Abstract
Since the range of comfort zone or thermo neutral zone of domestic chickens is narrow, they become easily susceptible to heat and cold environmental stress. We evaluated Brahma Rasayana (BR) supplementation on concentrations of certain oxidative stress markers associated with heat stress. A total of 48 egg type male chickens of local strain were divided into six groups (n = 8) for the study. Three groups were fed with BR orally at the rate of 2 g/kg bw daily for 10 days prior to and during the period of experiment. Two of the four groups that were exposed to heat stress (HST i.e. to a temperature of 40 ± 1°C and relative humidity of 80 ± 5% in an environmental chamber) for 4 h daily for 5 or 10 days, received BR orally. The other two groups remained as BR treated and untreated non-heat stressed (NHST) controls. There was a significant (P < 0.05) increase in the activities of antioxidant enzymes in blood such as catalase (CAT) and superoxide dismutase (SOD), as well as liver CAT, glutathione peroxidase (GPX) and glutathione reductase (GR) in NHST-BR treated and HST-BR treated (both 5 and 10 days) chickens when compared with untreated controls. A great deal of significant (P < 0.05) variations were seen in serum and liver reduced glutathione (GSH) concentration in NHST-BR treated and HST-BR treated (both 5 and 10 days) chickens. Serum and liver lipid peroxidation levels were found to be significantly (P < 0.05) higher in HST-untreated (both 5 and 10 days) chickens when compared with other groups. Thus BR supplementation during HST brings about enhanced action of enzymatic and non-enzymatic antioxidants, which nullified the undesired side effects of free radicals that are generated during HST.
Keywords: antioxidant enzymes, Brahma Rasayana, chickens, heat stress
Introduction
Under the routine production conditions various types of stress are experienced by chicken such as heat/cold, transport, pre-slaughter holding, etc. Both high and low environmental temperatures stimulate the hypothalamo-hypophyseal-adrenocortical axis which may alter susceptibility of animals including the chicken to infectious diseases, resulting in production loss. At temperature above or below thermo neutral zone, corticosteroid secretion increases in response to stress (1). By decreasing synthesis and secretion of corticosteroids, vitamin C alleviates the negative side effects of stress. Although poultry can synthesize vitamin C it becomes inadequate under stressful conditions such as low or high environmental temperature, high humidity, and high egg production rate and parasite infestation (2). To overcome or to alleviate the harmful effects of heat stress, heat tolerance in birds has to be increased. The physiological mechanisms underlying the acclimation induced heat tolerance in birds has yet to be elucidated in detail.
Antioxidants play a major role in protecting cells from the actions of reactive oxygen species (ROS) by reducing chemical radicals and preventing the process of lipid peroxidation (3,4). Earlier reports suggested that environmental stress diminishes in vivo antioxidant status (5,6). Lower plasma concentrations of antioxidant vitamins such as vitamin C, E and folic acid, and minerals like zinc and chromium has been inversely correlated to increased oxidative damage in stressed poultry (7,8). Vitamin E is a crucial antioxidant that protects unsaturated fatty acids and its concentration in tissues is inversely correlated to lipid peroxidation (9). Studies have shown that supplementation of Vitamin C, E and A, zinc and chromium in feed, can attenuate the side effects inflicted by extreme environmental stress (10–12).
Ayurveda, the traditional Indian system of medicine has given great emphasis to the promotion of health. Rasayanas are a group of non-toxic single or polyherbal preparation commonly used in indigenous medical practice in India to improve the health and longevity. Rasayanas improve memory, intelligence, and youthfulness, good luxture, complexion and efficiency (13–15). Brahma Rasayana (BR) is a non-toxic polyherbal preparation, which is made by the extracts from plants, claimed to be a potent immunomodulator in cancer patients undergoing chemotherapy and radiation therapy (16). Goose berry (Emblica officinalis) and Indian gall nut (Terminalia chebula) are the two major ingredients of BR accounting more than 75% w/w of the preparation (17). Earlier studies revealed that BR was found to be potent oxygen free radical scavenger in vitro and in vivo models (18) and also found to ameliorate the side effects in mice that undergo radiation and cyclophosphamide treatment (19–21). Other than BR, a number of standard Rasayanas such as Narasimha Rasayana, Ashwagandha Rasayana, Amruthaprasham and Chyavanprash have been reported to inhibit the oxidative stress and spontaneous mutations (22). Recently BR has been correlated with the protective effect against prostate tumor growth and lung metastasis in rats (23).
In the past two decades a number of Ayurvedic herbal preparations have been extensively used in poultry industry in India. Preparations like Livol and Zeestress have been found to possess hepatoprotective property (24), immunopotentiative action in vaccinated birds (25) and reducing the stress in intensively housed chickens (26) as well as in layer chickens during summer (27).
The present experiment was performed with objectives to determine the antioxidant status of chickens subjected to heat stress (high temperature–high humidity environmental condition), and the effect of BR supplementation in achieving heat tolerance at an early date.
Methods
Birds
A total of 48 male day-old (local strain: Gramapriya) egg type domestic chicken (Gallus domesticus) were procured from University Poultry Farm, College of Veterinary and Animal Sciences, Kerala Agricultural University, Mannuthy and reared in a battery brooder. They were fed with commercial starter feed for the first 4 weeks of life. At the end of brooding life, chicks were fed with commercial adult layer mash till they attained 1 kg body weight. Weekly body weight gain of all birds was recorded from the day of hatch till the completion of experiment. Before commencing the work, permission from Institutional Animal Ethics Committee was obtained.
Composition of BR
BR is a mixture of Emblica officinalis (20%), Terminalia chebula (6.67%), Urarira pitca (0.4%), Desmodium gangeticum (0.4%), Gmelina arborea (0.4%), Solanum nigrum (0.4%), Tribulus terrestris (0.4%), Aegle marmelos (0.4%), Premna tomentosa (0.4%), Stereospermum suvaeolens (0.4%), Sida rhombilfolia (0.4%), Boerhaavia diffusa (0.4%), Ricinus communis (0.4%), Vigna vexilata (0.4%), Phaseolus adenanthus (0.4%), Asperagus racemosus (0.4%), Holostemma annulare (0.4%), Leptadenia reticulate (0.4%), Desmostachya bipinnata (0.4%), Saccharum officinarum (0.4%), Oryza malampuzhensis (0.4%), Cinnamomum iners (0.16%), Elettaria cardamomum (0.16%), Cyperus rotundus (0.16%), Curcuma longa (0.16%), Piper longum (0.16%), Aquilaria agallocha (0.16%), Santalum album (0.16%), Centella asciatica (0.16%), Mesua ferrea (0.16%), Clitoria ternate (0.16%), Acorus calamus (0.16%), Scirpus crossus (0.16%), Glycyrrhiza glabra (0.16%) and Embelia ribes (0.16%).
Method of Preparing of BR
BR was prepared as per standard recommended procedures (28,29) and purchased from Vaidyaratnam Oushadhasala, Ollur, Thrissur, Kerala. In short, the method of preparation includes: 19 items from U. pitca to O. malampuzhensis were made into small pieces and washed well. 16 part of water was added to the total quantity of drugs and allowed to boil to get one fourth of the original volume. The seeds from fruit of E. officinalis and T. chebula were removed and the pulp was roasted well by adding sufficient quantity of ghee and seasame oil. Then sufficient quantity of sugar was added to the above decoction to get a paste like form. The rest of 14 items from C. iners to E. ribes were cleaned and dried well under shade and made into fine powder and this powder was mixed with above paste and stirred well. When the preparation came to normal temperature, sufficient quantity of honey was added, mixed well and stored at room temperature.
Administration of BR
The oral administration of BR at various dose levels ranging from 0.5 to 6.0 g/kg bw of bird, daily for 20 days did not induce any drug-related toxicity. No significant weight loss or gain was observed reflecting the influence of BR administration on the well being of birds. Even at a highest concentration of 6.0 g/kg bw, the birds showed no visible toxicity as measured by loss of appetite, lack of movement and alertness. Therefore it was concluded that BR, as a non-toxic herbal preparation as BR administration did not influence weekly body weight gain in the treated birds. However, for all experiments the dose used was 2.0 g/kg bw which was in par with the dose administered to mice i.e. 50 mg for mouse of 25 g bw (30).The required quantity of BR was made into suspension using warm water (five parts) and it was mixed with about 30 g of poultry feed (one-third of total daily ration) and fed to the experimental birds as the first meal of the day.
Heat Stress Regime
The protocol followed in this study was a combination of two earlier reports (31,32). A controlled environmental unit with a holding capacity of 12 birds at a time in six chambers (area 1800 cm2/chamber), with provisions for adjusting humidity and temperature was used. Chamber temperature of 40 ± 1°C and relative humidity (RH) of 80 ± 5% was chosen for the present study. To the above conditions chickens were exposed for four consecutive hours per day for 5 or 10 days. During the period of heat stress chickens were neither provided with drinking water nor feed, in the chamber.
Experimental Design
Group I: It represented non-heat stressed (NHST) and untreated birds. Distilled water (DW) was orally administered for 20 days and reared randomly under ambient temperature of 30 ± 1°C and 65% RH.
Group II: NHST and BR treated (2 g/kg bw p.o) for 20 days. Birds were reared under similar conditions of temperature and humidity as group I.
Group III: Heat stressed (HST) at 40 ± 1°C and RH of 80 ± 5% for four consecutive hours per day for 5 days. DW was orally administered for 15 days.
Group IV: HST at 40 ± 1°C and RH of 80 ± 5% for four consecutive hours per day for 5 days and BR treated (2 g/kg bw p.o) for 15 days (10 days prior to and 5 days during the experimental period of heat exposure).
Group V: HST at 40 ± 1°C and RH of 80 ± 5% for four consecutive hours per day for 10 days. DW was orally administered for 20 days.
Group VI: HST at 40 ± 1°C and RH of 80 ± 5% for four consecutive hours per day for 5 days and BR treated (2 g/kg bw p.o) for 20 days (10 days prior to and 10 days during the experimental period of heat exposure).
Blood and Tissue Collections from Birds
Blood samples (3 ml) were collected from wing veins of birds with and without anticoagulant (heparin) from group III and V after 5 days of HST, and from birds of group IV and VI after 10 days of HST. Blood collection was done on day 20, from all birds of group I and II controls. Immediately after collection, serum was separated, aliquoted and used for assays. On the same day of blood collection, birds were sacrificed by cervical dislocation; liver was excised out and thoroughly washed in ice cold saline (0.9%). Liver was perfused with ice cold saline via portal vein before homogenization (33). Liver homogenate (10%) was made in ice cold Tris–HCl buffer (pH 7.4) and cytosolic sample of liver homogenate was obtained by centrifuging at 10,000 g for 30 min at 4°C.
Biochemical Analysis
Catalase (CAT) activity in fresh blood (erythrocytes) and liver was assessed by the decomposition of hydrogen peroxide; according to Aebi's (34) method and activities were expressed as Kat/g Hb and units/mg protein. Superoxide dismutase (SOD) activity in fresh blood (erythrocytes) and liver was determined by photoreducion of nitroblue tetrazolium (NBT) by McCord and Fridovich (35) method. One unit of SOD activity was defined as the amount of protein that inhibits the rate of reduction of NBT by 50% and expressed as units/g Hb and units/mg protein. Serum and liver reduced glutathione (GSH) content were determined by its reaction with 5-5′dithiobis (2-nitrobenzoic acid), according to Moron et al. (36) method and the concentration in serum and liver were expressed as nmol/ml and nmol/mg protein, respectively. Liver glutathione peroxidase (GPX) activity was assessed by degradation of hydrogen peroxide (H2O2) in the presence of reduced glutathione, according to Paglia and Valentine's (37) method and the activity expressed as units/mg protein. Liver cytosolic glutathione reductase (GR) activity determined by the amount of NADPH consumed in the conversion of oxidized glutathione (GSSG) to GSH by following Racker's (38) method and the activity were expressed as nanomole of NADPH consumed/min/mg protein. Serum and liver lipid peroxidation (LPO) levels were assessed by determining small amounts of malondialdehyde (MDA) produced during peroxidation, following Ohkawa et al. (33) method and the concentration in serum and liver were expressed as nanomole of MDA formed per millilitre and per milligram protein. Protein concentration in liver was estimated by Lowry's (39) method and expressed in terms of milligram protein.
Statistical Analysis
Data were expressed as mean ± SE. Statistical analysis was done by one-way classification of ANOVA followed by Duncan's multiple range test (DMRT) (40). P < 0.05 was considered as significant.
Results
Body Weight Gain Reduced by Heat Stress
During the period of heat stress, untreated birds showed significant reduction (>10%) in their body weight when compared with controls and treated birds, while, BR administration did not influence body weight and performance in treated birds.
Heat Stress Influenced Levels of Blood Antioxidant Enzymes
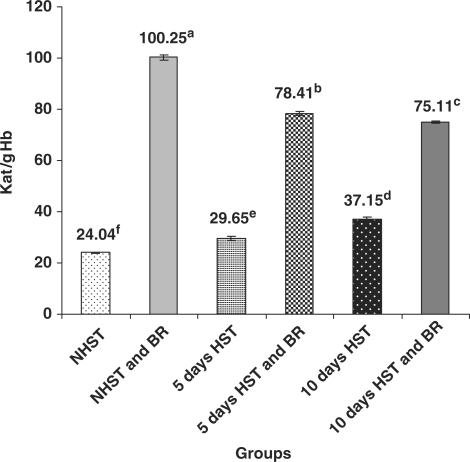
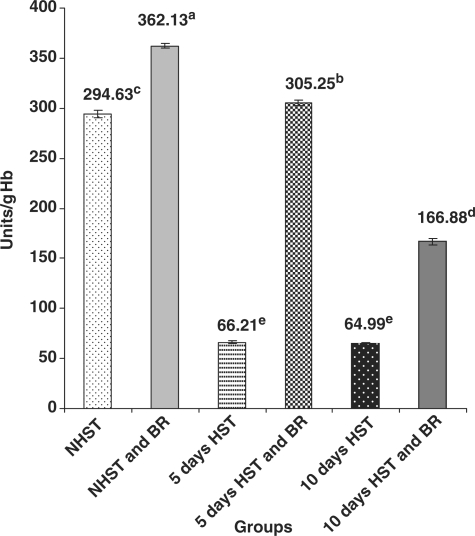
Untreated birds belonging to Group III (5 days HST) and Group V (10 days HST) exhibited significantly higher (P < 0.05) activity of blood erythrocyte CAT (Fig. 1) and significantly low (P < 0.05) activity of blood erythrocyte SOD (Fig. 2) when compared with NHST -untreated controls (Group I).
Figure 1.
Effect of heat stress on blood CAT activity in egg type male chicken (n = 8). Values are each mean ± SE, ANOVA followed Duncan's multiple range test, a–fP < 0.05; Means having same superscripts does not differ significantly at 5% level.
Figure 2.
Influence of heat stress on blood SOD activity in egg type male chicken (n = 8). Values are each mean ± SE, ANOVA followed Duncan's multiple range test, a–eP < 0.05; means having same superscripts does not differ significantly at 5% level.
Untreated Birds Showed Increase in Non-Enzymatic Antioxidants During Heat Stress
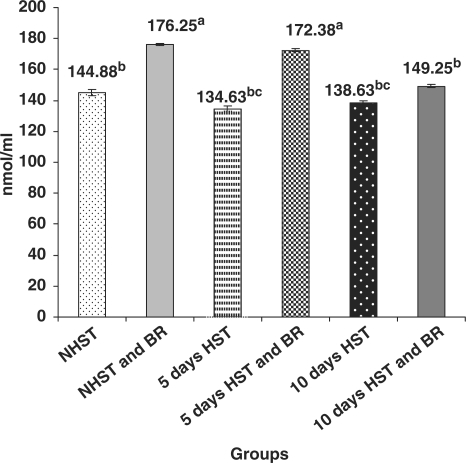
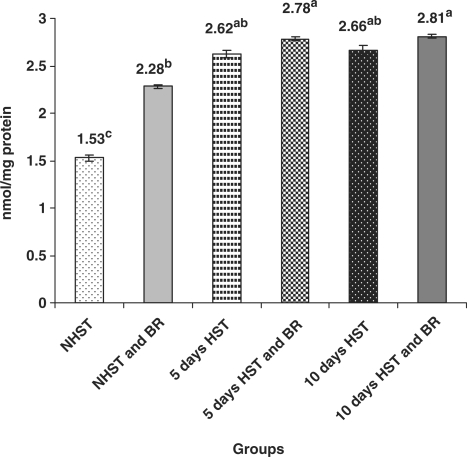
Untreated birds of Group III (5 days HST) and Group V (10 days HST) exhibited non-significant differences with respect to serum GSH level (Fig. 3) while liver GSH level showed a significant increase (P < 0.05) when compared with NHST-untreated controls (Fig. 4).
Figure 3.
Influence of heat stress on serum GSH content in egg type male chicken (n = 8). Values are each mean ± SE, ANOVA followed Duncan's multiple range test, abcP < 0.05; means having same superscripts does not differ significantly at 5% level.
Figure 4.
Influence of heat stress on liver GSH content in egg type male chicken (n = 8). Values are each mean ± SE, ANOVA followed Duncan's multiple range test, abcP < 0.05; means having same superscripts does not differ significantly at 5% level.
Heat Stress Damage to the Liver Averted in Treated Birds
Untreated birds of Group III (5 days HST) and Group V (10 days HST) showed significant increase (P < 0.05) in the activities of liver CAT, SOD and GPX when compared with NHST-untreated controls (Table 1). However there was a significantly lowered (P < 0.05) activity of GR in 5 days and 10 days HST-untreated birds when compared with NHST-untreated controls (Table 1).
Table 1.
Influence of heat stress on activities of liver CAT, GPX, SOD and GR in liver of egg type male chicken (n = 8)
| Groups | Liver CAT (U/mg protein) | Liver SOD (U/mg protein) | Liver GPX (U/mg protein) | Liver GR (nmol of NADPH consumed/min/mg protein) |
|---|---|---|---|---|
| Group I | 23.19f ± 0.29 | 132.88f ± 1.07 | 9.20e ± 0.03 | 504.75b ± 2.09 |
| Group II | 35.90c ± 0.42 | 184.00c ± 0.80 | 12.65c ± 0.10 | 1052.13a ± 5.46 |
| Group III | 31.89d ± 0.34 | 170.13e ± 2.50 | 10.23d ± 0.04 | 266.00d ± 2.58 |
| Group IV | 59.35b ± 0.42 | 201.00b ± 1.47 | 22.79a ± 0.23 | 504.88b ± 2.33 |
| Group V | 27.64e ± 0.34 | 177.88d ± 1.86 | 9.23e ± 0.04 | 215.99e ± 1.49 |
| Group VI | 64.28a ± 0.74 | 210.75a ± 2.36 | 15.54b ± 0.10 | 364.13c ± 3.46 |
Values are each mean ± SE, ANOVA followed Duncan's multiple range test, a–fP < 0.05; means having same superscripts does not differ significantly at 5% level.
BR Improved Enzymatic and Non-Enzymatic Antioxidants in Blood and Liver
When compared with HST-untreated birds (group III and V), BR treated birds (2 g/kg bw p.o for 15 or 20 days) of NHST and HST groups (groups II, IV and VI) exhibited significantly increased (P < 0.05) activities of erythrocyte CAT (3–4-fold) (Fig. 1), blood erythrocyte SOD (3–6-fold) (Fig. 2) and levels of serum GSH (10–30% increase) (Fig. 3). However, no such significant differences were encountered between NHST/HST-BR treated and HST-untreated groups (Fig. 4) with regard to liver GSH level. It was also observed that NHST/HST-BR treated groups (both 5 and 10 days) showed significantly higher (P < 0.05) activities/levels of enzymatic and non-enzymatic antioxidants when compared with NHST-untreated groups of birds (group I controls). Similarly almost 1.5–2-fold increase in the activity of all liver antioxidant enzymes such as CAT, SOD, GPX and GR could be noticed in NHST/HST-BR treated when compared with NHST/HST- untreated birds (Table 1).
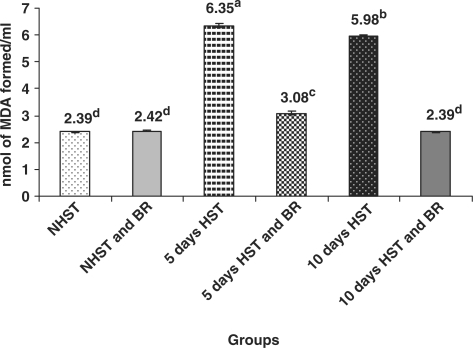
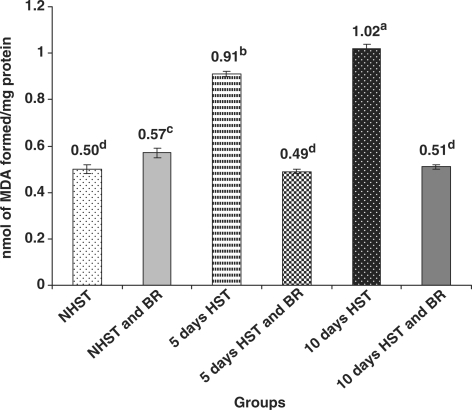
Lipid Peroxidation (LPO) Levels in Serum and Liver Increased in HST-Untreated Birds
All HST-untreated birds showed significant increase (P < 0.05) in LPO level in serum and liver when compared with NHST-untreated controls (Figs 5 and 6).
Figure 5.
Influence of heat stress on serum LPO level in egg type male chicken (n = 8). Values are each mean ± SE, ANOVA followed Duncan's multiple range test, a–dP < 0.05; means having same superscripts does not differ significantly at 5% level.
Figure 6.
Influence of heat stress on liver LPO level in egg type male chicken (n = 8). Values are each mean ± SE, ANOVA followed Duncan's multiple range test, a–dP < 0.05; means having same superscripts does not differ significantly at 5% level.
BR Administration Brought down LPO Levels in Serum and Liver
There was a significant decrease (P < 0.05) in the LPO levels in serum and liver of NHST/HST-BR treated (both 5 and 10 days) birds when compared with HST- untreated birds (both 5 and 10 days) (Figs 5 and 6). The LPO levels in NHST/HST-BR treated birds were comparable with LPO levels in NHST-untreated control birds.
Discussion
The stress of high ambient temperature may negatively influence the performance of broiler chickens by reducing feed intake, live weight gain and feed efficiency (41,42).
Several methods are available to alleviate the negative effects of high environmental temperature on performance of poultry. But of the high cost and impracticality of cooling animal buildings, interest on dietary manipulations has increased (11).
In the present study untreated birds exposed to heat stress (high temperature–high humidity environmental conditions) for 5 or 10 days invariably showed reduced activities of CAT and SOD as well as serum GSH levels when compared with both control and HST-BR treated birds. This kind of response in HST-untreated birds could be unfavorable to their body system as during heat stress because of panting there could be possibilities for oxidative stress, respiratory alkalosis and thus an overproduction of free radicals in the body. Maintenance of normal cell functions in the presence of oxygen largely depends on the efficiency of tissue protection against free radicals mediated oxidative stress. An earlier report (30) supported our present findings that the BR supplementation alone at 2g/kg bw p.o for 20 days in NHST birds (group II) brought about an increased activity of CAT and SOD. This could be beneficial for the birds as increased antioxidant activity in erythrocytes ensures proper and rapid elimination of ROS that could be formed during heat stress and thereby protecting the integrity of erythrocyte membrane.
Glutathione is considered to be the master antioxidant of the body and is found in almost all living cells. Body utilizes GSH chiefly for reducing oxidized vitamin C and vitamin E back to their reduced state, to detoxify many toxins, to maintain cellular redox potential, to maintain erythrocytes membrane integrity, etc. (43). Oral supplementations of vitamin C, Selenium and N-acetyl cysteine have a significant effect on raising glutathione levels in the liver and other tissues. Most of the ingredients used in preparing BR serve as rich source of vitamin C especially fruits of Emblica and Terminalia. In the present study, BR supplementation to HST/NHST birds resulted in an increased serum and liver GSH content when compared with control birds. Diminished content of GSH in cells ultimately results in cell death (44). Oxidative stressors that can deplete GSH includes ultra violet and other radiation, viral infections, extreme environmental conditions, heavy metals, surgery, inflammation, burns and septic shock (43,45,46).
GPX and GR are enzymes associated with conversion of GSH to oxidized form and back. Liver is the chief organ concerned with detoxification process and availability of increased amount of GSH in the liver improves this function. Results of the present study revealed that the GSH content of liver of NHST/HST-BR treated birds was elevated concomitant with the activities of GPX and GR enzymes significantly over control group. SOD enzyme has also a minor role to play in oxidizing GSH to GSSG. Liver SOD activity was increased in NHST/HST-BR treated birds when compared with NHST/HST-untreated birds because increased formation of GSH triggered the oxidation reactions mediated by GPX and SOD. However there were no such appreciable changes in CAT and SOD activities in liver tissue of HST-untreated birds. Moreover there was an insignificant increase in the activity of liver enzyme such as GPX and a great deal of decreased activity of GR in NHST/HST-untreated birds that resulted in the accumulation of GSH without being oxidized. In other words, the rate of forming of GSH and the rate at which it was oxidised by antioxidant liver enzymes did not match in HST-untreated birds while in NHST/HST-BR treated birds an appreciable balance between activities of oxidising and reducing enzymes of glutathione could be observed.
LPO level is a direct indication of oxidative damage of cells as seen in aging, atherosclerosis and other pathological disorders. LPO of serum lipids, especially those of HDL is highly unfavorable as it may end up with many vascular disorders. Results of the present study revealed that, LPO levels in serum and liver of NHST/HST-BR treated birds were significantly less than those encountered in HST-untreated birds. These results suggestive of beneficial aspects of BR supplementation for 10 days prior to HST and continued during the period of HST in birds; thereby undesired side effects produced by free radicals that were generated could be compromised successfully.
Because of increased activity and concentration of enzymatic and non-enzymatic antioxidants resulted by oral BR supplementation, birds could remain healthy, maintained body weight and were acclimated to the heat stressor quicker than untreated groups. In other words BR treatment facilitates adaptation of the chicken to stressful hot humid condition at an early date.
Acknowledgment
The present investigation is a part of Adhoc ICAR project implemented through Department of Physiology. The authors are thankful to the Dean, College of Veterinary and Animal Sciences, Mannuthy, for the facilities provided to conduct the work and to the Indian Council of Agricultural Research, Government of India, and New Delhi for funding the project.
References
- 1.Brown KI, Nedtor KE. Some physiological responses of turkeys selected for high and low adrenal response to cold stress. Poult Sci. 1973;52:948–54. doi: 10.3382/ps.0521948. [DOI] [PubMed] [Google Scholar]
- 2.Sykes AH. Vitamin C for poultry; some recent research. Proceedings of the Roche Symposium; 1978. pp. 5–15. [Google Scholar]
- 3.Nishigaki I, Kuttan R, Oku H, Ashoori F, Abe H, Yagi K. Suppressive effect of curcumin on lipid peroxidation induced in rats by carbon tetrachloride or Cobalt-60 irradiation. J Clin Biochem Nutr. 1992;13:23–9. [Google Scholar]
- 4.Yu BP. Cellular defences against damage from reactive oxygen species. Physiol Rev. 1994;74:139–62. doi: 10.1152/physrev.1994.74.1.139. [DOI] [PubMed] [Google Scholar]
- 5.Klasing KC. Comparative Avian Nutrition. Cambridge: University Press; 1998. [Google Scholar]
- 6.Sahin K, Sahin N, Onderci M, Yaralıoglu S, Kucuk O. Protective role of supplemental Vitamin E on lipid peroxidation, Vitamins E, A and some mineral concentrations of broilers reared under heat stress. Vet Med Czech. 2001;46:140–4. [Google Scholar]
- 7.Cheng TK, Coon CN, Hamare ML. Effect of environmental stress on the ascorbic acid requirement of laying hens. Poult Sci. 1990;69:774–80. doi: 10.3382/ps.0690774. [DOI] [PubMed] [Google Scholar]
- 8.Sahin K, Sahin N, Yaralıoglu S. Effects of Vitamin C and Vitamin E on lipid peroxidation, blood serum metabolites and mineral concentrations of laying hens reared at high ambient temperature. Biol Trace Elem Res. 2002;85:35–45. doi: 10.1385/BTER:85:1:35. [DOI] [PubMed] [Google Scholar]
- 9.Machlin LJ, Vitamin E. In: Handbook of Vitamins. Marcel Dekker Inc, New York:; 1991. pp. 100–44. [Google Scholar]
- 10.Kafri I, Cherry JA. Supplemental ascorbic acid and heat stress in broiler chicks. Poult Sci. 1984;63(Suppl 1):125–6. [Google Scholar]
- 11.Njoku PC. Effect of dietary ascorbic acid (Vitamin C) supplementation on the performance of broiler chickens in a tropical environment. Anim Feed Sci Technol. 1986;16:17–24. [Google Scholar]
- 12.McDowell LR. In: Comparative Aspects to Human Nutrition. Academic Press: New York:; 1989. Vitamins in animal nutrition: vitamin C, folacin; pp. 298–322. and 365–87. [Google Scholar]
- 13.Govindasa . Bhaisajyaratnavali. Varanasi: Chaukambha Sanskrita Academy; 1884. [in Sanskrit] [Google Scholar]
- 14.Hanumanthachar J, Milind P. Brahmi rasayana improves learning and memory in mice. eCAM. 2006;3:79–85. doi: 10.1093/ecam/nek014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohandas Rao KG, Muddanna Rao S, Gurumadhva Rao S. Centella asiatica (L.) leaf extract treatment during growth spurt period enhances hippocampal CA3 neuronal dendritic arborisation in rats. eCAM. 2006;3:349–57. doi: 10.1093/ecam/nel024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joseph CD, Praveen Kumar V, Kuttan G, Kuttan R. Myeloprotective effect of non-toxic indigenous preparation Rasayana in cancer patients receiving chemotherapy and radiation therapy. J Exp Clin Cancer Res. 1999;18:3–12. [PubMed] [Google Scholar]
- 17.Acharya Vaidya Jadavaji Trikanji. 4th edition. New Delhi: Munshiram Manoharlal Publishers Pvt. Ltd; 1981. Rasayana part (Chapter 1), Charakasamhitha –Chilsasthanam; p. 41. [Google Scholar]
- 18.Rekha PS, Kuttan G, Kuttan R. Antioxidant activity of Brahma Rasayana. Indian J Exp Biol. 2001;39:447–52. [PubMed] [Google Scholar]
- 19.Rekha PS, Kuttan G, Kuttan R. Effect of Brahma Rasayana on antioxidant system after radiation. Indian J Exp Biol. 2001;39:1173–5. [PubMed] [Google Scholar]
- 20.Rekha PS, Kuttan G, Kuttan R. Effect of Brahma Rasayana on antioxidant systems and cytokine levels in mice during cyclophosphamide administration. J Exp Clin Cancer Res. 2001;20:219–23. [PubMed] [Google Scholar]
- 21.Praveen Kumar V, Kuttan G, Kuttan R. Protective effects of Rasayanas on cyclophosphamide and radiation induced damage. J Altern Complement Med. 2002;8:787–96. doi: 10.1089/10755530260511801. [DOI] [PubMed] [Google Scholar]
- 22.Vayalil PK, Kuttan G, Kuttan R. Rasayanas: evidence for the concept of prevention of diseases. Am J Chinese Med. 2002;30:155–71. doi: 10.1142/S0192415X02000168. [DOI] [PubMed] [Google Scholar]
- 23.Jaya PG, Rajesh Kumar NV, Rajesh LT, Anuj S, Anoop KS, Radha KM. Protective effects of polyherbal preparation-Brahma Rasyana against tumour growth and lung metastasis in rat prostrate model system. J Exp Ther Oncol. 2004;4:203–12. [PubMed] [Google Scholar]
- 24.Parida RNS, Bisoi PC, Sahoo PK, Mohapatra M. Protective effect of Livol during experimental aflatoxicosis in chicks: a histomorphological approach. Indian J Indg Med. 1995;17:19–25. [Google Scholar]
- 25.Rao AT, Panda SK, Sahu RK. Effect of zeetress on infectious bursal disease vaccinated & challenged birds. Indian J Indg Med. 1995;17:43–6. [Google Scholar]
- 26.Wheeler GE. Use of a herbal supplement to reduce the effects of stress in intensively housed chickens. Indian J Indg Med. 1994;11:51–60. [Google Scholar]
- 27.Roy R, Maiti SK, Ali SL, Raju S. Study on the efficacy of Zeetress, an antistress in layers during summer. Indian Vet J. 1996;73:662–4. [Google Scholar]
- 28.Sharma PV. Dravyaguma Vijnam. Medhya Varga, Varanasi: Chaukambha Bharati Academy; 1987. [in Hindi] [Google Scholar]
- 29.Anonymous . The AyurvedicFormulatory of India. New Delhi: Ministery of Health and Family Planning; 1978. [Google Scholar]
- 30.Rekha PS, Kuttan G, Kuttan R. Effect of herbal preparation, Brahma Rasayana, in amelioration of radiation induced damage. Indian J Exp Biol. 2000;38:999–02. [PubMed] [Google Scholar]
- 31.Paul Thaxton. Influence of temperature on the immune responses of birds. Poultry Sci. 1978;57:1430–40. doi: 10.3382/ps.0571430. [DOI] [PubMed] [Google Scholar]
- 32.Kulkarni Shrikant, Varshney VP. Enhanced adrenocorticotropic hormone and nitric oxide production from caprine peripheral lymphocytes during heat stress. In: Compendium of National Symposium on Recent Advances in Cryopreservation of Livestock Germplasm; XIVth Annual Conference of Society of Animal Physiologists of India; 2005. pp. 348–58. [Google Scholar]
- 33.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–58. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 34.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods in Enzymatic Analysis. New York: 1983. pp. 276–86. [Google Scholar]
- 35.McCord JM, Fridovich I. The utility of superoxide dismutase in studying free radical reaction. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969;244:6056–63. [PubMed] [Google Scholar]
- 36.Moron MA, DePierre JW, Mannervick B. Levels of glutathione, glutathione reductase, glutathione-s-transferase activities in rat liver. Biochem Biophys Acta. 1979;582:67–69. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 37.Paglia DE, Valentine WN. Studies on the qualitative and quantitative characterisation of erythrocytes glutathione peroxidase. J Lab Clin Med. 1967;70:158–59. [PubMed] [Google Scholar]
- 38.Racker E. Glutathione reductase (liver and yeast) In: Sidney PC, Nathan OK, editors. Methods in enzymology. Vol. 3. New York: Academic Press; 1955. pp. 722–25. [Google Scholar]
- 39.Lowry DH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 40.Snedecor GW, Cochran WG. Calcutta: Oxford and IBH publishing corporation; 1994. Statistical Methods. [Google Scholar]
- 41.Donkoh A. Ambient temperature: a factor affecting performance and physiological response of broiler chickens. Int J Biometeoro. 1989;33:259–65. doi: 10.1007/BF01051087. [DOI] [PubMed] [Google Scholar]
- 42.Siegel HS. Stress, strains and resistance. Br Poult Sci. 1995;36:3–22. doi: 10.1080/00071669508417748. [DOI] [PubMed] [Google Scholar]
- 43.Kidd PM. Glutathione: systemic protectant against oxidative and free radical damage. Altern Med Rev. 1997;1:155–6. [Google Scholar]
- 44.Cook GC, Sherlock S. Results of a controlled clinical trial of glutathione in cases of hepatic cirrhosis. Gut. 1965;6:472–76. doi: 10.1136/gut.6.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosower NS, Kosower EM. The glutathione status of cells. Intl Rev Cytol. 1978;54:109–57. doi: 10.1016/s0074-7696(08)60166-7. [DOI] [PubMed] [Google Scholar]
- 46.Sen CK. Nutritional biochemistry of cellular glutathione. Nutr Biochem. 1997;8:660–72. [Google Scholar]