Abstract
Caffeine elevates cortisol secretion, and caffeine is often consumed in conjunction with exercise or mental stress. The interactions of caffeine and stress on cortisol secretion have not been explored adequately in women. We measured cortisol levels at eight times on days when healthy men and women consumed caffeine (250 mg × 3) and underwent either mental stress or dynamic exercise protocols, followed by a midday meal, in a double blind, placebo-controlled, crossover design. Men and women had similar cortisol levels at the predrug baselines, but they responded differently to mental stress and exercise. The cortisol response to mental stress was smaller in women than in men (p=.003). Caffeine acted in concert with mental stress to further increase cortisol levels (p=.011), the effect was similar in men and women. Exercise alone did not increase cortisol, but caffeine taken before exercise elevated cortisol in both men and women (ps<.05). After a postexercise meal, the women had a larger cortisol response than the men, and this effect was greater after caffeine (p<.01). Cortisol release in response to stress and caffeine therefore appears to be a function of the type of stressor and the sex of the subject. However, repeated caffeine doses increased cortisol levels across the test day without regard to the sex of the subject or type of stressor employed (p<.00001). Caffeine may elevate cortisol by stimulating the central nervous system in men but may interact with peripheral metabolic mechanisms in women.
Keywords: Cortisol, Caffeine, Stress, Diet, Men, Women
Caffeine is a widely consumed pharmacological substance that alters cortisol responses at rest and in response to various stressors. In a U.S. sample, 96% of adults reported having consumed caffeine at some time in their lives, and 83% were currently consuming 186 mg of caffeine per day (Hughes and Oliveto, 1997). Reported consumption is similar in women and men (James, 1991). Caffeine acts as an important modulator of cardiovascular and central nervous system activity (Hoyle, 1992). Its most prominent cardiovascular effect is to elevate blood pressure for up to several hours after intake (Pincomb et al., 1985; Robertson et al., 1978). This pressor effect occurs both at rest and during exercise (Sung et al., 1990) or mental exertion (Lane and Williams, 1985; Pincomb et al., 1988).
Caffeine also increases cortisol and epinephrine levels both at rest and during periods of stress (al'Absi and Lovallo, 2004). The cortisol response to stress varies across individuals (al'Absi et al., 1997), raising the question of variability in caffeine's effect on cortisol secretion. A recent meta-analysis found that men have larger cortisol responses to mental stress than do women (Dickerson and Kemeny, 2004). Caffeine effects on cortisol have been studied systematically in men but not in women, indicating a need for comparisons of men and women exposed to caffeine while undergoing stress protocols.
Under basal conditions, cortisol release is regulated by the hypothalamic paraventricular nucleus responding to a combination of diurnal and metabolic inputs (Weitzman et al., 1981). These signals are responsible for the typical peak of cortisol secretion at awakening and for its gradual decline through the day, with a nadir in the early morning hours during the human sleep period. This pattern can be altered by a variety of homeostatic challenges. Exercise, for example, may evoke a rise in cortisol resulting in liberation of glucose and stores of fatty acids (al'Absi and Lovallo, 2004). Cortisol can respond to purely mental stressors in the absence of physical effort. This psychological stress response is regulated by inputs from limbic structures and prefrontal cortex acting on the hypothalamus, resulting in an HPAC cascade (Herman et al., 2003). Because food intake is also known to cause a cortisol response (Beebe et al., 1990; Gibson et al., 1999), consuming a meal may modify the diurnal cortisol cycle alone and possibly in relation to caffeine intake. Given the potential for caffeine to increase cortisol secretion during a range of stressors and in relation to food intake and given the relative lack of information on men and women at these times, the present study examined the roles of sex and the type of stressor on cortisol release after caffeine consumption. To elucidate possible mechanisms of sex difference in cortisol release we measured glucose and free fatty acids in a subgroup of these subjects.
1. Methods
1.1. Subjects
Ninety-six healthy normotensive volunteers were recruited by newspaper ads, posters in the University community, and word of mouth. Volunteers were screened for good health by medical history, physical exam and electrocardiogram. By inclusion and exclusion criteria, volunteers were: normotensive, within 20% of ideal body weight by Metropolitan Life Insurance Company norms, in self-reported good health, not taking prescription medications, including oral contraceptives, not under treatment for hypertension, and had alcohol consumption <15 drinks/week, were nonsmokers, and reported equivalent caffeine use of 1–5 cups of coffee/day by structured interview, and were not pregnant. Subjects with reported or suspected caffeine intolerance were excluded. Approximately half of the volunteers were tested in Buffalo, NY (N=47, 23 Females), and the remainder in Oklahoma City, OK (N=49, 24 Females). At the time of screening each subject signed an informed-consent form approved by the respective Institutional Review Boards of the State University of New York at Buffalo, Buffalo, NY and the University of Oklahoma Health Sciences Center and the Veterans Affairs Medical Center, Oklahoma City, OK. Subjects were paid for their participation.
1.2. Study design
The primary study design was a 4-week randomized, placebo-controlled, double blind, crossover trial of caffeine (C) vs. placebo (P) effects on cardiovascular and endocrine function. Each study week began with 5 days of home self-administration of P (0 mg/day) or C (300 mg/day or 600 mg/day taken in 3 equal doses) followed by a laboratory test day during which the subject received P on one week (3 × 0 mg=0 mg) and C on the other three weeks (3 × 250 mg=750 mg) with a crossover day (C=100, 0, and 0 mg) to buffer sudden changes in intake between study weeks. The development of tolerance and its effect on cortisol response to caffeine across the day has been reported elsewhere (Lovallo et al., 2005).
Data reported here are from 2 of these weeks: the P home-maintenance, P test week (P–P week) vs. the P home-maintenance, C test week (P–C week). This allowed us to compare cortisol levels and stress responses in the absence caffeine (P–P week) and after acute caffeine administration following a placebo diet (P–C week). The exercise protocol was conducted at the Buffalo, NY site and mental stress testing in Oklahoma City, OK. Aside from a difference in the stressors, the study protocols were identical in all other respects at the two sites.
1.3. Caffeine administration and compliance
Home maintenance P doses were supplied as gelatin capsules containing lactose (College of Pharmacy, University of Oklahoma, Oklahoma City, OK). Acute doses in the lab were provided in capsules containing lactose or lactose plus 250 mg of USP caffeine (Gallipot, St. Paul, MN). The 250 mg dose is sufficient to produce reliable cardiovascular and neuroendocrine effects, is within the range persons normally consume in their daily lives, and is within the dose range used in other studies (James, 1991). Saliva samples were collected at home each maintenance day and at the beginning and end of each protocol day to verify compliance and accuracy of dose administration. Compliance was assessed by capsule counts in bottles brought in on lab days, by caffeine assay of saliva specimens collected at home each day at 7:00 p.m. (Lee et al., 1996; Lelo et al., 1986; Zylber-Katz et al., 1984), and from saliva specimens collected each morning upon entering the lab. Subjects found to be noncompliant by any of these criteria were dropped and replaced.
1.4. Study procedures and test protocol
Subjects were given lists of foods and printed instructions on how to abstain from all dietary sources of caffeine during maintenance days and evenings prior to lab testing on each study week. Mental stress testing and was done on 49 of the subjects (24 Females) and exercise testing on 47 (24 Females). Blood samples were obtained for measurement of free fatty acids and glucose levels in 12 volunteers (6 Females) in the exercise protocol.
Test days at both sites began at 8:00 a.m. in the lab and ended in the home at 7:00 p.m. The timing of cortisol sampling was held constant across subjects to control for diurnal effects. Upon entering the laboratory, subjects confirmed being in good health and complying with dietary requirements. Volunteers were given a standardized breakfast to control for glycemic status and were instrumented for cardiovascular monitoring (reported elsewhere). Caffeine or placebo was administered at three times (9:00 a.m., 1:00 p.m., and 6:00 p.m.). Cortisol was measured in eight repeated saliva specimens taken across each test day. The daily protocol started at 9:00 a.m. and the protocol time periods, saliva collection points, and their designations were: predrug resting baseline and saliva (30 min; Baseline), P or C capsule, drug absorption and saliva (60 min; C+60), mental or exercise stress and saliva (30 min; PostStress), recovery and saliva (40 min; Post+40), a midday meal and saliva (30 min; PostMeal), P or C capsule, drug absorption and a saliva (60 min; C+60), departure from lab and normal afternoon activities and by a saliva sample taken at home (6:00 p.m.), followed by the third P or C capsule, and a final saliva sample taken at 7:00 p.m. (C+60).
Mental stress consisted of 15-min of work on a demanding reaction time task followed by 15-min of mental arithmetic. The reaction time test consisted of rapidly depressing a response key to an unsignaled visual cue (the word “Go”) presented 60 times by video monitor at unpredictable intervals ranging from 4–30 s (M=15 s). A performance incentive was produced by giving the subject a $20 “bank” at the beginning of the task and deducting $0.25 for each response slower than 270 ms. The value of the remaining bank was displayed continuously in a corner of the monitor, and the final amount was added to the subject's incentive pay. Mental arithmetic consisted of three 5-min blocks of serial addition. Each block started when the subject was given a 3-digit number and told to add the digits together, add the sum to the original number and to use that answer as the starting point for the next calculation. Each answer was spoken via intercom to the experimenter, who informed the subject when an incorrect response was given. Prior work has shown this task combination to be mildly aversive (al'Absi et al., 1998).
Exercise consisted of a supine bicycle-ergometer protocol performed for 30 min at a moderate workload. We used different workloads for men and women to compensate for differences in body weight and aerobic work capacity. Males warmed up during the first 9 min at graded 3-min workloads of 50, 75, and 100 W, followed by 21 min at 75 W of steady-state work output. Women, warmed up for the first 9 min in steps of 40, 60 and 80 W followed by 21 min at 60 W.
One hour after the end of the stress period, the subjects consumed a standard microwave meal chosen from a selection of equivalent Healthy Choice (ConAgra Foods, Inc., Omaha, NE) brand frozen dinners.
1.5. Saliva cortisol and caffeine measurements
Saliva samples were collected using a self-contained collection and storage device (Salivette, Sarstedt, Germany) consisting of a cellulose pledget in a storage tube. The subject held the pledget in his or her mouth until saturated with saliva and then replaced it into the tube. Samples were centrifuged, transferred to cryogenic storage tubes, and held at −70 °C until assay. Unbound cortisol concentrations available in saliva were then determined by radioimmunoassay kit (Spectria Cortisol) by Orion Diagnostica (Espoo, Finland). The reliability and sensitivity of saliva cortisol measures have been documented elsewhere (Aardal and Holm, 1995; Kirschbaum and Hellhammer, 1989; Read et al., 1990; Riad-Fahmy et al., 1983). In order to test for compliance with caffeine restrictions subjects collected one saliva specimen each day at home at 7:00 p.m. To ensure accuracy of dosing in the lab, caffeine was extracted from 200 μL aliquots of saliva taken from specimens collected before and after dosing on test days. Caffeine concentrations were determined using high performance liquid chromatography as described previously (Christensen and Whitsett, 1979).
1.6. Data analysis
Demographic and physiometric characteristics of men and women were compared by Student's t test. Cortisol values were tested in a repeated measures Multivariate Analysis of Variance (MANOVA) model including 2 Sexes (Male, Female) and 2 Sites (Oklahoma City, Buffalo) as between subjects factors and 2 Drugs (P, C, corresponding to the two study weeks) and 8 Periods on each week (Baseline, 60 min post C or P=C+60, PostStress, 40 min PostStress=Post+40, PostMeal, 60 min PostMeal and C or P=C+60, 6:00 p.m., 7:00 p.m.=C+60) as within-subjects repeated measures. The cortisol effects of caffeine prior to mental stress, exercise, and the meal were tested with separate MANOVAs and by Student t tests with Bonferroni corrections. All analyses were done using SAS (SAS system for Windows, ver. 8.2, SAS Institute Inc., Cary, NC) or SPSS (SPSS for Windows, rel. 11, SPSS, Inc., Chicago, IL).
2. Results
The subject characteristics are shown in Table 1. Men had greater body surface areas than women (p<.01) but their body mass indexes did not differ (NS). Men also had significantly higher systolic blood pressure (p<.001) and lower resting heart rate (p<.01), but diastolic blood pressure was similar for men and women.
Table 1.
Subject characteristics
| Total group
(n=96) |
Males
(n=48) |
Females
(n=48) |
p | |
|---|---|---|---|---|
| Age (yr) | 29 (0.6) | 27 (0.8) | 30 (0.9) | 0.02 |
| Body weight (kg) | 72 (12.2) | 80 (1.8) | 65 (1.0) | 0.0001 |
| Body surface area (m2) | 1.86 (.02) | 2.00 (.02) | 1.72 (.02) | 0.0001 |
| Body mass index (kg/m2) | 23.8 (.3) | 24.1 (.4) | 22.5 (.5) | NS |
| Caffeine intake (mg/day) | 458 (48) | 451 (71) | 464 (65) | NS |
| Screening blood pressure (mmHg) | ||||
| Systolic | 112 (1.1) | 117 (1.6) | 107 (1.1) | 0.0001 |
| Diastolic | 66 (0.6) | 66 (0.9) | 66 (0.9) | NS |
| Heart rate | 69 (1.1) | 68 (1.4) | 70 (1.6) | NS |
Note: Entries show Mean (± SE). Caffeine intake reflects usual consumption from all sources based on structured interview. Screening blood pressure is the average of three readings taken over 5 min after 5 min of being seated.
Saliva caffeine levels taken at home on placebo maintenance days and from morning samples in the lab were near 0 μg/mL on the two study weeks, indicating compliance with dietary caffeine restrictions. Saliva caffeine levels after 250 mg caffeine in the lab ranged from 3.5 to 5.8 μg/mL, indicating expected concentrations for this dose and verifying accuracy of administration on the caffeine test day. There was a significant increase in systolic/diastolic blood pressure (p<.001/.001) after the morning caffeine dose, that was equal for the men and women. A detailed report has been provided elsewhere (Lovallo et al., 2004).
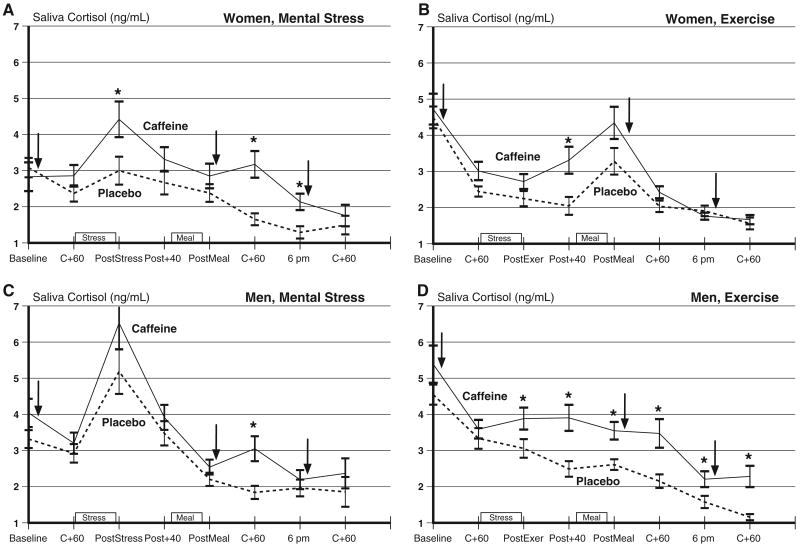
Cortisol data are shown in Fig. 1. As expected, cortisol varied significantly across the eight measurement periods, F(7, 86)=64, p<.00001, but it did not differ between the two test sites, F(1, 92)=.04, p=.85. Men had higher cortisol levels than women, F(1, 92)=4.83, p=.03, with no difference as a function of site, F(1, 92)=.39, p=.54 (Sex by Site).
Fig. 1.
Cortisol concentrations in saliva. Cortisol concentrations in saliva collected from women and men on days when they were administered capsules containing caffeine (250 mg × 3) or placebo, shown by arrows, and underwent either mental stress testing or bicycle exercise before consuming a noon meal. Stress refers to the time of mental stress or exercise. Meal refers to time of midday meal. Axis labels refer to protocol timepoints when saliva was collected: C60=60 min following morning placebo or caffeine intake. PostStress = immediately at the end of mental stress or exercise. Post+40=40 min after end of stress. PostMeal = immediately following midday meal. 6 p.m. = 6:00 p.m. at home. C+60=60 min following third caffeine administration of the day. Asterisks indicate significant differences between placebo and caffeine based on 1-tailed, paired Student's t test with Bonferroni correction and significance set at p<.05.
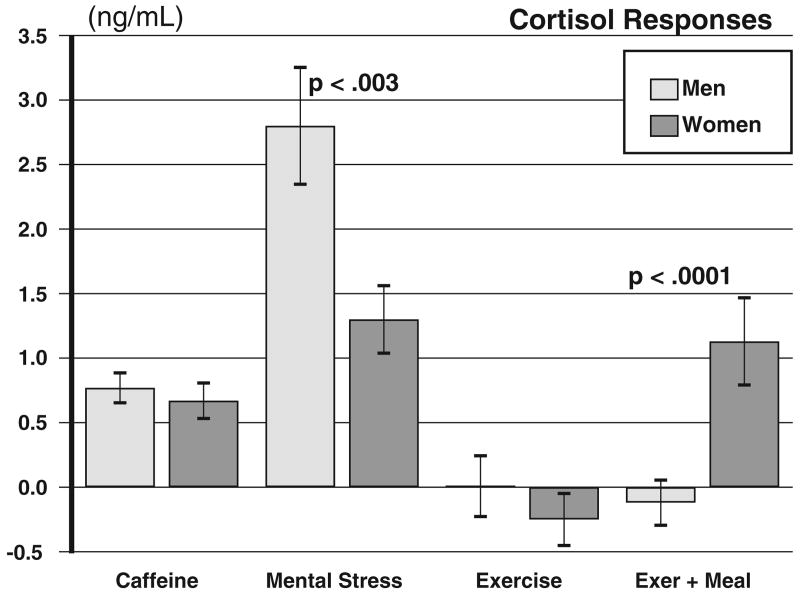
Caffeine did not acutely elevate cortisol levels in the absence of stress. As seen at the C+60 time point following the morning caffeine capsule, the caffeine-day values are equivalent to the placebo values in both settings and for both men and women (Fig. 1). However, the repeated administration of caffeine in conjunction with stress and food intake caused a robust elevation in cortisol levels relative to the placebo day, F(1, 92)=54, p<.00001 (Drug main effect from the primary MANOVA), and accordingly modified the pattern of cortisol secretion across the day, F(7, 86)=3.12, p=.005 (Drug by Period), an effect that differed between sites, F(7, 86)=2.84, p=.011. Caffeine had similar effects on cortisol in men and women in that all interactions in the primary MANOVA involving both the Drug and Sex factors were nonsignificant, all Fs≤1.60, ps≥.21. Fig. 2, left bars, shows the cortisol elevations on the caffeine day relative to placebo values in men and women averaged over both study sites and the seven time periods after the initial predrug baseline.
Fig. 2.
Mean changes in cortisol levels for men and women. Bars labeled Caffeine show difference between placebo and caffeine averaged over all time points starting at 60 min after first caffeine dose in the morning. Bars labelled Mental Stress, Exercise, and Meal indicate changes in cortisol from the value preceding the manipulation to immediately afterward, averaged over placebo and caffeine days. Bars for Meal reflect only the meal eaten after exercise. Significance values reflect Sex by Period interaction terms.
Inspection of Fig. 1 indicates that men were more responsive to the mental stressors than the women (panels A and C), while the women had greater responses to the meal, especially following exercise (panel B). The analysis also revealed interaction terms that suggested that mental stress and the meal affected cortisol differentially for men and women, although they responded similarly to exercise itself. First, as expected, the cortisol patterns across the day differed in the mental stress and exercise settings, F(7, 86)=14.5, p<.0001 (Site by Period). Second, men and women had different patterns of cortisol secretion across the day, F(7, 86)= 4.54, p<.0005 (Sex by Period), suggesting different reactions by men and women to the stressors and the meal. Third, caffeine affected cortisol secretion differently in relation to mental stress, exercise, and the meal, F(7, 86)=2.84, p=.011 (Drug by Site by Period). These results from the primary MANOVA were therefore explored in subsidiary analyses comparing men and women in response to mental stress, exercise challenge, and the noon meal following exercise. Fig. 2 compares the respective cortisol responses for men and women.
We examined mental stress effects in men and women using a MANOVA including Sex, Drug, and Period (C+60, PostStress). The men had a larger response than the women, F(1, 47) =9.69, p=.003 (Sex by Period interaction). Caffeine potentiated the mental stress effect, F(1, 47)=7.09, p=.011 (Drug by Period), but to a similar degree in both sexes, F(1, 47)=.031, p=.86 (Sex by Drug by Period). This agrees with other work showing that men have larger cortisol responses to mental stress than women do, but that men and women respond equally to caffeine (Fig. 2).
Exercise effects were tested using the same MANOVA design in the exercise setting, including Sex, Drug, and Period (C+60, PostExer). Neither men nor women had an acute cortisol response to exercise (Fig. 2), indicated by a nonsignificant Sex by Period interaction, F(1, 45)=0.597, p=.44, and a nonsignificant Period effect, F(1, 45)=0.524, p=.47. Caffeine-day values were higher than with placebo, F(1, 45)=7.45, p=.008, but caffeine did not acutely alter the cortisol response during exercise, F(1, 45)=1.34, p=.96 (Drug by Period), and it did not affect men differently from women as shown by a nonsignificant Sex by Drug by Period interaction, F(1, 45)=2.64, p=.11. However, the metabolic demands of exercise in conjunction with caffeine did produce a delayed cortisol rise, seen as higher cortisol levels on C day vs. P Day at the Post+40 time point for both the men and the women, ts>3.01, ps<.05 (Fig. 1B and D).
We then examined the effect of the meal on cortisol secretion in a MANOVA including Sex, Site, Drug, and Period (Post+40, PostMeal). Women, but not men, increased their cortisol levels to the meal challenge, F(1, 92)=16.71, p<.0001 (Sex by Period), with no difference in the presence of caffeine, F(1, 92)=.05, p=.81 (Sex by Drug by Period). The effect of the meal on cortisol was more pronounced following exercise, F(1, 92)=25, p<.0001 (Site by Period), and this effect was greater among women than men, t=3.08, p=.003 (Fig. 2).
The cortisol response to the meal suggested a possible difference between men and women in the glucocorticoid response to food intake following exercise. In the exercise protocol, we obtained limited free fatty acid and glucose data from 12 subjects (6 females) after drug administration, exercise, and the meal. Free fatty acid levels after caffeine were significantly higher in both sexes compared to the placebo day (0.4 vs. 0.18 MEQ/L, p<.001) and remained so after exercise. Women had a larger decrease in glucose levels immediately after exercise than did the men (−14 vs. −7 mg/ml, respectively), and this effect was greater on the caffeine day than the placebo day (−25% vs. −10%, p<.01, by t test). Glucose levels were increased in both sexes after the meal but in women the increase was more pronounced on the caffeine day than on the placebo day (47% vs. 36%, p<0.05). The women therefore appeared to be more responsive than the men to the metabolic challenge imposed by exercise, and caffeine enhanced this effect, which was then compensated when food was provided.
3. Discussion
This study documents four effects of caffeine and sex on cortisol responses that support earlier findings and indicate areas that require further study. First, exercise and mental stress have clearly different effects on cortisol secretion. Second, men and women respond differently to psychological stress. Third, caffeine enhances stress responses in both men and women. Fourth, metabolic factors can modify the above relationships.
Figs. 1 and 2 readily indicate that mental stress and exercise are different in the magnitude and time course of their effects on cortisol secretion. Mental stress had a much larger and rapid effect than did exercise under placebo conditions. Stressors like public speaking or mental arithmetic are effective because they evoke limbic system activity and interactions with the prefrontal cortex that in turn generate descending influences on the hypothalamus (De Kloet and Reul, 1987; Lovallo, 2005). Consistent with this analysis, cortisol responses during mental stress are associated with reports of negative affect (al'Absi et al., 1997), and cortisol output is lowered when negative affect is reduced from control levels by a humorous and uplifting video (Buchanan et al., 1999). This top-down influence of affective state is a potent manipulator of the HPA in the absence of metabolic demands and accordingly may be short lived once the stressor is over and the person returns to a baseline state. In contrast, the moderate-intensity exercise that we used had no immediate effect on cortisol output. Fig. 1 shows declining cortisol levels on the placebo day from the preexercise (C+60) time period through 40 min postexercise (Post+40). Exercise that is of short duration and moderate intensity may not activate a cortisol response if glucose levels remain normal (Sung et al., 1990), although long duration and intense exercise will increase cortisol output to high levels as a function of metabolic demands on fuel homeostasis (Laurent et al., 2000; Luger et al., 1987; Scavo et al., 1991). Despite the moderate intensity of the exercise protocol we used, the much larger HPA response to the mental stressors is striking.
Second, the sex of the subjects influenced how cortisol levels were regulated during stress. The placebo data in Fig. 1 show a large cortisol response to mental stress in men and only a small rise in women. Following exercise on the placebo day, the women had a response to the meal that was not seen in the men. These findings suggest that men may more readily activate an HPA response to mental stressors, and perhaps to the negative affect accompanying these events, while women are relatively more reactive to metabolic influences. This finding is consistent with published reports (Kirschbaum et al., 1992; Dickerson and Kemeny, 2004).
Third, caffeine increased cortisol secretion over the day of observation. Caffeine increases cortisol levels during periods of stress (Lovallo et al., 1989, 1996; Lane et al., 1990; Sung et al., 1990) both in the lab and in daily life (Lane, 1994), during naturalistic stressors such as medical school examinations (Shepard et al., 2000), and in relation to exercise (Laurent et al., 2000; Sung et al., 1990). Caffeine's effect on cortisol may be greater in persons with higher levels of central nervous system activation, such as those at high risk for hypertension (al'Absi et al., 1998). In the present study, caffeine enhanced cortisol release during mental stress, but the effect was statistically significant only in women. Caffeine combined with exercise had a small transient effect on cortisol in women but a substantial and prolonged effect in the men. As Fig. 1D shows, caffeine-day cortisol levels in the men were higher than placebo levels at all postexercise time points until the final cortisol sample taken at 7:00 p.m.
In order to gain some insight into possible metabolic influences on the cortisol response to the postexercise meal, we performed a limited number of observations late in the data collection process. Glucocorticoids play an important role in regulating proteins, carbohydrates, and fats (Dallman et al., 2003), and cortisol is elevated in response to falling blood glucose levels (Van Cauter et al., 1992). We observed that free fatty acid levels were higher in men and women after caffeine, as reported by others (Fisher et al., 1986). Women showed decreased glucose levels after exercise, and the fall was greater after caffeine. This may be responsible for the larger cortisol response that the women showed to the meal. The interpretation of a metabolic effect of exercise on cortisol levels in women may be discounted by the absence of a rise in cortisol on placebo days in either men or women (Fig. 1B and D). However, comparison of the postprandial time point in women exposed to mental stress vs. exercise (Fig. 1A and B) shows an effect of the meal after exercise that is lacking after mental stress. Caffeine caused an increase in cortisol at 40 min postexercise that was similar in magnitude in men and women, again suggesting that a differential metabolic response was not a predominant factor in the cortisol response. The caffeine results most readily appear to be explained by a direct stimulation of the HPA by caffeine, as occurs in mental stress, but with a greater time delay.
However, two results are not fully explained by the present data. Men had a prolonged increase in cortisol following exercise and in response to repeated caffeine doses that persisted until the last sample at 7:00 p.m. that evening. This was not seen in men exposed to mental stress. Therefore, some long lasting interaction between exercise and caffeine intake appears to occur in the men, and a metabolic effect of exercise cannot be fully discounted. Second, women had a pronounced cortisol response to the noon meal on both placebo and caffeine days. Cortisol is known to respond to food intake (Van Cauter et al., 1992) and it does so in comparable fashion in men and women in the absence of exercise (Gibson et al., 1999). Caffeine can increase the rate of glucose utilization in exercise (Ivy et al., 1979) thereby contributing to the cortisol rise during the meal in women. We are not aware of studies specifically comparing men and women in cortisol response to a meal given after exercise, as we did here. Given the interest in caffeine as an ergogenic aid in athletics, the effects of caffeine in conjunction with food intake and exercise in men and its possible differences in women may be an interesting topic for further study in relation to sports performance.
The men in this study had pronounced cortisol responses to mental stress, an effect that was much smaller in women. On the other hand, women increased cortisol more in response to the metabolic challenge of a meal following a period of exercise. These findings suggest that men undergoing mental stress elevate cortisol more readily than women, suggesting differences in central nervous system activity impinging on the hypothalamic–pituitary–adrenocortical axis. In contrast, men and women have similar cortisol responses to exercise. Caffeine increases cortisol levels in conjunction with mental stress and exercise challenge, but the time course is different, again suggesting interaction with central mechanisms during mental stress and with metabolic processes during exercise. These sex differences in cortisol response to stress and caffeine may have implications for caffeine use in sports and in relation to health outcomes.
Acknowledgments
This study was supported by Grants HL 32050, HL-32050-S2, and RR-14467 from the National Institutes of Health, Bethesda, MD and by the Medical Research Service of the Department of Veterans Affairs, Washington, D.C. We thank M. L'Hermité-Baleriaux, Université Libre de Bruxelles, Belgium, for her dedicated efforts in performing the saliva cortisol assays. We also thank Kim Sung, Ph.D. for her work with the exercise portion of the protocol.
References
- Aardal E, Holm AC. Cortisol in saliva-reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem. 1995;33:927–32. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Lovallo WR. Caffeine effects on the human stress axis. In: Nehlig A, editor. Coffee, tea, chocolate and the brain. Boca Raton, FL: CRC Press; 2004. pp. 113–31. [Google Scholar]
- al'Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–75. doi: 10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Lovallo WR, Pincomb GA, Sung BH, Wilson MF. Hypothalamic–pituitaryadrenocortical responses to psychological stress and caffeine in men at high risk for hypertension. Psychosom Med. 1998;60:521–7. doi: 10.1097/00006842-199807000-00021. [DOI] [PubMed] [Google Scholar]
- Beebe CA, Van Cauter E, Shapiro ET, Tillil H, Lyons R, Rubenstein AH, et al. Effect of temporal distribution of calories on diurnal patterns of glucose levels and insulin secretion in NIDDM. Diabetes Care. 1990;13:748–55. doi: 10.2337/diacare.13.7.748. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, al'Absi M, Lovallo WR. Cortisol fluctuates with increases and decreases in negative affect. Psychoneuroendocrinology. 1999;24:227–41. doi: 10.1016/s0306-4530(98)00078-x. [DOI] [PubMed] [Google Scholar]
- Christensen HD, Whitsett TL. Measurements of xanthenes and their metabolites by means of high pressure liquid chromatography. In: Hawk GL, editor. Biological/biomedical application of liquid chromatography science. Vol. 10. New York: Marcel Dekker; 1979. pp. 507–38. [Google Scholar]
- Dallman MF, Akana SF, Laugero KD, Gomez F, Manalo S, Bell ME, et al. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003;79:3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Reul JM. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Fisher SM, McMurray RG, Berry M, Mar MH, Forsythe WA. Influence of caffeine on exercise performance in habitual caffeine users. Int J Sports Med. 1986;7:276–80. doi: 10.1055/s-2008-1025774. [DOI] [PubMed] [Google Scholar]
- Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med. 1999;61:214–24. doi: 10.1097/00006842-199903000-00014. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamopituitary–adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–80. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hoyle CHV. Transmission: purines. In: Burnstock G, Hoyle CHV, editors. Autonomic neuroeffector mechanisms. Reading, England: Harwood Academic Publishers; 1992. pp. 367–408. [Google Scholar]
- Hughes JR, Oliveto AH. A systematic survey of caffeine intake in Vermont. Exp Clin Psychopharmacol. 1997;5:393–8. doi: 10.1037//1064-1297.5.4.393. [DOI] [PubMed] [Google Scholar]
- Ivy JL, Costill DL, Fink WJ, Lower RW. Influence of caffeine and carbohydrate feeding on endurance performance. Med Sci Sports. 1979;111:6–11. [PubMed] [Google Scholar]
- James JE. Caffeine and Health. New York: Academic Press; 1991. [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–69. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer DH. Consistent sex differences in cortisol responses to psychological stress. Psychosom Med. 1992;54:648–57. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Lane JD. Neuroendocrine responses to caffeine in the work environment. Psychosom Med. 1994;56:267–70. doi: 10.1097/00006842-199405000-00014. [DOI] [PubMed] [Google Scholar]
- Lane JD, Williams RB., Jr Caffeine affects cardiovascular responses to stress. Psychophysiology. 1985;22:648–55. doi: 10.1111/j.1469-8986.1985.tb01662.x. [DOI] [PubMed] [Google Scholar]
- Lane JD, Adcock RA, Williams RB, Kuhn CM. Caffeine effects on cardiovascular and neuroendocrine responses to acute psychosocial stress and their relationship to level of habitual caffeine consumption. Psychosom Med. 1990;52:320–36. doi: 10.1097/00006842-199005000-00006. [DOI] [PubMed] [Google Scholar]
- Laurent D, Schneider KE, Prusaczyk WK, Franklin C, Vogel SM, Krssak M, et al. Effects of caffeine on muscle glycogen utilization and the neuroendocrine axis during exercise. J Clin Endocrinol Metab. 2000;85:2170–5. doi: 10.1210/jcem.85.6.6655. [DOI] [PubMed] [Google Scholar]
- Lee TC, Charles BG, Steer PA, Flenady VJ. Saliva as a valid alternative to serum in monitoring intravenous caffeine treatment for apnea of prematurity. Ther Drug Monit. 1996;18:288–93. doi: 10.1097/00007691-199606000-00012. [DOI] [PubMed] [Google Scholar]
- Lelo A, Miners JO, Robson R, Birkett DJ. Assessment of caffeine exposure: caffeine content of beverages, caffeine intake, and plasma concentrations of methylxanthines. Clin Pharmacol Ther. 1986;39:54–9. doi: 10.1038/clpt.1986.10. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Stress and Health: Biological and Psychological Interactions. 2nd. Thousand Oaks, CA: Sage; 2005. p. 178. [Google Scholar]
- Lovallo WR, Pincomb GA, Sung BH, Wilson MF. Caffeine may potentiate adrenocortical stress responses in hypertension-prone men. Hypertension. 1989;14:170–6. doi: 10.1161/01.hyp.14.2.170. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, al'Absi M, Blick K, Whitsett TL, Wilson MF. Stress-like adrenocorticotropin responses to caffeine in young healthy men. Pharmacol Biochem Behav. 1996;55:365–9. doi: 10.1016/s0091-3057(96)00105-0. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Wilson MF, Sung BH, Vincent A, McKey B, Whitsett T. Blood pressure response to caffeine shows incomplete tolerance with regular consumption. Hypertension. 2004;43:760–5. doi: 10.1161/01.HYP.0000120965.63962.93. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Whitsett TL, al'Absi M, Sung BH, Vincent AS, Wilson MF. Caffeine stimulation of cortisol secretion across the waking hours in relation to caffeine intake levels. Psychosom Med. 2005;67:734–9. doi: 10.1097/01.psy.0000181270.20036.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger A, Deuster PA, Kyle SB, Gallucci WT, Montgomery LC, Gold PW, et al. Acute hypothalamic–pituitary–adrenal responses to the stress of treadmill exercise. N Engl J Med. 1987;316:1309–15. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- Pincomb GA, Lovallo WR, Passey RB, Whitsett TL, Silverstein SM, Wilson MF. Effects of caffeine on vascular resistance, cardiac output and myocardial contractility in young men. Am J Cardiol. 1985;56:119–22. doi: 10.1016/0002-9149(85)90578-8. [DOI] [PubMed] [Google Scholar]
- Pincomb GA, Lovallo WR, Passey RB, Wilson MF. Effects of behavior state on caffeine's ability to alter blood pressure. Am J Cardiol. 1988;61:798–802. doi: 10.1016/0002-9149(88)91069-7. [DOI] [PubMed] [Google Scholar]
- Read GF, Walker RF, Wilson DW, Griffiths K. Steroid analysis in saliva for the assessment of endocrine function. Ann NY Acad Sci. 1990;595:260–74. doi: 10.1111/j.1749-6632.1990.tb34300.x. [DOI] [PubMed] [Google Scholar]
- Riad-Fahmy D, Read GF, Walker RF. Salivary steroid assays for assessing variation in endocrine activity. J Steroid Biochem. 1983;19(1A):265–72. [PubMed] [Google Scholar]
- Robertson D, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, et al. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N Engl J Med. 1978;298:181–6. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- Scavo D, Barletta C, Vagiri D, Letizia C. Adrenocorticotropic hormone, beta-endorphin, cortisol, growth hormone and prolactin circulating levels in nineteen athletes before and after half-marathon and marathon. J Sports Med Phys Fitness. 1991;31:401–6. [PubMed] [Google Scholar]
- Shepard JD, al'Absi M, Whitsett TL, Lovallo WR. Additive pressor effects of caffeine and stress in male medical students at risk of hypertension. Am J Hypertens. 2000;13:475–81. doi: 10.1016/s0895-7061(99)00217-4. [DOI] [PubMed] [Google Scholar]
- Sung BH, Lovallo WR, Pincomb GA, Wilson MF. Effects of caffeine on blood pressure response during exercise in normotensive healthy young men. Am J Cardiol. 1990;65:909–13. doi: 10.1016/0002-9149(90)91435-9. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol. 1992;262:E467–75. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Czeisler CA, Zimmerman JC, Moore-Ede MC. Biological rhythms in man: relationship of sleep-wake, cortisol, growth hormone, and temperature during temporal isolation. Adv Biochem Psychopharmacol. 1981;28:475–99. [PubMed] [Google Scholar]
- Zylber-Katz E, Granit L, Levy M. Relationship between caffeine concentrations in plasma and saliva. Clin Pharmacol Ther. 1984;36:133–7. doi: 10.1038/clpt.1984.151. [DOI] [PubMed] [Google Scholar]