Abstract
Agaricus blazei Murrill (ABM) popularly known as ‘Cogumelo do Sol’ in Brazil, or ‘Himematsutake’ in Japan, is a mushroom native to Brazil, and widely cultivated in Japan for its medicinal uses, so it is now considered as one of the most important edible and culinary-medicinal biotechnological species. It was traditionally used to treat many common diseases like atherosclerosis, hepatitis, hyperlipidemia, diabetes, dermatitis and cancer. In vitro and in vivo ABM has shown immunomodulatory and antimutagenic properties, although the biological pathways and chemical substances involved in its pharmacological activities are still not clear. The polysaccharides phytocomplex is thought to be responsible for its immunostimulant and antitumor properties, probably through an opsonizing biochemical pathway. Clinical studies are positive confirmations, but we are still at the beginning, and there are perplexing concerns especially relative to the content of agaritine. Argantine is a well-known carcinogenic and toxic substance in animals, that must be completely and fully evaluated.
Keywords: Agaricus blazei Murrill (ABM), cancer prevention, immune response, agartine, medicinal mushroom
Introduction
Mushrooms and primarily basidiomycetous fungi are a popular and valuable food, low in calories and high in minerals, essential amino acids, vitamins and fibers (1); some of them produce substances having potential medical effects, and are called medicinal mushrooms. Agaricus blazei Murrill (ABM) is known in Brazil as Cogumelo do sol or medicinel, in Japan as Himematsutake, Agarikusutake or Kawarihiratake and in China as Ji Song Rong. It was brought to Japan due to alleged health effects and is widely used today in Oriental countries both as an edible mushroom, considered a functional food, and as natural therapy in the form of a medicinal extract mostly for prevention and treatment of cancer. In accordance with Brazilian tradition, it would be useful against a variety of diseases like diabetes, atherosclerosis, hepatitis, hypercholesterolemia, heart disease and so on. In Japan, researchers demonstrated immunostimulant and anticancer effects of ABM extracts experimentally, and due to the improving consumption of this mushroom in recent years, a considerable effort investigated the putative effects with interesting, but still insufficient clinical studies. Experimental studies increased commercial interest for ABM because of many requests as popular remedy especially in Japan, stimulating not only the production, but also the registration of new names and brands with new popular names. This makes it difficult for the public to identify pure ABM strains.
History and Ethnopharmacology
Agaricus blazei Murrill is a mushroom originally native to a small village, name Piedade, in the highland areas of Atlantic forest, near Tauape, in the province of Sao Paolo, Brazil. It was discovered in 1960 by Takatoshi Furumoto a grower and researcher who sent it to Japan in 1965 for investigation. It was identified as ABM by the Belgian botanist Heinemann in 1967 (2). Later it was given the common name of Himematsutake in Japan, while in Brazil it was named Cogumelo Piedade. The mushroom is traditionally believed to fight physical and emotional stress, stimulate immune system, improve the quality of life in diabetics, reduce cholesterol, prevent osteoporosis and peptic ulcer, treat circulatory and digestive problems and fight cancer (2). All traditional and not-proved beliefs, as often happens, are intentionally used and publicized on the web and mass media for commercial purposes often without any real scientifically demonstrated clinical benefit for patients (3). Over the last decade, the mushroom has been studied as a novel functional food in Japan, Korea, China and Taiwan. The fruiting bodies are still quite expensive to grow, so a relatively cheap and stable source for commercial purpose is still sought. Medicinal mushrooms have an established history of use in traditional oriental therapies: historically, hot-water soluble fractions from medicinal mushroom were used as medicine in the Far East from where this knowledge and practice seem to have been originated. The first historical description about the use of mushroom of Agaricus genus for medicinal purposes is probably described by Byzantine medical treatises in the Mediterranean area, from the 4th century AD to the 15th century AD by Orivasios and Apuleius for treating malignant ulcers of the larynx (4).
Botanics
Agaricus L.: Fr. Emend Karst. is the type genus of the family Agariaceae in the order Agariales (5), and is generally described as having small to large fruit bodies with white, yellow or brown pileus; free lamellae that are pallid or pinkish when young, later becoming chocolate-brown; and also dark-brown, smooth basidiospores (6). This highly diverse genus was divided into three subgenera by Heinemann (7): Agaricus, Conioagaricus and Lanagaricus. In this subdivision, the subgenus Agaricus contains the most typical species.
Agaricus spp. are saprophytes widely distributed over geographical areas from the tropics to the boreal regions, inhabitating a variety of habitats from alpine meadows, to salty and sandy seashores, to deciduous and conifer woodlands (6) The most economically important species is A. bisporus Imbach that is the most widely cultivated edible mushroom, accounting for 32% of the more than million metric tons of mushrooms produced worldwide in 1997 (8). ABM (Fig. 1) is a large Agaricus species with a brownish-gold cap (7–2 cm broad), convex, fleshy, the stem short and hard, with chocolate brown basidiospores (5 × 4 µm) and is closely related to A. subrufescens (9,10). The mushroom grows with a stalk length and a cap diameter that are about equal (campestroid type). As a litter-decomposing fungus, it naturally grows well in soils rich in lignicolous debris, in mixed woods, along forest edges and manures. Nowadays main cultivation centers are established in Japan, China and Brazil, where the fungus is cultured in enriched composts or pasteurized substrates supplemented with nitrogenous additives (10). New data indicate that the medicinal mushroom from Brazil and Japan could be biologically and phylogenetically the same species as A. subrufescens Peck from North America, although a search on the web and a review of diverse commercial product literature indicate that association of the name ABM with the Brazilian mushroom is attributed to P. Heinemann (11). So there would be an interfertility between North American A. subrufescens and the ‘medicinal Agaricus’; the presence in hybrids of genetic materials from two progenitors and novel phenotypes indicate that members of these geographically distant mushroom populations might constitute a single ‘biological species’ (11). This could be confirmed by the paper of Colauto (12) showing little genetic variability among commercialized strains based on results of RAPD analysis data of 20 primers from fungi cultivated in malt-agar medium for DNA extraction. The paper showed that some commercialized A. blazei spawns in Brazil have identical genotypes, and are probably clones having the same origin, which could be Japan (12). Nevertheless, recently Wasser has published (13) an historical-botanical analysis of the mushroom concluding that ABM differs from A. blazei ss. Heinemann in (i) size, shape of fruit bodies and pileal surface; (ii) type of pileal covering; (iii) presence of cheilocystidia; and (iv) spore size. That is, North American endemic species A. blazei ss. Murrill and the widely cultivated medicinal A. blazei ss. Heinemann would be two different species; and A. blazei ss. Heinemann should be considered a new species: A. brasiliensis (13,14). This problem is probably to be considered still open until an official international consensus statement will end this botanical dispute.
Figure 1.
Agaricus blazei Murill mushroom.
Phytochemical Constituents
Chemical Composition
In general, the gross composition of mushrooms is water (90%), protein (2–40%), fat (2–8%), carbohydrates (1–55%), fiber (3–32%) and ash (8–10%) (ash is mainly composed of salts, metals and so forth). Active metabolites can be isolated from fruiting bodies, pure culture mycelia and culture filtrate, and nowadays many attempts are being made to obtain active metabolites from mycelia through submerged fermentation culture to obtain cheaper preparations. Kawagishi was the first to separate an active anticancer compounds purified from the sodium hydroxide extract of the fruit body of ABM (15). The author detected polysaccharides with apparent antitumor activity, the major fraction being FIII-2-b, which comprised a protein complex composed of 43.4% protein and 50.2% carbohydrates (15). The FIII-2-b fraction contained simple (1-6)-β-D-glucopyranosyl chains. A significant contribution to the anticancer activity of the protein moiety of FIII-2-b was also speculated following the complete loss of antitumor activity after formolysis. ABM fruiting bodies in different stages of maturity contain α-glucans and β-glucans: the yield and structural diversity of glucans increase as the fruiting bodies mature; so the time of the harvest and conservation is of great importance, to obtain the best extract, data that almost invariably are not reported in scientific articles.
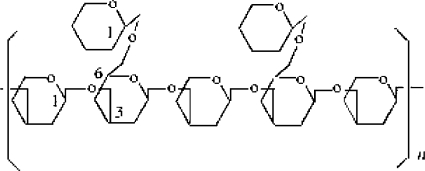
The α and β-glucan Structure
ABM glucans are side branches of a (1-6)-β-backbone as found by Dong and Ohno, who described that active fraction of β-glucans of ABM fruiting bodies had a (1-6)-β-backbone structure (or functional center) with (1-3)-β-side branches in the ratio of 1 : 2 (16); while the linear (1,6)-β-glucan seems to be inactive (17) (Fig. 2). The biochemical importance of (1-3)-β-side branches has been confirmed and has shown the enhancement of the immunomodulatory activity of polysaccharides (18); and Mizuno (19) reported an important antitumor activity linked to the water-soluble (1-6)-(1-3)-β-D-glucan. However, a significant increase of water-soluble (1-4)-α-glucan with apparent antitumor activity occurs during maturation (19); so probably cap-opened, more fragile mature fruiting bodies of ABM should be selected over immature ones for the production of nutraceuticals because they contain the most useful glucans (20). In addition, an α-1,6 and α-1,4 glucan complex (21) and a glucomannan with a main chain of β-1,2-linked d-mannopyranosyl residues have been isolated from this mushroom and found to inhibit tumorigenesis (22).
Figure 2.
(1–6)-β-backbone structure (or functional center) with (1–3)-β-side branches.
Mechanisms of Tumorigenesis and Carcinogens
These results suggest that whole-mushroom extracts contain compounds that may modulate tumorigenesis and carcinogenesis at different stages and/or may act at the same stage through different mechanisms. Responses to such highly different polysaccharides are likely to be mediated by different cell-surface receptors, which may be present only on specific subsets of cells, and may trigger distinct downstream responses. A combination of such responses involving different cell subsets could conceivably provide greater tumor inhibition than could be induced by a single polysaccharide. Nevertheless, a very important problem is the wide number of different and only partially homogeneous ABM extracts used to study the pharmacological activities of its constituents representing a difficult challenge to establish the best extract and active substances. Thus all these similar constituents could potentially provide additive, or even synergistic, effects in the prevention and treatment of cancer. Moreover they could interfere with other substances or healthy physiological functions. This has been shown by an in vitro study in which increasing fractionations of an ABM extract enhanced some biological activities but abolished others (23).
Immunologic Intervention
The specific mechanisms that contribute to an enhanced state of immunity remain partially understood. Recent insights in two rapidly expanding fields, the cytokine-mediated homeostasis of mature lymphocytes by cytokines, such as interleukins and autoreactive T cells by CD4+CD25+ regulatory T cells, provide the foundation for what might be occurring.
Recent advances in immunology have demonstrated the importance of local interactions between antigen-presenting cells and effector cells such as natural killer cells and T-lymphocytes for an effective immune reaction against tumors (24). Interferon stimulate such interactions, while interleukins play a central role in the activation of NK cells and T-lymphocytes. Interferons were investigated as potential anticancer agents because of their antiproliferative and cytotoxic effects, their ability to activate specific components of the immune system and their relatively modest toxicities. Increasing biological evidence supports the hypothesis that tumor-generated chemokines provide more than simply angiogenic signals. Tumor-derived chemokines may potentially act as inhibitors of anti-tumor immune responses as well as autocrine growth factors for the tumor. All these chemokines activating activities of A. blazei Miller remain to be completely evaluated both in animal model and in real clinical practice.
However, immunologically active glucans are (1-3)-β-d-linked glucose polymers, which occur as a primary component in the cell walls of bacteria and fungi or are secreted extracellularly by various fungi, and actually seem the most important active substance.
Studies in vitro
Biological Activities on the Immune System
The immunostimulant and immunomodulatory activity of both mycelial and fruiting bodies of ABM using water and ethanol extracts have been demonstrated in many in vitro experiments, although not always the results are concordant, but sometimes contradictory. Water extracts of the mycelial culture and fruiting bodies such as fractions B-4, B-5 obtained from ethanol precipitation (respectively 44% and 50%) of fruiting bodies, markedly induced TNF production and IL-8 of macrophages derived from rat bone-marrow (23). Fraction B-5 induced a significant increase in nitric oxide production, (23). In another paper, the same group using ethanolic fractions obtained from mycelia inhibited the occurrence of the viral cytopathic effect induced by Western equine encephalitis and herpes simplex (25). Other extracts containing lignin-based derivatives have shown the induction of TNF-γ, IL-8 and nitric oxide secretion by macrophages (26), anti-viral activity of different viruses (27), and direct anticancer activity (28); so lignin derivatives apparently have more different and important pharmacological activities. While, on the contrary, a down-regulation of IL-2, IL-4 and INF-γ in human peripheral blood mononuclear cells has also been documented (27). A hemicellulase-treated ABM fraction derived from mycelia composed of 63.3% carbohydrates, 30.9% proteins, 0.3% lipids and other minor components has shown to stimulate immature dendritic cell obtained from mice bone-marrow and up-regulate the expression of costimulatory molecules and MHC antigen, although did not increase the production of inflammatory inducible cytokines (30). ABM pre-treated dendritic cells inhibited some bacteria-mediated dendritic cells’ responses, ABM pre-treated macrophages reduced LPS-induced NF-κB activity, while ABM-mediated dendritic cells enhanced the Th1 response in allogenic mixed lymphocyte reaction. These antithetical effects may probably help to maintain immunological homeostasis, so the question is: can they be clinically effective in cancer patients? (30). Fine particles of ABM fruiting body and mycelium, respectively, prepared by mechanical disruption, activated the human complement system via the alternative pathway in human serum, is another proof of its activity in enhancing natural immunity in bacterial infections (31). From these studies it is clear that the ABM fractions act on many different biological receptors of the immune system but can also have antithetical pharmacological activity; so further studies are warranted to completely identify the real importance of this mushroom as an immunostimulant and/or immunomodulator.
Anticancer Activity
Anticancer Activity of Different Extracts
The ABM aqueous extract demonstrated no clastogenic activity whilst to have anticlastogenic properties with a 100% reduction of chromatid and 144.4% reduction of isochromatid breaks, is apparently really important for cancer prevention in humans since it is usually consumed in its natural form as tea or as food (32). Nevertheless, we emphasize that if in the same study methanolic and hexanic extracts were anticlastogenic, n-butanolic extract were both anticlastogenic and clastogenic (32); and in another article different hexane extracts of the fruiting body in culture of mammalian cells were at different concentrations genotoxic, cytotoxic and anticlastogenic; so these findings clearly suggest further studies are needed (33). Oliveira (34) studied ABM aqueous extracts, by simultaneous and pre-incubation administration, and demonstrated a strong protective effect based on the cytokinesis-blockmicronucleus (CBMN) assay under both conditions, suggesting a desmutagenic activity, and Menoli (35) observed a protective effect against CBMN induced by methyl methanesulfonate, when cells were treated with an aqueous extract of ABM strains mixture; and in the comet assay, the same authors also observed antigenotoxic potential. Moreover, resident human peripheral nucleated cells incubated in the presence of complement-opsonized complexes of fruiting bodies inhibited the proliferation of the human thyroid carcinoma cell line TPC-1 (31). Recently it has been shown on human gastric epithelial AGS cells that an aqueous extract can activate apoptosis through induction of caspase-3 and related cell cycle arrest at the G2/M phase (36).
Different Anticancer Activity of ABM Strains
ABM extracts have not always shown a protective effect against cancer. Delmanto (37) using the micronuclei test against genotoxicity induced by cyclophosphamide, found a decrease in the frequency of micronuclei after treating mice of mixed lineages with teas pre-treatment, but there was no lower micronuclei frequency with the isolated lineage AB 99/26. Luiz (38) did not find any antimutagenic activity in ABM aqueous extracts against methyl methanesulfonate in V79 cells, using the CBMN and comet assays. While using the comet assay, Guterrez (39) found no protective effect for ABM aqueous extracts in V79 cells, suggesting that differences in the cultivation, storage and extracts preparation could influence the effectiveness of preparations. In testing ABM aqueous extracts of three different origins (Botucatu-SP, Londrina-PR and Piedade-SP), Guterrez (39) observed any genotoxic potential, while an antigenotoxic activity only for ABM from Piedade-SP with pre-, post- and simultaneous treatments, and for ABM from Londrina-PR only following simultaneous treatment.
All these data implicate that lineages and pre-treatment types influence the pharmacological anticancer activity of ABM extracts and as confirmed by Manzi and Pizzoferrato (40) beta glucans, apparently the most important constituent, in mushrooms are distributed variably both in the soluble and in the insoluble dietary fraction. Luiz (41) has demonstrated that ethanol and chloroform/methanol extracts have anticlastogenic activity, although without a dose-response correlation, and the author suggests that because of a deficient repair of CHO-xrs5 cells, an activity as modulators of DNA replication and repair, and that probably fatty acids contained in the extract (especially linoleic and eicosapentanoic acid) could have a role in the antimutagenic activity of the mushroom.
In vitro Studies on Cytokines Stimulation of ABM
Examination of the cytokine-inducing activity of hemicellulase-derived mycelia extract on human peripheral mononuclear cells have shown induced expression of IL-12, a critical regulator of immune response against pathogens and tumors as it is the most potent promoter of type 1 responses in CD4 T cells; confirmed in the same article that oral administration to mice showed significant higher levels of NK cytotoxic activity of murine spleen cells (42). Stimulation of NK-cells is obtained by higher production of IFN-γ through hydroalcoholic extracts of fruiting body fractions, although the activity was significantly reduced after heat treatment of 2 h at 120°C in murine spleen cell (43). In an experimental research using a 9% solution of an aqueous ABM, extracts containing 300 µg ml−1 of β-glucans were examined to determine changes of gene expression caused by the extract on a human monocyte cell line, and drastic effects on gene expression were found: genes related to immune function were selectively up-regulated, particularly pro-inflammatory genes such as the interleukins IL-1β and IL-8. Although most genes induced by ABM were also induced by LPS, ABM produced a unique profile, e.g. as to a particular increase in mRNA for the chemokine ligands 1, 2 and 3, IL-1A, as well as prostaglandine-endoperoxidase synthase 2 (cyclooxygenase2); and gave rise to 63% inhibition of DNA synthesis and 30% inhibition of protein synthesis (44) (Table 1).
Table 1.
Chemokine-stimulated secretion
| TNF-α secretion by macrophages (23) |
| IL-8 (23), 12 (42), 1 (44) and 2B (44),10 (44) |
| Nitric oxide production by macrophages (23) |
| Stimulation of immature dendritic cells (30) |
| Enhanced Th1 response (30) |
| Activated the human complement system via the alternative pathway (39) |
| Stimulation of NK cytotoxic activity (42) |
| Higher production of IFN-γ (43) |
| mRNA increase for chemokine ligand 1, 2 and 3 (44) |
| mRNA increase for cyclossigenase 2 (44) |
| mRNA increase for regulator of G-protein signaling 1 (44) |
| Induction of caspase 3 (36) |
ABM extracts act mainly through modulation of the immune system activating macrophages, neutrophils and lymphocytes (21,45,46,47) confirming the possible anticancer activity by an enhanced immunostimulation. About protection against atherosclerotic vascular diseases, ABM has shown interesting antioxidant activity. In one study, ethanolic extract had a remarkable antioxidative substance effective in the auto-oxidation of linoleic acid (48); and as confirmed by an in vitro study through the trx1Δtrx2Δ mutant method, ABM has demonstrated to contain thermostable potent antioxidant substances, since the extract was prepared by boiling the mushroom in water for 120 min and autoclaving (49); and a wine produced by ABM contains 0.68% β-d-glucan and 8% alcohol showing fibrinolytic activity on artificial bovine thrombus, that, a preventive effect on thrombosis may pose the problem of administration in hypocoagulable defeated patients of more concentrated extracts (50).
On the basis of these discrepancies and the lack of homogeneity in the type of extracts used for experiments, it is difficult to now obtain reliable and definitive data about anticancer activity in vitro of ABM extracts, such as the immunological effects; so these studies are still in their infancy and probably a better definition of active principles is needed regarding the action mechanism in cell and interactions with cell physiological processes.
Anticancer Activity of β-Glucans
Early reports showed that β-glucans functioned by stimulating host defense mechanisms and were not toxic for tumors, but in following years β-1,3;1,6-glucans from fungi (e.g. mushrooms) and yeast became a new biological entity, so-called biologic response modifiers that function as immunostimulants against infectious diseases and showing a possible tumoricidal activity (51). Unlike most other natural products, purified β-1,3-glucans retain their bioactivity, and this has permitted the characterization of how β-1,3-glucans can work on a cellular and molecular level, showing that they function through stimulation of granulocytes (neutrophils and eosinophils), monocytes, macrophages and NK-cells (52). Certain data also suggested that β-glucans could promote T cell-specific responses, perhaps, through triggering the secretion of IFN-γ, IL-6, IL-8 and IL-12 from macrophages, neutrophils and NK-cells, and a role for T cells in β-glucan function was also proposed because of absent tumoricidal activity in nude or T-cell-depleted mice (53).
Anticancer Activity and β-Glucan Conformation
Polysaccharide antitumoral activity has been evaluated most often against allogenic sarcoma 180 in CD-I mice, a tumor sensitive to immunomodulating compounds. Of the polysaccharides with immunomodulating capacity, only those which consist of a (1→3)-linked β-glucan backbone with (l→6)-linked β-d-glucopyranosyl units as branches produce complete inhibition of tumor growth. (1→3)-β-glucans from fungi commonly have a tumor inhibition percentage of 99–l00%, while other polysaccharides exhibit l0–40% inhibition (54). Contradictory data exist on the influence of molecular weight, degree of branching, conformation and intermolecular associations of β-glucans on antitumor activity and on the mechanism(s) of their action (55). Most of the (1→3)-linked β-glucans with biological response modifier activity have been isolated from Basidiomycetes; a few with pronounced antitumor activity have come from Ascomycetes and Oomycetes (56).
Evidence suggests that the activity of these polysaccharides is also dependent on their size, with high molecular weight (100 000–200 000) fractions being most active, while fractions from the same source with molecular weights of 500–10 000 show no activity (55). The fact that there are polysaccharides with different chemical structures, but all of which have immunomodulating activity (56), suggests that the immune response is in part non-specific, determined by size rather than by chemical structure.
β-Glucan and Human Receptors
At least four receptors have subsequently been identified: complement receptor 3, lactosylceramide, scavenger receptors and Dectin-1. In addition to an iC3b binding site, complement receptor 3 possesses a lectin site for β-glucans that, in combination with iC3b, enhances phagocytic and cytotoxic responses (57). β-glucans can also prime the receptor for subsequent iC3b-mediated cytotoxic responses, including the iC3b-restricted antitumor activity (57). Lactosylceramide, a major glycosphingolipid of polymorphonuclear leukocytes, and selected scavenger receptors have also been identified as receptors for β-glucans, although their role in β-glucan-mediated responses is less clear (57).
Willment (56) has recently shown that the human receptor is widely expressed, functions as a pattern recognition receptor for β-glucans, and can also recognize T-lymphocytes (57). In contrast to the mouse receptor, the human receptor is alternatively spliced and splicing appears to be regulated in different cell types and various receptor isoforms generated by alternative splicing that differ in their ability to recognize β-glucans.
Although the two predominant isoforms are both expressed in multiple tissues they are expressed differently in various cell types, suggesting that the alternative splicing of these two isoforms can be regulated (57). While the significance of this is unclear, the presence or absence of a stalk does not seem to have significant effects on the ability of this receptor to recognize β-glucans. The other isoforms represent a minor population of the splice variants, and they may serve regulatory roles, a phenomenon described for other cell surface receptors such as CD40 (59) and scavenger receptor type A (60).
In addition to its ability to recognize glucans, the human β-glucan receptor also recognizes a subset of T cells. Given the similarity of the β-glucan receptors to those of the NK-cell-like C-type lectin-like domains (53,61) which normally recognize specific major histocompatibility complex class I molecules on target cells, it is possible that the ligands on T cells are major histocompatibility complex class I molecules. The ability of the human β-glucan receptor to recognize only one of the four T cell lines tested suggests that the ligand is restricted to a subset of T cells and we are currently exploring this possibility further (57). While the biological function of this interaction is unknown at present, it poses an intriguing role for this receptor in the recognition of self and non-self ligands (58).
Gastrointestinal Absorption of β-Glucans
There are also reports that some mushroom β-1,3;1,6-glucans could mediate tumor regression when given orally (62,63). In more recent studies using human tumor xenografts, orally administered soluble barley β-1,3;1,4-glucan or i.v. antitumor monoclonal antibodies were ineffective as single agents, but when combined, elicited a substantial antitumor effect (64,65). However, the mechanism by which large β-1,3-glucans could be taken up orally by the gastrointestinal tract and function to prime leukocyte CR3 was unknown.
Opsonizing Activity of β-1,3-Glucan
An important investigation showed that these large β-1,3-glucans were taken up by gastrointestinal macrophages and shuttled to reticuloendothelial tissues and bone marrow. Within the marrow, the macrophages degraded the β-1,3-glucan and secreted small soluble biologically active fragments that bound to CR3 of mature bone marrow granulocytes. Once recruited from the bone marrow by an inflammatory stimulus, these granulocytes with β-1,3-glucan-primed CR3 could kill iC3b-coated tumor cells. As had been found earlier with i.v. soluble yeast β-1,3;1,6-glucan therapy, oral β-1,3-glucan-mediated tumor regression required the presence of iC3b on tumors and CR3 on granulocytes, and therefore failed in mice deficient in C3 or CR3.
Yan (65) showed that the tumoricidal activity of soluble CR3-binding polysaccharides such as β-glucan was specific for neoplastic cells that had been opsonized with C3 through the action of naturally occurring tumor-reactive antibodies. Tumors that bore a sufficient density of C3 for recognition by the CR3 of circulating leukocytes responded to therapy with β-glucans, whereas tumors that were not opsonized with C3 did not respond to therapy (66). It is well known that β-glucan responses occur only in certain strains of mice bearing specific tumors, so this report (66) suggests that reports of the sensitivity or resistance of specific tumors to β-glucan corresponds to the presence or absence of antibodies capable of opsonizing the tumor with iC3b, opening new possible therapeutic options for treatment of immune-based therapies for human cancer.
β-glucans can potentially be used to generate a novel cell-mediated effector mechanism for tumor vaccines and antibodies to tumor antigens that otherwise rely mostly on the direct cytotoxic action of chemotherapy. This therapy appears to have the greatest applicability to metastatic tumors that have lost MHC class I and thus have escaped recognition cytotoxic lymphocytes (66,67). Such metastatic tumors frequently express polysaccharide or ganglioside tumor antigens for which there is an array of available vaccines and antibodies.
Studies in Animals
ABM Extracts as Anticancer Agents
Aqueous extracts of ABM given in the drinking water to rats and mice before chemical cancer induction exhibited antimutagenic effects, but were ineffective when administered in the post-induction period demonstrating protection only in the initiation step of liver carcinogenesis (37,68,69). These studies are of particular interest because the extraction consisted in preparing a ‘crude aqueous extract’ leaving a powdered dry fruiting body in water at room temperature for 2 h, which is the way ABM is popularly prepared (69).
Interestingly, rats fed with a dry powdered form of ABM using two strains (99/26 and 99/29) and prepared from two different moments of harvest (opened or closed basidiocarp) at 10% of the diet, on the basis of the strain and harvest (the more effective was strain Ab 26 closed basidiocarp) exhibited different significant antimutagenic activity, more evident when considering the reduction of both size and number of the preneoplastic lesions, even when given in the post-initiation period (70). Moreover, not only polysaccharides but also the lipid fraction of ABM was found to contain a compound with antitumor activity, subsequently identified as ergosterol (a precursor of ergocalciferol), that inhibited tumor-induced neovascularization in sarcoma 180-bearing mice by oral administration for 20 days without side effects, though the extract had no cytotoxic effect in vitro (71). Kimura (72) has identified from the lipid fraction sodium pyroglutamate, which has shown not only to have antiangiogenic (inhibition of von Willebrand Factor expression in tumors) and antitumor activity in Lewis lung carcinoma-bearing mice but also an inhibitory effect on the cancer-induced reduction of immune functions.
Ethanol fractions obtained from hot-water extract of mycelium or dried fruiting induced TNF-α and IL-8 secretion in rat bone-marrow macrophages. Further fractionation with increasing ethanol concentrations resulted in the reduction of this cytokine-inducing ability in mycelial extracts, but enhanced it in fruiting body extracts. Whereas mycelial fractions did not induce nitric oxide production, fractions obtained by precipitation of fruiting-body extract with high ethanol concentrations stimulated macrophages to produce significantly higher levels of nitric oxide than controls (23).
In a mouse model of peritonitis induced by i.p. injection of fecal stem solutions the pre-challenge oral administration of an aqueous ABM extract protected mice against lethal septicemia after fecal peritonitis as demonstrated by a reduction in bacteremia and increase in survival rate, which was comparable with the survival of a verum group to which were administered per os metronidazole and doxycicline (73); and confirming previous results of the same group in mice given i.p. infection with the virulent Streptococcus pneumoniae serotype 6B (74).
ABM Extracts as Immunostimulants
In an interesting article (75), experimenting intradermal injections of four different ABM extracts (ethanol, water, oxalate soluble and insoluble fractions) in a bilateral Meth-A tumor model, mice administered with the oxalate soluble fraction were tumor free after 21 days. In the same article, oral ad lib administration of the same fraction had no antitumor effect but enhanced that of the intratumorally injected fraction (P < 0.01 versus injection alone) (75). Significant macrophage chemotactic factor, but not neutrophil chemotactic factor activity, was detected, while serum levels of immunosuppressive chemotactic factor (a marker protein of activated macrophages and neutrophils in response to biological response modifier) increased dramatically suggesting an immunopotentiating activity besides a direct cytotoxic action on tumor cells (75).
The oxalate soluble fraction consisted of a large amount of carbohydrates (exclusively glucose as determined by HPLC) and small amounts of proteins; carbohydrates containing (1-4)-β-d-glucan and (1-6)-β-d-glucan in the ratio of approximately 1 : 2 (45,75).
Treatment with hot-water extracts of ABM fruiting bodies increased NK activity of spleen cells in naive BALB/c mice (75). In Meth A-bearing BALB/c mice, the same extracts enhanced the induction of antigen-specific cytotoxic T-lymphocytes and IFN-γ production. Up-regulation of NK and TC activity was triggered by IL-12 dependent activation (76) although it is not yet clear whether oral administration of ABM extract enhances IL-12 production in vivo (77).
These data are confirmed by a study of Itoh (78) showing moderate antiblastic activity in mice Meth-A tumor model but only by i.p. administration of FIII-2-b fraction, confirmed in another study where a new polysaccharide–protein complex (called ATOM: antitumor organic substance Mie) administered p.o. and i.p. appeared highly active against in mice sarcoma 180, Ehrlich ascites carcinoma, Shionogi carcinoma 42 and Meth A fibrosarcoma models, trough activation of immunostimulatory activity mediated by macrophage and complement (79).
Ehrlich carcinoma-bearing mice treated per os with the n-hexane (mainly unsaturated fatty acids), dichloromethane (sugar and amino acids), or methanol (mainly unidentified polymers) extracts from ABM fruiting bodies were able to maintain the NK activity of spleen cells during the first 10 days after tumor implantation (80). NK activity in these groups was similar to that of normal controls and higher than that of tumor-bearing mice treated with water. After 30 days, animals treated with n-hexane extract showed lower tumor growth than the other groups, but mice assuming dichloromethane extract presented signs of presumed toxicity (necrotic lesions in the extremity of the tail probably due to residues of organic solvents). The dichloromethane and methanol groups produced a more intense humoral response than the n-hexane and ethanol extract groups.
The results of NK activity on the 30th day after the injection of tumor cells suggest that none of the three extracts was able to maintain the lytic activity against Yac-1 target cells. And 30 days later, the Ehrlich carcinoma cells were enough to decrease the NK activity, perhaps, by the production of soluble factors like prostaglandins, TGF-β, or IL-10 (62).
Tumoricidal Acivity of β-Glucan
In a mouse model, for the first time in 2005, Kobayashi (81) has demonstrated that daily oral supplementation of β-glucan by a hydrochloric acid fraction (16.6% proteins, 90% glucose composed by 1-4-α-d-glucan and 1-6-β-d-glucan in the ratio 1 : 2) (82) seems the only orally active preparation in mice with respect to aqueous ammonium oxalate-soluble and ethanol-insoluble derivatives of ABM that are active only if administered intratumorally (83), probably due to a direct effect on tumor invasion and metastasis through a direct modulation of signalling cascades: inhibition of thymidine incorporation in a dose-dependent fashion in ovarian cancer cells in vitro although not in Lewis lung cancer cells. Data in vitro have been confirmed in a mouse model of peritoneally disseminated metastasis of human ovarian cancer by intraperitoneal injection and confirmed by oral administration, without influence on mean body weight or food consumption and the average number of formation of pulmonary nodules was lower on experimental lung metastasis of Lewis lung cancer (81). These actions are probably due to the suppression of cell proliferation, apoptosis and inhibition of urokinase-type plasminogen activator through promotion of p38 MAPK activation (81).
In reference to other popular supposed pharmacological activities, the only experimental data are those from a model (84) of rats with a streptozocin induced diabetes treated by oral administration of a dried fruiting body hot-water extracts showed anti-hyperglycemic, anti-hypertriglyceridemic, anti-hypercholesterolemic and anti-arteriosclerosis activity indicating overall anti-diabetic activity in diabetic rats, but oligosaccharides obtained by hydrolization of β-glucans by means of Bacillus megaterium showed higher activity than β-glucans.
So, many studies show in animals the potential activity of ABM extracts, and more than a direct antiblastic activity. Moreover on the basis of in vitro studies it is possible to assume that the most promising pharmacological activities are immunostimulatory and antiangiogenic probably by means of different extracts (Table 2).
Table 2.
Main active anticancerogenic substances
| Ergosterol | Antiangiogenic (71) |
|---|---|
| Pyroglutamate | Antiangiogenic, antiblastic, (immunostimulant?) (72) |
| Polysaccharides(1-6)-β-backbone | Immunostimulant, (antiblastic?) (81–83) |
| Lignin derivatives | Immunostimulant (TNF,IL8) (antiblastic?) (27,29) |
| Polysaccharide–protein complex | immunostimulant, (antiblastic?) (15,78,79) |
Clinical Studies
ABM in Cancer Patients
According to reports, 100 000–300 000 kg of the dried body of ABM is produced every year in Japan, and about 300 000–500 000 persons for the prevention or treatment of cancer assume the 3–5 g three times a day by a typical hot-water extract (71). In a small survey on the use of complementary therapies by patients affected by urological cancer in Japan, on a total of 293 patients surveyed, 52 were assuming ABM extracts representing a percentage of 31% and being the most commonly used ‘health food’ (85). Ahn (86) investigated the beneficial effects of the oral daily assumption of an extract of Agaricus blazei Murrill Kyowa (in the exact content and quantity of substances assumed by patients, and drop out for any cause were not described in the study) on immunological status and qualities of life in cancer patients undergoing chemotherapy. They observed that NK-cell cytotoxic activity, was significantly higher after a 6 weeks period compared with placebo, although there was no difference in white blood cells decrease in patients upon chemotherapy (carboplatin, etoposide and taxol). However, chemotherapy-associated side effects such as appetite, alopecia, emotional stability and general weakness were all improved on the base of the QLQ-30 Scoring Manual 2nd edition of EORTC modified by authors (86).
Clinical Studies in the Treatment of Hypertension, Hypercholesterolemia and Hepatic diseases
Administration of γ-aminobutyric acid (GABA)-enriched A. blazei (AG-GABA) to mild hypertensive human subjects, in an open test and double blind cross-over test, showed that during AG-GABA intake period, both systolic and diastolic blood pressure values decreased to statistically significant levels, if compared with those of the pretest period or placebo intake period. No significant difference was observed, neither in the values of cholesterol nor of hepatic transaminases and γ-GTP (87).
The effects of protein-bound polysaccharides (A-PBP and L-PBP) that were extracted from the mycelia of A. blazei on serum cholesterol and body weight were investigated in 90 female volunteers for 8 weeks: the weight-reduction effect (11.8%) and hypocholesterolemic effect (11.0%) was most significant, indicating their synergistic action. These data suggested that the weight-controlling and hypolipidemic effect of L-PBP and A-PBP protein-bound polysaccharides were involved, at least in part, in absorption of cholesterol as their role of dietary fiber, as well as cholesterol metabolism (88).
A study evaluated the clinical effects and safety on human volunteers with elevated γ-GTP activity of A. blazei Condensed Liquid (Agaricus Mushroom Extract; ABCL) in the treatment of C-hepatitis. A total of 20 patients (50% of men) with chronic C-type hepatitis received the ABCL orally, twice a day, for 8 weeks. Decreasing effect for serum γ-GTP activity was found in 80% of the patients in both sexes; without any toxicological findings and other side effects (89). These initial clinical data (Table 3) are interesting, but we think it is soon to establish definitely a real benefit from the assumption of ABM extracts although it is not known exactly which are the active substances, although actually only β-glucans can be considered as the more active substance.
Table 3.
Clinical studies
Toxicity
The Problem of Heavy Metals and Radioactive Substances
An important clinical-toxicological concern represented by mushrooms, especially wild ones, is the possible contamination with substantial levels of toxic metals such as arsenic, lead, cadmium and mercury as well as 137Cs, because many mushrooms species have the ability to accumulate radioactive substances such as relatively high concentrations of metals (90). So high levels of toxic compounds may offset whatever health benefits a diet rich in mushrooms or their extract could potentially confer (88,91,92,93).
Pharmacological Interferences
ABM extract can also down-regulate the expression of cytochrome P4501A and can be useful in reducing the production of metabolically activated procarcinogen from xenobiotics. It can consequently prolong the duration and intensity of drugs’ activity, and could give rise to unpredictable side effects or adverse drug reactions (94).
Toxicity in Animals
In a study to evaluate 90-day subchronic toxicity of an aqueous extract in F344 rats, there were no consistent treatment-related changes in clinical signs, body weight and food consumption at the dose of 2654 mg kg−1 b.w. day−1 for male and 2965 mg kg−1 b.w. day−1 for female rats, although there was an increase of blood urea nitrogen in males that was considered unlikely to be of toxicological significance (contemporary decrease of creatinine and no histopathological changes reported) (95). Although it has not been established the direct quantity toxic or cancerogenic for humans, a main problem for the administration of ABM remains the problem of aromatic hydrazines (i.e. agaritine and its derivatives) whose cancerogenicity and chronic systemic effects are well known in animals for many years (96); probably due to metabolized toxic intermediates capable of damaging cellular macromolecules and stimulating proteolysis giving rise to hydrazine-mediated DNA strand scissions (97). Toth demonstrated that the administration of hydrazine analogs administered s.c. in Swiss mice induced fibrosarcoma in 24% of males, and in both sexes soft tissue tumors (98); while in another article agaritine administered in drinking water at 0.062 and 0.031% did not give rise to cancer although a substantial number of animals (Swiss mice) developed convulsive seizures (99); while hydrazine analogs (from A. bisporus and Gyromitra esculenta) administered in drinking water in Swiss mice and Syrian hamsters gave rise to liver neoplasms (benign hepatomas, liver cell carcinomas, angiomas, angiosarcomas) and adenomas and adenocarcinomas of lungs (100).
Hydrazines in general have also been found potent irreversible inactivators of some hemoproteins (101). Although the stability of the molecule was examined and that agaritine degrades within 48 h in tap water and that degradation appeared to be oxygen-dependent (102), the presence in plasma from agaritine-administration in mice or rats as its definite toxicity remains unclear (103). The concentration of agaritine in methanol extracts of food was 112–1836 µg g−1 dry weight, in a commercial product of ABM arrived at 1791 g g−1 dry weight and the calculated one-day intake of the product was estimated to be 8955 g according to the label (104). For this reason the Ministry of Health, Labour and Welfare of Japan demanded a cessation of sales and voluntary recall of the product from K-Company after a request to the Food Safety Commission to assess the safety of products containing ABM. Nevertheless, in a clinical test placebo-controlled to verify human toxicity of ‘Freezing dryness A. blazei (Iwade strain 101) Himematsutake’, after 16 weeks, there were no clinical problems in the blood examination, urinalysis, physical examination and history taking (105).
Toxicity in Humans
Three cases of severe hepatic dysfunction in cancer patients have been reported recently. These are preliminary data, although one patient underwent rechallenge with the same extract that resulted in deterioration of liver function again (106). Nevertheless, other causes cannot be ruled out: there is an apparent probable relationship between ABM extract and liver damage that deserves full attention due to the large assumption of the mushroom as OTC remedy.
Conclusions
Careful clinical studies comparing the activity of isolated compounds, whole mushroom extracts and epidemiological data are still necessary to determine whether ABM provide real clinical benefits. Dose-response studies and isolation, as well as chemical identification and quantification of specific compounds responsible for the potential benefit from ABM mushroom ingestion should be fully developed, although there seems to be clear evidence that ABM extracts are rich in β-glucans that presumably contribute to the observed immunostimulatory activity.
Other substances are probably involved as well, the immunostimulation following ingestion of polysaccharides is possible and probably useful in cancer patients if it does not give rise to pharmacological interferences. A main safety concern is represented by the toxicity and cancerogenicity of agaritine and its derivatives that should be completely evaluated; and probably would be useful for these mushrooms like other herbal remedies, to completely define the problem of heavy metal contents. Due to the large consumption of ABM in popular medicine, probably more data are needed on action mechanisms of its component and safety before counseling the assumption for prevention and treatment of cancer and immunodepressive disorders.
References
- 1.Mattila P, Salo-Vaananen P, Konko K, Aro H, Jalava T. Basic composition and amino acid contents of mushrooms cultivated in Finlands. J Agric Food Chem. 2002;50:6419–22. doi: 10.1021/jf020608m. [DOI] [PubMed] [Google Scholar]
- 2.Mizuno TK. Agaricus blazei Murrill medicinal and dietary effects. Food Rev Int. 1995;11:167–72. [Google Scholar]
- 3.Firenzuoli F, Gori L, Di Simone L, Morsuillo M. Internet information about herbal products and dietary supplements. Recenti Prog Med. 2006;97:189–92. [PubMed] [Google Scholar]
- 4.Ramoutsaki IA, Ramoutsakis IA, Papadakis CE, Helidonis ES. Therapeutic methods for otolaryngological problems during the bzantine period. Ann Otol Rhinol Laryngol. 2002;111:553–7. doi: 10.1177/000348940211100612. [DOI] [PubMed] [Google Scholar]
- 5.Cappelli A, Agaricus L. Fungi Europaei. Vol. 1. Saronno, Italy: Libreria editrice Biella Giovanna; 1984. In: Fr. Ss Karsten (Psalliota Fr) [Google Scholar]
- 6.Geml J, Geiser DM, Royse DJ. Molecular evolution of Agaricus species based on ITS and LSU rDNA sequences. Mycol Prog. 2004;3:157–76. [Google Scholar]
- 7.Heinemann P. Essai d’une clé de détermination des genres Agaricus et Micropsalliota. Sydowia. 1977;30:6–37. [Google Scholar]
- 8.Chang ST. World production of cultivated edible and medicinal mushooms in 1997 with emphasis on Lentinus edodes (Berk.) Sing. in China. Int J of Med Mushrooms. 1999;1:291–300. [Google Scholar]
- 9.Arora D. Mushrooms Demystified. 2nd. Berkley: Ten speed; 1986. [Google Scholar]
- 10.Stamets P. Growing Gourmet and Medicinal Mushrooms. 3rd. Berkley: Ten Speed; 2000. [Google Scholar]
- 11.Kerrigan RW. Agaricus subrufescens, a cultivated edible and medicinal mushroom, and its synonyms. Mycologia. 2005;97:12–24. doi: 10.3852/mycologia.97.1.12. [DOI] [PubMed] [Google Scholar]
- 12.Colauto NB, Dias ES, Gimenes MA, da Eira AF. Genetic characterization of isolates of the basidiomycete Agaricus blazei by RAPD. Braz J Micobiol. 2002;33:131–3. [Google Scholar]
- 13.Wasser SP, Didukh MY, de Amazonas MAL, Nevo E, Stamets P, da Eira AF. Is a widely cultivated culinary-medicinal royal sun Agaricus (the Himematsutake Mushroom) indeed Agaricus blazei Murrill? Intern J of Med Mushrooms. 2002;4:267–90. [Google Scholar]
- 14.Lindequist U, Niedermeyer THJ, Jülich WD. The pharmacological potential of mushrooms. ECAM. 2005;2:285–99. doi: 10.1093/ecam/neh107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawagishi H, Ryuichi RI, Kanao T, Keishiro TM, Hitoshi S, Hagiwara IT, et al. Fractionation and antitumor activity of the water-insoluble residue of Agaricus blazei fruiting bodies. Carbohyd Res. 1989;186:267–73. doi: 10.1016/0008-6215(89)84040-6. [DOI] [PubMed] [Google Scholar]
- 16.Ohno N, Furukawa M, Miura NN, Adachi Y, Motoi M, Yadomae Tl. Antitumor-glucan from the cultured fruit body of A. blazei. Biol Pharm Bull. 2001;24:820–8. doi: 10.1248/bpb.24.820. [DOI] [PubMed] [Google Scholar]
- 17.Ohno N, Hayashi M, Iino K, Suzuki I, Oikawa S, Sato K, et al. Effect of glucans on the antitumor activity of grifolan. Chem Pharm Bull. 1986;34:2149–54. doi: 10.1248/cpb.34.2149. [DOI] [PubMed] [Google Scholar]
- 18.Dong Q, Yao J, Yang X. Structural characterization of water-soluble of β-D-glucan from fruiting bodies of Agaricus blazei Murr. Carbohyd Res. 2002;337:1417–21. doi: 10.1016/s0008-6215(02)00166-0. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno T, Hagiwara T, Nakamura T, Ito H, Shimura K, Sumiya T. Antitumor activity and some properties of water-soluble polysaccharides from “Himematsutake”, the fruiting body of Agaricus blazei Murril. Agric Biol Chem. 1990;54:2889–96. [Google Scholar]
- 20.Camelini CM, Maraschin M, de Mendonca MM, Zucco C, Ferreira AG, Tavares LA. Structural characterization of β-glucans of Agaricus brasiliensis in different stages of fruiting body maturity and their use in nutraceutical products. Biotechnol Lett. 2005;27:1295–9. doi: 10.1007/s10529-005-0222-6. [DOI] [PubMed] [Google Scholar]
- 21.Mizuno M, Morimoto M, Minato K, Tsuchida H. Polysaccharides from Agaricus blazei stimulate lymphocyte T-cell subsets in mice. Biosci Biotechnol Biochem. 1998;62:434–7. doi: 10.1271/bbb.62.434. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno M, Minato K, Ito H, Kawade M, Terai H, Tsuchida H. Antitumor polysaccharide from the mycelium of liquid-cultured Agaricus blazei mill. Biochem Mol Biol Int. 1999;47:707–14. doi: 10.1080/15216549900201773. [DOI] [PubMed] [Google Scholar]
- 23.Sorimachi K, Akimoto K, Inafuku K, Okubo A, Yamazaki S. Secretion of TNF-γ, IL-8 and nitric oxide by macrophages activated with Agaricus blazei Murril fractions in vitro. Cell Struct Funct. 2001;26:103–8. doi: 10.1247/csf.26.103. [DOI] [PubMed] [Google Scholar]
- 24.Cooper EL. Commentary on CAM and NK Cells by Kazuyoshi Takeda and Ko Okumura. eCAM. 2004;1:29–34. doi: 10.1093/ecam/neh011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorimachi K, Ikehara Y, Maezato G, Okubo A, Yamazaki S, Akimoto K, et al. Inhibitory effect of Agaricus blazei Murril fractions on cytophatic effect induced by Western equine encephalitis (WEE) virus on VERO cells in vitro. Biosci Biotech Biochem. 2001;65:1645–7. doi: 10.1271/bbb.65.1645. [DOI] [PubMed] [Google Scholar]
- 26.Sorimachi K, Akimoto K, Hattori Y, Ieiri T, Niwa A. Secretion of TNF-a, IL-8 and nitric oxide by macrophages activated with polyanions, and involvement of interferon-γ in the regulation of cytokine secretion. Cytokine. 1999;11:571–8. doi: 10.1006/cyto.1998.0472. [DOI] [PubMed] [Google Scholar]
- 27.Sorimachi K, Akimoto K, Niwa A, Yasumura Y. Delayed cytocidal effect of lignin derivatives on virally transformed rat fibroblasts. Cancer Detect Prev. 1997;21:111–7. [PubMed] [Google Scholar]
- 28.Sorimachi K, Niwa A, Yamazaki S, Toda S, Yasumura Y. Anti-viral activity of water-solubilized lignin derivatives in vitro. Agric Biol Chem. 1990;54:1337–9. [Google Scholar]
- 29.Kuo YC, Huang YL, Chen CC, Lin YS, Chuang KA, Tsai WJ. Cell cycle progression and cytokine gene expression of human peripheral blood mononuclear cells modulated by Agaricus blazei. J Lab Clin Med. 2002;140:176–87. doi: 10.1067/mlc.2002.126717. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura M, Kasai H, He L, Deng X, Yamashita A, Terunuma H, et al. Antithetical effects of hemicellulase-treated Agaricus blazei on the maturation of murine bone-marrow-derived dendritic cells. Immunology. 2005;114:397–409. doi: 10.1111/j.1365-2567.2004.02106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu S, Kitada H, Yokota H, Yamakawa J, Murayama T, Sugiyama K, et al. Activation of the alternative complement pathway by Agaricus blazei Murill. Phytomedicine. 2002;9:536–45. doi: 10.1078/09447110260573047. [DOI] [PubMed] [Google Scholar]
- 32.Bellini MF, Angeli JPF, Matuo R, Terezan AP, Ribeiro LR, Mantovani MS. Antigenotoxicity of Agaricus blazei mushroom organic and aqueous extracts in chromosomal aberration and cytokinesis block micronucleus assays in CHO-K1 and HTC cells. Toxicology in vitro. 2006;20:355–60. doi: 10.1016/j.tiv.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Machado MP, Filho ER, Terezan AP, Ribeiro LR, Mantovani MS. Cytotoxicity, genotoxicity and antimutagenicity of hexane extracts of Agaricus blazei determined in vitro by the comet assay and CHO/HGPRT gene mutation assay. Toxicology in vitro. 2005;19:533–9. doi: 10.1016/j.tiv.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Oliveira JM, Jordão BQ, Ribeiro LR, Eira AF, Mantovani MS. Anti-genotoxic effect of aqueous extracts of sun mushroom (Agaricus blazei Murill lineage 99/26) in mammalian cells in vitro. Food Chem Toxicol. 2002;40:15–20. doi: 10.1016/s0278-6915(02)00156-4. [DOI] [PubMed] [Google Scholar]
- 35.Menoli RCN, Mantovani MS, Ribeiro LR, Gunter S, Jordão BQ. Antimutagenic effects of the mushroom Agaricus blazei Murill extracts on V79 cells. Mutation Res. 2001;496:5–13. doi: 10.1016/s1383-5718(01)00227-3. [DOI] [PubMed] [Google Scholar]
- 36.Jin CY, Choi YH, Moon DO, Park C, Park YM, Jeong SC, et al. Induction of G2/M arrest and apoptosis in human gastric epithelial AGS cells by aqueous extract of Agaricus blazei. Oncol Rep. 2006;16:1349–55. [PubMed] [Google Scholar]
- 37.Delmanto RD, Alves de Lima PL, Sugui MM, da Eira AF, Salvadori DM, Speit G, et al. Antimutagenic effect of Agaricus blazei Murrill mushroom on the genotoxicity induced by cyclophosphamide. Mutat Res. 2001;496:15–21. doi: 10.1016/s1383-5718(01)00228-5. [DOI] [PubMed] [Google Scholar]
- 38.Luiz RC, Jordão BQ, Eira AF, Ribeiro LR, Mantovani MS. Non-mutagenic or genotoxic effects of medicinal aqueous extracts from the Agaricus blazei mushroom in V79 cells. Cytologia. 2003;68:1–6. [Google Scholar]
- 39.Guterrez ZR, Mantovani MS, Eira AF, Ribeiro LR, Jordão BQ. Variation of the antimutagenicity effects of water extracts of Agaricus blazei Murrill in vitro. Toxicology in Vitro. 2004;18:301–9. doi: 10.1016/j.tiv.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Manzi P, Pizzoferrato L. Beta-glucans in edible mushrooms. Food Chem. 2000;68:315–8. [Google Scholar]
- 41.Luiz RC, Jordão BQ, Eira AF, Ribeiro LR, Mantovani MS. Mechanism of anticlastogenicity of Agaricus blazei Murrill mushroom organic extracts in wild type CHO (K1) and repair deficient (xrs5) cells by chromosome aberration and sister chromatid exchange assays. Mutation Res. 2003;528:75–9. doi: 10.1016/s0027-5107(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 42.Kasai H, He LM, Kawamura M, Yang PT, Deng XW, Munkanta M, et al. IL-12 Production Induced by Agaricus blazei Fraction H (ABH) Involves Toll-like Receptor (TLR) Evid Based Complement Alternat Med. 2004;1:259–67. doi: 10.1093/ecam/neh043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong M, Akihiro T, Yamamoto I. In Vitro Augmentation of Natural Killer Activity and Interferon-γ Productionin Murine Spleen Cell with Agaricus blazei Fruiting Body Fractions. Biosci Biotechnol Biochem. 2005;69:2466–9. doi: 10.1271/bbb.69.2466. [DOI] [PubMed] [Google Scholar]
- 44.Ellertsen LK, Hetland G, Johnson E, Grinde B. Effect of a medicinal extract from Agaricus blazei Murill on gene expression in a human monocyte cell line as examined by microarrays and immuno assays. Int Immunopharmacol. 2006;6:133–43. doi: 10.1016/j.intimp.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 45.Fujimiya Y, Suzuki Y, Oshiman K, Kobori H, Moriguchi K, Nakashima H, et al. Selective tumoricidal effect of soluble proteoglucan extracted from the basidiomycete Agaricus blazei Murrill, mediated via natural killer cell activation and apoptosis. Cancer Immunol Immunother. 1998;46:147–59. doi: 10.1007/s002620050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimiya Y, Suzuki Y, Katakura R, Ebina T. Tumor-specific cytocidal and immunopotentiating effects of relatively low molecular weight products derived from the basidiomycete Agaricus blazei Murill. Anticancer Res. 1999;19:113–18. [PubMed] [Google Scholar]
- 47.Kakuta M, Tanigawa A, Kikuzaki H, Misaki A. Isolation and chemical characterization of antioxidative substance and glucans from fruting body of Agaricus blazei. Biryo Eiyouso Kenkyu. 2002;17:83–90. [Google Scholar]
- 48.Izawa S, Inoue Y. A screening system for antioxidants usin thioredoxin-deficient yeast: discovery of thermostable antioxidant activity from Agaricu blazei Murill. Appl Microbiol Biotechnol. 2004;64:537–42. doi: 10.1007/s00253-003-1467-4. [DOI] [PubMed] [Google Scholar]
- 49.Okamura T, Ogata T, Minamimoto N, Takeno T, Noda H, Fukuda S, et al. Characteristics of Wine Produced by Mushroom Fermentation. Biosci Biotechnol Biochem. 2001;65:1596–600. doi: 10.1271/bbb.65.1596. [DOI] [PubMed] [Google Scholar]
- 50.Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, et al. Mechanism by which orally administered β-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol. 2004;173:797–806. doi: 10.4049/jimmunol.173.2.797. [DOI] [PubMed] [Google Scholar]
- 51.Takeda K, Okumura K. CAM and NK Cells. ECAM. 2004;1:17–27. doi: 10.1093/ecam/neh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown GD, Gordon S. Immune recognition. A new receptor for β-glucans. Nature. 2001;413:36–7. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 53.Bohn JA, BeMiller JN. (l→3)-β-D-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohyd Polym. 1995;28:3–14. [Google Scholar]
- 54.Blaschek W, Kasbauer J, Kraus J, Franz G. Pythium aphanidermatum: culture, cell wall composition, and isolation and structure of antitumour storage and solubilised cell-wall (l→3) (l→6)-β-D-glucans. Carbohyd Res. 1992;231:293–307. doi: 10.1016/0008-6215(92)84026-o. [DOI] [PubMed] [Google Scholar]
- 55.Whistler RL, Bushway AA, Singh PP, Nakahara W, Tokuzen R. Noncytotoxic, antitumor polysaccharides. Adv Carbohydr Chem Biochem. 1976;32:235–75. doi: 10.1016/s0065-2318(08)60338-8. [DOI] [PubMed] [Google Scholar]
- 56.Willment JA, Gordon S, Brown GD. Characterization of the human β-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–23. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 57.Vetvicka V, Thornton BP, Ross GD. Soluble β-glucan polysaccharide binding to the lectin site of neutrophil or natural killer cell complement receptor type 3 (CD11b/CD18) generates a primed state of the receptor capable of mediating cytotoxicity of iC3b-opsonized target cells. J Clin Invest. 1996;98:50–61. doi: 10.1172/JCI118777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tone M, Tone Y, Fairchild PJ, Wykes M, Waldmann H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc Natl Acad Sci USA. 2001;98:1751–6. doi: 10.1073/pnas.98.4.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gough PJ, Greaves DR, Gordon S. A naturally occurring isoform of the human macrophage scavenger receptor (SR-A) gene generated by alternative splicing blocks modified LDL uptake. J Lipid Res. 1998;39:531–43. [PubMed] [Google Scholar]
- 60.Yokota K, Takashima A, Bergstresser PR, Ariizumi K. Identification of a human homologue of the dendritic cell-associated C-type lectin-1, dectin-1. Gene. 2001;11:51–60. doi: 10.1016/s0378-1119(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 61.Nanba H, Mori K, Toyomasu T, Kuroda H. Antitumor action of shiitake (Lentinus edodes) fruit bodies orally administered to mice. Chem Pharm Bull. 1987;35:2453–8. doi: 10.1248/cpb.35.2453. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki I, Sakurai T, Hashimoto K, Oikawa S, Masuda A, Ohsawa M, et al. Inhibition of experimental pulmonary metastasis of Lewis lung carcinoma by orally administered β-glucan in mice. Chem Pharm Bull. 1991;39:1606–8. doi: 10.1248/cpb.39.1606. [DOI] [PubMed] [Google Scholar]
- 63.Cheung NK, Modak S, Vickers A, Knuckles B. Orally administered β-glucans enhance anti-tumor effects of monoclonal antibodies. Cancer Immunol Immunother. 2002;51:557–64. doi: 10.1007/s00262-002-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung NK, Modak S. Oral (1→3),(1→4)-β-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin Cancer Res. 2002;8:1217–23. [PubMed] [Google Scholar]
- 65.Yan J, Vetvicka V, Xia Y, Coxon A, Carroll MC, Mayadas TN, et al. β-glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18) J Immunol. 1999;163:3045–52. [PubMed] [Google Scholar]
- 66.Porgador AO, Mandelboim NP, Restifo JL, Strominger S. Natural killer cell lines kill autologous ß2-microglobulin-deficient melanoma cells: implications for cancer immunotherapy. PNAS. 1997;94:13140. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hicklin DJ, Wang ZG, Arienti F, Rivoltini L, Parmiani G, Ferrone S. ß2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J Clin Invest. 1998;101:2720. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barbisan LF, Miyamoto M, Scolastici C, Salvadori DMF, Ribeiro LR, Eira AF, et al. Influence of aqueous extract of Agaricus blazei on a rat liver toxicity induced by different doses of diethylnitrosamine. J Ethnopharmacol. 2002;83:25–32. doi: 10.1016/s0378-8741(02)00171-x. [DOI] [PubMed] [Google Scholar]
- 69.Barbisan LF, Spinardi-Barbisan AL, Moreira EL, Salvadori DM, Ribeiro LR, da Eira AF, et al. Agaricus blazei (Himematsutake) does not alter the development of rat diethylnitrosamine-initiated hepatic preneoplastic foci. Cancer Sci. 2003;94:188–92. doi: 10.1111/j.1349-7006.2003.tb01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinheiro F, Faria RR, de Camargo JL, Spinardi-Barbisan AL, da Eira AF, Barbisan LF. Chemoprevention of preneoplastic liver foci by dietary mushroom Agaricus blazei Murril in the rat. Food Chem Toxicol. 2003;94:188–92. doi: 10.1016/s0278-6915(03)00171-6. [DOI] [PubMed] [Google Scholar]
- 71.Takaku T, Kimura Y, Okuda H. Isolation of an antitumor compound from Agaricus blazei Murril and its mechanism of action. J Nutr. 2001;131:1409–13. doi: 10.1093/jn/131.5.1409. [DOI] [PubMed] [Google Scholar]
- 72.Kimura Y, Kido T, Takaku T, Sumiyoshi M, Baba K. Isolation of an anti-angiogenic substance from Agaricus blazei Murril: its antitumor and antimetastatic actions. Cancer Sci. 2004;95:758–64. doi: 10.1111/j.1349-7006.2004.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernardshaw S, Hetland G, Grinde B, Johnson E. An extract of the mushroom Agaricus blazei Murill protects against lethal septicemia in a mouse model of fecal peritonitis. Shock. 2006;25:420–5. doi: 10.1097/01.shk.0000209526.58614.92. [DOI] [PubMed] [Google Scholar]
- 74.Bernardshaw S, Johnson E, Hetland G. An extract of mushroom Agaricus blazei Murrill administered orally protects against systemic Streptococcus pneumoniae infection in mice. Scand J Immunol. 2005;62:393–8. doi: 10.1111/j.1365-3083.2005.01667.x. [DOI] [PubMed] [Google Scholar]
- 75.Ebina T, Fujimiya Y. Antitumor effect of a peptide-glucan preparation extracted from Agaricus blazei in a double-grafted tumor system in mice. Biotherapy. 1998;11:259–65. doi: 10.1023/a:1008054111445. [DOI] [PubMed] [Google Scholar]
- 76.Emtage PC, Clarke D, Gonzalo-Daganzo R, Junghans RP. Generating potent Th1/Tc1 T cell doptive immunotherapy doses using human IL-12:harnessing the immunomodulatory potential of IL 12 without the in vivo-associated toxicity. [Published correction appears in J Immunother.] J Immunother. 2003;26:97–106. doi: 10.1097/00002371-200303000-00002. 2003;26:290. [DOI] [PubMed] [Google Scholar]
- 77.Takimoto H, Wakita D, Kawaguchi K, Kumazawa Y. Potentiation of cytotoxic activity in naive and tumor-bearing mice by oral administration of hot-water extracts from Agaricus blazei fruiting bodies. Biol Pharm Bull. 2004;27:404–6. doi: 10.1248/bpb.27.404. [DOI] [PubMed] [Google Scholar]
- 78.Itoh H, Ito H, Amano H, Noda H. Inhibitory action of a (1-6)-beta-D-glucan-protein complex (F III-2-b) isolated from Agaricus blazei Murrill (“himematsutake”) on Meth A fibrosarcoma-bearing mice and its antitumor mechanism. Jpn J Pharmacol. 1994;66:265–71. doi: 10.1254/jjp.66.265. [DOI] [PubMed] [Google Scholar]
- 79.Ito H, Shimura K, Itoh H, Kawade M. Antitumor effects of a new polysaccharide-protein complex (ATOM) prepared from Agaricus blazei (Iwade strain 101) “Himematsutake” and its mechanisms in tumor-bearing mice. Anticancer Res. 1997;17:277–84. [PubMed] [Google Scholar]
- 80.Kaneno R, Fontanari LM, Santos SA, Di Stasi LC, Rodrigues Filho E, Eira AF. Effects of extracts from Brazilian sun-mushroom (Agaricus blazei) on the NK activity and lymphoproliferative responsiveness of Ehrlich tumor-bearing mice. Food Chem Toxicol. 2004;42:909–16. doi: 10.1016/j.fct.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi H, Yoshida R, Kanada Y, Fukuda Y, Yagyu T, Inagaki K, et al. Suppressing effect of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murrill on spontaneous and peritoneal disseminated metastasis in mouse model. J Cancer Res Clin Oncol. 2005;131:527–38. doi: 10.1007/s00432-005-0672-1. [DOI] [PubMed] [Google Scholar]
- 82.Fujimiya Y, Sukuki Y, Oshima K, Kobori H, Moriguchi K, Nakashima H, et al. Selectivetumoricidal effect of soluble proteoglucan extrcated from the basidiomycete, Agaricus blazei Murril,mediated via natural killer cell activation and apoptosis. Cancer Immunology Immunotherapy. 1998;46:135–47. doi: 10.1007/s002620050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Oshiman K, Fujimiya Y, Ebina T, Suzuki I, Noji M. Orally administered β-1,6-D-polyglucose extracted from Agaricus blazei results in tumor regression in tumor-bearing mice. Planta Med. 2002;68:610–4. doi: 10.1055/s-2002-32904. [DOI] [PubMed] [Google Scholar]
- 84.Kim YW, Kim KH, Choi HJ, Lee DS. Anti-diabetic activity and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei. Biotechnol Lett. 2005;27:483–7. doi: 10.1007/s10529-005-2225-8. [DOI] [PubMed] [Google Scholar]
- 85.Yoshimura K, Ueda N, Ichioka K, Matsui Y, Terai A, Arai Y. Use of complementary and alternative medicine by patients with urologic cancer: a prospective study at a single Japanese institution. Support Care Cancer. 2005;13:685–90. doi: 10.1007/s00520-005-0842-3. [DOI] [PubMed] [Google Scholar]
- 86.Ahn WS, Kim DJ, Chae GT, Lee JM, Baes SM, Sin JI, et al. Natural killer cell activity and quality of life were improved consumption of a mushroom extract, Agaricus blazei Murill Kyowa, in gynecological cancer patients undergoing chemotehrapy. Int J Gynecol Cancer. 2004;14:589–94. doi: 10.1111/j.1048-891X.2004.14403.x. [DOI] [PubMed] [Google Scholar]
- 87.Toshiro W, Ayako K, Satoshi I, Kumar MT, Shiro N, Keisuke T. Antihypertensive effect of gamma-aminobutyric acid-enriched Agaricus blazei on mild hypertensive human subjects. Nippon Shokuhin Kagaku Kogaku Kaishi. 2003;50:167–73. [Google Scholar]
- 88.Kweon MH, Kwon ST, Kwon SH, Ma MS, Park YI. Lowering effects in plasma cholesterol and body weight by mycelial extracts of two mushrooms: Agaricus blazei and Lentinus edodes. Korean J Microbiol Biotechnol. 2002;30:402–9. [Google Scholar]
- 89.Inuzuka H, Yoshida T. Clinical utility of ABCL (Agaricus Mushroom Extract) treatment for C-type hepatitis. Jpn Pharmacol Ther. 2002;30:103–7. [Google Scholar]
- 90.Garcia MA, Alonso J, Fernandez MI, Melgar MJ. Lead content in edible wild mushrooms in Northwest Spain as indicator of enviromental contamination. Arch Environ Contam Toxicol. 1998;34:330–5. doi: 10.1007/s002449900326. [DOI] [PubMed] [Google Scholar]
- 91.Svoboda L, Kalac P. Contamination of two edible agaricus spp. Mushrooms growing in a town with cadmium, lead and mercury. Bull Environ Cotam Toxicol. 2003;71:123–30. doi: 10.1007/s00128-003-0138-6. [DOI] [PubMed] [Google Scholar]
- 92.Travnikova IG, Shutov VN, Bruck GY, Balonov MI, Skuterud L, Stradnd P, et al. Assessment of current exposure levels in different population groups of the Kola Peninsula. J Environ Radioact. 2002;60:235–48. doi: 10.1016/s0265-931x(01)00106-0. [DOI] [PubMed] [Google Scholar]
- 93.Hashimoto T, Nonaka Y, Minato K, Kawakami S, Mizuno M, Fukuda I, et al. Suppressive Effect of Polysaccharides from the Edible and Medicinal Mushrooms, Lentinus edodes and Agaricus blazei, on the Expression of Cytochrome P450 in Mice. Biosci Biotechnol Biochem. 2002;66:1610–4. doi: 10.1271/bbb.66.1610. [DOI] [PubMed] [Google Scholar]
- 94.Al-Fatimi MAA, Julich WD, Jansen R, Lindequist U. Bioactive Components of the Traditionally used Mushroom Podaxis pistillaris. ECAM. 2006;3:87–92. doi: 10.1093/ecam/nek008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuroiwa Y, Nishikawa A, Imazawa T, Kanki K, Kitamura Y, Umemura T, et al. Lack of subchronic toxicity of an aqueous extract of Agaricus blazei Murrill in F344 rats. Food Chem Toxicol. 2005;43:1047–53. doi: 10.1016/j.fct.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Back KC, Carter VL, Jr, Thomas AA. Occupational hazards of missile operations with special regard to the hydrazine propellants. Aviat Space Environ Med. 1978;49:591–8. [PubMed] [Google Scholar]
- 97.Runge-Morris M, Wu N, Novack RF. Hydrazine-mediated DNA damage: role of hemoprotein, electron transport, and organic free radicals. Toxicol Appl Pharmacol. 1994;125:123–32. doi: 10.1006/taap.1994.1056. [DOI] [PubMed] [Google Scholar]
- 98.Toth B, Nagel D. Studies ot the tumorigenesis potential of 4-substituted phenylhydrazines by the subcutaneous route. J Toxicol Environ Health. 1981;8:1–9. doi: 10.1080/15287398109530045. [DOI] [PubMed] [Google Scholar]
- 99.Toth B, Raha CR, Wallcave L, Nagel D. Attempted tumor induction with agaritine in mice. Anticancer res. 1981;1:255–8. [PubMed] [Google Scholar]
- 100.Toth B. Hepatocarcinogenesis by hydrazine mycotoxins of edible mushrooms. J Toxicol Environ Health. 1979;5:193–202. doi: 10.1080/15287397909529744. [DOI] [PubMed] [Google Scholar]
- 101.Flordeliza YB, Timkovich R. Inactivation of Cytochrome cd1 by Hydrazines. J Biological Chemistry. 1990;8:4247–53. [PubMed] [Google Scholar]
- 102.Hajslova J, Hajkova L, Schulzova H, Frandsen J, Gry J, Anderson HC. Stability of agaritine – a natural toxicant of Agaricus mushrooms. Food Addit Contam. 2002;19:1028. doi: 10.1080/02652030210157691. [DOI] [PubMed] [Google Scholar]
- 103.Kondo K, Watanabe A, Iwanga Y, Abe I, Tanaka H, Nagaoka MH, et al. Analysis of agaritine in mushrooms and in agaritine-administered mice using liquid chromatography-tandem mass spectrometry. J Chromatography B. 2006;834:55–61. doi: 10.1016/j.jchromb.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 104.Nagaoka MH, Nagaoka H, Kondo K, Akiyama H, Maitani T. Measurement of a genotoxic hydrazine, agaritine, and its derivatives by HPLC with fluorescence derivatization in the Agricus mushroom and its products. Chem Pharm Bull. 2006;54:922–24. doi: 10.1248/cpb.54.922. [DOI] [PubMed] [Google Scholar]
- 105.Kajimoto O, Ikeda Y, Yabune M, Sakamoto A, Kajimoto Y, Kajimoto O. The safety of extended consumption of freezing dryness Agaricus blazei (Iwade strain 101) Himematsutake. Jpn Pharmacol Ther. 2006;34:103–17. [Google Scholar]
- 106.Mukai H, Watanabe T, Ando M, Katsumata N. An alternative medicine, Agaricus blazei, may have induced severe hepatic dysfunction in cancer patients. Jpn J Clin Oncol. 2006 doi: 10.1093/jjco/hyl108. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]