Summary
The nonstructural NS3 protein of the Hepatitis C virus (HCV) is a multi-functional enzyme with an N-terminal serine-protease activity and a C-terminal helicase activity. The helicase is capable of unwinding both DNA and RNA duplexes; however, the overall processivity of the helicase is fairly low. We show here that single strand binding proteins (SSB) enhance the unwinding processivity of both the NS3 helicase domain (NS3h) and the full-length protease-helicase NS3-4A. The detailed study of the effect of SSB on the DNA unwinding activity of NS3h indicates that the SSB stabilizes the helicase at the unwinding junction and prevents its dissociation. These results suggest a potential role for either cellular or virus-encoded SSB in improving the processivity of the NS3 in vivo.
Keywords: HCV, Unwinding, NS3, SSB, Processivity
Introduction
Hepatitis C Virus (HCV) is a single-stranded, plus strand RNA virus and the causative agent of Non-A, Non-B Hepatitis, a condition known to affect ~180 million individuals worldwide 1; 2. The mono-cistronic message in the mRNA gets translated into a 9600 residue long polypeptide, which then undergoes post-translational cleavage to yield the structural (C, E1 and E2) and the Non-structural (NS2, 3, 4A, 4B, 5A and 5B) proteins. The non-structural proteins are important for viral replication and make up the viral replicative complex along with cellular host proteins whose roles are not well defined 2; 3.
The NS3 protein of the HCV is a multidomain, multifunctional protein. Its N-terminal domain contains the serine-protease activity, and the C-terminal domain contains the NTPase/helicase activity. The two domains are loosely connected and can be independently expressed, while retaining their activities 3–5. The NS3 helicase belongs to the SF2 superfamily of helicases. This superfamily of proteins is characterized by the presence of seven conserved motifs 6. Conserved motifs I and II referred to as Walker A and Walker B motifs, respectively, are important for ATP binding and hydrolysis 7. These helicases are defined by a conserved DexH/D sequence, which makes them members of the DEAD-box protein family that are involved in a variety of processes of RNA metabolism 8–10.
The HCV helicase is very unusual in that it is capable of binding single stranded DNA and RNA and unwinding both DNA and RNA duplexes 11–13. Previous studies have shown that NS3h (helicase domain) and NS3 (protease-helicase) have poor DNA unwinding processivity relative to the processivity of NS3-4A (protease-helicase-core peptide of 4A)14–16. Interestingly, the duplex RNA unwinding processivity is even poorer than the DNA unwinding processivity 16. Low processivity of unwinding is common to many SF1 and SF2 helicases (like RecQ, Dda) 17–19. One speculation for such poor unwinding processivity is that many processes of the RNA metabolism do not require helicases to travel along long stretches of the nucleic acid, and hence these helicases are evolved to function only for unwinding short stretches of RNA. Alternatively, since most of these helicases function as a part of large multi-protein assemblies, it is possible that accessory proteins are required to keep the helicase from dissociating by means of protein-protein interactions.
It has been shown in many helicases that the presence of single-strand binding proteins improves the unwinding efficiency 17; 20–23. The RecQ helicase from E.coli has physical and functional interaction with the E.coli SSB protein 17; 23; 24. The E.coli PriA helicase requires the SSB protein to mediate interactions with PriB and to form the functional PriA-PriB complex 25–27. PriA shows a functional interaction with the Replicative protein A, the human homologue of E.coli SSB 25. DNA helicases from E.coli, Helicase II (UvrD) and Helicase IV have both been shown to have improved activity when supplemented with SSB 28; 29. Similarly, many viral and archaebacterial helicases show improved unwinding efficiencies in the presence of either homologous or heterologous single strand binding proteins 30; 31. In this study, we have explored the effect of SSB proteins on the unwinding activity of the HCV helicases. We have used the bacterially expressed helicase domain (NS3h) and full-length protease-helicase (NS3-4A) covalently linked to a core peptide of NS4A to study the effect of single-strand binding proteins on the DNA unwinding activity of these helicases. Our results show that SSB proteins increase the unwinding activity of HCV helicases. SSB proteins do not alter the rates of unwinding, but substantially improve the unwinding processivity of these helicases.
Results
NS3h has a low processivity of DNA unwinding
NS3h is a 3′-5′ helicase that requires a ssDNA 3′-tail for initial loading and for subsequent strand separation of the duplex region. Previous work has shown that the presence of an additional 5′-tail in the unwinding substrate (fork substrate) does not hinder NS3h-catalyzed DNA unwinding 11; 32. Therefore, most of the studies reported here have been carried out with fork substrates (Table I) with a dT35 5′-tail and dT15 3′-tail. As reported previously15, when the strand separation of the 40bp substrate was measured under single turnover unwinding conditions, less than 1% ssDNA product was observed (Fig. 1A). The single turnover unwinding conditions were achieved by preincubating the DNA substrate with NS3h and adding polyU (protein trap) with ATP during initiation of the reactions. When strand separation reactions were carried out with a shorter 18bp DNA substrate, ~85% of the dsDNA was unwound under single turnover unwinding conditions. The average DNA unwinding rate of 3.7 bp/s at 22 °C (Fig 1B) was similar to the previously reported rates by NS3h15. When the strand separation of the 40bp substrate was measured under conditions where NS3h can cycle between DNA-bound and free states (in the absence of polyU), ~70% of the duplex was unwound (Fig. 1C). The rate of unwinding under these multiple turnover reaction conditions, again in agreement with the previously published results33, was 10-fold slower (~0.4 bp/s) compared to the single turnover rate of unwinding, indicating that strand separation rate is limited by NS3h cycling between DNA bound and free states. These results demonstrate that NS3h has a low processivity of DNA unwinding.
Table I.
 |
Fig. 1.
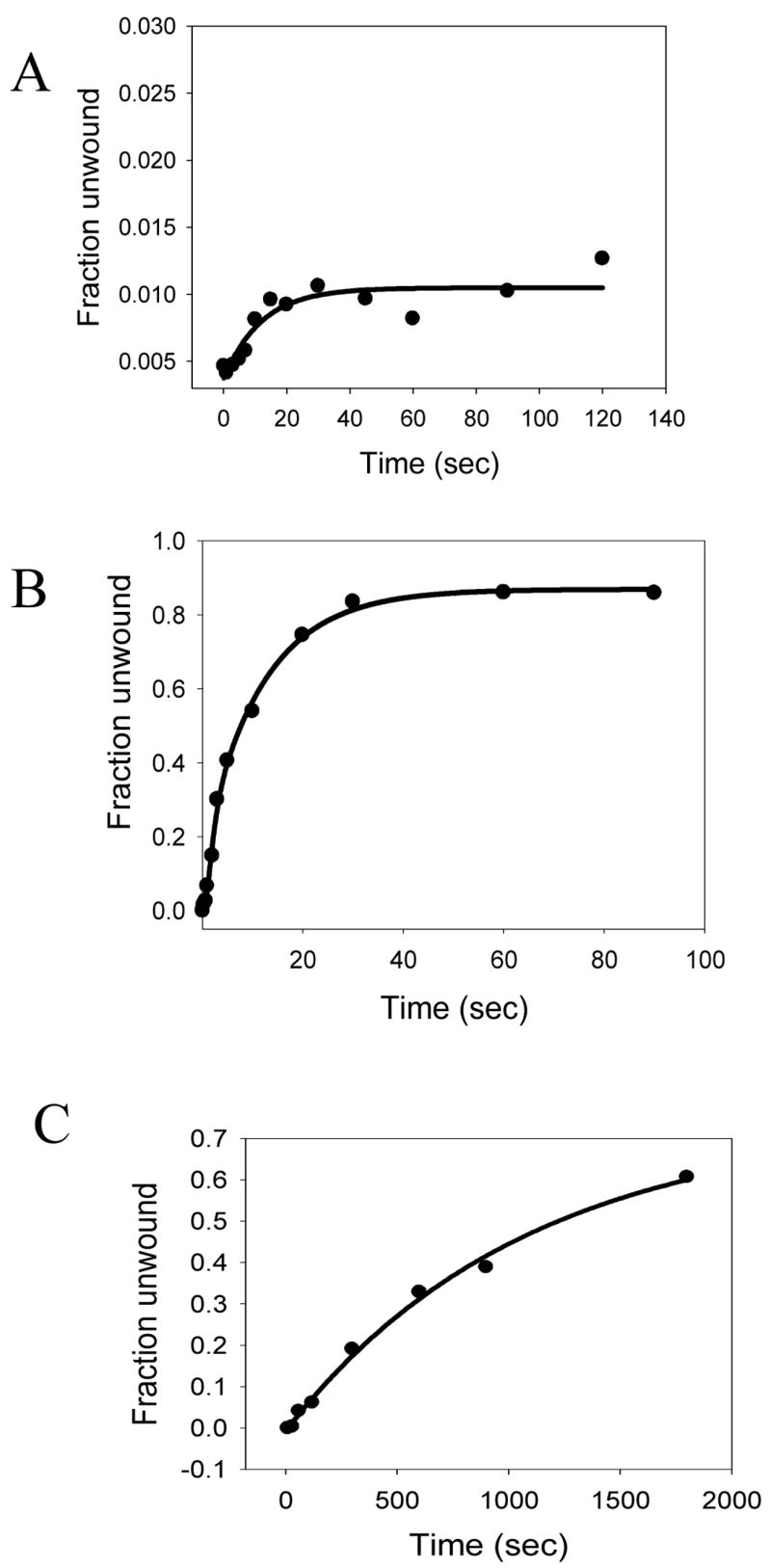
HCV NS3h has a low intrinsic processivity of DNA unwinding. (A) Unwinding kinetics of a 40bp forked DNA substrate (Table I, 3) under single turnover conditions. NS3h (200nM) was incubated with 5 nM ss/dsNA substrate in buffer in one syringe, and mixed with equal volume of 10 mM ATP, 1μM PolyU and 400nM DNA trap in the same buffer from the other syringe, for the times indicated. The unwinding reaction was stopped by the addition of a 1.5 fold volume of quenching solution. The reactions were carried out in a rapid quenched-flow instrument. NS3h unwinds less than 1% of the total DNA. (B) Unwinding kinetics of the 18bp forked DNA substrate (Table I, 6) under of single turnover conditions. Reactions were performed as explained above, but a 18bp forked DNA substrate was used instead of the 40bp forked DNA. NS3h unwinds about 80% of the 18bp substrate with a rate of 3.7 bp/s. (C) Unwinding kinetics of the 40bp forked DNA substrate under multiple turnover conditions. Unwinding reactions were carried out by incubating 200nM of NS3h with 5 nM ss/dsNA substrate in buffer in one syringe, and mixing with equal volume 10 mM ATP but no trap, in the same buffer from the other syringe for the times indicated. NS3h unwinds about 60% of the substrate at a rate of 0.4 bp/s.
Single strand binding proteins enhance the unwinding processivity of NS3h
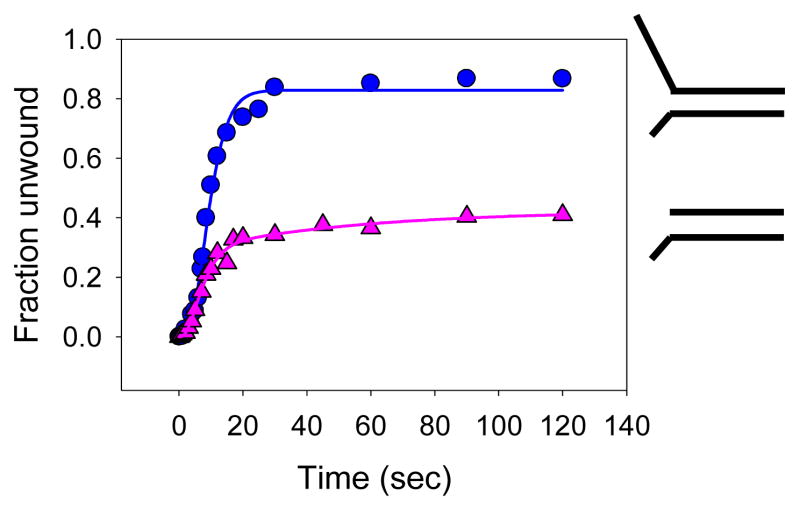
Many helicases like PriA, Werner’s syndrome helicase WRN, RecQ etc. have been shown to unwind duplex DNA efficiently only in the presence of single stranded DNA binding proteins 23; 25; 26; 34; 35. To investigate if the unwinding efficiency of HCV NS3h protein would be enhanced by the presence of a heterologous single stranded binding protein, helicase assays were performed in the presence of the E. coli SSB. The 40 bp forked substrate (3′15Tds40-60%, Table-1) was preincubated with NS3h, and ATP was added to initiate the unwinding reaction. When the reaction was supplemented with SSB, robust unwinding of the 40bp substrate was observed (Fig. 2A,B). Therefore, even if NS3h was initially bound to both the 3′- and the 5′-tail, addition of ATP likely results in the dissociation of the enzyme bound at the 5′-tail (either because of reduced affinity or due to translocation to the end of the tail and falling off) allowing for SSB to bind. This effect was not peculiar to E. coli SSB, as similar results were observed with the bacteriophage T7 single strand binding protein, gp2.5 (Fig 2B). SSB protein by itself was incapable of unwinding the duplex (data not shown). The unwinding rate in the presence of SSB (3.4 bp/s) was much faster than the unwinding rate under multiple turnover conditions (in the absence of polyU and SSB) and very close to the single turnover unwinding rate (in the presence of polyU) of NS3h. Therefore, the 40bp fork DNA was unwound to nearly 80% completion at the single turnover unwinding reaction rate in the presence of SSB. These results indicate that the unwinding of the 40bp DNA in the presence of SSB is not rate-limited by NS3h cycling between DNA bound and free states. Increasing NS3h concentration by 5-fold did not increase the unwinding rate any further (data not shown). The results show that the SSB proteins dramatically increase the processivity of unwinding. In these reactions involving E.coli SSB, no PolyU (protein trap) was added. This was because, it has been previously demonstrated that E.coli SSB binds to ssRNA as well 36; 37. Thus adding PolyU together with SSB would result in SSB getting trapped by PolyU and hence will not be available to bind to the substrate.
Fig. 2.
DNA unwinding processivity of NS3h unwinding is enhanced by single strand binding proteins. (A) The 40bp fork substrate and the unwound single stranded DNA (resolved by native PAGE) after various times of reaction with NS3h in the presence of E.coli SSB. NS3h (200nM) was incubated with 5 nM ss/dsNA substrate in buffer in one syringe, and mixed with equal volume of 10 mM ATP, 2μM E.coli SSB in the same buffer from the other syringe, for the times indicated (B) Fraction of the 40bp DNA unwound in the presence of E. coli SSB ( ) or T7 SSB, gp2.5 ( ) as a function of reaction time. NS3h (200nM) was incubated with 5 nM ss/dsNA substrate in buffer in one syringe, and mixed with equal volume of 10 mM ATP, 2μM E.coli SSB or 10μM T7 SSB (gp2.5) in the same buffer from the other syringe, for the times indicated. The unwinding rates range between 3.4 bp/s and 3.7 bp/sec and the amount of products formed range between 83–87% in the presence of E. coli SSB. The unwinding rate in the presence of gp2.5 ranges between 5.7bp/sec and 5.9 bp/sec and the amount of products formed range between 36%–42%.
The 40bp forked DNA substrate contains a 5′-dT35 tail that can bind a molecule of E. coli SSB tetramer. To determine if the 5′-tail was required for the stimulation of unwinding processivity by the SSB, experiments were carried out with a 40bp unwinding substrate lacking the 5′-tail (1Tds40-40%, Table-1). The fraction of products formed at the end of the reaction in the presence of SSB dropped by 50% when the 5′-tail was removed (Fig. 3). These results indicate that the 35 nt long 5′-tail, although not a requirement, when present enhances the NS3h activity most likely by promoting efficient SSB binding to the displaced strand. Though SSB shows tightest binding to longer stretches of DNA (≥33nt), the protein also binds to shorter DNAs albeit with reduced affinity 38. In the experiment with the partial duplex substrate, even though SSB does not bind initially, unwinding by the helicase would generate a ss-tail, which can be bound by SSB. Since the initial substrate-SSB interaction is not as strong as in the case of the forked substrate, the percentage of enzyme that does dissociate from the junction is higher in this case (as indicated by the decreased amplitude).
Fig. 3.
Effect of the 5′-ssDNA tail on the unwinding efficiency of NS3h in the presence of SSB. The unwinding reaction by NS3h was carried out in the presence of SSB using substrates that either possessed the 5′-tail (Table I, 3) or lacked it (Table I, 4). NS3h (200nM) was incubated with 5 nM ss/dsNA substrate in buffer in one syringe, and mixed with equal volume of 10 mM ATP, 2μM E.coli SSB in the same buffer from the other syringe, for the times indicated The fraction of DNA unwound in each case was plotted as a function of reaction time. The 40bp substrate with the 5′-tail was unwound to a greater extent ( ) than the DNA with out the 5′-tail ( ), but with similar DNA unwinding rates in the range of 3.4 – 3.7 bp/s and 2.9 – 3.1 bp/s, respectively.
DNA reannealing trap does not mimic the action of SSB
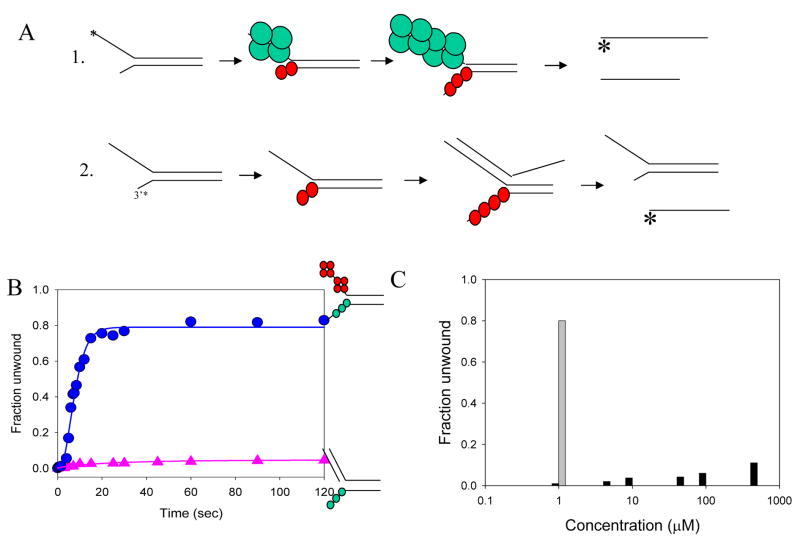
The above-mentioned results demonstrate a dramatic effect of SSB on the unwinding processivity of NS3h. We therefore addressed the question of what SSB was doing that improved the unwinding processivity of NS3h. One reason for NS3h not being able to unwind long duplexes is that the initially separated strands re-anneal behind the helicase during the unwinding time course, and SSB prevents DNA re-annealing by binding to the displaced strand.
To test this hypothesis, we added a large excess of the 3′-strand to the reaction along with the initiating ATP. The rationale behind doing this experiment was that, if all that SSB was doing was preventing re-annealing behind the helicase during the unwinding time course, then the situation can be mimicked by the addition of excess amount of the strand that is complementary to the displaced strand (Fig 4A). The excess of the 3′ loading strand (non-radioactive) would trap the displaced strand as it is generated by the action of NS3h, thereby preventing the displaced strand from re-annealing to the loading strand. Addition of excess 3′-loading strand showed a marginal increase in the amount of products formed (~5%); however, this fold increase did not match that of SSB (Fig 4B). The bimolecular rate constant of the two strands of DNA annealing is of the order of 105 sec−1. It is hence possible that in the time scale of the unwinding reaction, the externally added 3′-strand annealing to the displaced strand is not fast enough. One way to increase the rate of annealing is to increase the concentration of the DNA strand such that the lifetime of annealing of the displaced strand is now well within the time scale of the reaction under study. Thus, increasing the concentration of the 3′-loading strand to as high as 500 μM would mean that 50% of the DNA trap would have annealed to the displaced strand in 20msec. These reaction conditions resulted in 10% product formation, which was still only 1/8th of the products formed with SSB (Fig. 4C). We conclude that extensive reannealing of the strands does not occur during the unwinding reaction, and that the mode of action of SSB is not merely to prevent re-annealing of strands behind the helicase.
Fig. 4.
Mechanism of SSB action –DNA re-annealing trap. Experiments designed to investigate if SSB was preventing DNA re-annealing behind the helicase. (A) Schematic representation of the unwinding reaction in A-1 shows that the SSB tetramers (in green) bind to the displaced strand and prevent DNA re-annealing as NS3h molecules (in red) unwind the DNA. The unwinding reaction in A-2 shows that an excess of the DNA reannealing trap (strand complementary to the displaced strand) prevent DNA re-annealing. (B) The kinetics of 40bp substrate unwinding in the presence of SSB ( ) and in the presence of the DNA re-annealing trap ( ). (C) Fraction of DNA unwound in the presence of an increasing amount of the DNA re-annealing trap, from 1 – 500 μM (black bar) compared to the reaction with SSB (grey bar).
SSB promotes stable interactions of NS3h with the fork junction
Based on the above results, we propose that NS3h-catalyzed unwinding activity has a low processivity due to the tendency of the helicase to dissociate frequently from the unwinding substrate before reaching the end of a long stretch of dsDNA. One way SSB can increase the unwinding efficiency is by stabilizing the binding of NS3h to the fork junction; that is, keeping the helicase at the fork junction and preventing it from dissociating into solution. Another way SSB can increase the unwinding processivity is by stabilizing the partially unwound DNA intermediates and allowing NS3h to continue the unwinding process by undergoing multiple rounds of dissociation from and reassociation to these intermediates. Since we do not have a protein trap in the reaction, multiple NS3h molecules can bind to the loading strand in the intermediates, which can also increase the efficiency of unwinding by a functional cooperation mechanism15, also observed in the T4 Dda helicase19. To distinguish between the above scenarios, we designed the following mutant poisoning experiments.
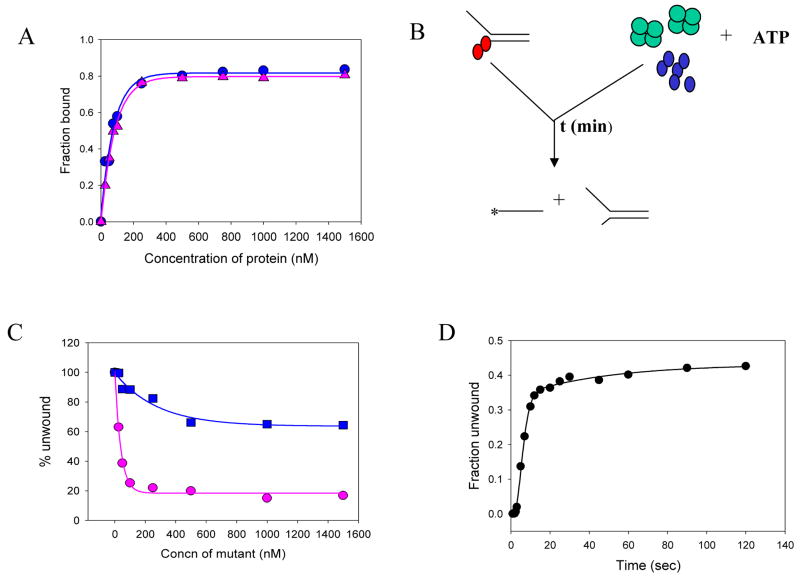
We have shown previously that mutant D261A NS3h lacks ATPase activity and hence cannot unwind DNA. The mutant NS3h binds DNA with the same affinity as WT NS3h (Fig. 5A) both in the absence and in the presence of ATP 33. WT NS3h was pre-incubated with the 40bp forked substrate (2Tds40-60%, Table-1) and an excess of mutant D261A NS3h (with or without SSB) was added with the initiating ATP. In the absence of SSB, unwinding is severely inhibited by the presence of excess mutant NS3h (Fig 5B). This indicates that in the absence of SSB, NS3h cycles between DNA bound and free states and after the first round of unwinding and dissociation, the WT NS3h is replaced by the mutant NS3h that blocks DNA unwinding. This inhibition is quite severe and unwinding activity drops to almost zero when the ratio of the mutant to WT NS3h is 1:1. Interestingly, in the presence of SSB, almost no decrease in unwinding was observed at 1:1 mutant to WT NS3h ratio. At very high ratios of mutant to WT NS3h (10:1) the activity decreased to only 50% (Fig. 5B). Measurement of the kinetics of unwinding (Fig 5C) showed that the decrease in activity was due to the decrease in the unwinding amplitude and the rate of unwinding by WT NS3h remained unchanged in the presence of the mutant NS3h. These results indicate that most of the initially DNA-bound WT NS3h does not freely exchange with the mutant NS3h present in solution, when SSB is present. We conclude from these experiments that SSB increases the unwinding processivity by somehow preventing the NS3h bound at the junction from dissociating into solution.
Fig. 5.
Mechanism of SSB action - mutant poisoning experiments. (A) Wild-type NS3h ( ) and mutant D261A NS3h ( ) bind to single-stranded DNA (Table I, 6) with equal affinity as assessed by the nitrocellulose filter binding assays. 20nM of 25mer DNA was incubated with increasing concentrations of either WT-NS3h or mutant D261A NS3h for 30 min at room temperature. The fraction of protein bound was plotted as a function of protein concentration. (B) Schematic representation of the unwinding experiments done in the presence of the mutant D261A. The red circles represent the WT NS3h, while the blue circles (on the right) represent the mutant D261A. The green tetramer represents the SSB protein. In the mutant poisoning experiments, The wild-type enzyme is pre-incubated with the DNA substrate and the reaction is initiated with the SSB-mutant NS3h-ATP mixture. The reaction is then quenched with SDS-EDTA quenching solution after appropriate times. (C) The unwinding of the 40bp substrate was measured using a constant amount of WT NS3h (100 nM) and increasing amounts of the mutant NS3h in the absence ( ) or in the presence of SSB ( ). 5nM forked substrate was pre-incubated with 200nM WT-NS3h. The unwinding reaction was initiated by mixing with equal volume of 10mM ATP and increasing concentrations of mutant D261A, either in the presence or absence of 1μM E.coli SSB. The amount of products formed in each case was plotted as a function of increasing mutant concentration. (D) The kinetics of 40bp substrate unwinding by NS3h in the presence of SSB and excess of the mutant D261A. WT-NS3h (200nM) was incubated with 5 nM ss/dsNA substrate in buffer in one syringe, and mixed with equal volume of 10 mM ATP, 2μM E.coli SSB and 1μM mutant NS3h D261A, in the same buffer from the other syringe for the times indicated. About 40% of the substrate is unwound at a rate of 3.9 bp/s.
NS3h monomer is capable of unwinding DNA duplexes
The forked DNA substrate that was used in the above experiments contained a dT15 3′-tail that according to our previous studies would bind two molecules of NS3h at the start of the unwinding reaction 39. To investigate if the same effect of SSB would be observed with a monomer of NS3h, we used a forked substrate with a 30bp duplex region and a dT5 3′-loading tail (3′-5Tds30, Table I). The short tail length ensures that only one molecule of NS3h is bound to the loading tail. The unwinding reactions were carried out by pre-incubating NS3h with the short tail fork substrate and starting the reaction by adding ATP, SSB, and excess mutant D261A. Excess of the mutant added in the reaction would drastically reduce the possibility of another WT helicase from loading behind the pre-bound WT NS3h as the substrate gets progressively unwound. Given these stringently controlled conditions, we observed substantial unwinding of the 30bp substrate (Fig. 6A). The kinetics of unwinding were very similar to those with the 15nt 3′-tail, an unwinding rate of 4 bp/sec, indicating that a single molecule of NS3h is sufficient for unwinding DNA with a high processivity in the presence of SSB. These results also demonstrate that a monomer of NS3h helicase is sufficient for DNA unwinding. Experiments were also done, where the 30bp substrate (3′-5Tds30, Table 1) was incubated with the DNA in a 1:1 ratio and the reaction was initiated using ATP from the other syringe in the presence of ATP. A burst type kinetics is observed, with ~12% products formed at the end of the burst-phase (Fig. 6B). This seems consistent with the fact that given equal proportion of enzyme and substrate, only 1/6th of the total enzyme population is favorably bound initially to generate the products. The rate of product formation is slightly slower (1.8 bp/sec) than the earlier observed single-turnover rate of unwinding.
Fig. 6.
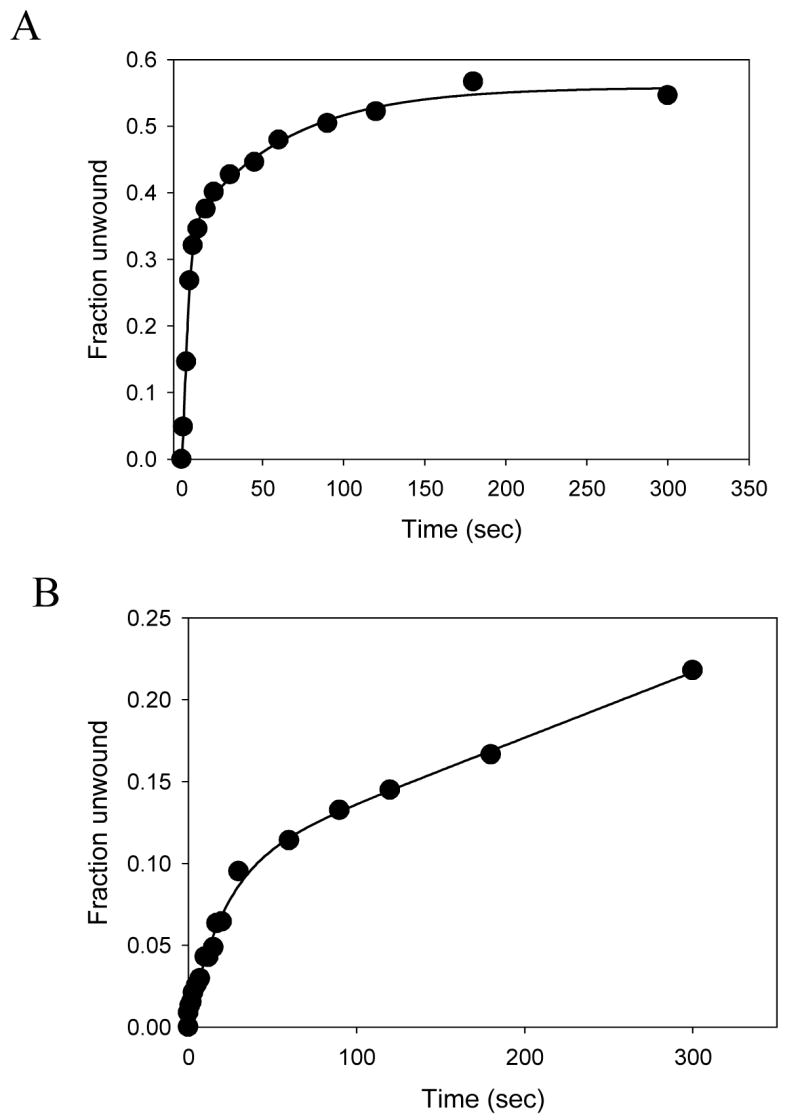
Unwinding activity of the NS3h monomer. (A) Unwinding of the 30bp substrate with a dT5 3′-loading tail and dT35 5′-tail (Table I, 5) by NS3h was carried out with in the presence of SSB and excess of the mutant NS3h. The fraction of substrate unwound is plotted as a function of time. About 45% of the substrate is unwound by NS3h at an average rate 4 bp/s. (B) Unwinding reaction was carried out using 10 nM NS3h and 10 nM 30bp forked DNA substrate. About 12% of the DNA is unwound (1.8 bp/s) in the burst phase, which is very close to the expected 16% (1/6th of the total enzyme, since the average enzyme binding site on the DNA is 7 bases).
SSB increases the unwinding processivity of full-length HCV helicase
The unwinding kinetics of the full-length protease-helicase construct NS3-4A enzyme was studied under similar conditions. NS3-4A was pre-incubated with the 40bp forked substrate (2Tds40-40%, Table-1), and the reaction was initiated with ATP from the other syringe either in the presence of SSB or PolyU. Using PolyU in the reaction bound up any free NS3-4A that either did not form the enzyme-substrate complex or dissociated from the DNA during unwinding. This ensured single turnover conditions for unwinding. As was the case with the helicase domain (NS3h), unwinding by NS3-4A in the presence of SSB resulted in nearly 4-fold higher product formation than in the presence of PolyU (Fig. 7). Thus, SSB increases the processivity of DNA unwinding by the full-length NS3-4A construct as well. As was the case with NS3h, the unwinding rate was similar in the presence or absence of SSB.
Fig. 7.

SSB enhances the unwinding processivity of the full length helicase-protease NS3-4A. The 40bp forked substrate (Table I, 3) is unwound by the full length helicase-protease NS3-4A in the presence ( ) and absence ( ) of SSB at average rates of 0.22 bp/s and 0.4 bp/s, respectively.
Discussion
RNA helicases are involved in various aspects of RNA metabolism such as transcription, splicing, translation, RNA degradation, etc 40. The low unwinding processivity of these proteins is often correlated to their functions in the RNA manipulation. That, however, cannot explain the role of many of these RNA helicases in replication. Although HCV NS3 protein has not established as the replicative helicase, knocking-out NS3 inhibits viral replication in vitro 41; 42. This enforces the fact that NS3 is essential for viral replication in HCV virions and also makes NS3 a strong candidate for the replicative helicase of HCV. In a similar scenario amongst the DNA helicases, where the replicative helicase has low unwinding processivity (eg. DnaB, T7 gp4A, T4 Dda), the problem gets alleviated when the unwinding is carried out in the presence of single strand binding proteins43–45. Our results presented here indicate an identical situation where single strand binding proteins enhance the unwinding processivity by keeping the unwinding helicase longer on its track.
Under the conditions of enhanced processivity, we have been able to demonstrate that NS3h monomer is active in unwinding DNA. It has already been established that NS3h has a binding site of about 7 bases 39. By using a forked substrate with a dT5 loading tail, we could restrict the number of NS3h molecules bound initially to the 3′-overhang to one. By carrying out the unwinding reaction in the presence of SSB and an excess of the NS3h helicase-deficient mutant, we ensured that the mutant NS3h rather than additional WT NS3h would bind to the intermediates. Since the mutant does not possess any unwinding activity, the observed DNA unwinding reaction is the outcome of activity of the NS3h monomer.
Although, the exact mechanism by which SSB stabilizes the helicase at the fork junction is not known, we can speculate on two scenarios: 1) there is a physical interaction between the helicase and SSB, which prevents helicase dissociation from the fork junction, or 2) the binding of SSB alters the nucleic acid structure at the junction in such a way that the helicase now binds the junction with a higher affinity. Even though the first situation sounds straightforward, in the context of E.coli SSB and HCV NS3h, it does not hold any direct physiological relevance. The physical interaction between the HCV helicase and SSB can be nonspecific or electrostatic in nature, such as acidic regions of SSB interacting with basic regions of the HCV helicase. Our observation, however, open the possibility that there could be some contenders in the cell that play a role of SSB either encoded from within the HCV genome or host proteins. One of the cellular RNA binding factors that was shown to be associated with the HCV genome, and required for its replication was the poly-pyrimidine tract binding protein (PTB)46–48. PTB was also shown to interact with the proteins of the HCV replication complex, notably NS3 helicase and NS5B polymerase49. Another strong contender could be the HCV protein that has been identified to have RNA binding properties, NS5A50. Within the cell, the replication complex is membrane-bound. In such a situation, presence of accessory proteins functionally homologous to SSB could ensure the required processivity by mediating protein-protein interactions with other proteins of the complex and preventing dissociation of the helicase from the replication complex and RNA.
Materials and Methods
Proteins
The helicase domain of genotype 1b HCV NS3 protein was over-expressed in E. coli carrying the pET21b-NS3HCV plasmid 51. NS3h protein was purified by metal-immobilization chromatography through its C-terminal hexa-His tag, and stored as described previously 33 The GenBank accession number of the protein is M62321. NS3-4A used in the experiments is a single chain construct where the NS4A fragment is covalently attached to the N-terminus of the NS3 protein52. The NS3-4A protein was purified as previously described52. Purified NS3-4A was obtained from Dr. Madhura Gurjar (Robert Wood Johnson Medical School, NJ). The protein concentration was determined from absorbance at 280nm in 8M urea. The extinction coefficients of NS3h (47,600 M−1 cm−1) and NS3-4A (102,000 M−1cm−1) were calculated by adding the molar extinction coefficients of tryptophan (5690 M−1 cm−1) and tyrosine (1280 M−1 cm−1) residues. The protein concentration was also checked using the Bradford method using BSA as the standard53. NS3h D261A (previously referred to as D1316A based on the polyprotein reading frame) carried an Asp to Ala mutation at position 261 in the Walker B motif, which made the enzyme deficient in ATP hydrolysis 33. The D261A mutant was purified and stored using the same procedure as for the wild-type NS3h.
The E.coli single strand binding protein (SSB) was purified as described by Lohman et al 54. The bacteriophage T7 single strand binding protein, gp2.5 was overexpressed by induction with IPTG to a final concentration of 0.4mM in E.coli BL21(DE3) containing the plasmid pAR3505. The cells were lysed using lysozyme/freeze-thaw treatment and the lysate was precipitated with 1M ammonium sulphate. The protein pellet was resuspended in buffer and passed through 3 subsequent ion-exchange columns (DE-52, Phosphocellulose-P11 and Hydroxyapatite). The final eluent was concentrated on a DEAE-column to yield pure gp 2.5, which was free of any detectable nucleases (Dr. Studier, personal correspondence).
Nucleic acids and other reagents
Oligodoxynucleotides were synthesized by Integrated DNA technologies (Coralville, IA) and purified by PAGE in 5M urea, 50°C. The oligodeoxynucleotide concentrations were determined from absorbance at 260nm, using the extinction coefficient obtained by adding the extinction coefficients of the individual bases. Forked DNA substrates were generated by annealing together the 3′- and the 5′-strands. The unwinding substrates used in this study are listed in Table I. PolyU, average length of 210 nucleotides, was purchased from Amersham Biosciences, and polyoxyethelenesorbitanmonolaurate (Tween 20), purchased from Sigma, was purified by passing through activated charcoal.
NC-DEAE double filter binding assay
BA-S 83 nitrocellulose membranes (Whatman-Schleicher & Schuell) were washed in 0.5 M NaOH for 10 min, equilibrated in the reaction buffer and assembled with DE-81 DEAE-cellulose membranes into a dot-blot apparatus with the nitrocellulose membrane on top of the DEAE membrane. Binding reactions containing WT-NS3h protein or NS3h D261A mutant protein, radiolabeled ssDNA substrate, 0.1 mg/ml BSA, and reaction buffer were incubated for 30 min, and were filtered through the wet membranes followed by washing with the reaction buffer 55; 56. The amount of radioactivity retained by each membrane was measured using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Helicase unwinding assay
The kinetics of dsDNA unwinding was measured at 22°C using RQF-3 Quench-Flow apparatus (KinTek Instruments, Austin,TX). NS3h (200nM) was incubated with 5 nM ss/dsNA substrate in buffer (50 mM MOPS-NaOH, 5 mM MgCl2, 5 mM DL-dithiothreitol, and 0.1% Tween 20, pH 7.0) in one syringe, and mixed with 10 mM ATP and 2.0 μM SSB in the same buffer from the other syringe, for the times indicated. The unwinding reaction was stopped by the addition of a 1.5 fold volume of quenching solution containing 100 mM EDTA, 1% SDS, 500nM PolyU and 200nM DNA trap. The duplex and unwound DNA substrates were resolved on a native 10% polyacrylamide gel, and the fraction of DNA unwound was determined using a PhosphorImager (Molecular Dynamics, Sunnyvale, CA). The fraction of unwound substrate was calculated and corrected for the presence of ssDNA at time 0 using Eq. 1.
| (Eq. 1) |
where F is the fraction of unwound substrate, DS and SS are intensities of duplex and unwound substrate bands at a given time, respectively, and DS0 and SS0 are intensities of duplex and unwound substrates at time 0, respectively.
Data Analysis
The unwinding time courses were fit as described previously 11; 15; 32 to the incomplete gamma function (Eq. 2).
| (Eq. 2) |
where F is a fraction of unwound DNA substrate molecules, A is the amplitude of unwinding, k is stepping rate, and t is reaction time. The number of steps, n, taken by the helicase to unwind the substrate was calculated as
| (Eq. 3) |
where Lds is the number of base pairs in the DNA substrate duplex, La is the length of the shortest DNA duplex that can stay together under the experimental conditions (10 bp), and s is the step size. The average unwinding rate is k × s. Software MATLAB with Optimization toolbox (The MathWorks, Inc., Natick, MA) was used for all calculations.
Acknowledgments
We thank Dr. Madhura Gurjar for purified NS3-4A protein. We would also like to thank Dr. William F. Studier for the technical advice on gp2.5 purification. This work was supported by the NIH grant GM55310.
Abbreviations
- HCV
hepatitis C virus
- ss
single strand
- bp
base pair
- WT
Wild-Type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blight KJ, Kolykhalov AA, Reed KE, Agapov EV, Rice CM. Molecular virology of hepatitis C virus: an update with respect to potential antiviral targets. Antivir Ther. 1998;3:71–81. [PubMed] [Google Scholar]
- 2.De Francesco R. Molecular virology of the hepatitis C virus. J Hepatol. 1999;31(Suppl 1):47–53. doi: 10.1016/s0168-8278(99)80374-2. [DOI] [PubMed] [Google Scholar]
- 3.Neddermann P, Tomei L, Steinkuhler C, Gallinari P, Tramontano A, De Francesco R. The nonstructural proteins of the hepatitis C virus: structure and functions. Biol Chem. 1997;378:469–76. [PubMed] [Google Scholar]
- 4.Kwong AD, Kim JL, Rao G, Lipovsek D, Raybuck SA. Hepatitis C virus NS3/4A protease. Antiviral Res. 1998;40:1–18. doi: 10.1016/s0166-3542(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 5.Kwong AD, Kim JL, Lin C. Structure and function of hepatitis C virus NS3 helicase. Curr Top Microbiol Immunol. 2000;242:171–96. doi: 10.1007/978-3-642-59605-6_9. [DOI] [PubMed] [Google Scholar]
- 6.Gorbalenya AaKEV. Helicases: amino acid sequence comparison and structure-function relationship. Current Opinion in Structural Biology. 1993;3:419–429. [Google Scholar]
- 7.Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. Embo J. 1982;1:945–51. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linder P. Quick guide: DEAD-box proteins. Curr Biol. 2000;10:R887. doi: 10.1016/s0960-9822(00)00857-5. [DOI] [PubMed] [Google Scholar]
- 9.Linder P, Daugeron MC. Are DEAD-box proteins becoming respectable helicases. Nat Struct Biol. 2000;7:97–9. doi: 10.1038/72464. [DOI] [PubMed] [Google Scholar]
- 10.Cordin O, Banroques J, Tanner NK, Linder P. The DEAD-box protein family of RNA helicases. Gene. 2006;367:17–37. doi: 10.1016/j.gene.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Tai CL, Chi WK, Chen DS, Hwang LH. The helicase activity associated with hepatitis C virus nonstructural protein 3 (NS3) J Virol. 1996;70:8477–84. doi: 10.1128/jvi.70.12.8477-8484.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gwack Y, Kim DW, Han JH, Choe J. DNA helicase activity of the hepatitis C virus nonstructural protein 3. Eur J Biochem. 1997;250:47–54. doi: 10.1111/j.1432-1033.1997.00047.x. [DOI] [PubMed] [Google Scholar]
- 13.Donmez I, Rajagopal V, Jeong YJ, Patel SS. Nucleic Acid unwinding by hepatitis C virus and bacteriophage t7 helicases is sensitive to base pair stability. J Biol Chem. 2007;282:21116–23. doi: 10.1074/jbc.M702136200. [DOI] [PubMed] [Google Scholar]
- 14.Frick DN, Rypma RS, Lam AM, Gu B. The nonstructural protein 3 protease/helicase requires an intact protease domain to unwind duplex RNA efficiently. J Biol Chem. 2004;279:1269–80. doi: 10.1074/jbc.M310630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin MK, Wang YH, Patel SS. The functional interaction of the hepatitis C virus helicase molecules is responsible for unwinding processivity. J Biol Chem. 2004;279:26005–12. doi: 10.1074/jbc.M403257200. [DOI] [PubMed] [Google Scholar]
- 16.Pang PS, Jankowsky E, Planet PJ, Pyle AM. The hepatitis C viral NS3 protein is a processive DNA helicase with cofactor enhanced RNA unwinding. Embo J. 2002;21:1168–76. doi: 10.1093/emboj/21.5.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umezu K, Nakayama H. RecQ DNA helicase of Escherichia coli. Characterization of the helix-unwinding activity with emphasis on the effect of single-stranded DNA-binding protein. J Mol Biol. 1993;230:1145–50. doi: 10.1006/jmbi.1993.1231. [DOI] [PubMed] [Google Scholar]
- 18.Cui S, Arosio D, Doherty KM, Brosh RM, Jr, Falaschi A, Vindigni A. Analysis of the unwinding activity of the dimeric RECQ1 helicase in the presence of human replication protein A. Nucleic Acids Res. 2004;32:2158–70. doi: 10.1093/nar/gkh540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrd AK, Raney KD. Increasing the length of the single-stranded overhang enhances unwinding of duplex DNA by bacteriophage T4 Dda helicase. Biochemistry. 2005;44:12990–7. doi: 10.1021/bi050703z. [DOI] [PubMed] [Google Scholar]
- 20.Stano NM, Jeong YJ, Donmez I, Tummalapalli P, Levin MK, Patel SS. DNA synthesis provides the driving force to accelerate DNA unwinding by a helicase. Nature. 2005;435:370–3. doi: 10.1038/nature03615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kato M, Frick DN, Lee J, Tabor S, Richardson CC, Ellenberger T. A complex of the bacteriophage T7 primase-helicase and DNA polymerase directs primer utilization. J Biol Chem. 2001;276:21809–20. doi: 10.1074/jbc.M101470200. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Chastain PD, 2nd, Griffith JD, Richardson CC. Lagging strand synthesis in coordinated DNA synthesis by bacteriophage t7 replication proteins. J Mol Biol. 2002;316:19–34. doi: 10.1006/jmbi.2001.5325. [DOI] [PubMed] [Google Scholar]
- 23.Harmon FG, Kowalczykowski SC. RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination. Genes Dev. 1998;12:1134–44. doi: 10.1101/gad.12.8.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shereda RD, Bernstein DA, Keck JL. A central role for SSB in Escherichia coli RecQ DNA helicase function. J Biol Chem. 2007;282:19247–58. doi: 10.1074/jbc.M608011200. [DOI] [PubMed] [Google Scholar]
- 25.Cadman CJ, McGlynn P. PriA helicase and SSB interact physically and functionally. Nucleic Acids Res. 2004;32:6378–87. doi: 10.1093/nar/gkh980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cadman CJ, Lopper M, Moon PB, Keck JL, McGlynn P. PriB stimulates PriA helicase via an interaction with single-stranded DNA. J Biol Chem. 2005;280:39693–700. doi: 10.1074/jbc.M508521200. [DOI] [PubMed] [Google Scholar]
- 27.Huang CY, Hsu CH, Sun YJ, Wu HN, Hsiao CD. Complexed crystal structure of replication restart primosome protein PriB reveals a novel single-stranded DNA-binding mode. Nucleic Acids Res. 2006;34:3878–86. doi: 10.1093/nar/gkl536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yancey-Wrona JE, Wood ER, George JW, Smith KR, Matson SW. Escherichia coli Rep protein and helicase IV. Distributive single-stranded DNA-dependent ATPases that catalyze a limited unwinding reaction in vitro. Eur J Biochem. 1992;207:479–85. doi: 10.1111/j.1432-1033.1992.tb17074.x. [DOI] [PubMed] [Google Scholar]
- 29.Matson SW, George JW. DNA helicase II of Escherichia coli. Characterization of the single-stranded DNA-dependent NTPase and helicase activities. J Biol Chem. 1987;262:2066–76. [PubMed] [Google Scholar]
- 30.Carpentieri F, De Felice M, De Falco M, Rossi M, Pisani FM. Physical and functional interaction between the mini-chromosome maintenance-like DNA helicase and the single-stranded DNA binding protein from the crenarchaeon Sulfolobus solfataricus. J Biol Chem. 2002;277:12118–27. doi: 10.1074/jbc.M200091200. [DOI] [PubMed] [Google Scholar]
- 31.Marsh VL, McGeoch AT, Bell SD. Influence of chromatin and single strand binding proteins on the activity of an archaeal MCM. J Mol Biol. 2006;357:1345–50. doi: 10.1016/j.jmb.2006.01.074. [DOI] [PubMed] [Google Scholar]
- 32.Du MX, Johnson RB, Sun XL, Staschke KA, Colacino J, Wang QM. Comparative characterization of two DEAD-box RNA helicases in superfamily II: human translation-initiation factor 4A and hepatitis C virus non-structural protein 3 (NS3) helicase. Biochem J. 2002;363:147–55. doi: 10.1042/0264-6021:3630147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin MK, Patel SS. The helicase from hepatitis C virus is active as an oligomer. J Biol Chem. 1999;274:31839–46. doi: 10.1074/jbc.274.45.31839. [DOI] [PubMed] [Google Scholar]
- 34.Shen JC, Gray MD, Oshima J, Loeb LA. Characterization of Werner syndrome protein DNA helicase activity: directionality, substrate dependence and stimulation by replication protein A. Nucleic Acids Res. 1998;26:2879–85. doi: 10.1093/nar/26.12.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brosh RM, Jr, Orren DK, Nehlin JO, Ravn PH, Kenny MK, Machwe A, Bohr VA. Functional and physical interaction between WRN helicase and human replication protein A. J Biol Chem. 1999;274:18341–50. doi: 10.1074/jbc.274.26.18341. [DOI] [PubMed] [Google Scholar]
- 36.Kuil ME, Holmlund K, Vlaanderen CA, van Grondelle R. Study of the binding of single-stranded DNA-binding protein to DNA and poly(rA) using electric field induced birefringence and circular dichroism spectroscopy. Biochemistry. 1990;29:8184–9. doi: 10.1021/bi00487a028. [DOI] [PubMed] [Google Scholar]
- 37.Shimamoto N, Ikushima N, Utiyama H, Tachibana H, Horie K. Specific and cooperative binding of E. coli single-stranded DNA binding protein to mRNA. Nucleic Acids Res. 1987;15:5241–50. doi: 10.1093/nar/15.13.5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krauss G, Sindermann H, Schomburg U, Maass G. Escherichia coli single-strand deoxyribonucleic acid binding protein: stability, specificity, and kinetics of complexes with oligonucleotides and deoxyribonucleic acid. Biochemistry. 1981;20:5346–52. doi: 10.1021/bi00521a040. [DOI] [PubMed] [Google Scholar]
- 39.Levin MK, Patel SS. Helicase from hepatitis C virus, energetics of DNA binding. J Biol Chem. 2002;277:29377–85. doi: 10.1074/jbc.M112315200. [DOI] [PubMed] [Google Scholar]
- 40.Linder P. Dead-box proteins: a family affair--active and passive players in RNP-remodeling. Nucleic Acids Res. 2006;34:4168–80. doi: 10.1093/nar/gkl468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prabhu R, Khalap N, Burioni R, Clementi M, Garry RF, Dash S. Inhibition of hepatitis C virus nonstructural protein, helicase activity, and viral replication by a recombinant human antibody clone. Am J Pathol. 2004;165:1163–73. doi: 10.1016/S0002-9440(10)63377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam AM, Frick DN. Hepatitis C virus subgenomic replicon requires an active NS3 RNA helicase. J Virol. 2006;80:404–11. doi: 10.1128/JVI.80.1.404-411.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Biswas EE, Chen PH, Biswas SB. Modulation of enzymatic activities of Escherichia coli DnaB helicase by single-stranded DNA-binding proteins. Nucleic Acids Res. 2002;30:2809–16. doi: 10.1093/nar/gkf384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefebvre SD, Wong ML, Morrical SW. Simultaneous interactions of bacteriophage T4 DNA replication proteins gp59 and gp32 with single-stranded (ss) DNA. Co-modulation of ssDNA binding activities in a DNA helicase assembly intermediate. J Biol Chem. 1999;274:22830–8. doi: 10.1074/jbc.274.32.22830. [DOI] [PubMed] [Google Scholar]
- 45.He ZG, Richardson CC. Effect of single-stranded DNA-binding proteins on the helicase and primase activities of the bacteriophage T7 gene 4 protein. J Biol Chem. 2004;279:22190–7. doi: 10.1074/jbc.M401100200. [DOI] [PubMed] [Google Scholar]
- 46.Chang KS, Luo G. The polypyrimidine tract-binding protein (PTB) is required for efficient replication of hepatitis C virus (HCV) RNA. Virus Res. 2006;115:1–8. doi: 10.1016/j.virusres.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Ali N, Siddiqui A. Interaction of polypyrimidine tract-binding protein with the 5′ noncoding region of the hepatitis C virus RNA genome and its functional requirement in internal initiation of translation. J Virol. 1995;69:6367–75. doi: 10.1128/jvi.69.10.6367-6375.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clerte C, Hall KB. Characterization of multimeric complexes formed by the human PTB1 protein on RNA. Rna. 2006;12:457–75. doi: 10.1261/rna.2178406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Domitrovich AM, Diebel KW, Ali N, Sarker S, Siddiqui A. Role of La autoantigen and polypyrimidine tract-binding protein in HCV replication. Virology. 2005;335:72–86. doi: 10.1016/j.virol.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 50.Huang L, Hwang J, Sharma SD, Hargittai MR, Chen Y, Arnold JJ, Raney KD, Cameron CE. Hepatitis C virus nonstructural protein 5A (NS5A) is an RNA-binding protein. J Biol Chem. 2005;280:36417–28. doi: 10.1074/jbc.M508175200. [DOI] [PubMed] [Google Scholar]
- 51.Kim DW, Gwack Y, Han JH, Choe J. C-terminal domain of the hepatitis C virus NS3 protein contains an RNA helicase activity. Biochem Biophys Res Commun. 1995;215:160–6. doi: 10.1006/bbrc.1995.2447. [DOI] [PubMed] [Google Scholar]
- 52.Howe AY, Chase R, Taremi SS, Risano C, Beyer B, Malcolm B, Lau JY. A novel recombinant single-chain hepatitis C virus NS3-NS4A protein with improved helicase activity. Protein Sci. 1999;8:1332–41. doi: 10.1110/ps.8.6.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 54.Lohman TM, Green JM, Beyer RS. Large-scale overproduction and rapid purification of the Escherichia coli ssb gene product. Expression of the ssb gene under lambda PL control. Biochemistry. 1986;25:21–5. doi: 10.1021/bi00349a004. [DOI] [PubMed] [Google Scholar]
- 55.Wong I, Lohman TM. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc Natl Acad Sci U S A. 1993;90:5428–32. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hingorani MM, Patel SS. Interactions of bacteriophage T7 DNA primase/helicase protein with single-stranded and double-stranded DNAs. Biochemistry. 1993;32:12478–87. doi: 10.1021/bi00097a028. [DOI] [PubMed] [Google Scholar]