Abstract
The final envelopment of herpesviruses during assembly of new virions is thought to occur by the budding of core viral particles into a late secretory pathway organelle, the trans-Golgi network (TGN), or an associated endosomal compartment. Several herpesvirus envelope glycoproteins have been previously shown to localize to the TGN when expressed independently from other viral proteins. In at least some cases this TGN localization has been shown to be dependent on clusters of acidic residues within their cytoplasmic domains. Similar acidic cluster motifs are found in endogenous membrane proteins that also localize to the TGN. These acidic cluster motifs interact with PACS-1, a connector protein that is required for the trafficking of proteins containing such motifs from endosomes to the TGN. We show here that PACS-1 interacts with the cytoplasmic domain of the HCMV envelope glycoprotein B (gB) and that PACS-1 function is required for normal TGN localization of HCMV gB. Furthermore, inhibition of PACS-1 activity in infected cells leads to a decrease in HCMV titer, whereas an increase in expression of functional PACS-1 leads to an increase in HCMV titer, suggesting that PACS-1 is required for efficient production of HCMV.
The betaherpesvirus human cytomegalovirus (HCMV) is a major cause of virally induced birth defects and also causes severe disease in immunosuppressed individuals (6). As with all members of the family Herpesviridae, HCMV is an enveloped DNA virus that can remain latent within host cells for life (32). The virion structure is composed of a DNA genome encased within an icosahedral protein lattice (the capsid) surrounded by an amorphous protein layer (the tegument), which is surrounded by a glycoprotein-rich lipid envelope (6, 13).
The assembly and egress of herpesviruses during infection is a complex process that is far from completely understood. The current model proposed for herpesviruses assembly involves the so-called “envelopment/de-envelopment/re-envelopment” pathway. In this model, the core virion structure, the viral DNA-containing capsid, is assembled in the nucleus, after which it buds across both the inner and outer nuclear membranes to be released into the cytoplasm (envelopment/de-envelopment). The capsid becomes coated with tegument proteins in the cytoplasm and then acquires its final lipid envelope (together with viral glycoproteins) by budding into a late secretory pathway organelle, the trans-Golgi network (TGN), or an associated endosomal compartment (re-envelopment) (11, 12, 14, 17, 25, 40). For the successful assembly of herpesviruses in this model, the envelope proteins of the mature herpesviruses need to be localized to the re-envelopment compartment (the TGN) independently of the capsid so that they can be incorporated into the final enveloped viruses. As with cellular membrane glycoproteins, the herpesvirus envelope glycoproteins are transported through the cell's secretory pathway, where the correct glycosylation groups are attached and modified. Therefore, to become incorporated into de novo assembled virions, the viral glycoproteins need to be transported far enough along the secretory pathway to reach the TGN and to be maintained there. Indeed, studies have shown many herpesvirus envelope proteins are localized to a TGN-like compartment, such as the glycoprotein B (gB) from HCMV and the glycoprotein E/I complex and the Us9 protein from various alphaherpesviruses (1, 2, 5, 14, 17, 19, 24, 28, 30, 35). Since the herpesvirus envelope proteins follow a similar pathway to various endogenous secretory pathway proteins, it seems likely that the viral envelope proteins would utilize the endogenous trafficking and localization machinery present in cells. However, the cellular mechanisms that herpesvirus envelope proteins use to maintain themselves in the TGN are poorly understood at present.
One mechanism utilized by secretory pathway proteins in eukaryotic cells to control their localization and trafficking itineraries is the presence of discrete trafficking motifs within their cytoplasmic domains. Examples of such trafficking motifs include tyrosine-based motifs (-YXXΦ-, where Φ is a bulky hydrophobic residue), dileucine motifs (generally -LL- or -LI-), and acidic cluster motifs (various numbers of Asp and Glu residues) (16). These motifs control the localization of the proteins they are found within by mediating interaction with various components of cellular machinery responsible for transporting proteins between different membrane compartments. For example, both the tyrosine-based and dileucine motifs are known to interact with adaptor complexes (e.g., AP-1, -2, and -3), which control the incorporation of protein cargo into clathrin-coated vesicles, for delivery to subsequent compartments (22).
The acidic cluster motif was first identified as playing a role in controlling the trafficking of furin, a prohormone processing enzyme that cleaves numerous protein substrates throughout the late secretory pathway (34). The acidic cluster in furin was found to be phosphorylated by casein kinase 2 (CK2) and that the phosphorylated acidic cluster was essential for the localization of furin to the TGN (8, 21, 33). Using the phosphorylated furin acidic cluster in screening strategies, a novel protein, phosphofurin acidic cluster sorting protein-1 (PACS-1) was discovered that interacts directly with acidic cluster motifs (37). PACS-1 acts as a connector protein, linking acidic cluster motifs to the adaptor complex AP-1, and this function of PACS-1 is required for the retrieval of integral membrane proteins, such as furin, from endosomal compartments to the TGN (7). Acidic cluster motifs, often (but not always) containing consensus CK2 phosphorylation sites, have subsequently been identified in many membrane proteins that localize to the TGN. These include several viral proteins such as human immunodeficiency virus type 1 Nef, which combines with PACS-1 to downregulate major histocompatibility complex class I molecules (3), and many herpesvirus envelope proteins such as HCMV gB, as well as gE and Us9 from alphaherpesviruses (1, 2, 5, 15, 27, 35, 39). The acidic cluster motif in HCMV gB has been shown to be phosphorylated by CK2, and the phosphorylation state affects gB trafficking (10). Given these data, we were interested in determining the role of PACS-1 in the trafficking of HCMV gB and in the production of infectious HCMV. We show here that HCMV gB interacts directly with PACS-1 in a phosphorylation-dependent manner and that PACS-1 function is required to transport gB from endosomes to the TGN. Furthermore, we show that disrupting PACS-1 function leads to a decrease in the amount of infectious HCMV produced in cell culture, whereas increasing the expression of wild-type PACS-1 above endogenous levels leads to an increase in infectious HCMV production.
MATERIALS AND METHODS
DNA constructs.
pRev-TRE-PACS-1 and pRev-TRE-Admut plasmids were constructed as follows. The DNA encoding for hemagglutinin (HA) epitope-tagged PACS-1 and PACS-1Admut were excised from pZVneo constructs (described in reference 7) with a partial HindIII digest. The appropriate fragments were ligated into pRev-TRE (Clontech). The pGEX-gB construct was generated by PCR amplification of the gB cDNA region encoding residues R853 to V906 with oligonucleotides A (GGAATTCCGTCTGGACGCAGAG) and B (GGGATCCTCAGACGTTCTCTTCTTC). PCR products were digested with BamHI and EcoRI and subcloned into pGEX-3X. pGEX-gBS900D and pGEXgBS900A were generated by PCR amplification of gB cDNA with 3′ PCR primers incorporating a TCC-GAC or TCC-GCC mutation of the S900 codon (oligonucleotides A plus C [GGGATCCTCAGACGTTCTCTTCTTCGTCGTCGTCTTTC] or D [GGGATCCTCAGACGTTCTCTTCTTCGTCGGCGTCTTTC], respectively). pET32-PACS-1FBR has been previously described (7).
Cell lines.
A7, U373, primary human foreskin fibroblasts (HFF) cells, and immortalized HFF cell lines (4) were cultured according to standard protocols.
U373 cells expressing HA-tagged PACS-1 and PACS-1Admut were generated as follows. U373 cells were transfected with pRevTet-ON (Clontech), and stable clones were selected with 1 mg of G418/ml. Tet-ON inducible cells were assayed by using a Tet-regulated green fluorescent protein reporter construct. A chosen positive Tet-ON clone was then transfected with pRev-TRE-PACS-1, pRev-TRE-PACS-1Admut, or pRev-TRE alone. Stable cells were selected with 500 μg of hygromycin/ml and expressing clones were isolated. Inducible protein expression was assayed by Western blotting with the HA.11 antisera (Berkley Antibody). Stable U373 clones were maintained with 500 μg of G418/ml plus 250 μg of hygromycin/ml.
Viruses and HCMV titer determination.
Adenoviruses expressing FLAG-tagged HCMV gB (Ad-gB/FLAG) have been described (20). Vaccinia viruses expressing HA-tagged PACS-1 and PACS-1Admut have also been described (7). All viral infections were carried out as described elsewhere (7, 20). HCMV Towne viruses were grown in HFF cells, and titers were determined by plaque assay as previously described (20).
All HCMV growth assays were performed as follows. Triplicate cell samples infected with HCMV were harvested by scraping in Dulbecco modified Eagle medium (DMEM) plus 2% fetal calf serum (FCS), followed by centrifugation (700 rpm for 5 min at room temperature). Cells were resuspended in DMEM plus 2% FCS and then probe sonicated (Fisher model 100), three times for 10 s each time at power level 3, on ice to release cell-associated virions. Samples were then clarified by centrifugation (1,500 rpm for 10 min at room temperature) and frozen at −80°C until samples from all time points were harvested. Samples were thawed at 37°C, water bath sonicated for 60 s (Fisher model FS20), and 10-fold serially diluted into DMEM plus 2% FCS. HFF cells grown to ∼95% confluence in 24-well plates were washed with phosphate-buffered saline (PBS), and 500-μl aliquots of each dilution series were added to triplicate wells of the HFF cells. The 24-well plates were centrifuged (3,000 rpm for 30 min at room temperature) and incubated at 37°C for >2 h. Inocula were aspirated, and cells were overlaid with DMEM plus 10% FCS plus 0.4% SeaPlaque agarose. Once the agarose was set, titer plates were incubated for 10 to 14 days at 37°C until a visible cytopathic effect was observed. Titer plates were fixed in 3.7% formaldehyde in PBS for 10 min at 37°C. Agarose plugs were removed, and fixed cells were washed twice with distilled water. Cells were stained with 0.5% methylene blue for 15 min at room temperature, rinsed three times with distilled water, and air dried. Plaques were counted under a microscope at 4× magnification. The data presented are the means and standard deviations (SD) from triplicate experiments, with each titer determined in triplicate.
Recombinant protein purification and in vitro interaction assays.
Glutathione S-transferase (GST)-gB proteins and TRX-PACS-1FBR were produced and purified as previously described (7). GST-gB proteins were phosphorylated with purified bovine CK2 as previously described (21). Then, 10 μg of GST-gB proteins (with or without phosphorylation) was incubated with 20 μg of TRX-PACS-1FBR in GST binding buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 2 mM MgCl2, 1% NP-40) for 1 h at room temperature. Binding reactions were centrifuged at 5,000 rpm for 5 min, and the supernatants were incubated with a 25-μl bed volume of glutathione-agarose beads (Sigma) for 30 min at room temperature. Glutathione-agarose beads were harvested by centrifugation at 3,000 rpm for 3 min at room temperature and then washed three times with 500 μl of GST binding buffer. Proteins samples were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and blots were developed with antisera specific for the TRX tag on PACS-1FBR (Anti-Thio; Invitrogen) by using chemiluminescence. Interaction signals were quantified after scanning by using the NIH Gel Imaging software (version 1.62).
Immunofluorescence.
A7 cells were infected with Ad-gB/FLAG viruses at a multiplicity of infection (MOI) of 2 in growth medium for 16 to 24 h and subsequently infected with either control vaccinia viruses or viruses expressing PACS-1 or PACS-1Admut at an MOI of 10. At 5 h postinfection, cells were fixed and processed for immunofluorescence as previously described (7) by using primary antisera to the N-terminally exposed FLAG tag MAb M1 (1:50; IBI-Kodak), TGN46 (1:200; Affinity Bioreajents), or CI-MPR (1:200; S. Pfeffer). Prior to fixation some cells were incubated with 50 mg of rhodamine-dextran (Molecular Probes)/ml at 37°C for 1 h.
U373 cells stably expressing PACS-1, Admut, or control cells were infected with HCMV at an MOI of 3. At 3 days postinfection, cells were fixed and processed for immunofluorescence by using antisera to HCMV gB (polyclonal antibody R2448, 1:50 [W. Britt]), γ-adaptin (100/3, 1:50 [Sigma]), and gH (MAb 14-4b, 1:150 [W. Britt]). After incubation with fluorescently labeled secondary antisera (Molecular Probes), images were obtained by using a 63× oil immersion objective lens on a Zeiss Axioplan 2 microscope and then processed by using Openlab 3.0 software (Improvision) or Photoshop 5.5 (Adobe).
siRNA treatment.
Small interfering RNA (siRNA) molecules were designed based on the PACS-1 cDNA sequence (corresponding to nucleotides A415ACTCAGTGGTCATCGCTGTGAA437 from human PACS-1) or an unrelated sequence (AAGAGGGAAGGCAACAAGCTTCA) according to the manufacturer's guidelines (Dharmacon). U373 or HFF cells were transfected with 20 μM siRNA by using oligofectamine (Invitrogen) according to the manufacturer's guidelines. At 2 days posttransfection, cells were infected with Ad-gB/FLAG viruses at an MOI of 2 or with HCMV at an MOI of 3, and cells were either fixed and processed for immunofluorescence or viral samples were harvested for titration as described above.
RESULTS
HCMV gB interacts with PACS-1 in a phosphorylation-dependent manner.
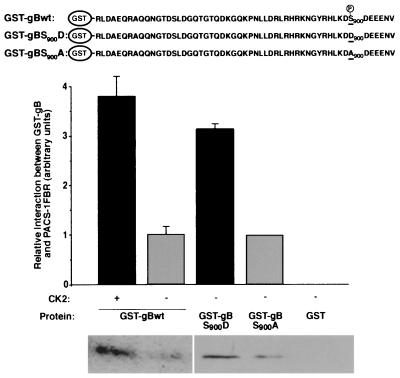
The cytoplasmic domain of HCMV gB protein has previously been shown to be phosphorylated in vivo, and this phosphorylation is dependent on the presence of serine 900 (S900) found within a consensus acidic cluster motif containing a CK2 site (10). HCMV gB has also more recently been shown to localize to the TGN in mammalian cells (19). Given that membrane proteins such as furin are localized to the TGN through interaction of acidic cluster motifs with PACS-1, we tested whether HCMV gB could interact with PACS-1. The C-terminal region of HCMV gB was expressed as a GST fusion protein (Fig. 1, top panel), phosphorylated with CK2, and incubated with a soluble thioredoxin-tagged PACS-1 fusion protein. Protein complexes were isolated with glutathione-agarose and analyzed by Western blotting with a thioredoxin-specific antibody. GST-gB interacted with PACS-1 in vitro, and this interaction was significantly stronger when GST-gB was phosphorylated with CK2 (Fig. 1, lanes 1 and 2). To confirm the dependence of the interaction between PACS-1 and gB on the phosphorylation of the S900 residue, two mutant forms of the GST-gB protein were made: one with a S900 to aspartate substitution to mimic phosphorylation and one with a S900 to alanine substitution to abolish phosphorylation (Fig. 1, top panel). Similar to the CK2 phosphorylation of GST-gB, the interaction of PACS-1 was significantly stronger with GST-gB900D than with GST-gBS900A (Fig. 1, lanes 3 and 4). GST alone demonstrated no detectable interaction with PACS-1 (Fig. 1, lane 5). These data show HCMV gB can interact with PACS-1 and that this interaction is enhanced by phosphorylation of S900.
FIG. 1.
HCMV gB interacts with PACS-1 in a phosphorylation-dependent manner. GST-tagged wild-type HCMV gB cytoplasmic domain (with or without [+ or −] CK2 phosphorylation), mutant gB cytoplasmic domains containing S900D or S900A mutations, or GST alone were incubated with TRX-tagged PACS-1FBR. Protein complexes were recovered with glutathione-agarose, and extracts were analyzed for associated TRX-PACS-1FBR by Western blotting with TRX-specific antisera. Interaction signals from separate experiments were quantified by using National Institutes of Health gel analysis software and are shown as mean interactions ± the SD.
Effect of dominant-negative PACS-1 on HCMV gB localization.
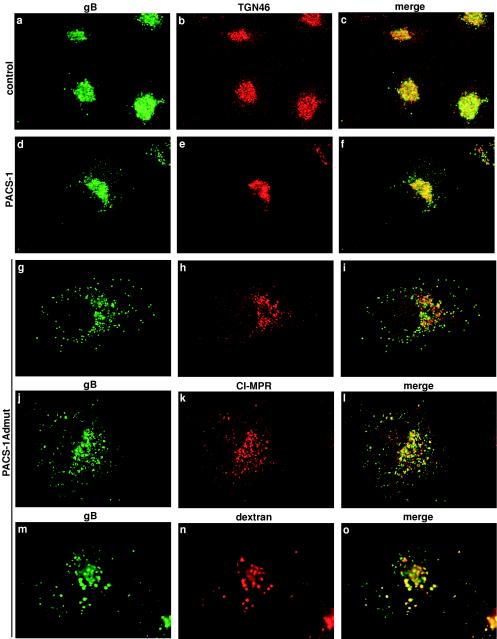
PACS-1 is a sorting connector protein that is required for the trafficking of membrane proteins with acidic cluster motifs (such as furin) within their cytoplasmic domains from endosomes to the TGN. PACS-1 can interact concomitantly with acidic cluster motifs and the AP-1 adaptor complex, physically connecting these proteins to form a ternary complex (7). We have previously constructed a dominant-negative form of PACS-1 (PACS-1Admut) that can no longer interact with AP-1 but can still bind to acidic cluster motifs. When expressed in cells, PACS-1Admut causes the mislocalization of proteins containing acidic cluster motifs to endosomes (7). FLAG-tagged HCMV gB was expressed in cells on its own or together with native PACS-1 or PACS-1Admut. In cells expressing gB-FLAG alone, HCMV gB was localized to a juxtanuclear compartment, where it colocalized well with the TGN marker TGN46 (Fig. 2a to c). In cells coexpressing gB-FLAG and wild-type PACS-1, HCMV gB similarly colocalized well with TGN46 (Fig. 2d to f). In cells coexpressing gB-FLAG and PACS-1Admut, HCMV gB was localized primarily in a dispersed, punctate staining pattern in which it no longer colocalized with TGN46 (Fig. 2g to i). TGN46 localization was unaffected by expression of PACS-1Admut because TGN46 localizes to the TGN in a PACS-1-independent manner (Fig. 2g to i) (37). The cation-independent mannose-6 phosphate receptor (CI-MPR) is an endogenous membrane protein that cycles between the TGN to early and late endosomes and requires PACS-1 for TGN localization (7, 37). In cells coexpressing gB/FLAG and PACS-1Admut, HCMV gB demonstrated colocalization with MPR, suggesting that PACS-1Admut causes both HCMV gB and MPR to be redistributed, at least partially, to the same endosomal compartment(s) (Fig. 2j to l). The compartment(s) into which HCMV gB is redistributed in the presence of PACS-1Admut are part of the endosomal system because, in the presence of PACS-1Admut, HCMV gB partially colocalized with internalized dextran, a marker of fluid phase endocytosis (Fig. 2m to o). However, the mislocalized HCMV gB in the presence of PACS-1Admut showed little or no colocalization with lamp-2, a marker of lysosomes and late endosomes (data not shown). This suggests that the endosomal compartment to which HCMV gB is mislocalized is earlier in the endocytic pathway than lamp-2-positive endosomes. The mislocalization of HCMV gB into endosomes is consistent with our previous work showing that CI-MPR is also mislocalized into dextran-containing endosomes in PACS-1 antisense cells (37).
FIG.2.
PACS-1Admut causes mislocalization of HCMV gB to endosomal compartments. FLAG-tagged HCMV gB was expressed in A7 cells by using replication-defective adenovirus vectors at an MOI of 2 for 24 h. Cells were subsequently infected with control vaccinia viruses (a to c) or vaccinia virus expressing PACS-1 (d to f) or PACS-1Admut (g to o) at an MOI of 10 for 5 h. Cells were fixed and developed with specific antisera to the FLAG tag (M1; a, d, g, j, and m) and assessed for colocalization with either TGN46 (b, e, and h), CI-MPR (k), or internalized dextran (n). M1 staining is shown in green, and colocalization markers are shown in red.
Effect of PACS-1 depletion by siRNA on HCMV gB localization.
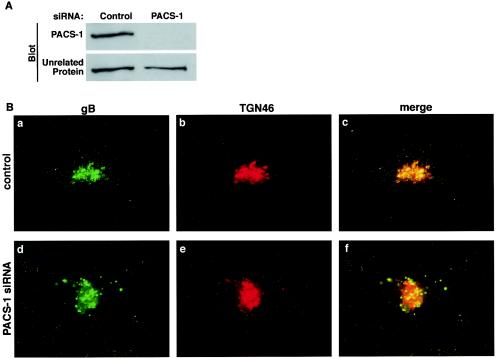
An alternative mechanism of disrupting the function of a protein, rather than the use of dominant negatives, is the depletion of the endogenous protein in cells. Such experiments are important because the absence of a particular protein may have a phenotype very different from the expression of defective versions of the same protein. A recent technique for depleting endogenous proteins within cells is the use of siRNA molecules. This technique silences gene expression by introducing short interfering 21- to 23-mer double-stranded RNA segments into cells that initiate mRNA degradation in a sequence-specific fashion (9). 23-mer siRNA molecules were designed for PACS-1 and transfected into U373 cells. At 3 days posttransfection, cellular PACS-1 protein levels were assayed by Western blotting with PACS-1-specific antisera. In cells transfected with PACS-1 siRNA, the level of PACS-1 protein was decreased by >90% compared to control levels (Fig. 3A). The transfection of cells with control siRNA molecules had no effect on the level of PACS-1 protein (data not shown). When gB-FLAG was expressed in control cells, HCMV gB colocalized with TGN46 in a juxtanuclear compartment (Fig. 3Ba to c). In cells with levels of PACS-1 protein reduced by siRNA transfection, HCMV gB was localized to a punctate endosome-like pattern (Fig. 3Bd to f) similar to the gB mislocalization in the presence of PACS-1Admut. The localization of HCMV gB was not affected in cells transfected with control siRNA molecules (data not shown). These data suggest that interfering with PACS-1 function by either overexpression of dominant-negative PACS-1Admut or reduction of endogenous PACS-1 levels by siRNA mislocalizes HMCV gB to endosomal compartments.
FIG. 3.
Depletion of endogenous PACS-1 protein by siRNA causes a mislocalization of HCMV gB. U373 cells were transfected with or without PACS-1-specific siRNA. (A) PACS-1 protein levels were assessed by Western blotting 3 days posttransfection with PACS-1-specific antisera. (B) FLAG-tagged HCMV gB was expressed in control (a to c) or PACS-1 siRNA-treated (d to f) cells by using adenovirus vectors at an MOI of 2 for 24 h. Cells were fixed and developed with antisera specific for the FLAG tag (M1; a and d) and TGN46 (b and e). M1 staining is shown in green, and TGN46 staining is shown in red.
Effect of PACS-1Admut expression on gB localization during HCMV infection.
The experiments described above were performed on cells expressing HCMV gB in isolation, independent of the expression of other HCMV proteins. In order to determine the role of PACS-1 for the localization of gB in the context of HMCV infection, stable clones of the HCMV permissive U373 cell line expressing wild-type PACS-1, PACS-1Admut, or empty vector controls were constructed. The Tet-ON system was used to allow regulated protein expression.
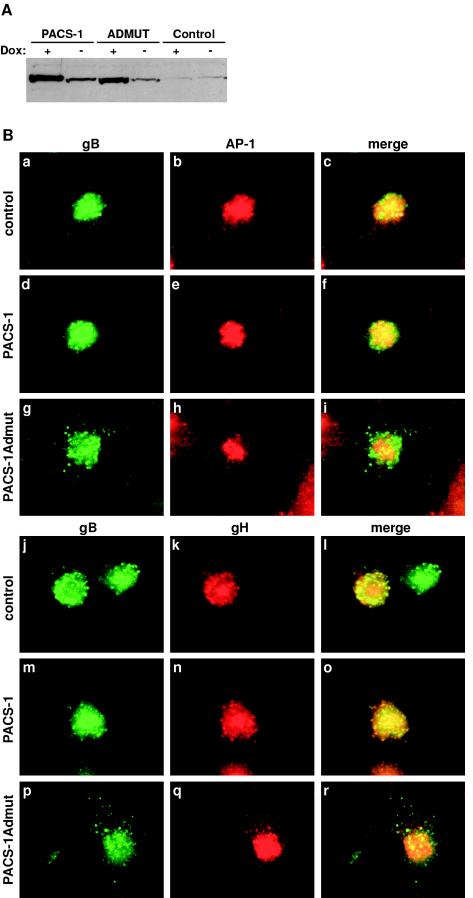
Inducible protein expression in PACS-1, PACS-1Admut, and control U373 stable cell lines was assayed by Western blotting with PACS-1 specific antisera. Both PACS-1 and PACS-1Admut cell lines showed a high level of protein expression in the presence of doxycycline (19- and 14-fold, respectively, above endogenous PACS-1 levels). Some leakage of expression was observed with a small increase of PACS-1 or PACS-1Admut over endogenous PACS-1 protein levels in the absence of doxycycline (nine- and fourfold, respectively) (Fig. 4A). However, the low-level expression of PACS-1 or PACS-1Admut in the absence of doxycycline had no effect on the localization of PACS-1-dependent cargo compared to control cells (data not shown). The U373 cell lines were induced with doxycycline, infected with HCMV, fixed, and processed for immunofluorescence 3 days postinfection. In control U373 cells infected with HCMV, gB was localized to a juxtanuclear compartment, where it colocalized with AP-1 (the TGN-localized clathrin adaptor complex; Fig. 4Ba to c). In U373-PACS-1 cells, HCMV expressed gB was also found to colocalize with AP-1 in a TGN-like compartment (Fig. 4Bd to f). In U373-PACS-1Admut cells however, HCMV expressed gB was found within a dispersed endosome-like punctate pattern, where it no longer colocalized with AP-1 (Fig. 4Bg to i). These data suggest that expression of dominant-negative PACS-1Admut causes HCMV gB to be mislocalized to an endosomal compartment during HCMV infection. To determine the effect of PACS-1 and PACS-1Admut expression on another HCMV envelope protein, the localization of gH was analyzed. gH is found within mature HCMV envelopes in complex with gL and gO (18, 23), and none of these three proteins contain acidic cluster motifs within their cytoplasmic domains. In control U373 cells infected with HCMV, gB and gH were found to colocalize in a juxtanuclear compartment, a finding reminiscent of the TGN (Fig. 4Bj to l). In PACS-1-expressing U373 cells, gB and gH were also found to colocalize in a TGN-like compartment (Fig. 4Bm to o). In PACS-1Admut-expressing U373 cells, gB was found in dispersed punctate endosomal structures, whereas gH appeared unaffected and was localized to a TGN-like compartment (Fig. 4Bp to r). These data suggest that the presence of dominant-negative PACS-1 causes a mislocalization of gB to endosomal compartments but does not affect gH during HCMV infection and so separates these two envelope proteins into different membrane-bound organelles.
FIG.4.
Stable expression of PACS-1Admut causes gB but not gH to be mislocalized during HCMV infection. (A) Stable U373-PACS-1, PACS-1Admut, or control cell lines were treated with or without 1 μg of doxycycline/ml for 2 days. Protein expression was assessed by Western blotting with PACS-1-specific antisera. (B) Stable U373 cell lines expressing empty vector controls (a to c and j to l), wild-type PACS-1 (d to f and m to o), or PACS-1Admut (g to i and p to r) were infected with HCMV at an MOI of 3 for 3 days in the presence of 1 μg of doxycycline/ml. Cells were fixed and developed with antiserum specific for HCMV gB (R2448; a, d, g, j, m, and p), together with antiserum specific for γ-adaptin (100/3; b, e, and h) or HCMV gH (14-4b; k, n, and q). HCMV gB staining is shown in green, and γ-adaptin or HCMV gH staining is shown in red.
Effect of PACS-1Admut expression and PACS-1 depletion by siRNA on HCMV growth.
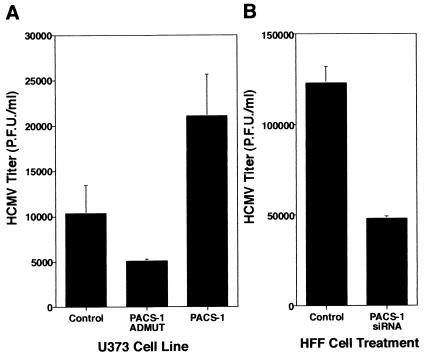
To test the effect of wild-type or dominant-negative (Admut) PACS-1 expression on the growth of HCMV with cells, stable U373 cells expressing PACS-1, Admut, or empty vector controls were infected with HCMV (Towne strain). Viral samples were harvested, and titers were determined 5 to 7 days postinfection. In U373 cells expressing PACS-1Admut there was a small but reproducible decrease in infectious HCMV produced compared to control cells (∼2-fold decrease; Fig. 5A, columns 1 and 2). Interestingly, an increase in infectious HCMV production was observed in U373 cells overexpressing wild-type PACS-1 compared to control cells (∼2- to 2.5-fold increase; Fig. 5A, column 3). These data suggest that inhibition of PACS-1 function via dominant-negative expression inhibits infectious HCMV production, whereas increasing wild-type PACS-1 levels above endogenous levels enhances production of infectious HCMV in U373 cells. Furthermore, these data suggest that in the U373 cell line PACS-1 may be a limiting component for HCMV assembly.
FIG. 5.
Inhibition of PACS-1 function causes a reduction in infectious HCMV production. (A) Stable U373 cell lines expressing empty vector controls, PACS-1, or PACS-1Admut were infected with HCMV at an MOI of 3 in the presence of 1 μg of doxycycline/ml. Cell-associated viral samples were harvested at 6 days postinfection, and the infectious HCMV titer was assessed by plaque assay. Titers from triplicate experiments are represented as mean titers ± the SD. (B) HFF cells were transfected with or without PACS-1-specific siRNA. At 1 day posttransfection, cells were infected with HCMV at an MOI of 3. Cell-associated viral samples were harvested at 6 days postinfection, and the infectious HCMV titer was assessed by plaque assay. Titers from triplicate experiments are represented as mean titers ± the SD.
To determine whether depletion of endogenous PACS-1 protein could cause a similar effect as dominant-negative PACS-1 expression on HCMV growth in culture, an siRNA approach was used. HFF cells were transfected with or without PACS-1 siRNA and infected with HCMV; viral samples were harvested, and titers were determined 5 to 7 days postinfection. In HFF cells with depleted PACS-1 (due to siRNA transfection) a similarly small but reproducible decrease in infectious HCMV production was observed compared to control cells (∼2.5-fold decrease; Fig. 5B). Taken together, these data suggest that PACS-1 function is necessary for efficient production of infectious HCMV in cell culture systems.
DISCUSSION
An important element for the successful assembly of herpesviruses in the envelopment/de-envelopment/re-envelopment model of herpesvirus egress is the correct spatial and temporal localization of virus envelope proteins to the site of final envelopment. It has been shown previously that several herpesvirus envelope proteins localize to a juxtanuclear compartment in cultured cells and show various degrees of colocalization with markers of the TGN (1, 2, 5, 17, 19, 24, 30, 36, 40). This compartment is thought to be a possible site where final herpesvirus envelopment occurs (12, 25, 38, 40). For certain herpesvirus envelope proteins, a cluster of acidic residues in their cytoplasmic domains has been shown to be important for their TGN localization (1, 2, 5, 10, 36). We have previously shown that this type of acidic cluster motif in cellular proteins such as furin and CI-MPR interacts with the connector protein PACS-1 and that PACS-1 is necessary for efficient retrieval of these proteins from endosomal compartments to the TGN (7, 37). We now show that, as with furin and other cellular proteins, PACS-1 interacts with HCMV gB in a CK2 phosphorylation-dependent manner. Furthermore, disruption of PACS-1 function by either dominant-negative PACS-1 expression or siRNA depletion techniques leads to a mislocalization of HCMV gB to endosomal compartments. This mislocalization occurs whether HCMV gB is expressed separately or during HCMV infection. Moreover, the mislocalization appears to be specific for proteins with acidic cluster motifs, since there is no effect on the localization of the endogenous glycoprotein TGN46 or gH, an HCMV glycoprotein without an acidic cluster. In HCMV permissive cell lines, disruption of PACS-1 function by dominant-negative PACS-1 expression or protein reduction by siRNA techniques leads to a small but reproducible reduction in infectious HCMV titer (to ca. 40 to 50% of control levels). Unexpectedly, there was an increase in infectious HCMV titer in U373 cells that expressed higher levels of wild-type PACS-1 protein (to ca. 200% of control levels), suggesting that PACS-1 may be a limiting molecule for HCMV replication in some cultured cell lines.
Previous reports have shown that HCMV gB cycles from the TGN to the plasma membrane, undergoes internalization, and then recycles back to the TGN where it can be incorporated into mature virions (10, 19, 29). Interestingly, blocking the internalization of gB with dominant-negative dynamin (a protein essential for clathrin-dependent endocytosis) (31) did not affect infectious HCMV titer, suggesting that gB molecules that leave the TGN, arrive at the plasma membrane, and then recycle to the TGN (for whatever unknown function) are not required for virion formation (19). The observation that blocking internalization of gB has no effect on HCMV titer does not correlate with our observation that inhibiting the PACS-1 based retrieval of gB from endosomes to the TGN (a later step in the plasma membrane to TGN pathway) does in fact reduce the levels of infectious HCMV produced. These data suggest PACS-1 has a more important role in gB localization (and infectious HCMV production) than dynamin-mediated endocytosis. One explanation for this disparity in the effects of PACS-1 and dynamin inhibition can be gleaned from observations of the cellular protein furin. Populations of furin have been shown to be present within a PACS-1-dependent local cycling loop between the TGN and endosomes, and furin molecules contained within this loop are only able to leave and reach the plasma membrane after dephosphorylation of the acidic cluster motif (26, 37). It is therefore quite possible that a proportion of HCMV gB traffics in a similar local cycling loop between the TGN and endosomes and that only a subset of these gB molecules reach the cell surface. If so, then interfering with PACS-1 function would prevent a greater amount of gB from returning to the TGN than interfering with dynamin function. Presumably, this results in a reduction of the level of gB in the TGN, causing infectious HCMV assembly to be inhibited.
Given the significant mislocalization of HCMV gB, as opposed to gH (and presumably the other subunits of the gH complex, such as gL and gO), when PACS-1 dominant negative is expressed, the fact that the HCMV titer is only reduced by 40 to 50% in cell culture when PACS-1 is inhibited is somewhat surprising. One possible reason the drop in titer is not greater is that sufficient levels of de novo-synthesized gB molecules are arriving at the TGN from the Golgi complex to allow half the normal infectious viral production to proceed. Alternatively, if gB and other acidic-cluster-containing HCMV envelope proteins are stuck in endosomal compartments and if the other glycoproteins also flux through the same endosomal compartments (in a PACS-1-independent manner), then viral envelopment could be taking place in these endosomes, albeit with a 50% lower efficiency than normal. Interestingly, it has recently been shown by electron microscopy that both gB and gH are present in multivesicular bodies (late endosomes) during HCMV infection (11), suggesting possible envelopment of HCMV in endosomal compartments. With respect to the relatively small reduction in HCMV titer in PACS-1-deficient cells, it is important to note that the relatively high rate of viral replication and protein expression in cell culture is not necessarily an accurate representation of viral replication in normal human hosts. It is therefore conceivable PACS-1 may play a more prominent role in HCMV replication in humans than in cell culture. Such possibilities warrant further investigation of the role of PACS-1 through the use or animal models of herpesvirus replication and pathogenesis, wherein the function of PACS-1 can be manipulated in a temporal and spatial manner.
One question that still remains from these studies is whether the drop in titer observed when PACS-1 is inhibited represents a drop in the physical number of enveloped particles (due to a lower concentration of gB causing final envelopment to be inhibited) or the same number of particles but with lower concentration of gB in their envelopes, causing the assembled HCMV virions to be less able to infect target cells. Conversely, the increase in infectious HCMV produced in the presence of higher PACS-1 protein levels could be due to either an increase in the physical number of enveloped particles or the same number of particles but with a greater concentration of gB in their envelopes giving an enhanced infectivity. Hopefully, insight into these questions will be obtained through the ongoing studies of the protein composition of virion envelopes produced in cells with disrupted PACS-1 function and by electron microscopy to assess the relative proportions of enveloped and nonenveloped virus particles produced.
In summary, we have shown here that the cellular trafficking protein, PACS-1, is required for the correct localization of HCMV gB to the TGN in cells but not for HCMV gH localization and that disruption of PACS-1 function causes a modest but reproducible inhibition of infectious HCMV production.
Acknowledgments
We thank W. Britt (University of Alabama), S. Pfeffer (Stanford University), and J. A. Nelson (Oregon Health and Science University) for generous gifts of reagents. We also thank members of the Thomas lab and the Nelson lab, as well as D. Johnson (Oregon Health and Science University), for helpful discussions and advice.
This work was supported by National Institutes of Health grants (AI48584, AI49793, and DK37274) and by the Wellcome Trust (Traveling Research Fellowship to C.M.C.).
REFERENCES
- 1.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15: 6096-6110. [PMC free article] [PubMed]
- 2.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C. H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853-866. [DOI] [PubMed] [Google Scholar]
- 4.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brideau, A. D., T. del Rio, E. J. Wolffe, and L. W. Enquist. 1999. Intracellular trafficking and localization of the pseudorabies virus Us9 type II envelope protein to host and viral membranes. J. Virol. 73:4372-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 7.Crump, C. M., Y. Xiang, L. Thomas, F. Gu, C. Austin, S. A. Tooze, and G. Thomas. 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dittie, A. S., L. Thomas, G. Thomas, and S. A. Tooze. 1997. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO J. 16:4859-4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbashir, S. M., J. Harborth, K. Weber, and T. Tuschl. 2002. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 26:199-213. [DOI] [PubMed] [Google Scholar]
- 10.Fish, K. N., C. Soderberg-Naucler, and J. A. Nelson. 1998. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J. Virol. 72:6657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraile-Ramos, A., A. Pelchen-Matthews, T. N. Kledal, H. Browne, T. W. Schwartz, and M. Marsh. 2002. Localization of HCMV UL33 and US27 in endocytic compartments and viral membranes. Traffic 3:218-232. [DOI] [PubMed] [Google Scholar]
- 12.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39:389-400. [DOI] [PubMed] [Google Scholar]
- 14.Grose, C. 1990. Glycoproteins encoded by varicella-zoster virus: biosynthesis, phosphorylation, and intracellular trafficking. Annu. Rev. Microbiol. 44:59-80. [DOI] [PubMed] [Google Scholar]
- 15.Grose, C., W. Jackson, and J. A. Traugh. 1989. Phosphorylation of varicella-zoster virus glycoprotein gpI by mammalian casein kinase II and casein kinase I. J. Virol. 63:3912-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, F., C. M. Crump, and G. Thomas. 2001. Trans-Golgi network sorting. Cell Mol. Life Sci. 58:1067-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Homman-Loudiyi, M., K. Hultenby, W. Britt, and C. Soderberg-Naucler. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-Golgi network 46, and mannosidase II. J. Virol. 77:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber, M. T., and T. Compton. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarvis, M. A., K. N. Fish, C. Soderberg-Naucler, D. N. Streblow, H. L. Meyers, G. Thomas, and J. A. Nelson. 2002. Retrieval of human cytomegalovirus glycoprotein B from cell surface is not required for virus envelopment in astrocytoma cells. J. Virol. 76:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jean, F., L. Thomas, S. S. Molloy, G. Liu, M. A. Jarvis, J. A. Nelson, and G. Thomas. 2000. A protein-based therapeutic for human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 97:2864-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones, B. G., L. Thomas, S. S. Molloy, C. D. Thulin, M. D. Fry, K. A. Walsh, and G. Thomas. 1995. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 14:5869-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirchhausen, T. 1999. Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 15:705-732. [DOI] [PubMed] [Google Scholar]
- 23.Li, L., J. A. Nelson, and W. J. Britt. 1997. Glycoprotein H-related complexes of human cytomegalovirus: identification of a third protein in the gCIII complex. J. Virol. 71:3090-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molloy, S. S., L. Thomas, C. Kamibayashi, M. C. Mumby, and G. Thomas. 1998. Regulation of endosome sorting by a specific PP2A isoform. J. Cell Biol. 142:1399-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norais, N., J. A. Hall, L. Gross, D. Tang, S. Kaur, S. H. Chamberlain, R. L. Burke, and F. Marcus. 1996. Evidence for a phosphorylation site in cytomegalovirus glycoprotein gB. J. Virol. 70:5716-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson, J. K., R. A. Santos, and C. Grose. 1998. Varicella-zoster virus glycoprotein gE: endocytosis and trafficking of the Fc receptor. J. Infect. Dis. 178(Suppl. 1):S2-S6. [DOI] [PubMed] [Google Scholar]
- 29.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hubinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmid, S. L., M. A. McNiven, and P. De Camilli. 1998. Dynamin and its partners: a progress report. Curr. Opin. Cell Biol. 10:504-512. [DOI] [PubMed] [Google Scholar]
- 32.Sissons, J. G., M. Bain, and M. R. Wills. 2002. Latency and reactivation of human cytomegalovirus. J. Infect. 44:73-77. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi, S., T. Nakagawa, T. Banno, T. Watanabe, K. Murakami, and K. Nakayama. 1995. Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues within the cytoplasmic domain. J. Biol. Chem. 270:28397-28401. [DOI] [PubMed] [Google Scholar]
- 34.Thomas, G. 2002. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell. Biol. 3:753-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan, L., S. S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. L. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205-216. [DOI] [PubMed] [Google Scholar]
- 38.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao, Z., W. Jackson, and C. Grose. 1993. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J. Virol. 67:4464-4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu, Z., M. D. Gershon, Y. Hao, R. T. Ambron, C. A. Gabel, and A. A. Gershon. 1995. Envelopment of varicella-zoster virus: targeting of viral glycoproteins to the trans-Golgi network. J. Virol. 69:7951-7959. [DOI] [PMC free article] [PubMed] [Google Scholar]