Abstract
Isolated growth hormone deficiency (IGHD) may be of genetic origin. One of the few genes involved in that condition encodes the growth hormone releasing hormone receptor (GHRHR) that, through its ligand (GHRH), plays a pivotal role in the GH synthesis and secretion by the pituitary. Our objective is to describe the phenotype of two siblings born to a consanguineous union presenting with short stature (IGHD) and Magnetic Resonance Imaging (MRI) abnormalities, and to identify the molecular basis of this condition. Our main outcome measures were clinical and endocrinological investigations, MRI of the pituitary region, study of the GHRHR gene sequence and transcripts. In both patients, the severe growth retardation (−5SD) was combined with anterior pituitary hypoplasia. In addition to these classical phenotypic features for IGHD, one of the patients had a Chiari I malformation, an arachnoid cyst, and a dysmorphic anterior pituitary. A homozygous sequence variation in the consensus donor splice site of intron 1 (IVS1 + 2T > G) of the GHRHR gene was identified in both patients. Using in vitro transcription assay, we showed that this mutation results in abnormal splicing of GHRHR transcripts. In this report, which broadens the phenotype associated with GHRHR defects, we discuss the possible role of the GHRHR in the proper development of extrapituitary structures, through a mechanism that could be direct or secondary to severe GH deficiency.
INTRODUCTION
Growth hormone deficiency (GHD) has been estimated to occur with an incidence of 1/4,000 to 1/10,000 live births; most cases are sporadic, but 5% to 30% are familial, an observation consistent with a genetic etiology of the disease (1–2). The growth hormone deficiency can be isolated (IGHD), combined to other pituitary hormone deficiencies (CPHD), or, in the extreme form, be part of a panhypopituitarism. The degree and severity of GH deficit is variable; moreover, GHD may be syndromic or not, depending on the gene implicated. Several genes have been shown to be involved in endocrine pituitary deficiency, with, to date, five of them related to IGHD: the growth hormone gene (GH-N); the gene encoding the receptor of the growth hormone releasing hormone GHRHR (1–2); recently, a mutation in the GHSR gene encoding the ghrelin receptor (an hypothalamic factor that stimulates GH secretion and appetite) has been identified in patients with short stature (3); IGHD has also been shown to result from heterozygous mutations in the homeobox gene expressed in embryonic stem cells, called HESX1 (4–6); or in X-linked GH deficiency (7) from molecular defects of the sex-determining region of the Y chromosome-related high-mobility group box gene (SOX3).
The critical role that GHRH plays, through GHRH receptor binding, in regulating GH pituitary synthesis and secretion has been confirmed in humans through the discovery of homozygous null mutations in the GHRHR gene that inactivate receptor function and cause severe GH deficiency and dwarfism (for review,1). The human GHRHR, mapped to chromosome 7p15, consists of 13 exons spanning approximately 15 Kb and encodes a protein of 423 amino acids. GHRHR is a seven-transmembrane domains receptor coupled to Gαs, predominantly expressed in the pituitary gland (8). To date, 16 GHRHR mutations have been identified in patients with severe growth retardation: one impairing a POU1F1 binding site in the promoter region, seven missense and two nonsense mutations, two microdeletions, and four splice site mutations (introns 1, 3, 7 and 12) (1,9). All but one mutations are recessive; they were found in the homozygous or compound heterozygous state. The remaining mutation (c.Del1121-1124) has a dominant negative effect (10). All the missense mutations have proven to cause the inability of the mutated receptor to bind the ligand, therefore affecting GHRH signaling (11). The other mutations (nonsense, splice mutation, and c.Del1140-1144), expected to lead to the synthesis of severely truncated receptor, represent 8/16 of the GHRHR molecular defects (12–16).
In the present study, we report the particular phenotypic characteristics of two Moroccan siblings with IGHD born to a consanguineous union in whom we identified a novel splice site mutation in the GHRHR gene.
MATERIALS AND METHODS
Patients
Two children were referred to the Department of Endocrinology, Diabetology, and Nutrition of the Ibn Sina hospital in Rabat, Morocco for evaluation of short stature. The patient’s parents gave informed consent for genetic studies.
Hormonal Assays
GH and insulin-like growth factor 1 (IGF-1) concentrations were evaluated by RIA (Beckman Coulter, France). GH plasma values were measured after two pharmacological stimulations: an insulin-induced hypoglycaemia test (0.1 U/kg); and a LevoDopa test (0.15 mg/mL). Plasma TSH, FSH, LH, prolactin (PRL), free T4 and T3, and cortisol (at 8:00 a.m.) were evaluated under baseline conditions by RIA.
Magnetic Resonance Imaging (MRI)
Pituitary MRI was performed on a 1.5-T magnet (Gyroscan ACS-II) with spin echo T1-T2 weighted images (repetition time = 450 msec; echo time = 20 msec; slice thickness = 6 mm). Pre- and post-gadolinium examinations were performed in the coronal and sagittal planes. MRI was interpreted by three neuroradiologists (Gueddari and Kabbaj in Morocco and L Hertz-Panier in France).
Mutation Screening
Genomic DNA was isolated from peripheral blood leukocytes and was screened for mutations in GH-N and GHRHR genes by direct sequencing of PCR products spanning exons and flanking intronic sequences. PCR products were purified (Nucleospin extract 2 in 1 columns, Macherey Nagel, Hoerdt, France) and sequenced on ABI PRISM 3100 Automated Sequencer (Applied Biosystems, Foster City, CA). All the primers’ sequences used in this study are available on request.
Restriction Endonuclease Analysis of PCR Products
Intrafamilial segregation analysis of the identified GHRHR mutation was accomplished by Taa-I digestion (Fermentas, St Rémy les Chevreuses, France) of the PCR products spanning the mutation site and generated with following primers: forward, 5′-CCAGAGTAGAAGGCGATGAGG-3′; and reverse, 5′-TCTCCAAGCCACAGC CTGCC-3′. The IVS1 + 2T > G mutation abolishes a Taa-I restriction site (ACNGT). Digestion products were then separated by electrophoresis on a 3% agarose gel.
Plasmid Constructs
The genomic DNA from a control and one patient was used as a template to amplify by PCR a genomic fragment spanning exons 1 to 4 of the GHRHR gene using primers P1 (forward: 5′-CCAGAGTAGA AGGCGATGAGG-3′) and P2 (reverse: 5′-AGAATGGCGGCCCCTACAGT-3′). The resulting PCR products were subsequently cloned into the pcDNA3.1/V5-His Topo TA expression vector (Invitrogen, Cergy-Pontoise, France) to generate the pcDNA3.1-GHRHR_WT and pcDNA3.1-GHRHR_Mut plasmids. The two plasmid constructs were checked by direct DNA sequencing.
Transfections and RT-PCR Analyses
The transfection assays were carried out in HeLa cells maintained in DMEM medium supplemented with 10% fetal-calf serum. The two plasmids containing the wild-type or mutated GHRHR minigene were transfected using the Lipofectamine-Plus method (Invitrogen) in OptiMEM medium according to the manufacturer’s protocol. Cells were harvested 24 h after transfection and total RNAs were then extracted by the RNA-Plus method (Q.Biogen, IUkirch, France). Reverse transcriptions with SuperScript II (Invitrogen) were performed with hexamer random primers followed by PCR amplifications between exons 1 and 3 with primers P3 (forward: 5′-CTTAC TGAGGCTGGTGGAGG-3′) and P4 (reverse: 5′-GAGAGAAGAAATCCG GGCAG-3′). Aliquots of RT-PCR products were separated by electrophoresis on a 1.5 % agarose.
RESULTS
Disease Phenotype
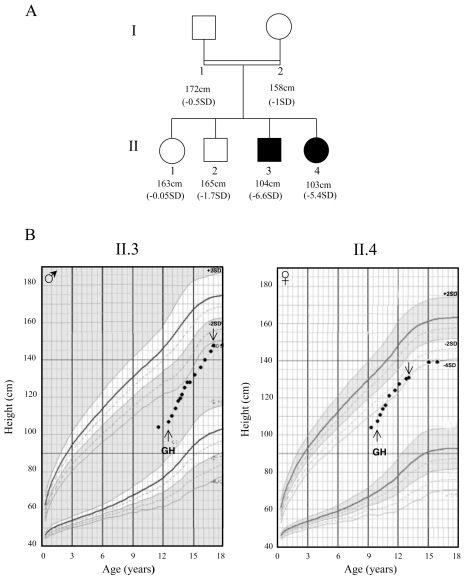
Patient II.3 (a boy) and patient II.4 (a girl) are the third and fourth children of a consanguineous union. Their parents and siblings are of normal stature (ranging from −1.7 to −0.05 SD) (Figure 1A). The two index cases (II.3 and II.4) were born at term after normal pregnancies and deliveries; their birth weights were 4,000 g and 2,500 g, respectively. On first examination, both patients were prepubertal (Tanner stage I). Patient II.3, who was 11 years, 6 months old, had a height of 104 cm (−6.6 SD) and his bone age (calculated with Greulich and Pyle atlas) was 10 years. Patient II.4, who was 9 years, 4 months old, had a height of 103.3 cm, (−5.4 SD) (Figure 1B) and her bone age was 8 years. The two patients showed frontal bossing, marked nasal bridge, and delayed dentition; moreover, patient II.4 presented acromicria and retrognatism. There was no abdominal obesity or history of neonatal hypoglycemia in either of them, and no micropenis for the boy.
Figure 1.
(A) Pedigree of the IGHD studied family with affected children represented by filled symbols. Heights are those measured at first examination for affected subjects. SD is evaluated according to the French standards of Children’s International Center and Sempé (41). (B) Height curves for patients II.3 and II.4. The duration of GH treatment is depicted as follows: ↑beginning, ↓ end.
All endocrinological values are summarized in Table 1. The patients underwent two GH stimulation tests by insulin-induced hypoglycemia and L-Dopa. Their response to these stimuli was very low: 0.1 ng/mL for patient II.3, and 1.2 and 4.2 ng/mL for patient II.4 (normal values > 10 ng/mL). IGF-1 values were also markedly low. There was an appropriate cortisol secretion for both children: 181 ng/mL in patient II.3 and 116 ng/mL in patient II.4 (normal values 90–260 ng/mL). Free T4 and T3, TSH, FSH, LH, and PRL were normal.
Table 1.
Hormonal data of patients II.3 and II.4 before GH therapy
| GH (ng/mL)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (year) | Tanner stage | L-Dopa | IHG | FT3 (pg/mL) | FT4 (pg/mL) | TSH (μU/mL) | FSH (mU/mL) | LH (mU/mL) | PRL (μU/mL) | IGF1 (ng/mL) | Cortisol (ng/mL) | |
| II.3 | M | 11.5 | I | 0.1a | 0.1a | 2.3 | 12.4 | 3.2 | 0.95 | 1.3 | 188 | 21a | 181 |
| II.4 | F | 9.3 | I | 1.2a | 4.2a | 3.5 | 12 | 3 | 1.3 | <0.5 | 275 | 17.4a | 116 |
IHG: insulin-induced hypoglycaemia.
Normal range for hormonal values: GH: > 10 ng/mL; FT3: 1.6–3.8 pg/mL; FT4: 9–18 pg/mL; TSH: 0.2–4 μU/mL, FSH: 1–8 mU/mL; LH: 0.6–12 mU/mL for boys and 0.5–5 mU/mL for girls; PRL: 78–216 μU/mL for boys and 90–400 μU/mL for girls; IGF-I: 107–310 ng/mL; cortisol: 90–260 ng/mL.
This item denotes a deficiency.
Both patients showed good responses to GH therapy with a growth velocity of 11 cm/year for patient II.3, and 10.5 cm/year for patient II.4 after one year of treatment. For patient II.4, GH treatment was stopped at the age of 13 because she gained only 0.6 cm/year and her bone age was 12 (Figure 1B).
They both underwent spontaneous puberty: at the age of 17, patient II.3 presented a Tanner stage III-IV and a height of 147.5 cm (−4.3 SD), whereas target height is 171.5 cm (−0.5 SD). As for patient II.4, who reached a height of 138 cm (−4 SD) at age 15 (target height of 158.5 cm, −1 SD), puberty had progressed to Tanner stage IV with stage B4 (B1, before GH treatment).
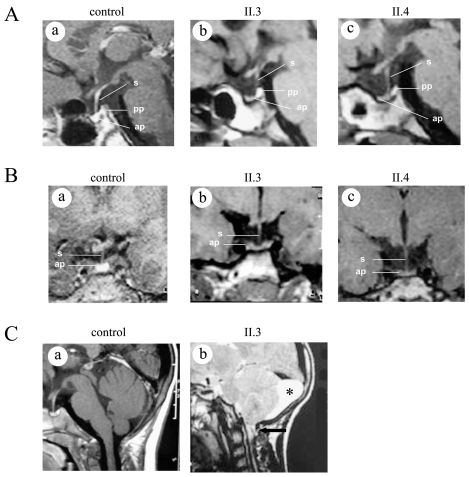
Imaging investigations, performed by magnetic resonance (MRI), showed anterior pituitary hypoplasia in both siblings: height of 3 mm and 2 mm in patient II.3 (at 14.9 years) and II.4 (at 9.8 years) respectively, on a sagittal medial view (Figure 2A, b and c). It was largely less than −2 SD by age-adjusted criteria as assessed by reference data (17–18). Moreover, the morphology of the anterior pituitary was found to be unusual in patient II.3, very thin in the center (3mm) with two small lateral lobes (5mm) (Figure 2B, b). Although the ends are larger, they remain under the mean. In addition, the same patient had a Chiari I malformation (tonsillar herniation of 4–5mm below the foramen magnum) and a left retrocerebellar arachnoid cyst (Figure 2C, b). The posterior pituitary is in place and the pituitary stalk is normal in both patients (Figures 2A and 2B).
Figure 2.
Cerebral MRI. (A) Sagittal medial section focused on the pituitary region of control (a), patient II.3 (b), and patient II.4 (c), showing an hypoplasia of anterior pituitary (ap). The pituitary stalk (s) is seen and the posterior pituitary (pp) is in normal position. (B) Coronal sections focused on pituitary region for control (a), patients II.3 (b), and patient II.4 (c) showing the anterior pituitary hypoplasia. Note the dysmorphy of the anterior pituitary (b, c). (C) Sagittal sections for patient II.3 (b) showing a Chiari type I malformation (herniated cerebellar tonsils through the foramen magnum showed by an arrow), and a left retrocerebellar arachnoid cyst (asterisk), (a) control MRI.
Mutation Detection
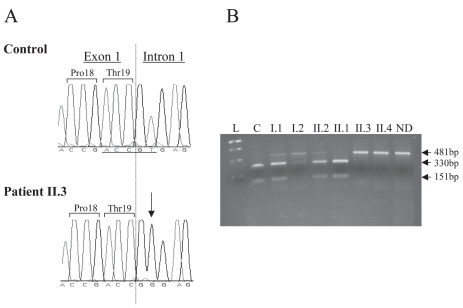
We first analyzed the sequence of the GH-N gene. No variation was detected in either patient (II.3 and II.4), except for the two previously reported single nucleotide polymorphisms (SNP) in exon 1 (c.40T > G and c.50A > G, pThr3Ala, GeneBank accession number BC090045). Then, we sequenced the GHRHR gene. This analysis revealed a homozygous T to G transversion at the second nucleotide of the donor splice site of intron 1 (IVS1 + 2T > G) (GeneBank accession number AY557192) (Figure 3A). This mutation affects the highly conserved dinucleotide guanine-thymine of the donor splice site. No other sequence variation was found except a SNP previously described in exon 6 (c.564C > T, p.His188His).
Figure 3.
(A) Electrophoregrams of the normal and mutated GHRHR sequences. Sequence analysis of portion of exon 1 and intron 1 of the GHRHR gene from an unaffected individual (control) and from the proband II.3 (patient). The homozygous T > G transversion (IVS1 + 2 T > G) is indicated by an arrow and the exon 1/intron 1 junction by a dotted vertical line. (B) Segregation analysis of the splice-donor-site mutation of GHRHR in the studied family. A 481-bp GHRHR genomic DNA fragment was digested by Taa-I restriction enzyme that recognizes only the wild type allele (underlined under the electrophoregram), then analyzed on a gel electrophoresis (3% nusieve agarose). This confirmed that the two patients (II.3 and II.4) are homozygous for the mutated allele (481-bp). The parents and the healthy brother (I.1, I.2, and II.2) are heterozygous (three fragments: 418, 330, and 151-bp), the sister (II.1) bears two wild type alleles, and the control (C) has two wild type alleles. L: “1Kb plus” ladder (Invitrogen); ND: not digested, bp: base pair.
As the identified T > G mutation abolishes a restriction site for Taa-I, its intrafamilial segregation was subsequently analyzed through digestion of PCR products spanning the mutation site. The two healthy parents (I.1 and I.2) were found to be heterozygous for the mutation, a result in keeping with the consanguinity documented in this family. This analysis also revealed that the patients’ brother (II.2), with normal height, is heterozygous whereas the healthy older sister (II.1) is homozygous for the wild-type allele (Figure 3B). These results were confirmed by DNA sequencing.
Consequence of the IVS1 + 2T > G Mutation on the Splicing of GHRHR Transcripts
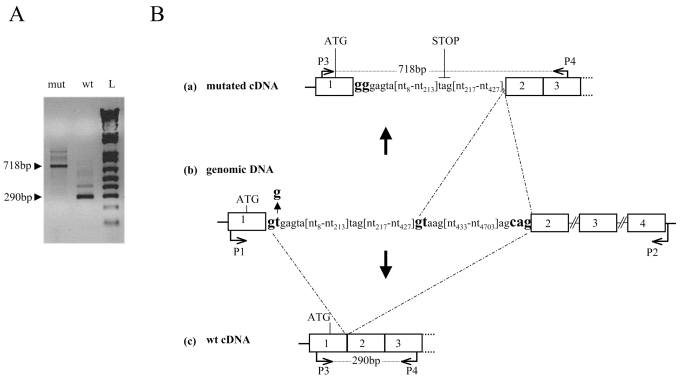
To determine the consequences of the IVS1 + 2T > G mutation on the splicing of GHRHR transcripts, HeLa cells were transfected with expression vectors containing the normal or the mutant GHRHR minigene consisting of a genomic fragment spanning exon 1 to exon 4. RT-PCR amplification of GHRHR transcripts isolated from cells transfected with the wild-type minigene generated a major product of 290 bp consistent with normal splicing of introns 1 and 2, whereas the IVS1 + 2T > G allele produced a larger product of 718 bp (Figure 4A). Sequencing of these fragments indeed showed that the 290-bp molecular species correspond to normally spliced GHRHR transcripts (Figure 4B), whereas, the 718-bp fragment contains a 427-bp insertion corresponding to the 5′ intronic sequence following exon 1. This latter observation indicates the use of a cryptic donor splice site in this intron at position 428 (Figure 4B). If translated, this abnormal transcript would give rise to a frameshift at codon 19 (end of exon1) that introduces 71 novel amino acids before a premature stop codon (TAG), thereby leading to a severely truncated protein.
Figure 4.
Transcription assay. (A) RT-PCR amplification of the GHRHR transcripts obtained from cells transfected with plasmids pcDNA3.1-GHRHR_WT (wild type minigene, amplified between P1–P2) (Lane wt) and pcDNA3.1-GHRHR_mut (mutated minigene, amplified between P1–P2) (Lane mut) using two internal exonic primers (P3 and P4), indicated by arrows in B (a, c). (B), (b) Schematic representation of the genomic GHRHR region where the IVS1 + 2T > G mutation at the donor splice site (gtgagta > gggagta) and the cryptic 5′ splice site (gtaag) (428bp downstream the first nucleotide of intron 1) are in bold characters. The 290-bp product corresponds to the normal transcript and the 718-bp fragment to the mutated transcript with a retention of a part of intron 1 because of activation of the intronic cryptic donor splice site (bold characters). (a) and (c): schematic representation of mutated and wild type partial cDNAs, respectively.
DISCUSSION
Description of a New GHRHR Mutation in Two Moroccan Patients, One of Them Displaying Chiari I Malformation
The IVS1 + 2T > G transition identified here is predicted to prevent normal splicing of GHRHR intron 1, because the GT dinucleotide at the beginning of each intron is 100% conserved in mammalian genes and is necessary for removal of intron sequences from the mature mRNA (19). Only four splice mutations in GHRHR have been identified in IGHD, and one of them is located in the same splice site (IVS1 + 1G > A) (1,12), presumably with identical molecular consequences. However, in all these cases, the effect of mutations on RNA splicing has not been studied, the GHRHR being expressed only in the pituitary and the placenta (20). In this study, we demonstrate—after generation of appropriate minigene constructs, transfection assays, RT-PCR, and sequencing—that this mutation causes the retention of a part of intron 1. A cryptic splice site (tgtg/gtaag) is mainly used 428 nucleotides downstream the beginning of intron 1, leading to the insertion of 71 new amino acids followed by a premature stop codon, which, most likely, causes nonsense-mediated degradation of the mRNA (21). Even if this does not occur, the putative receptor would be severely truncated at the beginning of the extracellular N-terminus lacking the seven transmembrane domains, resulting in complete loss of receptor function. Such defect may, therefore, have severe consequences on GH synthesis in accordance with the extreme small size of the two patients before GH treatment.
Patients carrying two mutated GHRHR alleles usually have severe IGHD revealed by severe short stature, but normal weight at birth and none presents micropenis nor neonatal hypoglycemia (1). Their GH and serum IGF-1 levels are reduced markedly, but good responsiveness and immunological tolerance to exogenous GH therapy are commonly reported (1–2). The two patients herein studied responded well to GH therapy. At the last examination, height was around −3.4 SD for patient II.3 (at the age of 20) and −4.5 SD for patient II.4 (at the age of 16). Unfortunately, the target height (−0.5 SD and −1 SD for patients II.3 and II.4 respectively) was not reached; due to social issues, the treatment was late. Also GHRHR-deficient patients may (22) or may not (15,23–24) display some dysmorphic features such as frontal bossing and marked nasal bridge; the two patients herein described have this phenotype.
It was thought that patients with a GHRHR molecular defect invariably have anterior pituitary hypoplasia (15,25–29), and that GHRHR mutations can be excluded in the absence of this feature because GHRHR is critical for pituitary development and function of somatotroph cells (30–31). However, patients homozygous for a GHRHR splice site mutation (IVS1 + 1G > A), presumably with molecular consequences identical to those documented in our patients, and with normal anterior pituitary have been reported recently (29). Both patients reported here showed anterior pituitary hypoplasia, and one of them had an abnormal aspect of the pituitary.
This is the first Chiari I malformation described in more than 40 subjects with bi-allelic GHRHR mutation (1). As the patient also presents a left arachnoid cyst, in the absence of previous MRI, it is not possible to determine if the Chiari I malformation is due to the cyst (mass effect) or represents a primitive malformation. A spectrum of intracranial-associated malformations has been observed in patients with IGHD and CPHD, including anatomic abnormalities of the hypothalamic-pituitary region and other cerebral structures. These malformations may be acquired or of developmental origin. As shown in a study reviewing over 22,000 unselected MRIs, Chiari I malformation occurs in 0.77% of cases (32); on the other hand, a Chiari I malformation has been shown to be much more prevalent in different IGHD-CPHD populations, affecting 9% (9/100) (33) or 20% (7/35) (34) of those patients in whom no molecular investigation was done. Fujita et al. (35) reported seven patients with idiopathic GH deficiency, hypopituitarism, small anterior pituitary, stalk interruption, and Chiari I malformation; however, as all of them were born after breech delivery, they postulated that this constellation of abnormalities might be explained by traction on the brain and spinal cord at birth. This hypothesis is not supported by the report of patients with GH deficiency and Chiari I malformation (7/35) who were delivered in vertex position (36), as is the case for patient II.3. Therefore, a genetic origin of Chiari I malformation represents an attractive hypothesis. The development of the cerebellar tonsils occurs from the fourth to eighth week of gestation, a critical period for the hypothalamo-pituitary region development during which genetic factors are implicated; a sequential expression of several transcription factors is indeed required for pituitary ontogeny (37). The hypothesis of a defect in embryogenesis as the cause for the Chiari I malformation is also supported by the fact that mutations in genes involved in early organogenesis (such as LHX4 and HESX1), critical for pituitary embryogenesis and differentiation, have been shown to be associated with this abnormality (38–39). Chiari I malformation could also be secondary to the severe GHD that was documented in all those patients, a hypothesis supported by the presence of a GH-N mutation in two siblings with severe GHD and Chiari I malformation (40). In addition, as all these mutations were identified in patients born to a consanguineous union, the implication of other genes cannot be excluded.
ACKNOWLEDGMENTS
We are grateful to the members of the family for their participation in this study. This work was supported by the Moroccan-French Convention CNRST/INSERM 2005-2006 and 2007-2008; the Assistance Publique-Hôpitaux de Marseille, Programme Hospitalier de Recherche Clinique (AP-HM n°2003-25, convention 20040080) and the Bonus Qualité Recherche from University Paris 6.
Footnotes
Online address: http://www.molmed.org
REFERENCES
- 1.Alba M, Salvatori R. Familial growth hormone deficiency and mutations in the GHRH receptor gene. Vitam Horm. 2004;69:209–20. doi: 10.1016/S0083-6729(04)69007-8. [DOI] [PubMed] [Google Scholar]
- 2.Bona G, Paracchini R, Giordano M, Momigliano-Richiardi P. Genetic defects in GH synthesis and secretion. Eur J Endocrinol. 2004;151:S3–9. doi: 10.1530/eje.0.151s003. [DOI] [PubMed] [Google Scholar]
- 3.Pantel J, et al. Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest. 2006;116:760–8. doi: 10.1172/JCI25303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho LR, et al. A homozygous mutation in HESX1 is associated with evolving hypopituitarism due to impaired repressor-corepressor interaction. J Clin Invest. 2003;112:1192–201. doi: 10.1172/JCI18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen RN, et al. Enhanced repression by HESX1 as a cause of hypopituitarism and septooptic dysplasia. J Clin Endocrinol Metab. 2003;88:4832–9. doi: 10.1210/jc.2002-021868. [DOI] [PubMed] [Google Scholar]
- 6.Thomas PQ, et al. Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septooptic dysplasia. Hum Mol Genet. 2001;10:39–45. doi: 10.1093/hmg/10.1.39. [DOI] [PubMed] [Google Scholar]
- 7.Laumonnier F, et al. Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet. 2002;71:1450–5. doi: 10.1086/344661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayo KE. Molecular cloning and expression of a pituitary-specific receptor for growth hormone-releasing hormone. Mol Endocrinol. 1992;6:1734–44. doi: 10.1210/mend.6.10.1333056. [DOI] [PubMed] [Google Scholar]
- 9.Haskin O, et al. A new mutation in the growth hormone-releasing hormone receptor gene in two Israeli Arab families. J Endocrinol Invest. 2006;29:122–30. doi: 10.1007/BF03344084. [DOI] [PubMed] [Google Scholar]
- 10.Horikawa R, Fujiita K, Nakajima R, Gaycinn BD, Tanaka T. A novel growth hormone-releasing hormone (GHRH) receptor mutation as a cause for isolated GH deficiency in Japanese boy with severe short-stature. Proceedings of the 82nd Meeting of the Endocrine Society; Toronto, Canada. 2000. abstract 1995. [Google Scholar]
- 11.Alba M, Salvatori R. Naturally-occurring missense mutations in the human growth hormonereleasing hormone receptor alter ligand binding. J Endocrinol. 2005;186:515–21. doi: 10.1677/joe.1.06213. [DOI] [PubMed] [Google Scholar]
- 12.Alba M, Hall CM, Whatmore AJ, Clayton PE, Price DA, Salvatori R. Variability in anterior pituitary size within members of a family with GH deficiency due to a new splice mutation in the GHRH receptor gene. Clin Endocrinol (Oxf) 2004;60:470–5. doi: 10.1111/j.1365-2265.2004.02003.x. [DOI] [PubMed] [Google Scholar]
- 13.Alba M, Salvatori R. Growth hormone-releasing hormone receptor mutations in familial growth hormone deficiency. Endocrinologist. 2003;13:422–7. [Google Scholar]
- 14.Roelfsema F, et al. Growth hormone (GH) secretion in patients with an inactivating defect of the GH-releasing hormone (GHRH) receptor is pulsatile: evidence for a role for non-GHRH inputs into the generation of GH pulses. J Clin Endocrinol Metab. 2001;86:2459–64. doi: 10.1210/jcem.86.6.7536. [DOI] [PubMed] [Google Scholar]
- 15.Salvatori R, Fan X, Mullis PE, Haile A, Levine MA. Decreased expression of the GHRH receptor gene due to a mutation in a Pit-1 binding site. Mol Endocrinol. 2002;16:450–8. doi: 10.1210/mend.16.3.0785. [DOI] [PubMed] [Google Scholar]
- 16.Salvatori R, et al. Familial dwarfism due to a novel mutation of the growth hormone- releasing hormone receptor gene. J Clin Endocrinol Metab. 1999;84:917–23. doi: 10.1210/jcem.84.3.5599. [DOI] [PubMed] [Google Scholar]
- 17.Argyropoulou M, Perignon F, Brunelle F, Brauner R, Rappaport R. Height of normal pituitary gland as a function of age evaluated by magnetic resonance imaging in children. Pediatr Radiol. 1991;21:247–9. doi: 10.1007/BF02018614. [DOI] [PubMed] [Google Scholar]
- 18.Binder G, Nagel BH, Ranke MB, Mullis PE. Isolated GH deficiency (IGHD) type II: imaging of the pituitary gland by magnetic resonance reveals characteristic differences in comparison with severe IGHD of unknown origin. Eur J Endocrinol. 2002;147:755–60. doi: 10.1530/eje.0.1470755. [DOI] [PubMed] [Google Scholar]
- 19.Faustino NA, Cooper TA. Pre-mRNA splicing and human disease. Genes Dev. 2003;17:419–37. doi: 10.1101/gad.1048803. [DOI] [PubMed] [Google Scholar]
- 20.Gaylinn BD. Growth hormone releasing hormone receptor. Receptors Channels. 2002;8:155–62. [PubMed] [Google Scholar]
- 21.Byers PH. Killing the messenger: new insights into nonsense-mediated mRNA decay. J Clin Invest. 2002;109:3–6. doi: 10.1172/JCI14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wajnrajch MP, Gertner JM, Harbison MD, Chua SC, Jr, Leibel RL. Nonsense mutation in the human growth hormone-releasing hormone receptor causes growth failure analogous to the little (lit) mouse. Nat Genet. 1996;12:88–90. doi: 10.1038/ng0196-88. [DOI] [PubMed] [Google Scholar]
- 23.Maheshwari HG, Silverman BL, Dupuis J, Baumann G. Phenotype and genetic analysis of a syndrome caused by an inactivating mutation in the growth hormone-releasing hormone receptor: Dwarfism of Sindh. J Clin Endocrinol Metab. 1998;83:4065–74. doi: 10.1210/jcem.83.11.5226. [DOI] [PubMed] [Google Scholar]
- 24.Netchine I, Talon P, Dastot F, Vitaux F, Goossens M, Amselem S. Extensive phenotypic analysis of a family with growth hormone (GH) deficiency caused by a mutation in the GH-releasing hormone receptor gene. J Clin Endocrinol Metab. 1998;83:432–6. doi: 10.1210/jcem.83.2.4528. [DOI] [PubMed] [Google Scholar]
- 25.Carakushansky M, et al. A new missense mutation in the growth hormone-releasing hormone receptor gene in familial isolated GH deficiency. Eur J Endocrinol. 2003;148:25–30. doi: 10.1530/eje.0.1480025. [DOI] [PubMed] [Google Scholar]
- 26.Murray RA, Maheshwari HG, Russell EJ, Baumann G. Pituitary hypoplasia in patients with a mutation in the growth hormone-releasing hormone receptor gene. AJNR Am J Neuroradiol. 2000;21:685–9. [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira HA, Salvatori R, Krauss MP, Oliveira CR, Silva PR, Aguiar-Oliveira MH. Magnetic resonance imaging study of pituitary morphology in subjects homozygous and heterozygous for a null mutation of the GHRH receptor gene. Eur J Endocrinol. 2003;148:427–32. doi: 10.1530/eje.0.1480427. [DOI] [PubMed] [Google Scholar]
- 28.Osorio MG, Kopp P, Marui S, Latronico AC, Mendonca BB, Arnhold IJ. Combined pituitary hormone deficiency caused by a novel mutation of a highly conserved residue (F88S) in the homeodomain of PROP-1. J Clin Endocrinol Metab. 2000;85:2779–85. doi: 10.1210/jcem.85.8.6744. [DOI] [PubMed] [Google Scholar]
- 29.Osorio MG, et al. Pituitary magnetic resonance imaging and function in patients with growth hormone deficiency with and without mutations in GHRH-R, GH-1, or PROP-1 genes. J Clin Endocrinol Metab. 2002;87:5076–84. doi: 10.1210/jc.2001-011936. [DOI] [PubMed] [Google Scholar]
- 30.Billestrup N, Swanson LW, Vale W. Growth hormone-releasing factor stimulates proliferation of somatotrophs in vitro. Proc Natl Acad Sci U S A. 1986;83:6854–7. doi: 10.1073/pnas.83.18.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364:208–13. doi: 10.1038/364208a0. [see comments] [DOI] [PubMed] [Google Scholar]
- 32.Meadows J, Kraut M, Guarnieri M, Haroun RI, Carson BS. Asymptomatic Chiari Type I malformations identified on magnetic resonance imaging. J Neurosurg. 2000;92:920–6. doi: 10.3171/jns.2000.92.6.0920. [DOI] [PubMed] [Google Scholar]
- 33.Arifa N, Leger J, Garel C, Czernichow P, Hassan M. [Cerebral anomalies associated with growth hormone insufficiency in children: major markers for diagnosis?] Arch Pediatr. 1999;6:14–21. doi: 10.1016/s0929-693x(99)80067-0. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton J, Blaser S, Daneman D. MR imaging in idiopathic growth hormone deficiency. AJNR Am J Neuroradiol. 1998;19:1609–15. [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita K, et al. The association of hypopituitarism with small pituitary, invisible pituitary stalk, type 1 Arnold-Chiari malformation, and syringomyelia in seven patients born in breech position: a further proof of birth injury theory on the pathogenesis of “idiopathic hypopituitarism. Eur J Pediatr. 1992;151:266–70. doi: 10.1007/BF02072226. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton J, Chitayat D, Blaser S, Cohen LE, Phillips JA, 3rd, Daneman D. Familial growth hormone deficiency associated with MRI abnormalities. Am J Med Genet. 1998;80:128–32. [PubMed] [Google Scholar]
- 37.Dasen JS, et al. Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes Dev. 2001;15:3193–207. doi: 10.1101/gad.932601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machinis K, et al. Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4. Am J Hum Genet. 2001;69:961–8. doi: 10.1086/323764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sobrier ML, et al. Novel HESX1 mutations associated with a life-threatening neonatal phenotype, pituitary aplasia, but normally located posterior pituitary and no optic nerve abnormalities. J Clin Endocrinol Metab. 2006;91:4528–36. doi: 10.1210/jc.2006-0426. [DOI] [PubMed] [Google Scholar]
- 40.Iughetti L, et al. Complex disease phenotype revealed by growth hormone deficiency associated with a novel and unusual defect in the GH-1 gene. Clin Endocrinol (Oxf) 2007 doi: 10.1111/j.1365-2265.2007.03157.x. [DOI] [PubMed] [Google Scholar]
- 41.Sempé M, Pedron G, Roy-Pernot M. Auxologie: méthode et séquences. Paris: Théraplix; 1979. pp. 205.1–205. [Google Scholar]