models, such as the ovalbumin (OVA)-induced model of allergic airway disease (AAD), are utilized to investigate the underlying mechanisms and potential therapies for asthma. Asthma is complex with characteristic airway hyperresponsiveness, airway inflammation, increased mucus and airway remodeling; measurable outcomes are produced similar to those found in humans. These outcomes include increases in inflammatory Th2 cytokines (IL-4, IL-5, IL-6 and IL-13), enhanced airway hyperresponsiveness, and increased total white blood cells, eosinophils and CD4+ T lymphocytes recovered from the bronchoalveolar lavage (BAL) (23–25).
The effect of intraperitoneally (i.p.) injected bromelain has been previously characterized in an OVA-induced murine model AAD (23). Bromelain treatment was found to be well-tolerated and non-toxic in both naive and AAD mice. Bromelain significantly reduced total BAL leukocytes, eosinophils and CD4+ T cells and lowered the concentration of IL-13 in the BAL. In order to better mimic the clinical environment and consider the effects of digestion and degradation on enzymatic activity, the oral administration of bromelain was adapted in this AAD model. The present study was designed to determine whether oral bromelain treatment had an anti-inflammatory or immunoregulatory effect in an OVA-induced murine model of acute AAD.
Methods
Mice
Female C57BL/6J mice, 3–6 months of age and weighing 17–24 g, were purchased from the Jackson Laboratory (Bar Harbor, ME, USA), and housed conventionally in plastic cages with corncob bedding. The mouse room was maintained at 22–24°C with a daily light/dark cycle (light from 0600 to 1800 h). Chow and water were supplied ad libitum. Seven to eight mice were used per group. The protocols for mice used were approved by the Animal Care Committee at the University of Connecticut Health Center.
Ovalbumin Sensitization and Aerosol Exposure Protocol
Mice were immunized with three weekly i.p. injections of a suspension containing 25 µg of OVA (grade V, Sigma Chemical Co., St. Louis, MO, USA) and 2 mg of aluminum hydroxide (alum) in 0.5 ml of 0.9% sodium chloride (pH 5.5, 308 mOsmol L−1; Baxter Healthcare Corporation, Deerfield, IL, USA). One week after the last injection the mice were exposed to 1% aerosolized OVA in sodium chloride, 1 h per day, for 3 days (acute AAD model) (23,24). The mice were placed in plastic restraint tubes (Research and Consulting Co., Basel, Switzerland) for nose-only aerosol exposure. The aerosols were generated by a BANG nebulizer (CH Technologies, Westwood, NJ, USA) into a 7.6 l inhalation exposure chamber to which restraint tubes were attached. Chamber airflow was 6 l min−1, and aerosol particle size of OVA was monitored by gravimetric analysis with a Mercer cascade impactor (In-Tox Products, Moriarty, NM, USA). The mass median aerodynamic diameter and geometric standard deviations were 1.4 and 1.6 µm, respectively. The estimated daily inhaled OVA dose ∼30–40 µg per mouse. Twenty-four hours after the final aerosol exposure, the mice were sacrificed by overdose (0.15 ml i.p. injection per 20 g mouse of: 13 mg Ketamine HCL, Ketaset-III™ Fort Dodge Animal Health, Fort Dodge IA, USA, and 0.4 mg of xylazine, Tranquived™ Vedco, St Joseph, MO, USA) and exsanguination.
Airway Hyperresponsiveness
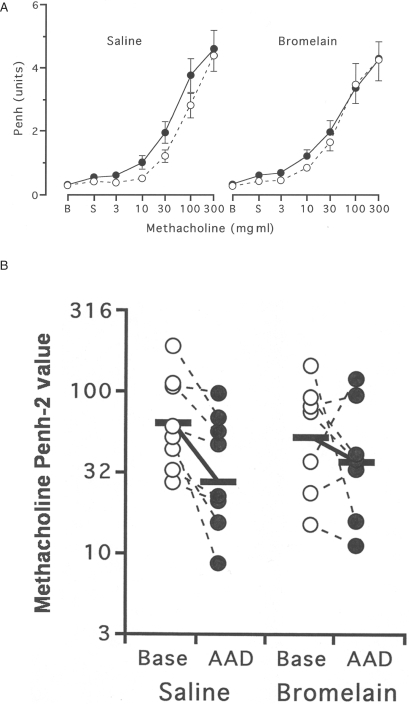
Airway responses to methacholine were assessed by whole-body barometric plethysmography, using the Buxco system (Buxco Electronics, Troy, NY, USA), as previously described (23). Briefly, mice were evaluated for maximal enhanced pause (Penh), 24 h before treatment and 12 h after the third OVA aerosol was administered. Mice were placed in individual chambers and exposed for 2 min to aerosolized saline or increasing concentrations of methacholine from 3 to 300 mg ml−1. Respiratory system variables including tidal volume, respiratory frequency, inspiratory and expiratory times and changes in box pressure were recorded before and during aerosolization and for 4 min after each exposure. The Penh value response to methacholine was recorded at each dose. The entire dose–response relationship to methacholine was compared in individual animals before and after OVA aerosol exposures. In addition, individual animal sensitivity to methacholine was measured by the interpolated concentration of methacholine needed to increase the Penh value to two units (the ‘Penh-2’ value). The Penh-2 value was selected as the portion of the dose–response curve in which the greatest changes in sensitivity would be manifested (25). Increased sensitivity to methacholine during AAD was demonstrated by a decreased Penh-2 value (i.e. less methacholine needed to elicit the response).
BAL Fluid Analysis
At time of sacrifice the lungs were lavaged in situ with five 1 ml aliquots of sodium chloride. The BAL fluid was centrifuged at 200 g for 10 min, the cellular pellet was resuspended in sodium chloride, and the total nucleated cells were counted with a hemocytometer using nigrosin exclusion as a measure of viability. Leukocyte differentials were determined in BAL fluid using cytocentrifuged (at 900 rpm for 5 min, Cytospin-4® Thermo Shandon, Astmoor, Runcorn, Cheshire, England, UK) preparations stained with May-Grünwaldstain and Giemsa (Accustain®, Sigma, St Louis, MO, USA). Briefly the slides were allowed to air dry for ∼10 min, immersed in methanol, May-Grünwald and May Grunwald Buffer (pH 7.2, Sigma, St Louis, MO, USA) for 5 min each, and stained with Giemsa for 15 min. Stained BAL slide differentials were counted in a blind manner by 3 individuals. The remaining cells were analyzed phenotypically for T cell subpopulations using specific antibodies and fluorescence flow cytometry. BAL protein concentrations were measured in the supernatants by bicinchoninic acid (BCA) protein assay using bovine serum albumin as a standard (Pierce Biotechnology, Rockford, IL, USA).
BAL-Cytokine Analysis
After collection of BAL, samples were centrifuged at 200 g for 10 min to remove cells. The BAL fluid component was concentrated using an Amicon Centriplus YM-10 filtration device (Millipore Corp., Bedford, MA, USA). Concentrated samples were then analyzed for Th2 cytokines IL-5 and IL-13 using enzyme-linked immunosorbent assay (ELISA) kits (Pierce Biotechnology Rockford, IL; R&D Systems Minneapolis, MN, USA) according to the manufacturer's directions. The limits of detection for IL-5 and IL-13 were 5 and 1.5 pg ml−1, respectively.
BAL-Flow Cytometry and Immunofluorescence
BAL samples were prepared as previously described (23). Briefly the samples were washed in PBS (Dulbecco's Phosphate Buffered Saline, pH 7.4, Sigma St Louis, MO, USA) containing 0.2% bovine serum albumin and 0.1% NaN3. Aliquots containing 104 to 105 cells were incubated with 100 µl of appropriately diluted antibodies for 30 min at 4°C. After staining, the cells were washed twice with the above PBS solution, and relative fluorescence intensities were determined via flow cytometry using a FACSCalibur and analyzed with BD FACSDiva™ Software v4.1 (Becton Dickinson, San Jose, CA, USA). Eosinophils were gated on forward and side scatter using a 4-decade log scale. The following fluorescence labeled monoclonal antibodies was used: CD19-PE (1D3), CD4-PerCP (RM4-5) and CD8a-FITC (53-6.7) (Pharmingen, San Jose, CA, USA).
Histology
After sacrifice, non-inflated lungs from separate animals were removed, fixed with 10% buffered formalin and processed in a standard manner. Tissue sections were stained with hematoxylin and eosin, Mallory's trichrome for collagen and periodic acid-schiff for mucus detection. All specimens were evaluated with a microscope-mounted Nikon Eclipse 400™ camera (Tokyo, Japan). Digital images were created using Spot RT Slider™ Software (Sterling Heights, MI, USA), and evaluated in Microsoft Photo Editor™ (Redmond, WA, USA).
Oral Bromelain administration
A stock solution of stem bromelain (EC 3.4.22.32), Lot # 1965 (Vital Nutrients, Middletown, CT, USA) was made using 60 mg of bromelain dissolved in 250 ml of PBS. Bromelain (200 mg kg−1) in 0.5 ml of PBS was prepared from the stock solution. Each animal received either bromelain or saline via gavage using a 22 gauge−1 inch straight stainless steel feeding needle with a 1.25 mm ball tip (Braintree Scientific inc., Braintree, MA, USA), twice a day. Bromelain or saline treatments were administered 6–8 h apart, beginning 1 day prior to aerosolization of OVA. The dosages used were based on in vivo dose response studies performed in our laboratory (23). Bromelain was independently tested for authenticity, potency (2400–2660 GDU g−1), microbial contamination, residual solvents, heavy metals and aflatoxins (Vital Nutrients, Middletown, CT; ChromaDex, Clearwater, FL and Pharmline, Florida, NY, USA). Ananas comosus (common pineapple) was identified by the raw material provider (Indonesia) using organoleptic techniques and bromelain identity was confirmed with an Fourier transform infrared spectrometer (FTIR; Pharmline, Florida, NY, USA).
Statistical Analysis
Statistical comparisons between groups were made with analysis of variance and unpaired t-tests using StatView 4.5 (Abacus Concepts, Inc., Berkeley, CA, USA) and JMP® Software (SAS Institute Inc., Cary, NC, USA). Dose-response data and cytokine levels were compared by repeated measures analysis of variance. Changes in Penh dose–response relationships were compared within and between groups by repeated measures analysis of variance. Changes in Penh-2 values before and after aerosol exposure within groups were made by paired t-tests and were compared between the control and bromelain-treated groups by repeated measures analysis of variance. All data were expressed as means ± standard error of the mean, and differences were considered significant at P ≤ 0.05.
Results
Treatment Protocol
Each animal received either bromelain (200 mg kg−1) in 0.5 ml of PBS or 0.5 ml PBS alone via gavage (orally) twice daily beginning 6 days after the third OVA–Alum sensitization injection (Fig. 1). The treatments were administered 6–8 h apart, and all animals were sacrificed ∼12 h after the last treatment and the primary AAD outcomes collected and assessed.
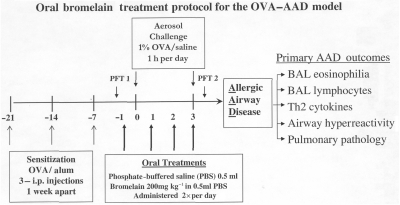
Figure 1.
Bromelain treatment protocol. Each animal was sensitized with 3 OVA-Alum i.p. injections 1 week apart (−21 days, −14 days, −7 days). Six days after the third injection (−1 day) each animal received either Bromelain (200 mg/kg) in 0.5 ml of phosphate buffered saline (PBS) or 0.5 ml PBS alone via gavage (orally). Treatment was administred twice daily (6–8 h apart) for 4 consecutive days. Animals were challenged with 1% OVA in saline for 1 h per day (0–3). All animals were sacrificed 12 h after the last treatment (day 3) and the primary AAD outcomes assessed.
Analytical Testing and Quality Control
Bromelain was extracted from mature pineapple stems with methanol and bound with ≤1% rice starch. Upon importation, the certificate of analysis of bromelain was independently verified for its purity (≥99%), identity (via FTIR spectrometer) and potency (with a GDU assay) (Table 1). Further analyses for quality and contamination were performed for aflatoxins, heavy metals, chemical solvents pesticides, herbicides and microbial contaminants. All samples were stored at 4°C in opaque containers and source samples were stored for future analysis of stability and degradation.
Table 1.
Bromelain Identity and Quality Control Data
| Bromelain quality control and analytical testing data | |||
|---|---|---|---|
| Item/test profile | Specification | Result | Method |
| Botanical—Pineapple | Ananas cosmosus | Ananas cosmosus | Visual |
| Plant parts used | Mature stem | Mature stem | Visual |
| Botanical extract | Bromelain (EC 3.4.22.31) | Bromelain (EC 3.4.22.31) | Methanol extraction |
| Identification | Standard match (Sigma) | Conforms to standard | FTIR, HPLC |
| Activity, potency | ≥2400 GDU/g | 2674 GDU/g | GDU assay |
| Solvent residues | |||
| Methanol | ≤3000 ppm | Negative | Gas chromatography |
| Ethanol | ≤5000 ppm | Negative | Gas chromatography |
| Acetone | ≤5000 ppm | Negative | Gas chromatography |
| Isopropanol | ≤5000 ppm | Negative | Gas chromatography |
| Methylene chloride | ≤600 ppm | Negative | Gas chromatography |
| Hexane | ≤290 ppm | Negative | Gas chromatography |
| Ethyl acetate | ≤5000 ppm | Negative | Gas chromatography |
| Microbial profile | Total plate count ≤3000/g | ≤50/g | |
| Mold | ≤300/g | ≤50/g | STVCA |
| E. Coli | Negative | Negative | STVCA |
| Salmonella | Negative | Negative | STVCA |
| Coliforms | ≤10/g | Negative | USP24p. 1814 |
| S. aureus | Negative | Negative | USP24p. 1814 |
| P. aeruginosa | Negative | Negative | USP24p. 1814 |
| Aflatoxin profile (G2, G1, B2, B1) | Aflatoxins ≤20 ppb | ≤1ppb | HPLC |
| Heavy metal profile | |||
| Aluminum | ≤1000 ppm | 16 ± 0.5 ppm | ICP–MS |
| Arsenic | ≤3ppm | ≤0.50 ± 0.5 ppm | ICP–MS |
| Lead | ≤10 ppm | 0.07 ± 0.05 ppm | ICP–MS |
| Cadmium | ≤3 ppm | ≤0.25 + 0.25 ppm | ICP–MS |
| Mercury | ≤2 ppm | ≤0.10 + 0.1 ppm | ICP–MS |
| Pesticide residues | CDFA | ||
| Organochlorines | ≤5 ppm | Negative | Gas and liquid |
| Organophosphates | ≤7 ppm | Negative | chromatography |
| Organonitrates | ≤5 ppm | Negative | coupled with mass |
| Carbamates | ≤5 ppm | Negative | spectrometry |
EC = enzyme classification; FTIR = Fourier transform infrared spectroscopy; HPLC = high-performance liquid chromatography; GDUs = gelatin dissolving units; STVCA = Soleris’ total viable count assay; ICP–MS = inductively coupled plasma – mass spectrometry; CDFA = California Department of Food and Agriculture.
Decreased Pulmonary Eosinophilia Following Oral Bromelain Administration
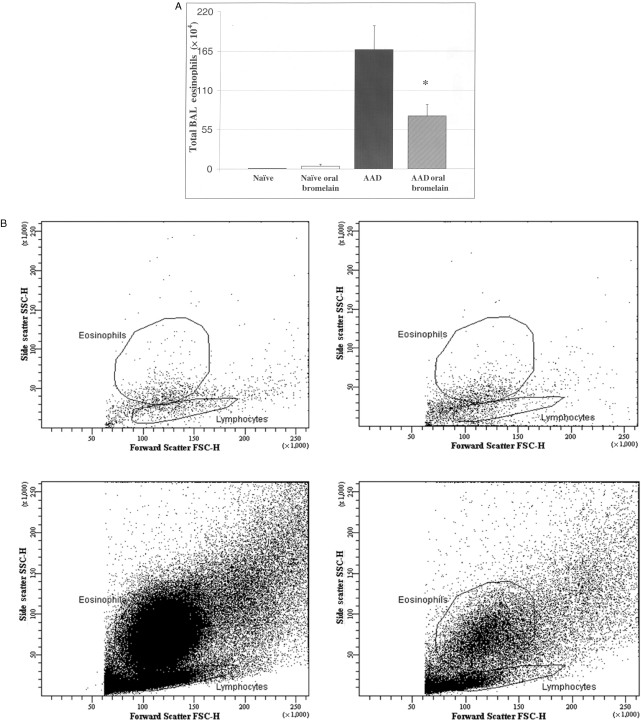
Total BAL eosinophils were significantly elevated in AAD mice (167 ± 33 × 104 cells; P ≤ 0.0001) as compared with naïve (1 ± 2 × 104 cells) or naïve oral bromelain-treated (4 ± 2 × 104 cells) mice (Fig. 2A). There was a significant reduction in total BAL eosinophils with bromelain treatment of AAD mice as compared with saline-treated AAD mice (P ≤ 0.02; Fig. 2A). Similar trends were noted on evaluation of BAL samples with flow cytometry (Fig. 2B). A representative FACS plot showing gated eosinophils on forward and side scatter from naïve saline-treated (upper left), naïve Bromelain-treated (upper right), AAD saline-treated (lower left), and AAD Bromelain-treated mice (lower right) are shown.
Figure 2.
The effect of oral Bromelain treatment on BAL eosinophils. Total BAL eosinophils were significantly elevated in AAD mice as compared to naïve or naïve oral Bromelain-treated mice (P ≤ 0.0001; A). There was a significant reduction in total BAL eosinophils with Bromelain treatment of AAD mice as compared to saline treated AAD mice. Similar trends were noted on evaluation of BAL samples with Flow cytometry (B). A representative FACS plot showing gated eosinophils on forward and side scatter from; naïve-saline treated (upper left), naïve-Bromelain treated (upper right), AAD-saline treated mice (lower left), and AAD-Bromelain treated mice (lower right) are shown. Statistical comparisons were made by un-paired t-test, *P ≤ 0.02; Data represent means ± SEM, n = 8 animals per group.
Bromelain Modulates Lung Lymphocytes during Acute Asthma
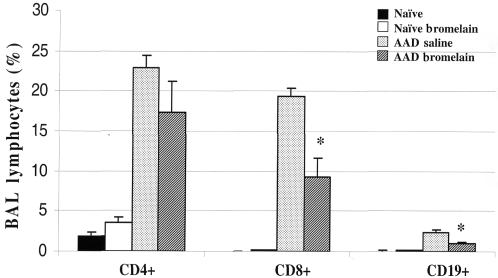
As compared with naïve and naïve bromelain-treated mice, AAD mice displayed significant increases in the percentage of BAL CD4+, CD8+ and CD19+ lymphocytes (Fig. 3). Bromelain-treated AAD mice resulted in significant reductions in CD8+ (19 ± 1 versus 9 ± 2; P ≤ 0.002), and CD19+ lymphocytes (2.4 ± 0.3 versus 2.4 ± 0.2; P ≤ 0.002) when compared with AAD-saline-treated mice. No significant changes in CD4+ lymphocytes were noted in similar comparisons.
Figure 3.
The effect of oral Bromelain treatment on BAL Lymphocytes. After 3 OVA aerosol exposures, there was a significant increase in the percentages of CD19 + B cells (A) CD8 + T cells (B) and CD4 + T cells (C) in the BAL of AAD mice, as compared to naïve controls. Oral Bromelain treatment significantly reduced CD19 + B cells (P ≤ 0.00003; A), and CD8 + T cells (P ≤ 0.00002; B). There was no change in the percentages of CD4 + T cells in the BAL between AAD and Bromelain treated AAD mice (P ≤ 0.095; C). Statistical comparisons were made by un-paired t-test, *P ≤ 0.0001; Data represent means + SEM, n = 8 animals per group.
Reduced IL-13 Levels in the BAL of Bromelain-treated Mice
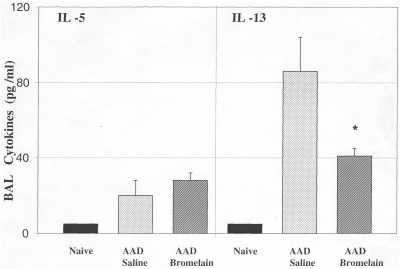
A significant decrease in IL-13 concentration was observed in bromelain-treated AAD mice as compared with the saline treated AAD mice (41 ± 12 versus 86 ± 18 pg/ml; Fig. 4). There were no significant changes in BAL IL-5 concentrations with oral bromelain treatment of AAD mice as compared with saline treated AAD mice (20 ± 4 versus 24 ± 2 pg/ml).
Figure 4.
The effect of oral Bromelain treatment on IL-5 and IL-13 concentrations in BAL. There were no significant changes in BAL IL-5 concentrations with oral Bromelain treatment of AAD mice as compared to saline treated AAD mice. A significant decrease in IL-13 concentration was observed in oral Bromealin treated AAD mice as compared to the saline treated AAD mice. Statistical comparisons were made by un-paired t-test, *P ≤ 0.04; Data represent means ± SEM, n = 8 animals per group.
Shifts in Airway Responsiveness Following Bromelain Treatment
Airway responses to methacholine were assessed by whole-body barometric plethysmography using the Buxco system (Sharon, CT, USA). At baseline, before bromelain or saline treatment (open circles), both groups of animals had similar overall responsiveness (Fig. 5A; P = 0.50) and sensitivity (Fig. 5B) to methacholine (Penh-2 values 48 ± 13 mg/ml in the bromelain treatment versus 64 + 15 mg/ml in saline treatment group; P = 0.47). At AAD (filled circles), saline-treated mice tended to be more generally responsive to methacholine (P = 0.069; A) and were twice as sensitive to methacholine (P = 0.01; B). No change occurred in bromelain-treated 3-day OVA mice terms of overall responsiveness (P = 0.52; A) or methacholine sensitivity (P = 0.45; B).
Figure 5.
The effect of oral Bromelain treatment on airway reactivity and methacholine sensitivity. At baseline (open circles), Bromelain-treated and saline-treated mice had similar reactivity (P = 0.50) and sensitivity to methacholine (p2 values 48 ± 13 mg/ml in Bromelain treated versus 64 ± 15 mg/ml in saline treated; P = 0.47). After 3 OVA aerosol exposures (filled circles), saline treated mice tended to be more reactive to methacholine (P = 0.069; A) and were twice as sensitive to methacholine (P = 0.01; B) whereas no change occurred in Bromelain treated mice in reactivity (P = 0.52; A) and sensitivity (P = 0.45; B). Comparisons were made by paired t-test and repeated measures ANOVA; n = 7–8 animals per group.
The Effect of Oral Bromelain Treatment on Lung Pathology
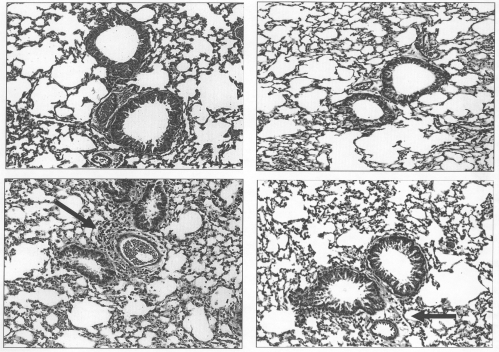
Histological evaluations and Pathology Scores (PS), were performed on unmanipulated, uninflated formalin-fixed lungs from separate groups (n = 4) of naïve, naïve bromelain-treated, AAD saline- and AAD bromelain-treated mice stained with hematoxylin and eosin (H & E), Mallory's trichrome and periodic acid-schiff. The H & E stain has been included for comparison (Fig. 6). As demonstrated in the top panels, no evidence of histological injury was noted in naïve animals (A) or oral bromelain-treated naïve animals (B). Three day AAD mice develop mild pathologic changes, characterized by perivascular and peribronchial (arrows) inflammation which includes cellular infiltrates (lymphocytes, plasma cells and eosinophils). These changes are present in the 3-day AAD saline-treated mice (C). Oral bromelain-treated AAD mice (D) appeared to have minimal histological injury. However, upon statistical comparisons of pathological scoring by four reviewers, there were no statistical changes in pathological scoring.
Figure 6.
The effect of oral Bromelain treatment on lung pathology. Top panels: No evidence of histological injury was noted in naïve animals (A) or oral Bromelain treated naïve animals (B). Lower panels: As previously described, 3 day AAD mice develop pathological changes characterized by perivascular and peribronchiolar infiltration of lymphocytes, eosinophils and plasma cells (C), which was reduced in oral Bromelain treated AAD mice (D).
Discussion
The current study is a continued characterization of the use of bromelain, a cysteine protease extracted from pineapple, in asthma. We have previously shown that the i.p. administration of bromelain exerts anti-inflammatory effects in an OVA-induced murine model of AAD (23). The present study was designed to determine the efficacy of oral bromelain treatment in a murine model of asthma. The data presented in this study demonstrate that oral bromelain therapy can attenuate inflammation in a well-characterized murine model of asthma (23–25) and support the existing literature that oral enzymes are bioavailable and efficacious for the treatment of inflammatory conditions (14,26–30).
The quality control and analytical verification of botanical products such as bromelain remain a concern as the use of these products continues to increase (6,31–33). Many botanicals are now undergoing intensive testing to ensure safety and quality for consumers and reproducibility for basic science and clinical research (34–36). The bromelain utilized for these experiments was obtained from a professional natural product manufacturing company that produces products for clinical use. The bromelain extract was independently evaluated for purity, potency and contamination to ensure quality and reproducibility (Table 1). There were no solvent residues, microbial contamination, or greater than acceptable heavy metals or pesticide residues. There was no observed toxicity of oral bromelain treatment in either naive or AAD animals (as assessed by body weight, BAL protein concentrations and lung pathology).
The presence of increased BAL eosinophils is a hallmark of asthma. Compared with saline-treated AAD mice, bromelain-treated AAD mice had significantly reduced lung eosinophilia as observed by BAL differential and flow cytometry (Fig. 2). This observed effect of bromelain treatment on eosinophils may be due to a reduction in lymphocyte populations or a decrease in the Th2 cytokines that are responsible for the recruitment of eosinophils into the lung during asthma. Therefore, we examined the effect of oral bromelain treatment on Th2 cytokines (IL-5, IL-13) and lymphocyte subsets (CD4+, CD8+ and CD19+). Bromelain treatment reduced the percentages of CD8+ T cells and CD19+ B cells in the BAL (Fig. 3) and BAL IL-13 concentrations (Fig. 4). CD19+ B cells can act as primary antigen presenting cells in Th2 conditions such as asthma. A bromelain-mediated reduction in this subset may also diminish T cell driven inflammation thereby inhibiting the progression of allergic disease. Though, primarily a CD4+ T cell mediated condition, CD8+ T cells which secrete Th2 cytokines, have also been implicated in asthma (37). A reduction in these CD8+ T cells via bromelain may have additional protective effects by modulating lymphocyte-mediated cytotoxic asthma mechanisms such as granzymes or perforins. The effects of bromelain on the lymphocyte subpopulations were observed locally as represented by the BAL and not systemically (in peripheral blood or spleen, data not shown) suggesting that bromelain activity is focused at sites of inflammation.
Interestingly, the percentages of CD4+ T cells were not significantly reduced. This may be due to bromelain altering the ratio of CD4+ T cell subsets, mainly the effector cells (CD4+CD25−Foxp3−) which cause airway inflammation, to regulatory cells (CD4+CD25+ Foxp3+) which suppress it. Bromelain may enhance regulatory cell numbers and their function leading to the resolution of disease. The characterization of the effects of bromelain on these regulatory T cells is currently being investigated in our laboratory.
In addition, we examined the effects of bromelain treatment in asthma on both pulmonary function (PFT) and pathology. As a measure of pulmonary function, both saline and bromelain treatment groups were challenged to increasing doses (0–300 mg/ml) of methacholine, a common lung smooth muscle irritant, for assessment of airway reactivity and sensitivity (Fig. 5). During the baseline challenge, there was no difference between the groups. After OVA aerosols, the saline-treated group became more sensitive to methacholine whereas the bromelain group did not vary from baseline. This protective effect on lung function correlates well with the observed reductions in eosinophils, lymphocytes and cytokines that have been implicated in the pathogenesis of airway reactivity (38–41). In regards to lung pathology (Fig. 6), bromelain-treated mice appeared to qualitatively have reduced pulmonary injury as compared with saline-treated controls; however, independent blinded scoring did not reveal significant differences between the groups.
Future studies will evaluate the effects of bromelain in a 7–10 day AAD model with more advanced disease and bromelain administration will be varied after the onset of asthma (3 days of aerosol) to better simulate a clinical presentation and given during or before OVA sensitization to evaluate its effect on the priming response. Experiments are currently being designed to evaluate the effect of bromelain treatment on key immunoregulatory CD4+ T cell subsets such as Foxp3+ T regulatory cells which are known to modulate allergic responses (42,43) and to measure the retained enzymatic activity of bromelain in the serum of naïve and AAD mice after oral administration. In conclusion, the results obtained from this study suggest that the oral administration of bromelain attenuates inflammation in an OVA-induced murine model of asthma. These results may translate well in clinical trials.
Acknowledgments
We thank the technical assistance of Dr Enrico P. Liva of Vital Nutrients for providing the bromelain extract and quality control expertise and Dr Charlie Wang for carrying out independent analysis for bromelain identification, quality and purity. This work was supported by sponsored research grants: NIH/NCCAM FG32-AT001569; NIH/AI R01 HL-43573.
References
- 1.Mickleborough TD, Lindley MR, Ionescu AA, et al. Protective effect of fish oil supplementation on exercise-induced bronchoconstriction in asthma. Chest. 2006;129:39–49. doi: 10.1378/chest.129.1.39. [DOI] [PubMed] [Google Scholar]
- 2.Mickleborough TD. Dietary omega-3 polyunsaturated fatty acid supplementation and airway hyperresponsiveness in asthma. J Asthma. 2005;42:305–14. doi: 10.1081/JAS-62950. [DOI] [PubMed] [Google Scholar]
- 3.Shaheen SO, Sterne JA, Thompson RL, et al. Dietary antioxidants and asthma in adults: population-based case-control study. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1823–8. doi: 10.1164/ajrccm.164.10.2104061. [DOI] [PubMed] [Google Scholar]
- 4.Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–17. doi: 10.1016/j.jaci.2004.12.1139. quiz 1118. [DOI] [PubMed] [Google Scholar]
- 5.Spector SL, Surette ME. Diet and asthma: has the role of dietary lipids been overlooked in the management of asthma? Ann Allergy Asthma Immunol. 2003;90:371–377. doi: 10.1016/S1081-1206(10)61817-0. quiz 377–8, 421. [DOI] [PubMed] [Google Scholar]
- 6.Brownie S. The development of the US and Australian dietary supplement regulations. What are the implications for product quality? Complement Ther Med. 2005;13:191–8. doi: 10.1016/j.ctim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Hijazi N, Abalkhail B, Seaton A. Diet and childhood asthma in a society in transition: a study in urban and rural Saudi Arabia. Thorax. 2000;55:775–9. doi: 10.1136/thorax.55.9.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaminogawa S, Nanno M. Modulation of immune functions by foods. Evid Based Complement Alternat Med. 2004;1:241–50. doi: 10.1093/ecam/neh042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes P, Powell-Griner E, McFann K, Nahin R. CDC Advance Data Report 2004. 2002. Complementary and alternative medicine use among adults: United States. [PubMed] [Google Scholar]
- 10.Miller AL. The etiologies, pathophysiology, and alternative/complementary treatment of asthma. Altern Med Rev. 2001;6:20–47. [PubMed] [Google Scholar]
- 11.Gupta I, Gupta V, Parihar A, et al. Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. Eur J Med Res. 1998;3(11):511–4. [PubMed] [Google Scholar]
- 12.Patwardhan B, Gautam M. Botanical immunodrugs: scope and opportunities. Drug Discov Today. 2005;10:495–502. doi: 10.1016/S1359-6446(04)03357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guggi D, Bernkop-Schnurch A. Improved paracellular uptake by the combination of different types of permeation enhancers. Int J Pharm. 2005;288:141–50. doi: 10.1016/j.ijpharm.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Hale LP, Greer PK, Trinh CT, et al. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin Immunol. 2005;116:135–42. doi: 10.1016/j.clim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Shinto L, Calabrese C, Morris C, et al. Complementary and alternative medicine in multiple sclerosis: survey of licensed naturopaths. J Altern Complement Med. 2004;10:891–7. doi: 10.1089/acm.2004.10.891. [DOI] [PubMed] [Google Scholar]
- 16.Sprava L. Effect of phlogenzym in long-term treatment of patients with multiple sclerosis; 2003;Apr–Jun:109–13; Mialovyts’ka OA. [PubMed] [Google Scholar]
- 17.Bellavite P, Ortolani R, Conforti A. Immunology and homeopathy. 3. Experimental studies on animal models. Evid Based Complement Alternat Med. 2006;3:171–86. doi: 10.1093/ecam/nel016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mynott TL, Engwerda CR, Peek K. Component of bromelain. United States: Mynott, TL; 2004. [Google Scholar]
- 19.Mynott TL, Ladhams A, Scarmato P, et al. Bromelain, from pineapple stems, proteolytically blocks activation of extracellular regulated kinase-2 in T cells. J Immunol. 1999;163:2568–75. [PubMed] [Google Scholar]
- 20.Munzig E, Eckert K, Harrach T, et al. Bromelain protease F9 reduces the CD44 mediated adhesion of human peripheral blood lymphocytes to human umbilical vein endothelial cells. FEBS Lett. 1994;351:215–8. doi: 10.1016/0014-5793(94)00860-4. [DOI] [PubMed] [Google Scholar]
- 21.Hale LP, Haynes BF. Bromelain treatment of human T cells removes CD44, CD45RA, E2/MIC2, CD6, CD7, CD8, and Leu 8/LAM1 surface molecules and markedly enhances CD2-mediated T cell activation. J Immunol. 1992;149:3809–16. [PubMed] [Google Scholar]
- 22.Hale LP, Greer PK, Sempowski GD. Bromelain treatment alters leukocyte expression of cell surface molecules involved in cellular adhesion and activation. Clin Immunol. 2002;104:183–90. doi: 10.1006/clim.2002.5254. [DOI] [PubMed] [Google Scholar]
- 23.Secor ER, Jr, Carson WFt, Cloutier MM, et al. Bromelain exerts anti-inflammatory effects in an ovalbumin-induced murine model of allergic airway disease. Cell Immunol. 2005;237:68–75. doi: 10.1016/j.cellimm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yiamouyiannis CA, Schramm CM, Puddington L, et al. Shifts in lung lymphocyte profiles correlate with the sequential development of acute allergic and chronic tolerant stages in a murine asthma model. Am J Pathol. 1999;154:1911–21. doi: 10.1016/S0002-9440(10)65449-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kabbur PM, Carson WFt, Guernsey L, et al. Interleukin-10 does not mediate inhalational tolerance in a chronic model of ovalbumin-induced allergic airway disease. Cell Immunol. 2006;239(1):67–74. doi: 10.1016/j.cellimm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Klein G, Kullich W, Schnitker J, et al. Efficacy and tolerance of an oral enzyme combination in painful osteoarthritis of the hip. A double-blind, randomised study comparing oral enzymes with non-steroidal anti-inflammatory drugs. Clin Exp Rheumatol. 2006;24:25–30. [PubMed] [Google Scholar]
- 27.White RR, Crawley FE, Vellini M, et al. Bioavailability of 125I bromelain after oral administration to rats. Biopharm Drug Dispos. 1988;9:397–403. doi: 10.1002/bod.2510090408. [DOI] [PubMed] [Google Scholar]
- 28.Castell JV, Friedrich G, Kuhn CS, et al. Intestinal absorption of undegraded proteins in men: presence of bromelain in plasma after oral intake. Am J Physiol. 1997;273(1 Pt 1):G139–46. doi: 10.1152/ajpgi.1997.273.1.G139. [DOI] [PubMed] [Google Scholar]
- 29.Gaspani L, Limiroli E, Ferrario P, et al. In vivo and in vitro effects of bromelain on PGE(2) and SP concentrations in the inflammatory exudate in rats. Pharmacology. 2002;65(2):83–6. doi: 10.1159/000056191. [DOI] [PubMed] [Google Scholar]
- 30.Martynenko AV. Wobenzym in the combined pathogenetic therapy of chronic urethrogenic prostatitis. Lik Sprava. 1998;6:118–20. [PubMed] [Google Scholar]
- 31.Gollin MA. New rules for natural products research. Nat Biotechnol. 1999;17:921–2. doi: 10.1038/12921. [DOI] [PubMed] [Google Scholar]
- 32.Crowley R, FitzGerald LH. The impact of cGMP compliance on consumer confidence in dietary supplement products. Toxicology. 2006;221:9–16. doi: 10.1016/j.tox.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Larimore WL, O'Mathuna DP. Quality assessment programs for dietary supplements. Ann Pharmacother. 2003;37:893–8. doi: 10.1345/aph.1D031. [DOI] [PubMed] [Google Scholar]
- 34.Dogheim SM, Ashraf el MM, Alla SA, et al. Pesticides and heavy metals levels in Egyptian leafy vegetables and some aromatic medicinal plants. Food Addit Contam. 2004;21:323–30. doi: 10.1080/02652030310001656361. [DOI] [PubMed] [Google Scholar]
- 35.Durgnat JM, Heuser J, Andrey D, et al. Quality and safety assessment of ginseng extracts by determination of the contents of pesticides and metals. Food Addit Contam. 2005;22:1224–30. doi: 10.1080/02652030500199439. [DOI] [PubMed] [Google Scholar]
- 36.Jiang B, Kronenberg F, Nuntanakorn P, et al. Evaluation of the botanical authenticity and phytochemical profile of black cohosh products by high-performance liquid chromatography with selected ion monitoring liquid chromatography-mass spectrometry. J Agric Food Chem. 2006;54:3242–53. doi: 10.1021/jf0606149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bratke K, Haupt F, Kuepper M, et al. Decrease of cytotoxic T cells in allergic asthma correlates with total serum immunglobulin E. Allergy. 2006;61:1351–7. doi: 10.1111/j.1398-9995.2006.01192.x. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Saito H, Crawford L, et al. Haemopoietic mechanisms in murine allergic upper and lower airway inflammation. Immunology. 2005;114:386–96. doi: 10.1111/j.1365-2567.2005.02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito H, Matsumoto K, Denburg AE, et al. Pathogenesis of murine experimental allergic rhinitis: a study of local and systemic consequences of IL-5 deficiency. J Immunol. 2002;168:3017–23. doi: 10.4049/jimmunol.168.6.3017. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, McCusker CT. Interleukin-13-dependent bronchial hyper-responsiveness following isolated upper-airway allergen challenge in a murine model of allergic rhinitis and asthma. Clin Exp Allergy. 2005;35:1104–11. doi: 10.1111/j.1365-2222.2005.02301.x. [DOI] [PubMed] [Google Scholar]
- 41.Ohkawara Y, Lei XF, Stampfli MR, et al. Cytokine and eosinophil responses in the lung, peripheral blood, and bone marrow compartments in a murine model of allergen-induced airways inflammation. Am J Respir Cell Mol Biol. 1997;16:510–20. doi: 10.1165/ajrcmb.16.5.9160833. [DOI] [PubMed] [Google Scholar]
- 42.Stock P, DeKruyff RH, Umetsu DT. Inhibition of the allergic response by regulatory T cells. Curr Opin Allergy Clin Immunol. 2006;6((1)):12–16. doi: 10.1097/01.all.0000200502.69672.44. [DOI] [PubMed] [Google Scholar]
- 43.Vojdani A, Erde J. Regulatory T Cells, a potent immunoregulatory target for CAM researchers: modulating allergic and infectious disease pathology (II) Evid Based Complement Alternat Med. 2006;3:209–15. doi: 10.1093/ecam/nel020. [DOI] [PMC free article] [PubMed] [Google Scholar]