Abstract
The role of leukocytes in the in vivo dissemination of cytomegalovirus was studied in this experiment. Rat cytomegalovirus (RCMV) could be transferred to rat granulocytes and monocytes by cocultivation with RCMV-infected fibroblasts in vitro. Intravenous injection of purified infected granulocytes or monocytes resulted in a systemic infection in rats, indicating that our model is a powerful tool to gain further insight into CMV dissemination and the development of new antivirals.
Primary infection or reactivation of human cytomegalovirus (HCMV) in immunocompromised hosts causes severe morbidity and mortality (18, 26, 27).
During active infection, the presence of the viral matrix protein pp65 (ppUL83) can be demonstrated in the nucleus of infected polymorphonuclear cells (PMN) and monocytes (MNC) by the antigenemia assay (11, 12, 23). PMN have been shown to be abortively infected, because transcription of the HCMV genome is blocked after expression of the immediate-early genes IE1 and IE2 (9, 11).
Although several animal models exist for the study of CMV infection, little information has been gathered on the in vivo dissemination of this virus. In a murine model for CMV infection, viral DNA was detected in mononuclear leukocytes in the blood of infected mice (1, 22).
We previously postulated that HCMV might employ MNC and PMN as a vehicle for its dissemination through the body (15, 21). Using clinical isolates of HCMV, we and others have shown that PMN and MNC can acquire infective HCMV in vitro by coculture on infected cells. Subsequently, these leukocytes were able to retransmit the virus to uninfected cells (10, 14, 15, 19, 25). The presence of intact viral particles in these leukocytes could also be demonstrated with electron microscopy (10, 15, 19).
We hypothesized that infected MNC and PMN facilitate the dissemination of CMV in vivo. To test our hypothesis, we transferred rat CMV (RCMV) by coculturing from infected fibroblasts to phagocytes. Subsequently, we investigated the potential of these infected phagocytes to induce a systemic RCMV infection in vivo.
Detection of virus in leukocytes of infected rats.
The local board for animal welfare approved all animal experiments described. Cell-free RCMV stocks were produced from salivary gland homogenates from RCMV-infected rats (Harlan, Zeist, The Netherlands) as described previously (5).
Male PVG rats (n = 6; Harlan), 8 weeks of age, received 5 Gy of total body irradiation (TBI), and 106 PFU of cell-free virus was administered intraperitoneally 16 h after TBI. Irradiation was performed consistently in all RCMV infection experiments to allow for maximal viral replication, which is less pronounced in immunocompetent rats.
At several time points after infection, heparinized blood samples (400 μl) were obtained by orbital punctures. Of each sample, 200 μl was used for DNA isolation according to standard procedures, and the presence of RCMV UL 54 was detected using a nested PCR as described previously (2) (Fig. 1). The remaining 200 μl of the blood samples was used to isolate the mononuclear and granulocyte cell fractions. These cell fractions were cocultivated with uninfected rat lung fibroblasts (RFL-6; European Collection of Cell Cultures, Salisbury, United Kingdom) and were checked for virus transmission.
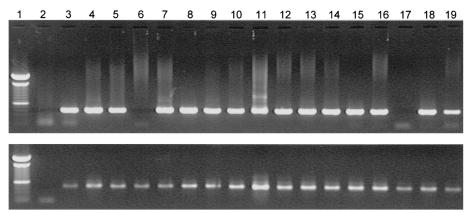
FIG. 1.
Presence of RCMV DNA in whole-blood samples of rats infected with cell-free RCMV. The figure shows viral DNA, amplified by nested PCR, after loading on an agarose gel and staining with ethidium bromide. Lane 1, marker; lane 2, negative control (H2O); lanes 3 to 19, time course of blood samples obtained at days 1, 2, 3, 4, 7, 18, 21, 25, 28, 32, 42, 46, 49, 53, 56, 60, and 63, respectively. The blood samples were RCMV negative at days 4 and 56 p.i. As a control, glyceraldehyde-3-phosphate dehydrogenase DNA was amplified for every sample.
At 1 day postinfection (dpi), all samples were RCMV DNA negative, meaning that input of virus in the rat circulation was below detection level. However, starting from day 3, RCMV DNA became detectable in peripheral blood samples, indicating that viral replication had taken place. At 7 dpi, RCMV DNA was detectable in a number of blood samples already after the first round of nested PCR, indicating a relatively high viral load. Moreover, the presence of virus DNA could be demonstrated up to 60 dpi after the second amplification round. Occasionally, blood samples were also RCMV DNA negative. This could partially be ascribed to the small amounts of blood (<200 μl) that were obtained after orbital puncture for these time points.
At 7 and 21 dpi, we were able to culture RCMV from both PMN and MNC, obtained from the rats infected with cell-free virus (data not shown). The presence of RCMV was immunohistochemically confirmed using monoclonal antibody (MAb) 8 or 35 (16).
These data suggest that, during active RCMV infection, rats have a DNAemia and viremia that are comparable to those of a clinical HCMV infection. Earlier studies with murine CMV (MCMV) also indicated a similar viremia in mice after injection of recombinant cell-free MCMV, with up to 0.1% of MNC being MCMV infected (1, 22).
Generation of RCMV-infected PMN and MNC.
Purified PMN and MNC, obtained from uninfected rats (Harlan), were cocultivated with RCMV-infected RFL-6 cells for 2 h. Subsequently, the leukocytes were transferred to empty Transwell plates (Corning Costar, Cambridge, United Kingdom). The PMN and MNC were allowed to transmigrate against a chemotactic gradient of 0.4 μM fMLP (Sigma, St. Louis, Mo.) or 1.0 μM histamine (Sigma), respectively, for 16 h. The transmigrated leukocytes were washed twice with phosphate-buffered saline, assessed for viability by using trypan blue, transferred to uninfected fibroblasts, incubated for another 2 h, and removed subsequently. After approximately 2 days, the fibroblasts showed cytopathic effect, and after approximately 5 days plaques had formed. Immunohistochemical staining with MAb 8 or 35 (16) confirmed that transfer of RCMV had taken place from the infected leukocytes to the fibroblasts (data not shown).
It proved to be essential that leukocyte populations were purified by transmigration, since nonpurified leukocyte fractions were contaminated with low levels (<0.5%) of infected, α-actin-positive fibroblasts. Cocultivation in vitro or intravenous injection of this low amount of infected fibroblasts into rats also resulted in a viral infection (data not shown). After transmigration, the leukocyte populations were devoid of α-actin-positive cells; thus, fibroblasts did not transmigrate.
The purity of the cell fractions was confirmed with immunohistochemical staining with the specific antibodies ED-1 and His-48 (Pharmingen) for MNC and PMN, respectively. Both the PMN and the MNC fraction proved to be >95% pure. Infective cell-free virus that had diffused through the Transwells was removed by washing the purified leukocyte fractions at least twice with phosphate-buffered saline. The absence of free virus after washing was confirmed by plaque assay (4).
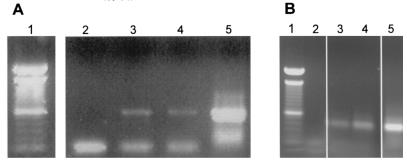
After cocultivation of PMN and MNC fractions with RCMV-infected fibroblasts and subsequent purification by transmigration, the presence of RCMV DNA in these leukocytes could be confirmed by PCR (Fig. 2). Both MNC and granulocyte fractions were RCMV DNA positive; RCMV DNA could be detected already after the first round of the nested PCR.
FIG. 2.
Presence of RCMV DNA in transmigrated PMN and MNC. (A) Viral DNA, amplified by nested PCR after loading on an agarose gel and staining with ethidium bromide. Lane 1, 100-bp marker; lane 2, negative control (H2O); lane 3, PMN; lane 4, MNC; lane 5, positive control (RCMV-infected fibroblasts). (B) As a control, glyceraldehyde-3-phosphate dehydrogenase DNA was amplified for every sample.
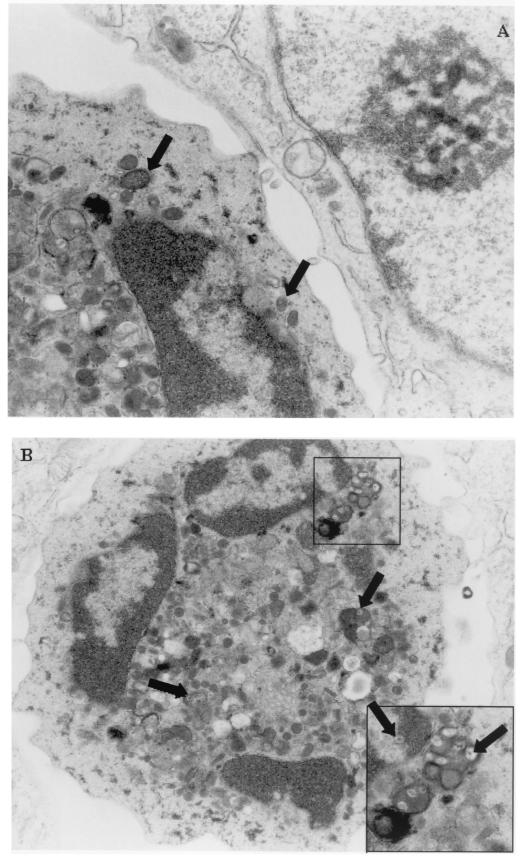
The presence of intact virus particles in infected fibroblasts and PMN and MNC was confirmed by transmission electron microscopy after 12 h of coculture (Fig. 3). Virus particles were detected in the nucleus and cytoplasm of infected fibroblasts. In addition, virus appeared to be present in vesicles in infected PMN and MNC, possibly indicating phagocytosis of virus particles. Moreover, virus particles were detected in the cytoplasm and in the nucleus of PMN and MNC, suggesting the possibility of a productive RCMV infection.
FIG. 3.
Detection of virus particles by transmission electron microscopy in RCMV-infected leukocytes after cocultivation with RCMV-infected fibroblasts. (A) Presence of virus particles in RCMV-infected MNC. Shown is a detail of a contact point of an MNC with an RCMV-infected fibroblast. Virus particles (in vesicles) are indicated by arrows. Magnification, ×32,000. (B) Overview of an RCMV-infected granulocyte; magnification, ×17,000. The inset shows a typical detail of virus particles present in vesicles in the cytoplasm and in the nucleus (indicated by arrows). Magnification, ×40,000.
Thus, the generation of RCMV-infected leukocytes, the presence of intact virus particles in these cells, and transfer of the virus to uninfected cells are comparable to data obtained with HCMV in vitro (9, 11, 12, 23, 25).
Infection of rats with PMN or MNC.
Male PVG rats (Harlan), 8 weeks of age, received 5 Gy of TBI and were injected in the tail vein with in vitro RCMV-infected PMN or MNC at 16 h after irradiation. All animals were sacrificed at 4 weeks postinfection (p.i.). Sera and organs were obtained for a plaque assay (4) and for histological evaluation on frozen and paraffin-embedded tissue sections (16).
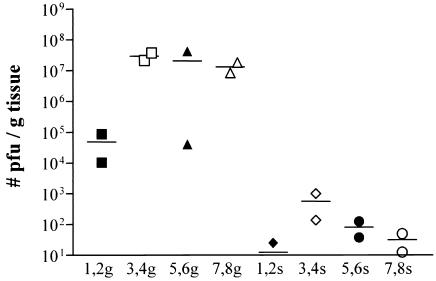
Intravenous injection of a low dose of infected PMN or MNC in irradiated rats resulted in a systemic infection. Figure 4 shows the virus titers in spleens (s) and salivary glands (g) at 4 weeks p.i. as determined by a plaque assay. Rats 1 to 4 were injected with infected PMN, whereas rats 5 to 8 were injected with infected MNC. Injection of 70,000 PMN resulted in higher virus titers than did injection with 35,000 PMN. Injection of 70,000 MNC resulted in similar virus titers as did injection with 150,000 MNC.
FIG. 4.
Virus titers in salivary glands (g) and spleens (s) of rats injected with RCMV-infected leukocytes, determined using a plaque assay. Rats 1 and 2 were injected with 35,000 PMN, and rats 3 and 4 received 70,000 PMN. Rats 5 and 6 were injected with 70,000 MNC, whereas rats 7 and 8 received 150,000 MNC. Virus titers in salivary glands are considerably higher than those in spleens of these animals.
Using a plaque assay, we established that approximately 3% of the PMN were infected with RCMV, i.e., 1,050 to 2,100 of the in vivo-administered PMN. During clinical HCMV infection, it is known that the percentage of infected leukocytes during active infection is up to 5% (19). Therefore, the in vivo transmission of RCMV by infected leukocytes proved to be efficient, because a single injection with a low dose of infected cells was sufficient to cause a systemic infection. The presence of RCMV was further confirmed using immunohistochemical staining on salivary glands with MAbs 8 and 35 (data not shown).
Generation of HCMV-infected MNC and PMN can be accomplished only when low-passage-number clinical isolates are used (14, 15, 19). Comparison of genomes of clinical isolates and laboratory strains of HCMV revealed the presence of approximately 20 additional open reading frames in clinical isolates, which have been only partially characterized so far (6). These virulence genes are dispensable for viral replication in vitro but are beneficial for replication and dissemination in vivo. Several virally encoded chemokines and chemokine receptors have been described for HCMV, RCMV, and MCMV (2, 3, 7, 8, 13, 17, 20, 24). These virally encoded chemokines are thought to specifically attract MNC and PMN and therefore may play a crucial role in the dissemination process. Based on these observations and the data that we have obtained about this leukocyte-mediated dissemination of RCMV in vivo, we concur with the hypothesis of Grundy et al. (14) that the dissemination of CMV by leukocytes, as demonstrated in our model, plays an important role not only during primary infections but also during reactivation of CMV.
In conclusion, our study has shown that leukocytes play an important role in the dissemination of RCMV in vivo. RCMV DNA was isolated from peripheral blood of rats that were infected with cell-free virus. RCMV-infected leukocytes could be generated in vitro, and transfer of these cells resulted in virus transfer both in vitro and in vivo.
It is likely that HCMV disseminates in humans in a similar manner during reactivations or primary infection. Therefore, our model is a powerful tool for further studies of the viral dissemination process and may prove valuable for the evaluation of new antiviral drugs that can interfere with this dissemination process and subsequent infection.
Acknowledgments
This research was financially supported by a grant from Numico Research B.V. (Wageningen, The Netherlands) and the Dutch Ministry of Economic Affairs (project BTS 97209).
We thank Marja Brinker for help with the PCR experiments.
REFERENCES
- 1.Bale, J. F., and M. E. O'Neil. 1989. Detection of murine cytomegalovirus DNA in circulating leukocytes harvested during acute infection of mice. J. Virol. 63:2667-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beisser, P. S., C. Vink, J. G. Van Dam, G. Grauls, S. J. Vanherle, and C. A. Bruggeman. 1998. The R33 G protein-coupled receptor gene of rat cytomegalovirus plays an essential role in the pathogenesis of viral infection. J. Virol. 72:2352-2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benedict, C. A., K. D. Butrovich, N. S. Lurain, J. Corbeil, I. Rooney, P. Schneider, J. Tschopp, and C. F. Ware. 1999. Cutting edge: a novel viral TNF receptor superfamily member in virulent strains of human cytomegalovirus. J. Immunol. 162:6967-6970. [PubMed] [Google Scholar]
- 4.Bruggeman, C. A., W. M. Debie, G. Grauls, G. Majoor, and C. P. van Boven. 1983. Infection of laboratory rats with a new cytomegalo-like virus. Arch. Virol. 76:189-199. [DOI] [PubMed] [Google Scholar]
- 5.Bruggeman, C. A., H. Meijer, P. H. Dormans, W. M. Debie, G. E. Grauls, and C. P. van Boven. 1982. Isolation of a cytomegalovirus-like agent from wild rats. Arch. Virol. 73:231-241. [DOI] [PubMed] [Google Scholar]
- 6.Cha, T. A., E. Tom, G. W. Kemble, G. M. Duke, E. S. Mocarski, and R. R. Spaete. 1996. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J. Virol. 70:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis-Poynter, N. J., D. M. Lynch, H. Vally, G. R. Shellam, W. D. Rawlinson, B. G. Barrell, and H. E. Farrell. 1997. Identification and characterization of a G protein-coupled receptor homolog encoded by murine cytomegalovirus. J. Virol. 71:1521-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fleming, P., N. Davis-Poynter, M. Degli-Esposti, E. Densley, J. Papadimitriou, G. Shellam, and H. Farrell. 1999. The murine cytomegalovirus chemokine homolog, m131/129, is a determinant of viral pathogenicity. J. Virol. 73:6800-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerna, G., E. Percivalle, F. Baldanti, S. Sozzani, P. Lanzarini, E. Genini, D. Lilleri, and M. G. Revello. 2000. Human cytomegalovirus replicates abortively in polymorphonuclear leukocytes after transfer from infected endothelial cells via microfusion events. J. Virol. 74:5629-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerna, G., E. Percivalle, M. Torsellini, and M. G. Revello. 1998. Standardization of the human cytomegalovirus antigenemia assay by means of in vitro-generated pp65-positive peripheral blood polymorphonuclear leukocytes. J. Clin. Microbiol. 36:3585-3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grefte, A., M. C. Harmsen, M. van der Giessen, S. Knollema, W. J. van Son, and T. H. The. 1994. Presence of human cytomegalovirus (HCMV) immediate early mRNA but not ppUL83 (lower matrix protein pp65) mRNA in polymorphonuclear and mononuclear leukocytes during active HCMV infection. J. Gen. Virol. 75:1989-1998. [DOI] [PubMed] [Google Scholar]
- 12.Grefte, J. M., B. T. van der Gun, S. Schmolke, M. van der Giessen, W. J. van Son, B. Plachter, G. Jahn, and T. H. The. 1992. The lower matrix protein pp65 is the principal viral antigen present in peripheral blood leukocytes during an active cytomegalovirus infection. J. Gen. Virol. 73:2923-2932. [DOI] [PubMed] [Google Scholar]
- 13.Gruijthuijsen, Y. K., P. Casarosa, S. J. Kaptein, J. L. Broers, R. Leurs, C. A. Bruggeman, M. J. Smit, and C. Vink. 2002. The rat cytomegalovirus R33-encoded G protein-coupled receptor signals in a constitutive fashion. J. Virol. 76:1328-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy, J. E., K. M. Lawson, L. P. Maccormac, J. M. Fletcher, and K. L. Yong. 1998. Cytomegalovirus-infected endothelial cells recruit neutrophils by the secretion of C-X-C chemokines and transmit virus by direct neutrophil-endothelial cell contact and during neutrophil transendothelial migration. J. Infect. Dis. 177:1465-1474. [DOI] [PubMed] [Google Scholar]
- 15.Kas-Deelen, A. M., T. H. The, N. Blom, B. W. A. van der Strate, E. F. de Maar, W. J. van Son, and M. C. Harmsen. 2001. Uptake of pp65 in in vitro generated pp65-positive polymorphonuclear cells mediated by phagocytosis and cell fusion? Intervirology 44:8-13. [DOI] [PubMed] [Google Scholar]
- 16.Meijer, H., P. H. Dormans, and C. P. van Boven. 1986. Studies on rat cytomegalovirus induced structural and non-structural proteins present at (immediate-)early and late times of infection. Arch. Virol. 89:45-56. [DOI] [PubMed] [Google Scholar]
- 17.Penfold, M. E., D. J. Dairaghi, G. M. Duke, N. Saederup, E. S. Mocarski, G. W. Kemble, and T. J. Schall. 1999. Cytomegalovirus encodes a potent alpha chemokine. Proc. Natl. Acad. Sci. USA 96:9839-9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plummer, G. 1973. Cytomegaloviruses of man and animals. Prog. Med. Virol. 15:92-125. [PubMed] [Google Scholar]
- 19.Revello, M. G., E. Percivalle, E. Arbustini, R. Pardi, S. Sozzani, and G. Gerna. 1998. In vitro generation of human cytomegalovirus pp65 antigenemia, viremia, and leukoDNAemia. J. Clin. Investig. 101:2686-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saederup, N., Y. C. Lin, D. J. Dairaghi, T. J. Schall, and E. S. Mocarski. 1999. Cytomegalovirus-encoded beta chemokine promotes monocyte-associated viremia in the host. Proc. Natl. Acad. Sci. USA 96:10881-10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinzger, C., A. Grefte, B. Plachter, A. S. Gouw, T. H. The, and G. Jahn. 1995. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 76:741-750. [DOI] [PubMed] [Google Scholar]
- 22.Stoddart, C. A., R. D. Cardin, J. M. Boname, W. C. Manning, and G. B. Abenes. 1994. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J. Virol. 68:6243-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The, T. H., M. van der Ploeg, A. P. van den Berg, A. M. Vlieger, M. van der Giessen, and W. J. van Son. 1992. Direct detection of cytomegalovirus in peripheral blood leukocytes—a review of the antigenemia assay and polymerase chain reaction. Transplantation 54:193-198. [DOI] [PubMed] [Google Scholar]
- 24.Vink, C., E. Beuken, and C. A. Bruggeman. 2000. Complete DNA sequence of the rat cytomegalovirus genome. J. Virol. 74:7656-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waldman, W. J., D. A. Knight, E. H. Huang, and D. D. Sedmak. 1995. Bidirectional transmission of infectious cytomegalovirus between monocytes and vascular endothelial cells: an in vitro model. J. Infect. Dis. 171:263-272. [DOI] [PubMed] [Google Scholar]
- 26.Weller, T. H. 1971. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N. Engl. J. Med. 285:203-214. [DOI] [PubMed] [Google Scholar]
- 27.Weller, T. H. 1971. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N. Engl. J. Med. 285:267-274. [DOI] [PubMed] [Google Scholar]