Abstract
Triphala Mashi is an ayurvedic formulation that was prepared in our lab. Aqueous and alcoholic extracts of both Triphala and Triphala Mashi were used, to evaluate antimicrobial activity. Comparative phytochemical profile of Triphala and Triphala Mashi was done by preliminary phytochemical screening, total phenolic content and thin layer chromatography (TLC). Antimicrobial activity includes isolation of pathogens from clinical samples, its characterization, testing its multiple drug resistance against standard antibiotics and antimicrobial activity of aqueous and alcoholic extracts of both Triphala and Triphala Mashi against these organisms by using agar gel diffusion method. Triphala Mashi containing phenolic compounds, tannins exhibited comparable antimicrobial activity in relation to Triphala against all the microorganisms tested. It inhibits the dose-dependent growth of Gram-positive and Gram-negative bacteria. In conclusion, it appears that Triphala Mashi has non-specific antimicrobial activity.
Keywords: antimicrobial activity, clinical sample and Tannin, Triphala, Triphala Mashi
Introduction
Current advancements in drug discovery technology and search for novel chemical diversity have intensified the efforts for exploring leads from Ayurveda the traditional system of medicine in India. Many plant extracts have been used as a source of medicinal agents to cure urinary tract infections, cervicitis vaginitis, gastrointestinal disorders, respiratory diseases, cutaneous affections, helmintic infections, parasitic protozoan diseases and inflammatory processes (1).
Although extremely effective, antibiotics are able to induce resistance in bacteria. For >50 years, bacterial resistance has been the main factor responsible for the increase of morbidity, mortality and health care costs of bacterial infections. This bacterial defense mechanism is widely present in bacteria (e.g. Pseudomonas, Klebsiella, Enterobacter, Acinetobacter, Salmonella, Staphylococcus, Enterococcus and Streptococcus) and became a world health problem worsened by developments in human, animal and plant transportation (2).
Triphala is a traditional Ayurvedic herbal formulation consisting of the dried fruits of three medicinal plants Terminalia chebula, Terminalia belerica and Phyllanthus embelica also known as ‘three myrobalan’. Triphala means ‘three’ (tri) ‘fruits’ (phala) (3).
Triphala is used in Ayurvedic medicine in treating a variety of conditions and also forms part of many other Ayurvedic formulations. Conditions for which Triphala is employed include headache, dyspepsia, constipation, liver conditions, ascites and leucorrhoea. It is also used as a blood purifier that can improve the mental faculties and is posseses anti-inflammatory, analgesic anti-arthritic, hypoglycaemic and anti-aging properties (4–8).
Phyllanthus embelica contains ascorbic acid (9) astragalin–flavonol (10), gallic acid–benzenoid, emblicol, phyllemblic acid (11), emblicanin A, emblicanin B, pedunculagin, punigluconin–tannin (12), ellagic acid coumarin (13). Terminalia chebula contains arjungenin, arjunglucoside I (14), gallic acid-benzenoid (15), chebulic acid, ellagic acid–coumarin, corilagin, punicalagin, terchebulin, terflavin A–tannin (16). β-Sitosterol, gallic acid, ellagic acid, ethyl acetate, galloyl glucose and chebulagic acid have been isolated from fruits of T. belerica (3).
Mashi (Black ash) is obtained, when any natural product from vegetable or animal sources is heated slowly, at lower temperature (generally below 450°C). If heating is continued further at higher temperatures (above 450°C) it forms Bhasma (White ash). Mashi is an intermediate product of Bhasma in which unlike Bhasma, both organic and inorganic constituents are present.
Mashi is a dosage form in which bulk of raw material is reduced to a greater extent by application of a certain quantum of energy. As a result of this treatment hidden chemical constituents become prominent and/or new chemical moieties are formed which are therapeutically active. Due to thermal degradation or decomposition thermo labile constituent are lost. Therapeutically active organic and inorganic chemical constituents can be prepared by simple heat treatment in a controlled manner. The black color indicates high percentage of carbon and oxides. Non-specific odor and charcoal like taste may be attributed to oxides, inorganic elements and carbon (17).
Ayurvedic System of Medicine has its long history of therapeutic potential. Ayurveda is already well accepted and used since thousand years. Now it is time to give it modern scientific proof. Most of the drugs used today are obtained from natural sources or semi synthetic derivatives of natural products as mentioned and used in the Traditional Systems of Medicine. Thus it is a logical approach to drug discovery to screen traditional natural products instead of randomly synthesized chemical moieties. So we undertook this Triphala Mashi as a prototype to give it scientific proof. References about Triphala Mashi were found in Bhaisajyaratnavali and Bharat Bhaisjya Ratnakar. The objective of the present investigation was to analyze antimicrobial potential of Triphala Mashi and also to assay its toxicities.
Material and Methods
Raw Material
Triphala was procured from local markets, Pune, Maharashtra, India.
Preparation of Triphala Mashi
Triphala Mashi was prepared by heating the Triphala in closed silica crucibles in a muffle furnace. Triphala was heated from 30°C to 450°C higher temperature by continuously increasing temperature to 10°C/min.
Preparation of Triphala and Triphala Mashi Extracts
Aqueous extracts (1:6) of Triphala and Triphala Mashi were prepared by hot maceration method and ethanolic extracts (1:6) of both were prepared using soxhlet apparatus. The extracts were filtered and the solvent was removed using rotary evaporator. The extracts were stored in an airtight glass bottle in a refrigerator.
Preliminary Phytochemical Screening (18)
Phytochemical evaluation of aqueous and ethanol extract of Triphala and Triphala Mashi: A stock solution was prepared by dissolving 500 mg extract in 20 ml of solvent that was subjected to preliminary phytochemical testing for the detection of major chemical groups.
Antimicrobial Activity using Agar Diffusion Method
We accomplished isolation of pathogens from clinical samples obtained at the hospital, Pune (India) with specific diagnosis of the different human pathologies. They were characterized through microscopic examination, Gram's character, and biochemical test profile. They were analyzed multiple drug resistance against standard antibiotics and antimicrobial activity with Agar Diffusion Method of plant extract against the organisms.
Isolation of Pathogens from Clinical Samples
The organisms were isolated from patients who visited clinical pathology for samples like sputum, wound swab, urine, pus, semen, bronchial lavage, etc. The standard method for isolation and identification based on microscopic examination, cultural characteristics and biochemical properties was followed (19). The samples were collected carefully, avoiding contamination with normal flora of the body and enriched in selective media and subjected to identification and characterization.
Characterization (19)
It was based on the microscopic examination, Gram's character and biochemical test, profile is presented in table 1.
Table 1.
Microscopic and biochemical profile of clinical isolates
| Isolate No. | Name of the organism | Biochemical tests | ||||||
|---|---|---|---|---|---|---|---|---|
| Indole | Urease | Citrate | TSI | Oxidase | Catalase | Coagulase | ||
| 2443 | Escherichia Coli (urine) | +ve | +ve | +ve | A/G | N/A | N/A | N/A |
| 2438 | Klebsiella Pneumoniae (extra ETT) secretion | −ve | +ve | +ve | A/G | N/A | N/A | N/A |
| 2379 | Pseudomonas Aeruginosa (Wound swab) | −ve | +ve | +ve | A/G | +ve | +ve | N/A |
| 2444 | Pseudomonas Aeruginosa (semen) | −ve | −ve | +ve | A/G | −ve | +ve | N/A |
| 2378 | Escherichia Coli (urine) | +ve | −ve | −ve | A/G | N/A | N/A | N/A |
| 2401 | Staphylococcus aureus (Urine) | −ve | +ve | −ve | A | −ve | +ve | +ve |
| 2384 | Klebsiella Pneumoniae (Pus from abdominal wound) | −ve | +ve | −ve | A/G | N/A | N/A | N/A |
| 2381 | Staphylococcus aureus (Urine) | −ve | +ve | −ve | A | −ve | +ve | −ve |
| 2445 | Staphylococcus aureus (urine) | −ve | +ve | −ve | A | −ve | +ve | −ve |
| 2447 | Staphylococcus aureus(urine) | −ve | +ve | −ve | A | −ve | +ve | −ve |
N/A, Not Applicable; A/G, Acid and Gas production; A, Acid production; +ve, Possitive; −ve, Negative.
Testing Multiple Drug Resistance against Standard Antibiotics
Isolated cultures were grown overnight in sterile nutrient broth and their optical density (O.D.) adjusted to 0.1. This O.D. adjusted culture was spread on sterile nutrients agar plates. With a sterile forcep the multi-antibiotic disc was placed on the spreaded plate. These plates were kept at 10°C to allow diffusion of the drug for half an hour. The plates were then incubated at 37°c for 24 h. Zone of inhibition around the particular drug was observed and recorded.
Testing Antimicrobial Activity of Triphala and Triphala Mashi Extracts (20)
Antimicrobial activity was checked by agar gel diffusion method. The cultures were grown in nutrient broth and incubated at 37°C, for 24 h. After incubation period is finished the O.D. of the culture was adjusted to 0.1 with sterile nutrient broth. The 0.1 ml of the culture was seeded in 25 ml molten nutrient agar butts, mixed and poured into sterile petri plate and allowed to solidify. The wells were bored with 8 mm borer in seeded agar. Then the particular concentration (50–1500 mg/ml) of the Triphala and Triphala Mashi extract (0.4 ml each containing 50–1500 mg/ml) was added in each well. Soon afterwards the plates were then kept at 10°C for 30 min. After it normalized to room temperature plates were incubated at 37°C for 24 h. After incubation period was finished the zone of inhibition was measured and recorded.
Acute Oral Toxicity
Acute oral toxicity refers to those adverse effects that occurred following oral administration of a single dose of a substance or multiple doses given within 24 h. OECD Guideline No. 425 (Acute Oral Toxicity–Acute Toxic Class Method) is undertaken as a test procedure to ascertain the acute oral toxicity, that occurred after administration of the test substance. The test procedure described in this OECD guideline is of value in minimizing the number of animals required to estimate the acute oral toxicity. In addition to the estimation of LD50 and confidence intervals, the test allows observation of signs of toxicity.
Test Report
We worked with six groups of six mice each, of the swiss albino mice species/strain, sourced from National Toxicology Center, Pune, of the female sex, weighing 25–30 g, maintained in standardized environmental conditions (animal house conditions) with free access to food and water and with water used as vehicle. We tested aqueous and alcoholic extracts of Triphala and Triphala Mashi, in conditions presented subsequently.
Test Conditions
Individual body weights of rats were determined just before dosing. All extracts were dissolved in water. No information was available on toxicity of the substance to be tested. As per AOT 425, initial dose of 175-mg/kg-body weight was administered. Rats were observed for 48 h. Based on the status (alive or died) of rats during the observation period the next dose of the test drug is decided as per OECD guidelines 425. Stopping criteria was met as three consecutive animals survived at the upper limit (5000 mg/kg) or three consecutive rats died. Oral administration of the drug solution was carried out using a ‘gavage’. Drug solution was prepared freshly just before dosing. Dosing volume was 1ml/100 g of body weight, at 9:30 am. After the period of fasting, performed rats were weighed and then the test substance was adminstered and after the administration of drug, food was withheld for 1–2 h.
Results
Antimicrobial activity
Effectiveness of extracts
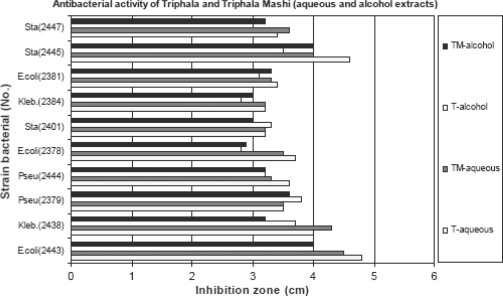
In general, the aqueous and ethanolic extracts of Triphala and Triphala Mashi exhibited a broad-spectrum antimicrobial activity against all the microorganisms from human secretions and from pathology lab with prior diagnosis. It inhibited the growth of all Gram-positive and Gram-negative bacteria. In agar diffusion method, with the broad range of concentrations of 50–1500 mg/ml of the extract, the growth of all microorganisms was inhibited. Aqueous extracts shows better activity than ethanolic extract for all strains. More inhibitory zone is observed for the strains E. coli, S. aureus. All extracts show dose-dependant activity (Fig. 1). All the microorganisms that presented resistance to certain tested antibiotics, showed good susceptibility to the extracts of Triphala and Triphala Mashi. Variations of susceptibilities and resistance existed among same microorganisms to the same antibiotics.
Figure 1.
Antibacterial activity of Triphala and Triphala Mashi.
Toxicity study
No signs of toxicity were observed in short-term analysis but mortality was seen in long-term study at the dose of 5000 mg/kg. The data collected for individual animals is presented in Table 4. Triphala aqueous and alcohol extract (50–1500 mg/ml)/Triphala Mashi aqueous and alcohol extract (50–1500 mg/ml). Escherichia coli (2443/2378/2381)/Klebsiella (2438/2384)/Pseudomas (2379/2444)/Staphylococcus aureus (2401/2445/2447).
Table 4.
Effect of Triphala and Triphala Mashi on mortality in Swiss albino mice
| Sr.No. | Animal ID (n = 6) | Dose (mg/kg) | Short-term result | Long-term result |
|---|---|---|---|---|
| 1 | H | 175 | O | O |
| 2 | B | 550 | O | O |
| 3 | T | 1750 | O | O |
| 4 | HB | 5000 | O | X |
| 5 | BT | 5000 | O | X |
| 6 | HBT | 5000 | O | X |
X, Died; O, Survived.
Discussion
The antimicrobial mechanisms of tannins can be summarized as follows (21). (i) The astringent property of the tannin may induce complexing with enzymes or substrates (22). Many microbial enzymes in raw culture filtrates or in purified forms are inhibited when mixed with tannins (23). (ii) Tannins toxicity may be related to their action on membranes of the microorganisms. (iii) Complexation of metal ions by tannins may account for tannin toxicity (24). The ester linkage between gallic acid and glucose was important to the antimicrobial potential of these compounds.
Some of the simplest bioactive phytochemicals consist of a single substituted phenolic ring. Catechol and pyrogallol both are hydroxylated phenols, shown to be toxic to microorganisms. Catechol has two 2OH groups, and pyrogallol has three. The site(s) and number of hydroxyl groups on and the phenol group are thought to be related to their relative toxicity to microorganisms, with evidence that increased hydroxylation results in increased toxicity. In addition, some authors have found that more highly oxidized phenols are more inhibitory. The mechanisms thought to be responsible for phenolic toxicity to microorganisms include enzyme inhibition by the oxidized compounds, possibly through reaction with sulfhydryl groups or through more non-specific interactions with the proteins (25).
Several studies have shown that purified catechin fractions from green and black tea, and Epicatechin gallate (ECG) and Epigallocatechin gallate (EGCG) in particular, inhibit the growth of many bacterial species and possess anticariogenic properties (26–29). ECG and EGCG, but not Epicatechin (EC) or EGC, have been reported to be powerful antagonists of human immunodeficiency virus reverse transcriptase, causing 50% inhibition at concentrations of 10–20 ng/ml (30). Ikigai et al. (31) showed that EC was much less active than EGCG. Staphylococcus aureus was more susceptible than E. coli, consistent with a much greater binding of EGCG to staphylococci. The MICs of EGCG and EC were 73 and 573 mg/ml, for S. aureus and 183 and 1140 mg/ml, for E. coli, respectively. The bactericidal effect of EGCG was attributed to membrane perturbation.
In Ayurvedic texts Triphala Mashi has been mentioned for its antimicrobial effect. To justify the claims made in Ayurveda, its antimicrobial activity was checked and results were expressed in terms of zone of inhibition (in cm). Bacterial strains were multiple drug resistance strains of both Gram-positive and Gram-negative (Table 2). Both aqueous and alcoholic extracts of Triphala and Triphala Mashi were used for antimicrobial activity. Different concentrations of samples from 50 mg/ml to 1500 mg/ml showed linear activity with increase in concentration. Total organic carbon content was determined to analyse the organic constituent's degradation in Triphala Mashi. Preliminary phytochemical screening of both Triphala and Triphala Mashi indicates the presence of tannins and ascorbic acid. Anthraquinone glycosides were present only in Triphala extract but these are absent in Triphala Mashi (Table 3). This shows that the degradation of anthraquinones takes place as temperature increases.
Table 2.
Multiple drug resistance pattern against standard antibiotics
| Isolate No. | Name of the organism | Amikacin | Bactrim | Ciprofloxacin | Cefotoxime | Cefatazidime | Cefuroxime | Ceftriaxone | Furadentin | Augmetin | Norfloxacin | Netromycin | Cefaparazone | Levofloxacin | Epocinin | Piperacillin | Ofloxacin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2443 | Escherichia Coli (urine) | S | S | R | R | R | R | R | S | R | R | R | R | R | R | R | R |
| 2438 | Klebsiella Pneumoniae (extra ETT) secretion | R | – | R | I | I | R | R | – | R | – | R | S | – | – | R | – |
| 2379 | Pseudomonas Aeruginosa (Wound swab) | S | R | R | R | S | R | R | R | R | R | S | R | R | S | – | R |
| 2444 | Pseudomonas Aeruginosa (semen) | S | – | R | R | R | R | R | – | R | – | R | R | – | – | R | – |
| 2378 | Escherichia Coli (urine) | S | R | R | S | S | R | R | S | I | R | R | R | I | S | – | R |
| 2401 | Staphylococcus aureus (Urine) | S | R | R | S | S | R | R | S | I | R | R | R | I | S | – | R |
| 2384 | Klebsiella Pneumoniae (Pus from abdominal wound) | S | R | R | R | R | R | R | R | R | R | R | R | R | I | – | R |
| 2381 | Staphylococcus aureus (Urine) | S | – | R | S | S | R | S | – | S | – | S | S | – | S | S | – |
| 2445 | Staphylococcus aureus (urine) | R | – | R | R | R | R | R | – | R | – | R | R | – | – | R | – |
| 2447 | Staphylococcus aureus (urine) | S | S | S | S | S | S | S | S | S | S | S | S | S | S | – | S |
S, Sensitive; R, Resistance; I, Insensitive. (i) Aminoglycosides (1. Amikacin, 2. Netromycin.) (ii) Cephalosporins (1. Cefotoxime, 2. Cefatazidime, 3. Cefuroxime, 4. Ceftriaxone, 5. Cefaparazone.) (iii) Quinolones (1.Ciprofloxacin, 2. Norfloxacin, 3. Ofloxacin, 4. Levofloxacin) (iv) Sulphamethoxazole with trimetropim (Bactrim) (v) Clavulanic acid with amoxicillin (Augmetin) (iv) Penicillin (Piperacillin) (vii) Furadentin (Nitrofuran).
Table 3.
Preliminary phytochemical screening
| Chemical test | Triphala aqueous | Triphala Mashi aqueous | Triphala alcoholic | Triphala Mashi alcoholic |
|---|---|---|---|---|
| Alkloids | − | − | − | − |
| Anthraquinone glycosides | + | − | + | − |
| Saponins | ++ | + | − | − |
| Steroids and triterpenoids | − | − | − | − |
| Phenolic compounds Tannins | ++ | ++ | ++ | ++ |
| Proteins | − | − | − | − |
| Ascorbic acid | + | + | + | + |
| Sugars | − | − | − | − |
−, Negative; +, Positive; ++, Positive.
Estimation of total phenolic content and total tannins give reproducible quantity of tannins and phenolics in both Triphala and Triphala Mashi. TLC study also reveals that Triphala Mashi contains comparable gallic acid, ellagic acid and other tannins as that of Triphala. In the present circumstances antimicrobial effect may be attributed to these constituents. Further detail analysis is required in order to confirm the prediction. Also during TLC research there are new bands formed in Triphala Mashi which are not present in Triphala that may be due to heat treatment. So further fractionating and studying antimicrobial effect is necessary to possibly reveal molecules from Triphala Mashi. There is need to pursue the characterization of active principles, to optimize the observed activity.
Such results are interesting, because bacteria was isolated from a hospital environment and its control is difficult by the usual therapeutic means. Studies regarding the mode of action for these compounds in the bacterial cell should be done.
Acknowledgments
The authors are thankful to AICTE for the financial assistance as Mr Yogesh S. Biradar got fellowship. The authors are deeply grateful to Dr B. A. Chopde, Head of the Department, Department of Microbiology, Poona University, Pune, for research facilities and motivation.
References
- 1.Sônia Pereira Leite, Jeymesson Raphael Cardoso Vieira, Paloma Lys de Medeiros, Roberta Maria Pereira Leite, Vera Lúcia de Menezes Lima, Haroudo Satiro Xavier, et al. Antimicrobial activity of Indigofera suffruticosa. Evid Based Complement Alternat Med. 2006;3:261–5. doi: 10.1093/ecam/nel010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deivy Clementino de Lima, Paula Alvarez Abreu, Cícero Carlos de Freitas, Dilvani Oliveira Santos, Rodrigo Oliveira Borges, Tereza Cristina dos Santos, et al. Snake venom: any clue for antibiotics and CAM? Evid Based Complement Alternat Med. 2005;2:39–47. doi: 10.1093/ecam/neh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hans Wohlmuth. Triphala–a short review. Information and research on botanical medicine. (Issue 16):2. [Google Scholar]
- 4.Jagetia GC, Baliga MS, Malagi KJ, Kamath M. The evaluation of the radioprotective effect of Triphala (an Ayurvedic rejuvenating drug) in the mice exposed to -radiation. Phytomedicine. 2002;9:99–108. doi: 10.1078/0944-7113-00095. [DOI] [PubMed] [Google Scholar]
- 5.Rege NN, Thatte UM, Dahanukar SA. Adaptogenic properties of six rasayana herbs used in Ayurvedic medicine. Phytother Res. 1999;13:275–91. doi: 10.1002/(SICI)1099-1573(199906)13:4<275::AID-PTR510>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Mehta BK, Shitut S, Wankhade H. in vitro antimicrobial efficacy of Triphala. Fitoterapia. 1993;64:371–2. [Google Scholar]
- 7.Vani T, Rajani M, Sarkar S, Shishoo CJ. Antioxidant properties of the Ayurvedic formulation Triphala and its constituents. Int J Pharmacogn. 1997;35:313–7. [Google Scholar]
- 8.Kaur S, Arora S, Kaur K, Kumar S. The in vitro antimutagenic activity of Triphala - an Indian herbal drug. Food & Chem Toxicol. 2002;40:527–34. doi: 10.1016/s0278-6915(01)00101-6. [DOI] [PubMed] [Google Scholar]
- 9.Prakash D, Niranjan A, Tewari SK. Vitamin C in Emblica officinalis (amla) and its products. J Med Aromatic Plant Sci. 2000;22:237–41. [Google Scholar]
- 10.El-Mekkawy S, Meselhy MR, Kusumoto IT, Kadota S, Hattori M, Namba T. Inhibitory effects of Egyptian folk medicines on human immunodeficiency virus (HIV) reverse transcriptase. Chem Pharm Bull. 1995;43:641–8. doi: 10.1248/cpb.43.641. [DOI] [PubMed] [Google Scholar]
- 11.Iyer KM, Pillay PP. A chemical examination of Emblica Officinalis, gaertn. Curr Sci. 1958;27:266–7. [Google Scholar]
- 12.Bhattacharya SK, Bhattacharya D, Muruganandam AV. Effect of Emblica Officinalis tannoids on a rat model of tardive dyskinesia. Indian J Exp Biol. 2000;38:945–7. [PubMed] [Google Scholar]
- 13.Jamwal KS, Sharma IP, Chopra CL. Pharmacological investigation of the fruit of Emblica Officinalis. J Sci Ind Res. 1959;18:180–1. [Google Scholar]
- 14.Reddy BM, Rao NK, Ramesh M, Appa Rao Avn, Lin LJ, Lin LZ, et al. Chemical investigation of the fruits of Terminalia Chebula. Int J Pharmacog. 1994;32:352–6. [Google Scholar]
- 15.Grampurohit ND. Gallic acid from myrobalans. Indian J Nat Prod. 1986;2:10–11. [Google Scholar]
- 16.Lin TC, Nonaka GI, Nishioka I, Ho FC. Tannins and related compounds. Cii. Structures of terchebulin, an ellagitannin having a novel tetraphenylcarboxylic acid (terchebulic acid) and biogenetically related tannins from Terminalia Chebula retz. Chem Pharm Bull. 1990;38:3004–8. [Google Scholar]
- 17.Ambike AA, Khandelwal KR, Jadhav BK. ‘Mashi’ an Ayurvedic dosage form. Deerg hayu International. 76;19:239–43. [Google Scholar]
- 18.Khandelwal KR. Practical Pharmacognosy. Pune: Nirali Prakashan; 2000. p. 149. [Google Scholar]
- 19.Jae Hak Sohn, Jung-Hyun Lee, Hana Yi, Jongsik Chun, Kyung Sook Bae, Tae-Young Ahn, et al. Kordia algicida gen. nov., sp. nov., an algicidalbacterium isolated from red tide. Int Syst Evol Microbiol. 2004;54:675–80. doi: 10.1099/ijs.0.02689-0. [DOI] [PubMed] [Google Scholar]
- 20.Vol. 2. 1996. Indian Pharmacopoeia. appendix 9.1: A- 100–3. [Google Scholar]
- 21.Chung KT, Wong TY, Wei CI, Hang YW, Lin Y. Tannin and human health: a review. Food Sci Nutr. 1998:421–64. doi: 10.1080/10408699891274273. [DOI] [PubMed] [Google Scholar]
- 22.Mason TL, Wasserman BP. Inactivation of red beet betaglucan synthase by native and oxidized phenolic compounds. Phytochemistry. 1987;26:2197–202. [Google Scholar]
- 23.Jones GA, McAllister TA, Muir AD, Cheng KJ. Effects of sainfoin (Onobrychis viciifolia scop.) condensed tannins on growth and proteolysis by four strains of ruminal bacteria. Appl Environ Microbiol. 1994;60:1374–8. doi: 10.1128/aem.60.4.1374-1378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung KT, Lu Z, Chou MW. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem Toxicol. 1998;36:1053–60. doi: 10.1016/s0278-6915(98)00086-6. [DOI] [PubMed] [Google Scholar]
- 25.Marjorie Murphy Cowan. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn YJ, Kawamura T, Kim M, Yamamoto T, Mitsuoka T. Tea polyphenols: selective growth inhibitors of Clostridium spp. Agric Biol Chem. 1991;55:1425–6. [Google Scholar]
- 27.Das DN. Studies on the antibiotic activity of tea. J Ind Chem Soc. 1962;39:849–54. [Google Scholar]
- 28.Kawamura J, Takeo T. Antibacterial activity of tea catechin to Streptococcus mutans. J Jpn Soc Food Sci Technol. 1989;36:463–7. [Google Scholar]
- 29.Otake S, Makimura M, Kuroki T, Nishihara Y, Hirasawa M. Anticaries effects of polyphenolic compounds from Japanese green tea. Caries Res. 1991;25:438–43. doi: 10.1159/000261407. [DOI] [PubMed] [Google Scholar]
- 30.Nakane H, Ono K. Differential inhibitory effects of some catechin derivatives on the activities of human immunodeficiency virus reverse transcriptase and cellular deoxyribonucleic and ribonucleic acid polymerases. Biochemistry. 1990;29:2841–5. doi: 10.1021/bi00463a029. [DOI] [PubMed] [Google Scholar]
- 31.Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993;1147:132–6. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]