Abstract
Decades of research have shown that, from an early age, proficient bilinguals can speak each of their two languages separately (similar to monolinguals) or rapidly switch between them (dissimilar to monolinguals). Thus we ask do monolingual and bilingual brains process language similarly or dissimilarly, and is this affected by the language context? Using an innovative brain imaging technology, functional Near Infrared Spectroscopy (fNIRS), we investigated how adult bilinguals process semantic information, both in speech and in print, in a monolingual language context (one language at a time) or in a bilingual language context (two languages in rapid alternation). While undergoing fNIRS recording, ten early-exposed, highly-proficient Spanish-English bilinguals completed a Semantic Judgment task in monolingual and bilingual contexts, and were compared to ten English monolingual controls. Two hypotheses were tested: the Signature Hypothesis predicts that early, highly proficient bilinguals will recruit neural tissue to process language differently from monolinguals across all language contexts. The Switching Hypothesis predicts that bilinguals will recruit neural tissue to process language similarly to monolinguals, when using one language at a time. Supporting the Signature Hypothesis, in the monolingual context, bilinguals and monolinguals showed differences in both hemispheres in the recruitment of DLPFC (BA 46/9) and IFC (BA 47/11), but similar recruitment of Broca’s area (BA 44/45). In particular, in the monolingual context, bilinguals showed greater signal intensity in channels maximally overlaying DLPFC and IFC regions as compared to monolinguals. In the bilingual context, bilinguals demonstrated a more robust recruitment of right DLPFC and right IFC. These findings reveal how extensive early bilingual exposure modifies language organization in the brain—thus imparting a possible “bilingual signature.” They further shed fascinating new light on how the bilingual brain may reveal the biological extent of the neural architecture underlying all human language and the language processing potential not fully recruited in the monolingual brain.
INTRODUCTION
Can early dual language exposure modify neural tissue? Early childhood is a key period in human development when neurological organization and reorganization takes place (Dawson & Fischer, 1994) and many early childhood experiences yield a life-long impact on brain organization (Fine, Finney, Boynton, & Dobkins, 2005; Johnson & Newport, 1989; Neville & Bavelier, 2001; Mayberry & Eichen, 1991; Newman, Bavelier, Corina, Jezzard, & Neville, 2002; Newport, 1990; Ohnishi, Matsuda, Asada, Aruga, Hirakata, Nishikawa, et al., 2001; Petersson, Reis, & Ingvar, 2001; Petitto, Zatorre, Gauna, Nikelski, Dostie, & Evans, 2000; Roder, Stock, Bien, Neville, & Rosler, 2002). Is early exposure to two languages one of these childhood experiences? Does extensive and maintained exposure to two languages from early life leave a “bilingual signature” on the human brain?
Early language experiences have been shown to result in permanent behavioral and neurological changes. Individuals not exposed to any language before puberty (or even before age 7) commonly fail to achieve monolingual-like language proficiency, experience enhanced difficulty learning language later in life (Lenneberg, 1967; Mayberry & Eichen, 1991; Mayberry & Fisher, 1989; Mayberry, Lock, & Kazmi, 2002; Neville et al., 1997), and display non-native language organization in the brain (Newman et al., 2002). On the opposite end of the spectrum, early, extensive, and maintained bilingual exposure appears to yield the greatest dual language proficiency and the most similar neural organization between the two languages (e.g., Johnson & Newport, 1989; Kovelman & Petitto, 2002; McDonald, 2000; Petitto & Kovelman, 2003; Weber-Fox & Neville, 1999).
Only a handful of researchers have conducted neuroimaging studies that directly compare bilingual and monolingual brains (Mechelli, Crinion, Noppeney, O'Doherty, Ashburner, Frackowiak, & Price, 2004; Rodriguez-Fornells, Rotte, Heinze, Noesselt, & Muente, 2002). In the only structural-anatomical MRI study comparing bilinguals and monolinguals (Mechelli et al., 2004), results showed that bilinguals had an increase in grey-matter volume in the left inferior parietal cortex, which was greatest in early-exposed, highly-proficient bilinguals. In one of the two functional studies, Rodriguez-Fornells et al. (2002) found that when bilinguals were required to ignore words in one of their languages, they showed greater recruitment of the left inferior frontal cortex (IFC; BA 44) and the left IFC area adjacent to the middle frontal gyrus (MFG; BA 46/9) than monolinguals. Given that the bilinguals (unlike the monolinguals) were required to ignore one of their languages in this study, the observed LIFC/MFG activation could be related to the switching and inhibition nature of the task. Finally, in an fMRI neuroimaging study conducted in our own laboratory, when performing a complex syntactic task in one language at a time, bilinguals showed greater activation within LIFC (BA 45) than monolinguals (Kovelman, Baker, Grafton & Petitto, 2005).
Although it is commonly observed that early, extensive, and maintained bilingual exposure typically results behaviorally in optimal dual language competence (Johnson & Newport, 1989; Kovelman & Petitto, 2002; Weber-Fox & Neville, 1999), there is controversy about the nature of the neural recruitment for the two languages, especially when exposure to two languages does not occur close in time (e.g., one language is acquired first, L1, followed by another language, L2). On one hand, some studies have shown both subcortical activation differences and greater frontal and bilateral recruitment for L2 using a variety of language tasks (text comprehension: Dehaene, Dupoux, Mehler, Cohen, Paulesu, Perani, et al., 1997; Kim, Relkin, Lee, & Hirsch, 1997; semantic and grammatical processing of sentences: Hahne & Friederici, 2001; Wartenburger, Heekeren, Abutalebi, Cappa, Villringer, & Perani, 2003; Weber-Fox & Neville, 1999; phonological & lexical processing of words and sounds: Klein, Watkins, Zatorre, & Milner, 2006; Klein, Zatorre, Milner, Meyer & Evans, 1995; Marian, Spivey, & Hirsch, 2003; Pillai, Araque, Allison, Sethuraman, Loring, Thiruvaiyaru, et al., 2003). On the other hand, some studies have shown no neural activation differences between bilinguals’ L1 and L2 (text comprehension: Perani, Paulesu, Galles, Dupoux, Dehaene, Bettinardi et al., 1998; semantic and grammatical processing of sentences: Friederici, Steinhauer, & Pfeifer, 2002; phonological & lexical processing of words and sounds: Chee, Tan, & Thiel, 1999; Klein, Milner, Zatorre, Zhao, & Nikelski, 1999; Klein, Milner, Zatorre, Meyer, & Evans, 1995). One reason for this controversy is that the extent and maintenance of dual language exposure can impact an individual’s proficiency in each language. In turn, proficiency can influence dual language representation in the brains of bilinguals; age of acquisition also has influence on the representation of language in bilingual brains (semantic and grammatical processing of sentences: Wartenburger et al., 2003; phonological & lexical processing of words and sounds: Chee, Soon, Lee, & Pallier, 2004; Chee, Soon, & Lee, 2001; Golestani, Alario, Meriaux, Le Bihan, Dehaene, & Pallier, 2006; Meschyan, & Hernandez, 2006; also, see review by Abutalebi, Cappa, & Perani, 2001).
Another factor to consider when comparing bilinguals and monolinguals is the types of language contexts in which a proficient bilingual adult routinely functions. Bilinguals can interact in different contexts or “modes” (Grosjean, 1997). For example, when a bilingual is with a monolingual, he or she speaks in only one language and is thus in Monolingual mode. When with other bilinguals, they often speak both of their languages or function in a Bilingual mode. Thus, bilinguals might find themselves using either one language at a time or both languages in rapid succession. High facility with this kind of switching across Bilingual and Monolingual language modes is observed even in very young bilingual children (Genesee, 1989; Genesee, Boivin, & Nicoladis, 1996; Holowka, Brosseau-Lapré, & Petitto, 2002; Paradis, Nicoladis, & Genesee, 2000; Petitto & Holowka, 2002; Petitto, Kovelman, & Harasymowycz, 2003; Petitto, Katerelos, Levy, Gauna, Tetreault, & Ferraro, 2001; Petitto & Kovelman, 2003; Poulin-Dubois & Goodz, 2001). In particular, research in our own laboratory has observed how even very young bilinguals can easily and systematically modulate their language choice when interacting with monolinguals of their two languages within the same context/environment (e.g., Petitto et al., 2001).
An additional intriguing factor to consider when comparing bilingual and monolingual language processing is the types of lexico-semantic usage that are possible for bilinguals but not monolinguals. One of the most common forms of “code switching” observed in bilinguals (often called “language mixing” when observed in children) is the swapping of lexico-semantic items belonging to one language when building a phrase in another language. For example, one might say “Yesterday we ate crème glassée” (“ice-cream ” in French spoken in Quebec; e.g., Grosjean, 2001; Paradis, Nicoladis, & Genesee, 2000; Petitto et al., 2001; Petitto & Kovelman, 2003; Poplack, 1980). Current theories of bilingual lexico-semantic representation have assumed the existence of a combined lexical store, in which each lexical item is connected to a number of semantic features in a common semantic store (Ameel, Storms, Malt, & Sloman, 2005; Dijkstra & Van Heuven, 2002; Green, 1998; Kroll & Sunderman, 2003; Monsell, Matthews, & Miller, 1992). Words in two languages that share overlapping semantic representations within the common semantic store are called “translation equivalents” (e.g., “mother” in English and “maman” in French). This idea is supported by the fact that bilinguals can be semantically primed in one language to produce a word in the other language (Dijkstra & Van Heuven, 2002; Kerkhofs, Dijkstra, Chwilla, & de Bruijn, 2006; Kroll & Sunderman, 2003) and can translate concrete words, whose semantic representations overlap, faster than abstract words, which are less likely to share semantic features across languages (Van Hell & De Groot, 1998).
How do bilinguals avoid confusing their two languages as they rapidly process their languages and/or move from one language context to the next? Language switching has been thought to be due to a mechanism, and possibly even an area in the brain, which can selectively activate only one language (Paradis, 1997). This idea was challenged both by the failure to locate a specific brain area that controls the choice of language (Paradis, 1997), and also because of the observation that language choice is not an “on”/”off” process. Rather, bilingual language usage appears to form a continuum in which bilinguals selectively activate or inhibit their two languages to a greater or lesser extent (Grosjean, 2001; Paradis, 1997). Currently, language switching is considered to be a dynamic process in which the degree that each language is activated and inhibited is modulated and dependent on the language context (Green, 1998; Grosjean, 1997; Paradis, 1997).
Behavioral studies alone cannot answer the question of whether bilingual language exposure yields neural differences as compared to monolingual language exposure. Behavioral studies have presented conflicting results, with some suggesting that even early-exposed, highly-proficient bilinguals perform significantly worse than monolinguals on semantic tasks across different language contexts (Thomas & Allport, 2000; von Studnitz & Green, 2002), and others suggesting no such bilingual deficit (Caramazza & Brones, 1980; Grosjean & Miller, 1994; Van Heuven, Dijkstra & Grainger, 1998). Additionally, as noted above, there are a limited number of neuroimaging studies on bilingual switching and semantic language processing across dual-language contexts.
With regard to language switching, bilinguals in Chee, Soon, & Lee’s (2003) study showed different amounts of neural activation within classic semantic processing brain areas (in particular, middle temporal gyrus, MTG) across different language contexts; while Klein et al. (1995) showed similar recruitment of both classic language-dedicated brain areas (left IFC) as well as dorsolateral prefrontal cortex (DLPFC) irrespective of whether bilinguals were using one or two languages. Still other studies have shown that bilinguals require increased recruitment of neural tissue classically associated with task switching and mediation of executive processes, such as DLPFC and anterior cingulate cortex (AC) in bilingual versus monolingual contexts (Fugelsang & Dunbar, 2005; Hernandez, Martinez, & Kohnert, 2000; Hernandez, Dapretto, Mazziotta, & Bookheimer 2001; Holtzheimer, Fawaz, Wilson, & Avery, 2005; Price, Green, & von Studnitz, 1999). As DLPFC might be involved in task monitoring, particularly during novel and/or attention demanding tasks, regardless of whether task switching is involved, the lack or presence of DLPFC activation during language switching tasks should be interpreted with caution (Duncan & Owen, 2000; Fugelsang, Green & Dunbar, 2006; Wager, Jonides, & Reading, 2004).
In the present study we examine and compare semantic processing in bilinguals and monolinguals. Using behavioral and neuroimaging techniques, we examine how bilinguals process printed and auditory semantic information presented to them across two types of typically encountered contexts: (i) Monolingual mode – one language in isolation, and (ii) Bilingual mode – two languages in rapid succession. We studied a group of early-exposed, highly-proficient bilinguals who were carefully screened for early dual language exposure, dual language proficiency, and dual language maintenance, as well as a group of monolingual controls matched for gender and age. Our analyses included comparisons of behavioral performance (accuracy and reaction time) and changes in hemodynamic response in participants as measured with an innovative brain imaging technology, functional Near Infrared Spectroscopy, fNIRS.
Two hypotheses about how the bilingual brain processes language were tested: the Neural Signature Hypothesis predicts that early-exposed, proficient bilinguals should process language differently from monolinguals and recruit different neural tissue across all contexts, including one language at a time (Monolingual mode), and two languages in rapid alternation (Bilingual mode). The difference would be expressed by bilinguals showing greater or lesser neural recruitment (greater or lesser intensity of the hemodymaic signal or presence versus absence of activation in a particular area) of the classic language, cognitive-attention/inhibition, and verbal working memory brain areas as compared to monolinguals, regardless of language mode. The Functional Switching Hypothesis predicts that early-exposed, proficient bilinguals should process language similarly to monolinguals and recruit similar neural tissue, but not across all contexts. Specifically, bilinguals and monolinguals should show similar neural profiles when processing one language at a time (Monolingual mode). Neural differences should emerge only in the context specific and unique to bilinguals: that is, when bilinguals are processing two languages in rapid alternation (Bilingual mode); in this mode bilinguals should show greater neural recruitment of the classic language areas, and/or brain areas involved in task switching. Classic language brain areas would include left IFC (BA 44/45), while classic verbal working-memory/attention brain areas would include left DLPFC (BA 46/9) and anterior IFC (BA 47/11).
Previous research has shown that there is no simple one-to-one correspondence between behavioral performance and neural activity. Such findings are particularly abundant in the bilingual literature, where different language groups can show the same behavioral performance, yet different neural profiles (Chee et al., 2004; Wartenburger et al., 2003). Therefore, our behavioral predictions are not specific to either of the two neurological hypotheses. For both “Neural Signature” and “Functional Switching” hypotheses, we predicted that bilinguals would make semantic decisions with the same or slower reaction time and the same or lower accuracy as monolinguals.
fNIRS is among the world's most innovative imaging technologies. Like fMRI, fNIRS measures changes in the brain's blood oxygen level density (BOLD) while a person is performing specific cognitive tasks. An advantage over fMRI is that, in addition to BOLD, fNIRS also are computes the deoxygenated and oxygenated hemoglobin from the absorption measured at different wavelengths using the modified Beer-Lambert equation. While fNIRS cannot record deep into the human brain (~4cm depth), it has good spatial resolution that is excellent for studies of human higher cognition and language, and it has better temporal resolution than fMRI (~<5s HR, sampling rate = 10 × per second). Unlike the large size of fMRI, fNIRS is very small, highly portable (the size of a desktop computer), and particularly child friendly (children and adults sit normally in a comfortable chair, and babies can be studied while seated on mom's lap). Further, fNIRS is virtually silent, unlike the loud whirring of the fMRI that can make language processing studies challenging. Most importantly, fNIRS tolerates movement. By contrast, fMRI does not tolerate movement and, thus, is difficult to use for studying language production. The fMRI's restriction on movement, its production of loud noises, and its restrictive testing chamber make it very challenging to use for studying infants and children as well as special populations of adults. With the advent of fNIRS, new insights into the human child's developing brain function with respect to higher cognition and language, as well as new insights into the aging brain, can now be laid bare.
To the best of our knowledge, the present study is unique in its direct comparison of bilingual and monolingual semantic processing while using modern neuroimaging technology and behavioral techniques. It is also unique in its focus on bilinguals’ semantic performance across multiple bilingual contexts, and in its use of both auditory and visually presented stimuli. By doing so we hope to provide insight into the nature of bilingual language processing, the impact that early and extensive bilingual exposure has on the bilingual brain, and the effectiveness of fNIRS in cognitive neuroscience research.
Materials & Methods
Participants
Bilingual Participants
Ten right-handed Spanish-English bilinguals participated in this experiment (4 men, 6 women, see Table 1). All bilingual participants started receiving extensive and systematic exposure to both English and Spanish before the age of 5. All bilingual participants had high, monolingual-like, language proficiency in each of their two languages (as established with participant screening methods described below, on which all participants achieved the required accuracy of at least 80%). Half of the participants were exposed to both Spanish and English at home from birth, and the other half of the participants were exposed to Spanish at home from birth and extensive and maintained exposure to English in daycare or kindergarten beginning by ages 3–5. All bilingual participants used English and Spanish consistently from the first onset of bilingual exposure to the present, had at least one Spanish-speaking parent (most of the parents were native speakers of Spanish and late learners of English), and learned to read in English within ages 5–7 and in Spanish within ages 5–12. Bilingual participants had no other exposure to a language outside of English and Spanish until after age 10 and only in the format of a “foreign” language class.
Table 1.
Participant groups.
| Group | Mean Age | Age of Language exposure | Age of Literacy Exposure | Parents’ native language(s) | Language proficiency score | |||
|---|---|---|---|---|---|---|---|---|
| Eng | Span | Eng | Span | Eng | Span | |||
| Bilinguals n=10 | 20 | birth-5 | birth | 5–7 | 5–13 | English & Spanish | > 80% | > 80% |
| Monolinguals n=10 | 21 | birth | 5–7 | English only | > 80% | |||
Monolingual Participants
Ten right-handed monolinguals participated in this experiment (4 men, 6 women, see Table 1). All monolingual participants completed language-screening tasks in English with the required accuracy of 80% and above and came from monolingual English families. Monolingual participants had no other exposure to a language outside of English until after age 10 and only in the format of a “foreign” language class.
All participants received compensation for their time. The treatment of all participants and all experimental procedures were in full compliance with the ethical guidelines of NIH and Dartmouth College’s Ethical Review Board.
Participant Screening
Assessment of Bilingual Language Background & Use
All participants first were administered an extensive and standardized Bilingual Language Background and Use screening questionnaire to ensure confidence in both our “bilingual” (early-exposed, highly-proficient) and our “monolingual” group assignments (Penhune, Cismaru, Dorsaint-Pierre, Petitto, & Zatorre, 2003; Petitto et al., 2001). This screening tool permitted us to determine the age of first bilingual exposure, language(s) used in the home by all caretakers and family members/friends, language(s) used during/throughout schooling, language(s) of reading instruction, cultural self-identification and language maintenance (language(s) of the community in early life and language(s) used throughout development up until the present).
Grammaticality Judgment Behavioral Task
A standardized grammaticality judgment task was administered in English to monolingual participants, and in English and in Spanish to bilingual participants. The goal of the task was to assess participants’ knowledge (or “competence”) of the systematic rules that bind key syntactic and morphological information in each of their two languages. In this grammaticality judgment task, modeled after ones used by Johnson and Newport (1989), McDonald (2000), and Winitz (1996), participants were presented with grammatical and ungrammatical sentences and instructed to read each sentence and indicate whether or not the sentence was grammatical. Examples: I see a book (grammatical); I see book (ungrammatical). This type of task has been used for decades, is effective at identifying individuals with low language proficiency and age of first exposure to the language (with only those exposed to the language before age 7 performing with high accuracy). All participants had to score at least 80% correct in their language(s) to be eligible.
Semantic Judgment Task Presented During Brain Imaging
The goal of this task was to assess bilingual language processing when each language was presented in isolation (Monolingual mode) or when the two languages were presented in rapid alternation (Bilingual mode). This task was modeled after the classic Pyramids & Palm Trees Task (Howard & Patterson, 1992), commonly used in both behavioral and neurological investigations (Chee et al., 2001; Chee, Weekes, Lee, Soon, Schreiber, Hoon et al., 2000). On each trial, the participant was presented with the first word (1s), followed by the second word (1s), followed by a picture (1s) that corresponded either to the first or second word (Figure 1). The participants were asked to indicate via button press whether the first or the second word corresponded to the picture. This task consisted of two parts: Auditory and Visual. During the Auditory part of the experiment participants heard the words via computer speakers. During the Visual part of the experiment participants read the words as they appeared on the computer screen. The two conditions were otherwise identical, including instruction, response type, and length of stimulus presentation.
Figure 1.

(a) Monolingual Mode: One language (English or Spanish) presented during the entire block of trials (sample of English block shown here). (b) Bilingual Mode, Language Integration: During each trial one word from Spanish and one word from English was presented. (c) Bilingual Mode, Language Alternation: Trials in English and trials in Spanish within the same block in a random order.
Language conditions
We employed a block design with two language conditions: Monolingual mode and Bilingual mode (Figure 1). Monolingual mode included two block types, where each language was presented in isolation: blocks of English trials only and blocks of Spanish trials only (Figure 1a). Bilingual mode was comprised of two types of experimental blocks, Bilingual mode Integration and Bilingual mode Alternation. In Bilingual mode Integration (Figure 1b) both languages were presented within each trial. Bilingual mode Alternation (Figure 1c) had randomly alternating trials in English and Spanish.
Stimuli
We selected a total of 112 pictures from the Peabody Picture Vocabulary Test (Dunn & Dunn, 1981) and the International Picture Naming project (Abbate & La Chappelle, 1984; Max Planck Institute for Psycholinguistics; Kaplan, Goodglass, & Weintraub, 1983; Oxford Junior Workbooks, 1965). The pictures and their definitions have been standardized over decades of research and over many languages (Szekely, D'Amico, Devescovi, Federmeier, Herron, Iyer et al., 2005). The two words presented in each trial were equated for syllable length. For the Auditory condition, the words in each pair were also matched for the number of phonemes. For the Visual condition, the words were matched for the number of letters. The average number of phonemes/letters per word was equal across languages (Table 2). For all same-language blocks in which the two words on each trial were in the same language (Spanish, English, Alternating), the words in the pair were matched for written frequency (frequency dictionaries: Juilland & Chang-Rodriguez, 1964; MRC Psycholinguistic Database). Exact frequency matches for Spanish and English words for Integration block trials were impossible given that the two language frequency dictionaries were compiled over a different total number of words. However, given that low versus high frequency in general is an important factor in word recognition (Rodriguez-Fornells et al., 2002), for the Integration trials these words were equated for their general frequency (high versus low; see frequency matching results in Table 2). These experimental stimuli were extensively piloted (n=30) to ensure that participants were comfortable/familiar with the pictures, their definitions and the trial lengths.
Table 2.
Semantic Judgment Task Stimuli. Word length and frequency.
| Language | phoneme/letters | syllable length | frequency |
|---|---|---|---|
| English | |||
| M | 4.5 | 1.7 | 178.8 |
| SD | 1.0 | 0.6 | 266.0 |
| Spanish | |||
| M | 4.5 | 1.9 | 124.7 |
| SD | 1.0 | 0.6 | 234.4 |
The use of cognates (words with similar form/sound and meaning across two languages) or homographs/homophones (words with similar form/sound but with different meanings across two languages) was minimal to avoid word selection facilitation or disruption due to the special properties of these words in the bilingual lexicon (Dijkstra & Van Heuven, 2002; Doctor & Klein, 1992; Klein & Doctor, 2003). For the Visual Integration trials (Figure 1) we entirely avoided words with Spanish-specific orthographic markers (e.g. jabón).
Words for the Auditory condition were recorded with Final Cut Express Software using a G4 Macintosh computer. Three different female voices were used. For English trials in English and Alternating blocks a Monolingual English speaker recorded all English words. For Spanish trials in Spanish and Alternating blocks a native Spanish-English bilingual recorded all the Spanish words. For Integration block trials a different native Spanish-English bilingual recorded both Spanish and English words. Different voices were used to ensure mode differentiation for bilinguals and to avoid inadvertent priming of an incorrect language condition.
Procedure
Bilingual participants completed all language conditions, including Monolingual mode (English and Spanish) and Bilingual mode (Integration and Alternation, Figure 1). For bilingual participants the order of blocks (English, Spanish, Integration, Alternation) was randomized across all participants. Monolingual participants completed Monolingual mode English blocks only. Participants received a 2 second warning before the beginning of each block, telling them the type of block they were about to complete. In order to ensure that both groups had the exact same amount of exposure to each block-type, we purposefully chose to give the same number of English blocks to each participant, without additional English blocks to equate the amount of testing time for two groups. Thus, we ensured that when comparing performance and brain activity during English blocks, the two groups of participants had the exact same amount of exposure to the English task. Participants were instructed to indicate their decision as quickly and as accurately as possible by pressing a left button if the first word corresponded to the picture, and pressing a right button if the second word corresponded to the picture. Right and left-hand responses were randomized for each condition. Accuracy, reaction time (RT), and hemodynamic response (signal measured by fNIRS) were measured simultaneously.
There were two runs of each Auditory and Visual condition with 14 trials per block, 56 trials per run, 112 trials total (3s per trial: 1s per word and per picture followed by a 1s fixation period between trials; 56s block duration and 24s inter-block rest/fixation period (consisting of a fixation cross), 4 blocks per run for bilinguals and 1 block per run for monolinguals (See Figure 2). There were a total of 112 pictures with one picture per trial. Each picture represented a distinct lexical item and none of the pictures were repeated during the two runs. This imaging paradigm has been standardized and successfully used in previous bilingual imaging studies (Chee et al., 2001; Kovelman, Baker, & Petitto, in press). The extended 56s block duration was chosen on the basis of previous research suggesting that increased task duration is more likely to reveal whether frontal lobe and particularly DLPFC recruitment is necessary (Sapir, Maimon, & Eviatar, 2002) and rest periods of 24s were intended to give participants a sufficient break before introducing a different language context. Taken together, the durations of both the block and the rest periods were designed to be commensurate with typical hemodynamic change and recovery (see Figure 3). We used an Apple G4 Laptop running PsyScope software and attached to a freestanding 17-inch monitor, in order to present the stimuli and record behavioral responses (MacWhinney, Cohen & Provost, 1997). All participants were trained in the task before brain scanning began. During training we used different words and images than those used during brain imaging.
Figure 2.
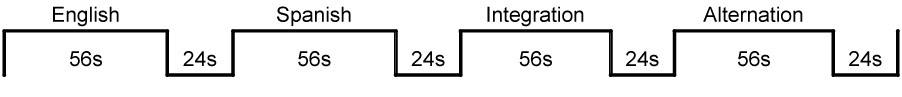
Semantic Judgment Task Imaging Paradigm. Example of bilinguals’ blocks (order of blocks was counterbalanced across participants).
Figure 3.

Typical time course of hemodynamic change and recovery over the experimental blocks and rest periods.
fNIRS Imaging
Apparatus & Procedure
To record the hemodynamic response we used a Hitachi ETG-4000 with 24 channels, acquiring data at 10 Hz (Figure 4a). The lasers were factory set to 690 nm and 830 nm. The 10 lasers and 8 detectors were segregated into two 3 × 3 arrays corresponding to 12 channels per array (Figure 4b). Once the participant was comfortably seated, one array was placed on each side of the participant’s head. Positioning of the array was accomplished using the 10–20 system (Jasper, 1957; Figure 4c) to maximally overlay regions classically involved in language, verbal and working memory areas in the left hemisphere and their homologues in the right hemisphere. In brief, the bottom back corner on 10–20 position T3 (left) or T4 (right), and the bottom front corner on 10–20 position F7 (left) or F8 (right). Channels were tested for noise prior to the beginning of the recording session. Digital photographs were taken of the array positioning prior to and after the recording session to identify if the arrays had moved during testing. An MPEG video recording was synchronized with the testing session, so any apparent movement artifacts could be confirmed during off-line analysis.
Figure 4.

Hitachi ETG-4000 Imaging System, Neuroanatomical Probe Positioning, and MRI Neuroanatomical Co-Registration. (a) Participant with Hitachi 24-channel ETG-4000, with lasers set to 698nm and 830nm, in place and ready for data acquisition. (b) The 3×3 optode arrays were positioned on participants’ heads using rigorous anatomical localization measures (see c–e). (c) 10–20 frontal and temporal coordinates (F7/F8 respectively) were identified and served as anchor points for the bottom front position of the 3×3 optode array. The T3/T4 positions served as anchor points for the bottom back positions of the optode array. (d) MRI co-registration was conducted by having participants (N=6) wear 2 3×3 arrays with vitamin-E capsules in MRI. (e) Neuroanatomical precision of NIRS probe placements: ROI/brain regions were identified using MRI co-registration so that channels were maximally overlaying Broca’s Area and Verbal Working-Memory/Attention Prefrontal Cortex areas.
ROI Identification
In the 3 × 3 recording array channels were considered as the area between adjacent lasers and detectors, as hardwired into the ETG-4000 system. Each channel had two components, attenuation values from the 690nm and 830nm lasers. The attenuation values were converted to deoxy- and oxy-Hb values using the Modified Beer-Lambert equation. Once converted from laser attenuation, channels referred to the deoxy- and oxy-Hb changes in the regions between the laser and detectors.
Our ROI included classic brain regions of interest for language processing: Language PFC (channels maximally overlaying Broca’s area BA 44/45) and Verbal Working-Memory/Attention PFC (channels maximally overlaying DLPFC BA 46/9 and IFC BA 47/11).
There regions were identified with the help of PCA analysis which grouped the channels into the Language PFC and Verbal Working-Memory/Attention PFC principal components. The same channels were grouped for every subject. Each channel was overlaying the same brain area for every subject, as established by 10–20 probe placement and MRI coregistration.
After the recording session, data were exported and analyzed using Matlab (The Mathworks Inc.). Conversion of the raw data to hemoglobin values was accomplished in two steps. Under the assumption that scattering is constant over the path length, we first calculated the attenuation for each wavelength by comparing the optical density of light intensity during the task to the calculated baseline of the signal. We then used the attenuation values for each wavelength and sampled time points to solve the modified Beer-Lambert equation to convert the wavelength data to a meaningful oxygenated and deoxygenated hemoglobin response (HbO and Hb respectively) (Kohl et al 1998).
MRI Co-registration
For MRI (anatomical) co registration, at another session, 2 3×3 arrays of Vitamin E tablets was constructed with the tablets placed precisely at each of the optode locations as described above. These Vitamin E arrays were then placed on to the participant’s head at precisely the same location as the optode array using the 10–20 coordinate system and secured in place with MRI safe tape and straps. Using a Philips 3T MRI, an anatomical scan was taken from 6 participants. The Vitamin E locations from these scans were used as landmarks for corregistration and hence the recorded channels, indicating that indeed the channels covered the anatomical locations anticipated by the 10–20 coordinate system (see Figures 4d and 4e).
Foam padding was placed in the head coil to limit subject head movement during image acquisition. T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo (3D-MPRAGE) sagittal images were obtained with a Phillips 3T scanner. Scanning parameters were as follows: echo time (TE) = 4.6 ms, repetition time (TR) = 9.8 ms, flip angle = 8 degrees, acquisition matrix = 256 × 256, 160 sagittal slices, and voxel size = 1 × 1 × 1 mm with no gap.
RESULTS
Semantic Judgment Task
Behavioral Results
Bilinguals versus Monolinguals
Bilinguals’ and monolinguals’ accuracy and reaction time in English was compared using two 2 × 2 mixed ANOVAs, one ANOVA for accuracy and one ANOVA for reaction time (groups (between factor) X Audio and Video conditions (within factor)). Bilinguals performed equally accurately (F(1, 18) = 0.8, p > 0.01) and equally fast as monolinguals (F(1, 18) = 0.2, p > 0.01). Both groups performed with the same accuracy, but faster (F(1, 18) = 58.2, p < 0.01) when they read the words, rather than when they heard the words. Behavioral scores for this task are presented in Table 3. We used a two-standard deviation cut-off method for analyzing our reaction time data. In particular, for each condition (Audio English, Audio Spanish, Audio Integration, Audio Alternation, Video English, Video Spanish, Video Integration, Video Alternation) we established a mean and standard deviation, and for each participant for each condition we eliminated reaction time data points, which were below or above two standard deviations for that condition.
Table 3.
Behavioral Scores for Semantic Judgment Task.
| Monolinguals | Bilinguals | Monolinguals | Bilinguals | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Condition | RT | RT | |||||||
| # Correct | (SD) | # Correct | (SD) | msec | (SD) | msec | (SD) | ||
| English | audio | 26.3 | (0.9) | 26.1 | (1.1) | 1000 | (86) | 919 | (282) |
| video | 26.8 | (1.5) | 27.1 | (0.9) | 628 | (124) | 654 | (132) | |
| Spanish | audio | 26.4 | (0.7) | 955 | (223) | ||||
| video | 27 | (0.8) | 639 | (98) | |||||
| Integration | audio | 25.4 | (0.5) | 945 | (220) | ||||
| video | 25.7 | (1.1) | 673 | (121) | |||||
| Alternation | audio | 24.6 | (1) | 912 | (236) | ||||
| video | 26.9 | (0.7) | 652 | (76) | |||||
Bilingual Language Modes
Bilinguals performed equally fast (F(1, 18) = 0.5, p > 0.01), but with unequal accuracy across trials (F (3, 27) = 8.7, p < 0.01) as was revealed by two 4 × 2 ANOVAs, one ANOVA for accuracy and one ANOVA for reaction time (Language conditions (within factor) X Audio and Video conditions (within factor)). Post-hoc investigation showed that bilinguals performed better during English as compared to Integration trials (Tukey Honestly Significant Difference (HSD) p < 0.05). However, as can be seen in Table 3, these scores are within one point of each other (English mean = 26 correct and Integration mean = 25 correct out of a total of 28 items, Audio and Video conditions taken together for each language context and numbers are rounded for clarity). Therefore, we suggest that the readers interpret this statistical difference with a degree of caution as the statistical difference may not be entirely meaningful and the participants may have essentially performed with the same accuracy across all language trials and modes. The participants performed more accurately (F (1, 9) = 51.4, p < 0.01) and faster (F (1, 9) = 54.4, p < 0.01) when they read the words, rather than when they heard them.
Imaging Results
Statistical analyses were performed on the concentration of HbO within a cortical region, as this chromophore provided the most robust contrast-to-noise ratio across participants. HbO values for each channel were plotted and inspected. The maximum positive peak values were determined for each channel from 5s before the onset of the trial until 10s after the end of the trial. These values and baseline values (mean of 50s preceding the first trial for each channel) were used for statistical analysis.
Task Versus Baseline
“Task” activation was defined as peak activation during Monolingual mode blocks (English for monolinguals, and English and Spanish averaged for bilinguals; Audio and Video conditions averaged for both groups) and “Baseline” was defined as the mean of the 50 second period prior to the first task for each channel. A 2 × 24 MANOVA (task versus baseline X 24 channels) analysis revealed that all participants had overall greater brain activity during the task as compared to baseline (F(15, 40) = 24, p < 0.0001). The test of individual task-baseline contrasts for each channel confirmed that this Task > Baseline difference was significant for each channel with significance levels ranging from p < 0.01 to p < 0.0001.
ROI Identification
We further used the Task activations (English for monolinguals, English & Spanish for bilinguals, Audio & Video averaged) in a Principal Component Analysis (PCA) for left hemisphere channels as the first exploratory procedure to identify regions of interest (ROI), the channels that overlay brain regions particularly involved with the current language task. The first principal component explained 50% of the variance in the data and indicated two clusters: channels 1–8 had high coefficient loadings (0.680– 0.880) and channels 9–12 had low coefficient loadings (0.280 – 0.580). Anatomical locations of channels 1–8 were examined using MRI anatomical co-registration scans. The anatomical co-registration analysis was conducted with MRIcro and Talairach Deamon anatomical localization software. Anatomical localization analysis suggested that most of the channels with high PCA coefficient loadings primarily overlay our originally hypothesized ROI areas. Channels 1 and 3 maximally overlay IFC (BA 44/45, including classic Broca’s area), channel 2 maximally overlays IFC (BA 47/11) and channels 4 and 7 maximally overlay DLPFC (BA 46/9). Thus, we further narrowed down our selection of ROI channels to only these 5 channels (1–4, 7). Henceforth in the analysis, we treat channels 1 and 3 as maximally overlaying Language PFC areas (BA 44/45), channels 2, 4, and 7 as maximally overlaying Verbal-Working Memory/Attention PFC areas (BA 47/11 & 46/9), and the remaining channels 5–6 and 8–12 as maximally overlaying control areas.
Audio versus Video
The first step in data analysis was to see whether there was a significant difference between the Audio and Video conditions that might preclude us from averaging these two conditions for subsequent analysis presented in the paragraphs below. We explored potential differences between Audio and Video conditions separately for each ROI and using the data from monolinguals (English condition) and bilinguals (all language conditions averaged). For each ROI we used a 2×2 repeated-measures ANOVA (Audio vs Video in Monolingual mode conditions (English for monolinguals; English & Spanish averaged for bilinguals) X hemispheres). For the purposes of this analysis and all the subsequent comparisons for each individual participant we averaged brain activation for the groups of channels belonging to the same ROI (channels 1 & 3 for Language PFC, channels 2, 4, 7 for Verbal Working-Memory/Attention PFC, and channels 5, 6, 8, 9, 10, 11, 12 for Control regions).
Language PFC
The results showed a significantly greater left hemisphere Language PFC (BA 44/45) recruitment (F(1, 19) = 13.9, p < 0.01), with no significant differences between Audio and Video conditions (F(1, 19) = 0.9, p > 0.05), or interaction between the two factors (F(1, 19) = 1.8, p > 0.05). Verbal Working-Memory/Attention PFC. The results showed no significant main effects of Audio versus Video comparison (F(1, 19) = 0.01, p > 0.05), no significant effect of hemisphere (F(1, 19) = 0.04, p > 0.05) or interaction between these two factors (F(1, 19) = 1.9, p > 0.05). Control areas. The results showed no significant main effects of Audio versus Video comparison (F(1, 19) = 0.01, p > 0.05), no significant effect of hemisphere (F(1, 19) = 0.02, p > 0.05), or interaction between the two factors (F(1, 19) = 0.03, p > 0.05). In summary, we observed no significant differences between the Audio and Video conditions, even though participants showed an overall greater recruitment of left hemisphere during Video conditions and a more even bilateral during the Audio condition (Video mean activation LH = 0.069, RH = 0.057; Audio mean activation LH = 0.063, RH = 0.063), which is a classic finding given that visual language processing should be more left-lateralized than auditory language processing that should be more bilateral due to the general auditory processing of sound in both hemispheres. Therefore, for all subsequent analyses, data were averaged across Audio and Visual conditions.
“Bilingual Signature” & Modes
Bilinguals versus Monolinguals
Do monolinguals differ from bilinguals in Monolingual mode, when both groups are using only one language at a time? Similarity: As can be seen in Figure 5a, when using one language at a time (Monolingual mode), both bilinguals and monolinguals had greater left than right hemisphere recruitment in the Language PFC (BA 44/45) brain area. This similarity between the groups is supported by absence of any main effects found in a 3 × 2 × 2 mixed-measures ANOVA (group: monolinguals, bilinguals in Monolingual mode (between factor) X hemispheres (within factor) X ROI (Language PFC & Working Memory/Attention PFC, Control areas, within factor)), averaged across Audio and Video conditions. Non-significant main effects are as follows: group F(1, 18) = 0.2, p > 0.05; hemispheres F(2, 18) = 2.6, p > 0.05; and ROI F(2, 17) = 0.2, p > 0.05. Differences: the significant 3-way interaction of group by ROI by hemisphere can be seen in Figure 5b (Wilks’ Lambda F(2; 18) = 5.0, p < 0.05), suggesting that there was a difference in both the left and right hemispheres in how the two groups recruited Verbal Working-Memory/Attention PFC. Tukey Honestly Significant Differences (HSD) post-hoc comparisons showed a significantly greater right homologue of Verbal Working-Memory/Attention PFC recruitment in bilinguals as compared to monolinguals (p < 0.05). The means for each language mode for each group can be found in Table 4.
Figure 5.

(a) Percent signal change in the left versus right hemispheres for Broca’s Area/Language PFC. There was no significant difference between bilinguals and monolinguals in the recruitment of Broca’s Area (p > 0.05), (b) Percent signal change in the left versus right hemispheres for Verbal Working-Memory/Attention PFC. For this ROI, there was a significant interaction between bilinguals and monolinguals in Monolingual mode, and Bilingual and Monolingual modes in bilinguals (p < 0.05).
Group & Language Mode Similarity: Bilinguals (Monolingual mode) and monolinguals showed similar patterns of activation in left Broca/Language area and right Broca homologue. Group & Language Mode Differences: Bilinguals (Monolingual mode) showed a different pattern of recruitment for left Verbal Working-Memory/Attention PFC and its right homologue as compared to monolinguals. Bilinguals (Bilingual mode) showed greater recruitment of the right hemisphere homologue of Broca’s area and right hemisphere Working-Memory/Attention PFC versus when in Monolingual mode.
Table 4.
Mean peak activation values (and SD) for Monolinguals in Monolingual mode and Bilinguals in Monolingual and Bilingual modes. In Monolingual mode bilinguals and monolinguals had similar recruitment of Language PFC regions. In Monolingual mode bilinguals also showed greater signal intensity in right Verbal Working-Memory/Attention PFC areas as compared to monolinguals (p < 0.05). In Bilingual mode as compared to Monolingual mode bilinguals also showed greater signal intensity in right Verbal Working-Memory/Attention PFC areas (p < 0.05).
| Group | Mode | Language PFC | Verbal Working-Memory/Attention PFC | Control Regions | |||
|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | ||
| 0.047 | 0.034 | 0.059 | 0.042 | 0.051 | 0.049 | ||
| Monolinguals | Monolingual | (0.030) | (0.026) | (0.038) | (0.015) | (0.024) | (0.020) |
| 0.065 | 0.051 | 0.046 | 0.057 | 0.047 | 0.050 | ||
| Bilinguals | Monolingual | (0.056) | (0.047) | (0.035) | (0.043) | (0.020) | (0.020) |
| 0.058 | 0.063 | 0.040 | 0.07 | 0.047 | 0.061 | ||
| Bilinguals | Bilingual | (0.082) | (0.093) | (0.026) | (0.045) | (0.012) | (0.020) |
Bilinguals in Monolingual versus Bilingual Modes
Do bilinguals differ in their use of neural resources towards processing one language at a time (Monolingual mode) versus processing two languages in rapid alternation (Bilingual mode)? We used a 2 × 2 × 3 repeated-measures ANOVA (Bilingual versus Monolingual modes X hemispheres x ROI (Language PFC, Verbal Working-Memory/Attention PFC, Control areas)), averaged across Audio and Video conditions. We found a significant main effect of hemisphere (F(1, 9) = 6.8, p < 0.05), all other main effects were non-significant (mode F(1, 9) = 0.3, p > 0.05; ROI F(2, 18) = 0.2, p > 0.05). Importantly, however, and as can be seen in Figure 5 (a & b), there were significant mode X hemisphere (F(1, 9) = 7.3, p < 0.05) and hemisphere X ROI (F(2, 18) = 14.5, p < 0.05) interactions, suggesting increased right hemisphere involvement during Bilingual mode as compared to Monolingual mode. Follow-up 2 × 2 repeated-measures ANOVAS (mode X hemisphere) for each ROI showed that hemisphere by mode interaction was significant only for Verbal Working-Memory/Attention PFC (F(1, 9) = 5.1, p < 0.05) and Control regions (F(1, 9) = 5.3, p < 0.05). Mode X hemisphere interaction was not significant for Language PFC (F(1, 9) = 3.0, p > 0.05), even though the means (Table 4) suggested that for this brain region there was also an increased right hemisphere involvement during bilingual mode. In summary and as can be seen in Table 4 and Figure 5, left hemisphere involvement remained relatively constant across language modes for each brain region of interest, while right hemisphere involvement significantly increased in the bilingual mode.
Monolingual mode in Bilinguals: English versus Spanish
In order to explore any differences between the two Monolingual mode conditions in bilinguals, Spanish and English, we used a 2 × 2 × 3 repeated-measures ANOVA (English & Spanish languages X hemispheres X brain area (Language PFC, Verbal Working-Memory/Attention PFC, Control areas)), averaged across Audio and Video conditions. There were no main effects of English versus Spanish languages suggesting that the two languages of early and highly proficient bilinguals yield the same intensity and extent of activation (F(1, 9) = 0.7, p > 0.05). There was a slight, yet significantly greater recruitment of Right Hemisphere for both languages, due to greater right versus left activation in the Verbal Working-Memory/Attention PFC area as can be seen from the means in Table 5 and follow-up post-hoc comparisons described below (hemispheres F(1, 9) = 5.4, p < 0.05). Similar to Bilingual conditions comparison (see paragraph below), there was a significant hemisphere by ROI interaction (F(2, 18) = 4.2, p < 0.05), showing greater left than right recruitment of Language PFC area (BA 44/45), greater right than left recruitment of Verbal Working-Memory/Attention PFC area (BA 47/11; 46/9), and a balanced bilateral recruitment of the Control areas. For Monolingual mode comparison post-hoc Tukey HSD comparison showed a significantly greater right versus left recruitment only for Verbal Working-Memory/Attention PFC (p < 0.05), see means and standard deviations in Table 5. There was no main effect of ROI (F(2, 18) = 0.2, p > 0.05).
Table 5.
Mean peak activation values (and SD) for Bilinguals in each language condition. Monolingual mode: English and Spanish; Bilingual mode: Integration and Alternation.
| Condition | Language PFC | Verbal Working-Memory/Attention PFC | Control Regions | |||
|---|---|---|---|---|---|---|
| L | R | L | L | R | L | |
| 0.065 | 0.051 | 0.046 | 0.057 | 0.048 | 0.050 | |
| English | (0.056) | (0.047) | (0.035) | (0.043) | (0.019) | (0.020) |
| 0.045 | 0.051 | 0.039 | 0.059 | 0.043 | 0.050 | |
| Spanish | (0.062) | (0.042) | (0.022) | (0.036) | (0.011) | (0.020) |
| 0.058 | 0.063 | 0.041 | 0.070 | 0.047 | 0.061 | |
| Integration | (0.082) | (0.093) | (0.026) | (0.045) | (0.012) | (0.020) |
| 0.054 | 0.051 | 0.042 | 0.057 | 0.047 | 0.052 | |
| Alternation | (0.043) | (0.047) | (0.021) | (0.033) | (0.017) | (0.027) |
Bilingual mode in Bilinguals: Integration versus Alternation
In order to explore any differences between the two Bilingual mode conditions, Integration and Alternation, we used a 2 × 2 × 3 repeated-measures ANOVA (Bilingual mode conditions X hemispheres X ROI (Language PFC, Verbal Working-Memory/Attention PFC, Control areas)), averaged across Audio and Video conditions. There was a marginally significant condition by hemisphere interaction which can be seen in Figure 6 (F(1, 9) = 10.0, p = 0.06, the means for the interaction are in Table 5), showing that while bilinguals maintained the same level of left hemisphere recruitment during both Bilingual mode conditions, there was greater right hemisphere involvement during the Integration condition as compared to the Alternation condition. Post-hoc Tukey HSD comparisons showed significantly greater right versus left Verbal Working-Memory/Attention PFC recruitment during Integration condition, but not during Alternation condition (p < 0.05). Same as in Monolingual conditions (see paragraph above), there was a significant ROI by hemisphere interaction (F(2, 18) = 4.5, p < 0.05). For Bilingual mode comparison Tukey HSD comparison showed a significantly greater right versus left recruitment only for Verbal Working-Memory/Attention PFC (p < 0.05). There were no main effects of either condition (F(1, 9) = 0.2, p > 0.05), hemispheres (F(1, 9) = 3.2, p > 0.05), or ROI (F(2, 18) = 0.1, p > 0.05).
Figure 6.

Percent signal change during Integration and Alternation conditions for each ROI. There was a significant hemisphere by condition interaction (p < 0.05), with greater right hemisphere recruitment during integration condition, and relatively similar left hemisphere recruitment during both conditions.
DISCUSSION
Here we examined whether early dual language exposure both neurally and behaviorally changes language processing in bilinguals as compared to monolinguals, and if so whether these changes support the Neural Signature or the Switching Hypothesis. The present findings show that bilinguals do neurally process language differently than monolinguals in both when using one language at a time and when using two languages in rapid alternation, which provides support for the Neural Signature hypothesis of how the bilingual brain processes language. In Monolingual mode, whereas bilinguals and monolinguals had similar recruitment of the classic Language PFC areas (BA 44/45), the two groups had differential recruitment of Verbal Working-Memory/Attention PFC (BA 46/9 & 47/11) and its right hemisphere homologue. In Bilingual mode, as compared to Monolingual mode, bilinguals had differential recruitment of classic Language PFC areas (BA 44/45) and Verbal Working-Memory/Attention PFC areas (BA 46/9 & 47/11), as well as their right hemisphere homologues. Taken together, these findings indicate that early, extensive and maintained exposure to two languages can modify the neural basis of language processing.
Language Behavioral Performance
Bilinguals and monolinguals overall performed with the same speed and accuracy. Moreover, bilinguals performed equally well in both of their languages during the two Monolingual modes. They also performed equally well during the Bilingual Alternation mode as they did during each of the Monolingual modes. The only observed performance difference was bilinguals’ better performance in the English-only condition as compared to the Integration condition. It previously has been found that during language comprehension, bilinguals are inadvertently processing some components of both of their languages in parallel (Dijkstra, Van Heuven, & Grainger, 1998; Doctor & Klein, 1992; Grosjean, 1997; Kroll & Sunderman, 2003). Therefore, it is notable that bilinguals maintained the same efficacy of language processing as monolinguals, and to some extent maintained the same successful performance as their task load became more complex during Bilingual mode.
Dijkstra and Van Heuven’s bilingual language model suggests that bilinguals’ should be able to equally effectively process one language at a time versus two languages in rapid alternation. It is the cognitive load incurred by the experimental task that might result in specific patterns of speed and accuracy during Bilingual versus Monolingual modes. Indeed, under some experimental conditions, bilinguals perform with the same speed and accuracy during both Bilingual and Monolingual modes (Caramazza & Brones, 1980; Grosjean & Miller, 1994; Van Heuven, Dijkstra & Grainger, 1998), whereas during other experimental conditions their performance declines during Bilingual mode tasks (Thomas & Allport, 2000; von Studnitz & Green, 2002). We captured these phenomena in our study: bilinguals performed with the same speed and accuracy during the Monolingual Mode and the Bilingual mode Alternating condition (Table 3). These results suggest that early-exposed and highly proficient bilinguals can perform with equal success in single and dual language contexts. However, experimental tasks such as the Bilingual mode Integration condition (Table 3), can take a toll on bilinguals’ accuracy of performance. Why might this be so? It is possibly due to an increased attentional and working-memory load that is required to keep semantic items in two different languages activated in memory at the same time.
Imaging Findings
To the best of our knowledge, the results of the few functional imaging studies outside of our laboratory to have directly compared language processing in healthy, early-exposed and highly-proficient bilinguals versus monolinguals are in agreement with our findings here. For example, Proverbio, Cok, & Zani (2002) compared Italian-Slovenian bilinguals and Italian monolinguals using Event-Related Potential (ERP) technology, and a sentence-processing task. Bilinguals were shown to have a different neural response as compared to monolinguals. Also consistent with our findings, Rodriguez-Fornells et al.’s (2002) fMRI work found that there was differential frontal lobe activation between bilinguals in Bilingual mode and monolinguals in Monolingual mode. Together, these ERP and fMRI studies have converged with our fNIRS study to suggest that the human neural organization and language processing capacity can be molded by extensive dual language exposure early in life.
When using only one language at a time, we found no difference in how bilinguals and monolinguals recruited classic Language area PFC (BA 44/45). However, there was a hemispheric difference in how bilinguals and monolinguals recruited Working-Memory/Attention PFC (BA 47/11 & 46/9). In particular, there was an overall greater bilateral recruitment of Working-Memory/Attention PFC area in bilinguals as compared to monolinguals, when both groups were presented with only one language at a time (Monolingual mode). This greater recruitment, described by the observed significantly greater signal intensity, of Working-Memory/Attention PFC in bilinguals was particularly significant in right hemisphere. This greater signal intensity (as measured by the change in oxygenated hemoglobin) was observed in fNIRS channels maximally overlaying Working-Memory/Attention PFC regions (DLPFC (BA 46/9) and IFC (BA 47/11)) in bilingual group as compared to monolingual group. These findings support the idea that dual language exposure can result in monolingual-like recruitment of the classic language brain areas, such as left IFC, which incorporates Broca’s area (BA 44/45). The observed group differences suggest that dual language processing may incur neural changes within brain regions that support working-memory and attention associated with language processing. Prior memory and language research has established that there are strong empirical links between DLPFC (BA 46/9) and working memory (Baddeley, 2000; D'Esposito, Postle, & Rypma, 2000; Gabrieli, Poldrack, & Desmond, 1998; Smith & Jonides, 1999). In particular, left DLPFC seems to be more associated with verbal working-memory (e.g., Gabrieli et al., 1998), while right DLPFC seems to be more associated with visuo-spatial working memory, as well as cross-modal information integration (e.g., Bushara, Grafman, & Hallett, 2001). Increased activation in left IFC (BA 47/11) has been found in association with verbal tasks, such as semantic decision-making (Roskies, Fiez, Balota, Raichle, & Petersen, 2001), while increased activation in right anterior IFC has been found in association with attention and selective inhibition for both verbal and non-verbal tasks (Aron, Robbins, & Poldrack, 2004). The differential recruitment of Verbal Working Memory/Attention PFC for bilinguals as compared to monolinguals that we found here is likely due to differences in the verbal working memory and attention resources required for dual language processing, such as selective attention allocation between competing linguistic information. Alternatively, this activation difference between the groups could simply be due to an overall greater effort exerted by bilinguals. Although we cannot rule out this possibility, bilinguals and monolinguals showed equally good performance on the task, which suggests the same level of difficulty for the two groups. Previous research has demonstrated equal level of performance and yet greater BOLD signal in aging and pathological populations, suggesting evidence of compensation strategies. However, pathological and aging populations also typically undergo changes in brain vasculature, which makes it difficult to interpret the exact nature of differences in BOLD signal, while here we studied young and healthy individuals with, hopefully, healthy and unaffected vascular system (see discussion by D’Esposito, Deouell, & Gazzaley, 2003).
In bilingual language modes a bilingual needs to integrate and negotiate cross-linguistic information. In our study, processing two languages in rapid succession was accompanied by an increase in both Verbal Working-Memory/Attention PFC (ROI analysis, Figure 5; BA 46/9 & 47/11) as well as Language PFC (ROI analysis, BA 44/45). This result is commensurate with our pilot fMRI findings, in which a comparable group of bilingual participants were scanned using an fMRI (Phillips 3T) with the very same Semantic Judgment task (using identical screening and task administration methods as described in the methods section above). Using fMRI, we observed greater right DLPFC (BA 46) and right IFC recruitment, for both BA 47 (Attention PFC) and BA 45 (Language PFC) in bilinguals during Bilingual versus Monolingual mode (see Figure 7, the data was analyzed with SPM 99 random-effects model, Friston, Holmes, Worsley, Poline, Frith, & Frackowiak, 1995). As previously mentioned, increased activation in right hemisphere brain areas responsible for executive processing has been observed in cross-domain information integration (Bushara et al., 2001). It also appears that semantic tasks which require atypical “outside-the-box” information search and integration also make selective reliance on right hemisphere frontal areas (e.g., generating unusual verbs in response to nouns, as in “dish” – “throw”; Seger, Desmond, Glover, & Gabrieli, 2000). If information integration is a strong characteristic of right hemisphere executive processing brain areas, then the observed right DLPFC and IFC activations might be due to the increased working memory and attention, as well as cross-linguistic integration processes required during Bilingual mode.
Figure 7.

Replication of NIRS findings with fMRI. As was found with NIRS, bilinguals in Bilingual mode showed greater right hemisphere recruitment of both Broca’s homologue and Verbal Working-Memory/Attention PFC areas versus in Monolingual mode. Here is shown greater right DLPFC (BA 46) activation during Bilingual mode > Monolingual mode (p<0.001;k>10).
A number of different bilingual research groups have hypothesized a link between DLPFC function and bilingual “language switching,” the ability to selectively inhibit one language while activating the other (Bialystok, 2001; Hernandez et al., 2001; Price et al., 1999; Rodriguez-Fornells et al., 2002). However, results from other studies have not shown an increase in DLPFC activation during task switching (Kane & Engle, 2002; Wager et al., 2004). Because our findings show increased activation in DLPFC during both Monolingual and Bilingual modes for bilinguals, they suggest that increased activation in DLPFC cannot be simply reduced to DLPFC being the site for task switching per se (as had been previously argued in the literature). Another intriguing possibility for further study is that, rather than inhibiting one language and activating the other (switching), the increased DLPFC activation may instead be due to both languages being active (i.e., simultaneous dual activation), or possibly be due to the increased demands of cross-linguistic integration of semantic information.
Right prefrontal activation has been previously observed in late- and low-proficiency bilinguals (Kim et al., 1997; Newman-Norlund, Frey, Petitto, & Grafton, 2006; Pillai et al., 2003; Weber-Fox & Neville, 1999; Hahne & Friederici, 2001). It has been previously suggested that low proficiency leads to heavier reliance on meta-linguistic cues processed within the right hemisphere (Caplan & Dapretto, 2001; Fabbro, 2001; Paradis, 1997; Jung-Beeman, 2005). In fact, right IFC activity decreases as L2 proficiency increases (Newman-Norlund et al., 2006). Our study included only early-exposed and highly-proficient bilinguals, thus, even if they did resort to meta-linguistic cues, it was not due to their lower language proficiency. Meta-linguistic mechanisms do include high levels of information integration as discussed above, including the ability to combine knowledge about discourse and pragmatics and multiple contextual cues. Thus, in accordance with views on right hemisphere involvement in information integration (Baddeley, 2000) and meta-linguistic language processing (Caplan & Dapretto; 2001; Paradis, 1997), we suggest that greater right PFC activation during Bilingual mode is associated with the cross-linguistic information integration and manipulation during Bilingual mode.
The present study is one of only a few studies to conduct a systematic investigation into the nature of dual language processing as compared to that of monolingual language processing. The key limitation to this work was that a monolingual Spanish-speaking control group was not available. The matched monolingual controls in this study were native speakers of English living and studying in the U.S.A. A comparable Spanish control group would have been a group of monolingual Spanish-speaking students scanned in a Spanish-speaking Latin American country. Although we did not include native monolingual Spanish speakers, our investigation included a bilingual group and an English monolingual group that were maximally comparable to each other with respect to language competence and proficiency, literacy exposure, and language maintenance.
For decades we studied language processing in monolinguals with the assumption that this research would lead us to the understanding of the true nature of language processing, language acquisition, and language organization in the brain. As many people are proficient speakers of more than one language, it may be that bilinguals are the group that uses the remarkable human language ability to its full potential, while monolinguals use this capacity to a more limited extent. Possibly, in an unexpected twist, it is the study of bilinguals that may reveal the language processing potential not fully recruited in monolinguals and lead us to the biological extent of the neural tissue underlying all human language.
In this study functional Near Infrared Spectroscopy showed itself to be a highly useful technology, much comparable to fMRI, for the study of language and higher cognition in adults. It is our hope that future research will expand the use of this technology to younger bilingual populations with the goal of uncovering the basis of neural development associated with all language acquisition and maturation of other higher cognitive functions in children.
CONCLUSION
Early and extensive dual language exposure appears to have an impact on how the bilingual brain processes language within classic language areas (IFC, BA), as well as brain areas that support language processing (DLPFC, BA 46/9 and IFC BA 47/11). The overall implication is that this neural change is entirely positive – bilinguals can read and listen to semantic information in each of their languages with the same effectiveness as monolinguals. The bilingual brain also develops mechanisms that allow for successful processing of two languages concurrently in a Bilingual mode. We therefore hope that scientists, educators, and bilingual policymakers, alike, will take note of the present findings--especially those who decide on educational settings for the nation’s young bilinguals and on whether early bilingual language learning as a child harms one’s dual language, reading, and cognitive processing as an adult. To be sure, we found no evidence of harm and instead found evidence that the bilingual brain processes each of its two languages with the aplomb of a monolingual brain processing one. We further hope that our findings may excite cognitive neuroscientists to view bilingual language processing as shedding new light on the full extent and variability of the brain’s neural architecture underlying the remarkable human language capacity.
ACKNOWLEDGEMENTS
We thank Kevin Dunbar and the Pettito laboratory members, particularly Rachael Degenshein, Katherine White, Douglas McKenney, and Melissa Melendez. We also extend our thanks to all the members of the Dartmouth Brain Imaging Center. L. A. Petitto (P.I.) thanks the National Institutes of Health (R01HD045822-01 and R21HD050558-01), and the Dana Foundation for funding this research. We especially thank the Hitachi Medical Systems Japan for their continuing technical support for our Hitachi functional Near Infrared Spectroscopy system. E-mail: Petitto@utsc.utoronto.ca
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbate MS, La Chappelle NB. Pictures, please! An articulation supplement. Tucson, AZ: Communication Skill Builders; 1984. [Google Scholar]
- Abutalebi J, Cappa FS, Perani D. The bilingual brain as revealed by functional neuroimaging. Biling Lang Cogn. 2001;4(2):179–190. [Google Scholar]
- Ameel E, Storms G, Malt BC, Sloman SA. How bilinguals solve the naming problem. J Mem Lang. 2005;53:60–80. [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bushara KO, Grafman J, Hallett M. Neural correlates of auditory-visual stimulus onset asynchrony detection. J Neurosci. 2001;21(1):300–304. doi: 10.1523/JNEUROSCI.21-01-00300.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. The episodic buffer: a new component of working memory? Trends Cog Sci. 2000;4(11):417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Bialystok E. Bilingualism in development: Language, literacy, and cognition. New York: Cambridge University; 2001. [Google Scholar]
- Caplan R, Dapretto M. Making sense during conversation: An fMRI study. NeuroReport. 2001;12(16):3625–3632. doi: 10.1097/00001756-200111160-00050. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Brones I. Semantic classification by bilinguals. Can J Psychology. 1980;34(1):77–81. [Google Scholar]
- Chee MWL, Soon CS, Lee HL, Pallier C. Left insula activation: A marker for language attainment in bilinguals. PNAS. 2004;101(42):15265–15270. doi: 10.1073/pnas.0403703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Soon CS, Lee HL. Relative language proficiency modulates BOLD signal change when bilinguals perform semantic judgments. NeuroImage. 2001;13(6):1155–1163. doi: 10.1006/nimg.2001.0781. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Soon CS, Lee HL. Common and segregated neuronal networks for different languages revealed using functional magnetic resonance adaptation. J Cogn Neurosci. 2003;15(1):85–97. doi: 10.1162/089892903321107846. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Tan EWL, Thiel T. Mandarin and English single word processing studied with functional magnetic resonance imaging. J Neurosci. 1999;19(8):3050–3056. doi: 10.1523/JNEUROSCI.19-08-03050.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MWL, Weekes B, Lee KM, Soon CS, Schreiber A, Hoon JJ, Chee M. Overlap and dissociation of semantic processing of Chinese characters, English words, and pictures: Evidence from fMRI. NeuroImage. 2000;12(4):392–403. doi: 10.1006/nimg.2000.0631. [DOI] [PubMed] [Google Scholar]
- Dawson G, Fischer KW. Human behavior and the developing brain. New York: Guilford Press; 1994. [Google Scholar]
- Dehaene S, Dupoux E, Mehler J, Cohen L, Paulesu E, Perani D, van de Moortele PF, Lehericy S, Le Bihan D. Anatomical variability in the cortical representation of first and second language. NeuroReport. 1997;8(17):3809–3815. doi: 10.1097/00001756-199712010-00030. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: A challenge for neuroimaging. Nature Reviews Neuroscience. 2003;4(11):863–871. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Exp Brain Res. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Dijkstra T, Van Heuven WJB. The architecture of the bilingual word recognition system: From identification to decision. Biling Lang Cogn. 2002;5(3):175–197. [Google Scholar]
- Dijkstra T, Van Heuven WJB, Grainger J. Simulating cross-language competition with the bilingual interactive activation model. Psychologica Belgica. 1998;38(3–4):177–196. [Google Scholar]
- Doctor EA, Klein D. Phonological processing in bilingual word recognition. In: Harris RJ, editor. Cognitive Processing in Bilinguals. Amsterdam: Elsevier; 1992. pp. 237–252. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23(10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody picture vocabulary test-revised. Circle Pines, MN: American Guidance Service; 1981. [Google Scholar]
- Fabbro F. The bilingual brain: Cerebral representation of languages. Brain Lang. 2001;79(2):211–222. doi: 10.1006/brln.2001.2481. [DOI] [PubMed] [Google Scholar]
- Fine I, Finney EM, Boynton GM, Dobkins KR. Comparing the effects of auditory deprivation and sign language within the auditory and visual cortex. J Cogn Neurosci. 2005;17:1621–1637. doi: 10.1162/089892905774597173. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Steinhauer K, Pfiefer E. Brain signatures of artificial language processing: Evidence challenging the critical period hypothesis. PNAS. 2002;99(8):529–534. doi: 10.1073/pnas.012611199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R. Statistical parametric maps: Linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Fugelsang JA, Dunbar KN. Brain-based mechanisms underlying complex causal thinking. Neuropsychologia. 2005;43(8):1204–1213. doi: 10.1016/j.neuropsychologia.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Fugelsang J, Green A, Dunbar K. Mapping the shift from controlled to automatic processing in the brain. Poster presented at the annual Cognitive Neuroscience conference; April.2006. [Google Scholar]
- Gabrieli JD, Poldrack RA, Desmond JE. The role of left prefrontal cortex in language and memory. PNAS. 1998;95(3):906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genesee F. Early bilingual development: One language or two? J Child Lang. 1989;16:161–179. doi: 10.1017/s0305000900013490. [DOI] [PubMed] [Google Scholar]
- Genesee F, Boivin I, Nicoladis E. Bilingual children talking with monolingual adults: A study of bilingual children's communicative competence. Appl Psycholinguist. 1996;17:427–442. [Google Scholar]
- Golestani N, Alario FX, Meriaux S, Le Bihan D, Dehaene S, Pallier C. Syntax production in bilinguals. Neuropsychologia. 2006;44(7):1029–1040. doi: 10.1016/j.neuropsychologia.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Green DW. Mental control of the bilingual lexico-semantic system. Biling Lang Cogn. 1998;1(2):67–81. [Google Scholar]
- Grosjean F. Processing mixed language: Issues, findings and models. In: de Groot AMB, Kroll JF, editors. Tutorials in bilingualism: Psycholinguistic perspectives. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 225–254. [Google Scholar]
- Grosjean F, editor. The bilingual's language modes. Malden, MA: Blackwell Publishing; 2001. [Google Scholar]
- Grosjean F, Miller JL. Going in and out of languages: An example of bilingual flexibility. Psychol Sci. 1994;5(4):201–206. [Google Scholar]
- Hahne A, Friederici AD. Processing a second language: Late learners' comprehension mechanisms as revealed by event-related brain potential. Biling Lang Cogn. 2001;4(2):123–141. [Google Scholar]
- Hernandez AE, Dapretto M, Mazziotta J, Bookheimer S. Language switching and language representation in Spanish-English bilinguals: An fMRI study. NeuroImage. 2001;14(2):510–520. doi: 10.1006/nimg.2001.0810. [DOI] [PubMed] [Google Scholar]
- Hernandez AE, Martinez A, Kohnert K. In search of the language switch: An fMRI study of picture naming in Spanish-English bilinguals. Brain Lang. 2000;73(3):421–431. doi: 10.1006/brln.1999.2278. [DOI] [PubMed] [Google Scholar]
- Holowka S, Brosseau-Lapré F, Petitto LA. Semantic and conceptual knowledge underlying bilingual babies' first signs and words. Lang Learn. 2002;52(2):205–262. [Google Scholar]
- Holtzheimer P, Fawaz W, Wilson C, Avery D. Repetitive transcranial magnetic stimulation may induce language switching in bilingual patients. Brain Lang. 2005;94(3):274–277. doi: 10.1016/j.bandl.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Howard D, Patterson K. The pyramid and palm trees test: A test of semantic access from words and pictures. Bury St. Edmunds: Thames Valley Test Co; 1992. [Google Scholar]
- Jasper HH. Report of the Committee on Methods of Clinical Examination in Electroencephalography. Electroencephalogr Clin Neurophysiol. 1957;10:371–375. [Google Scholar]
- Johnson JS, Newport EL. Critical period effects in second language learning: The influence of maturational state on the acquisition of English as a second language. Cognitive Psychol. 1989;21(1):60–99. doi: 10.1016/0010-0285(89)90003-0. [DOI] [PubMed] [Google Scholar]
- Juilland A, Chang-Rodríguez E. Frequency dictionary of Spanish words. The Hague: Mouton & Co; 1964. [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends Cogn Sci. 2005;9(11):512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual differences perspective. Psychon B Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lee & Febiger; 1983. [Google Scholar]
- Kerkhofs R, Dijkstra T, Chwilla DJ, de Bruijn E. Testing a model for bilingual semantic priming with interlingual homographs: RT and N400 effects. Brain Res. 2006;1068(1):170–183. doi: 10.1016/j.brainres.2005.10.087. [DOI] [PubMed] [Google Scholar]
- Kim KHS, Relkin NR, Lee K-M, Hirsch J. Distinct cortical areas associated with native and second languages. Nature. 1997;388(6638):171–174. doi: 10.1038/40623. [DOI] [PubMed] [Google Scholar]
- Klein D, Doctor EAL. Patterns of developmental dyslexia in bilinguals. In: Goulandris N, editor. Dyslexia in Different Languages: Cross-Linguistic Comparisons. London, England: Whurr Publishers, Ltd; 2003. pp. 112–136. [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Meyer E, Evans AC. The neural substrates underlying word generation: A bilingual functional-imaging study. PNAS. 1995;92(7):2899–2903. doi: 10.1073/pnas.92.7.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D, Milner B, Zatorre RJ, Zhao V, Nikelski J. Cerebral organization in bilinguals: A PET study of Chinese-English verb generation. NeuroReport. 1999;10(13):2841–2846. doi: 10.1097/00001756-199909090-00026. [DOI] [PubMed] [Google Scholar]
- Klein D, Watkins KE, Zatorre RJ, Milner B. Word and nonword repetition in bilingual subjects: A PET study. Hum Brain Mapp. 2006;27(2):153–161. doi: 10.1002/hbm.20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl M, Nolte C, Heekeren HR, Horst S, Scholz U, Obrig H, Villringer A. Determination of the wavelength dependence of the differential pathlength factor from near-infrared pulse signals. Phys Med Biol. 1998;43(6):1771–1782. doi: 10.1088/0031-9155/43/6/028. [DOI] [PubMed] [Google Scholar]
- Kovelman I, Petitto LA. Bilingual babies' maturational and linguistic milestones as a function of their age of first exposure to two languages. Poster session presented at the conference for the Society for Neuroscience; November; Orlando, FL. 2002. [Google Scholar]
- Kovelman I, Baker SA, Grafton ST, Petitto LA. Bilingual and monolingual brains compared: Is there a neurological signature of bilingualism?. Abstracts of the 5th International Symposium on Bilingualism; Barcelona: 2005. Mar, [Google Scholar]
- Kovelman I, Baker S, Petitto LA. Bilingual and Monolingual brains compared: An fMRI investigation of Syntactic processing and a possible "neural signature" of bilingualism. J Cogn Neurosci. doi: 10.1162/jocn.2008.20011. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll JR, Sunderman G. Cognitive processes in second language learners and bilinguals: The development of lexical and conceptual representations. In: Doughty C, Long M, editors. The handbook of second language acquisition. Oxford, England: Blackwell; 2003. pp. 104–129. [Google Scholar]
- Lenneberg EH. Biological Foundations of Language. New York, NY: Wiley; 1967. [Google Scholar]
- MacWhinney B, Cohen J, Provost J. The PsyScope experiment-building system. Spatial Vision. 1997;11:99–101. doi: 10.1163/156856897x00113. [DOI] [PubMed] [Google Scholar]
- Marian V, Spivey M, Hirsch J. Shared and separate systems in bilingual language processing: Converging evidence from eye tracking and brain imaging. Brain Lang. 2003;86(1):70–82. doi: 10.1016/s0093-934x(02)00535-7. [DOI] [PubMed] [Google Scholar]
- Mayberry RI, Eichen EB. The long-lasting advantage of learning sign language in childhood: Another look at the critical period for language acquisition. J Mem Lang. 1991;30(4):486–512. [Google Scholar]
- Mayberry RI, Fischer SD. Looking through phonological shape to lexical meaning: The bottleneck of non-native sign language processing. Mem Cogn. 1989;17(6):740–754. doi: 10.3758/bf03202635. [DOI] [PubMed] [Google Scholar]
- Mayberry RI, Lock E, Kazmi H. Linguistic ability and early language exposure. Nature. 2002;417(6884):38. doi: 10.1038/417038a. [DOI] [PubMed] [Google Scholar]
- McDonald JL. Grammaticality judgements in a second language: Influences of age of acquisition and native language. Appl Psychololinguist. 2000;21:395–423. [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, Price CJ. Neurolinguistics: Structural plasticity in the bilingual brain. Nature. 2004;431(7010):757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Meschyan G, Hernandez AE. Impact of language proficiency and orthographic transparency on bilingual word reading: An fMRI investigation. NeuroImage. 2006;29(4):1135–1140. doi: 10.1016/j.neuroimage.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Monsell S, Matthews GH, Miller DC. Repetition of lexicalization across languages: A further test of the locus of priming. Q J Exp Psychol- A. 1992;44(4):763–783. doi: 10.1080/14640749208401308. [DOI] [PubMed] [Google Scholar]
- MRC Psycholinguistic Database: Machine Usable Dictionary. Version 2.00 Informatics Division Science and Engineering Research Council Rutherford Appleton Laboratory Chilton. Didcot: [Google Scholar]
- Neville HJ, Bavelier D. Specificity of developmental neuroplasticity in humans: Evidence from sensory deprivation and altered language experience. In: Shaw CA, McEachern JC, editors. Toward a Theory of Neuroplasticity. Philadelphia, PA: Psychology Press/Taylor & Francis; 2001. pp. 261–274. [Google Scholar]
- Neville HJ, Coffey SA, Lawson DS, Fischer A, Emmorey K, Bellugi U. Neural systems mediating American sign language: Effects of sensory experience and age of acquisition. Brain Lang. 1997;57(3):285–308. doi: 10.1006/brln.1997.1739. [DOI] [PubMed] [Google Scholar]
- Newman AJ, Bavelier D, Corina D, Jezzard P, Neville HJ. A critical period for right hemisphere recruitment in American Sign Language processing. Natl J Neurosci. 2002;5(1):76–80. doi: 10.1038/nn775. [DOI] [PubMed] [Google Scholar]
- Newman-Norlund R, Frey S, Petitto LA, Grafton S. Anatomical substrates of visual and auditory miniature second language learning. J Cogn Neurosci. 2006;18:1984–1997. doi: 10.1162/jocn.2006.18.12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport EL. Maturational constraints on language learning. Cogn Sci. 1990;14(1):11–28. [Google Scholar]
- Norman DA, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness & Self-Regulation. New York, NY: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- Ohnishi T, Matsuda H, Asada T, Aruga M, Hirakata M, Nishikawa M, Katoh A, Imabayashi E. Functional anatomy of musical perception in musicians. Cereb Cortex. 2001;11(8):754–760. doi: 10.1093/cercor/11.8.754. [DOI] [PubMed] [Google Scholar]
- Oxford Junior Workbooks. UK: Oxford University Press; 1965. [Google Scholar]
- Paradis J, Nicoladis E, Genesee F. Early emergence of structural constraints on code-mixing: Evidence from French-English bilingual children. Biling Lang Cogn. 2000;3(3):245–261. [Google Scholar]
- Paradis M. The cognitive neuropsychology of bilingualism. In: de Groot AMB, editor. Tutorials in Bilingualism: Psycholinguistic Perspectives. Mahwah, NJ: Lawrence Erlbaum Associates; 1997. pp. 331–354. [Google Scholar]
- Penhune VB, Cismaru R, Dorsaint-Pierre R, Petitto LA, Zatorre RJ. The morphometry of auditory cortex in the congenitally deaf measured using MRI. NeuroImage. 2003;20(2):1215–1225. doi: 10.1016/S1053-8119(03)00373-2. [DOI] [PubMed] [Google Scholar]
- Perani D, Paulesu E, Galles NS, Dupoux E, Dehaene S, Bettinardi V, Cappa SF, Fazio F, Mehler J. The bilingual brain: Proficiency and age of acquisition of the second language. Brain. 1998;121(10):1841–1852. doi: 10.1093/brain/121.10.1841. [DOI] [PubMed] [Google Scholar]
- Petersson KM, Reis A, Ingvar M. Cognitive processing in literate and illiterate subjects: A review of some recent behavioral and functional neuroimaging data. Scand J Psychol. 2001;42(3):251–267. doi: 10.1111/1467-9450.00235. [DOI] [PubMed] [Google Scholar]
- Petitto LA, Holowka S. Evaluating attributions of delay and confusion in young bilinguals: Special insights from infants acquiring a signed and a spoken language. Sign Lang Stud. 2002;3(1):4–33. [Google Scholar]
- Petitto LA, Kovelman I. The Bilingual Paradox: How signing-speaking bilingual children help us to resolve it and teach us about the brain’s mechanisms underlying all language acquisition. Learn Lang. 2003;8:5–19. [Google Scholar]
- Petitto LA, Kovelman I, Harasymowycz U. Bilingual language development: Does learning the New damage the Old?. Poster session presented at the biennial meeting of the Society for Research in Child development; Tampa, FL. 2003. [Google Scholar]
- Petitto LA, Katerelos M, Levy BG, Gauna K, Tetreault K, Ferraro V. Bilingual signed and spoken language acquisition from birth: Implications for the mechanisms underlying early bilingual language acquisition. J Child Lang. 2001;28(2):453–496. doi: 10.1017/s0305000901004718. [DOI] [PubMed] [Google Scholar]
- Petitto LA, Zatorre R, Gauna K, Nikelski EJ, Dostie D, Evans A. Speech-like cerebral activity in profoundly deaf people while processing signed languages: Implications for the neural basis of human language. PNAS. 2000;97(25):13961–13966. doi: 10.1073/pnas.97.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JJ, Araque JM, Allison JD, Sethuraman S, Loring DW, Thiruvaiyaru D, Ison CB, Balan A, Lavin T. Functional MRI study of semantic and phonological language processing in bilingual subjects: Preliminary findings. NeuroImage. 2003;19(3):565–576. doi: 10.1016/s1053-8119(03)00151-4. [DOI] [PubMed] [Google Scholar]
- Poplack S. Sometimes I'll start a sentence in English y termino en espanol. Linguistics. 1980;18:581–616. [Google Scholar]
- Poulin-Dubois D, Goodz N. Language differentiation in bilingual infants: Evidence from babbling. In: Cenoz J, Genesee F, editors. Trends in Bilingual Acquisition. Trends in Language Acquisition Research. vol. 1. Amsterdam, Netherlands: John Benjamins Publishing Company; 2001. pp. 95–106. [Google Scholar]
- Price CJ, Green DW, von Studnitz R. A functional imaging study of translation and language switching. Brain. 1999;122(Pt 12):2221–2235. doi: 10.1093/brain/122.12.2221. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Cok B, Zani A. Electrophysiological measures of language processing in bilinguals. J Cogn Neurosci. 2002;14(7):994–1017. doi: 10.1162/089892902320474463. [DOI] [PubMed] [Google Scholar]
- Roder B, Stock O, Bien S, Neville H, Rosler F. Speech processing activates visual cortex in congenitally blind humans. Eur J Neurosci. 2002;16(5):930–936. doi: 10.1046/j.1460-9568.2002.02147.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fornells A, Rotte M, Heinze H-J, Noesselt T, Muente TF. Brain potential and functional MRI evidence for how to handle two languages with one brain. Nature. 2002;415(6875):1026–1029. doi: 10.1038/4151026a. [DOI] [PubMed] [Google Scholar]
- Roskies AL, Fiez JA, Balota DA, Raichle ME, Petersen SE. Task-dependent modulation of regions in the left inferior frontal cortex during semantic processing. J Cogn Neurosci. 2001;13(6):829–843. doi: 10.1162/08989290152541485. [DOI] [PubMed] [Google Scholar]
- Sapir S, Maimon T, Eviatar Z. Linguistic and nonlinguistic auditory processing of rapid vowel formant (F2) modulations in university students with and without developmental dyslexia. Brain Cogn. 2002;48(2–3):520–526. [PubMed] [Google Scholar]
- Seger CA, Desmond JE, Glover GH, Gabrieli JD. Functional magnetic resonance imaging evidence for right-hemisphere involvement in processing unusual semantic relationships. Neuropsychology. 2000;14(3):361–369. doi: 10.1037//0894-4105.14.3.361. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Szekely A, D'Amico S, Devescovi A, Federmeier K, Herron D, Iyer G, Jacobsen T, Arevalo AL, Vargha A, Bates E. Timed action and object naming. Cortex. 2005;41(1):7–25. doi: 10.1016/s0010-9452(08)70174-6. [DOI] [PubMed] [Google Scholar]
- Thomas MSC, Allport A. Language switching costs in bilingual visual word recognition. J Mem Lang. 2000;43(1):44–66. [Google Scholar]
- Van Hell JG, De Groot AMB. Conceptual representation in bilingual memory: Effects of concreteness and cognate status in word association. Bilingualism: Lang Cogn. 1998;1(3):193–211. [Google Scholar]
- Van Heuven WJB, Dijkstra A, Grainger J. Orthographic neighborhood effects in bilingual word recognition. J Mem Lang. 1998;39:458–483. [Google Scholar]
- Von Studnitz RE, Green DW. The cost of switching language in a semantic categorization task. Biling Lang Cogn. 2002;5(3):241–251. [Google Scholar]
- Wager TD, Jonides J, Reading S. Neuroimaging studies of shifting attention: A meta-analysis. NeuroImage. 2004;22(4):1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren RH, Abutalebi J, Cappa FS, Villringer A, Perani D. Early setting of grammatical processing in the bilingual brain. Neuron. 2003;37:159–170. doi: 10.1016/s0896-6273(02)01150-9. [DOI] [PubMed] [Google Scholar]
- Weber-Fox CM, Neville HJ. Functional neural subsystems are differentially affected by delays in second language immersion: ERP and behavioral evidence in bilinguals. In: Birdsong D, editor. Second language acquisition and the Critical Period Hypothesis. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. pp. 23–38. [Google Scholar]
- Winitz H. Grammaticality judgment as a function of explicit and implicit instruction in Spanish. Mod Lang J. 1996;80(1):32–46. [Google Scholar]


