Abstract
Natively disordered proteins are a growing class of anomalies to the structure–function paradigm. The natively disordered protein α-synuclein is the primary component of Lewy bodies, the cellular hallmark of Parkinson’s disease. We noticed a dramatic difference in dilute solution 1H-15N Heteronuclear Single Quantum Coherence (HSQC) spectra of wild-type α-synuclein and two disease-related mutants (A30P and A53T), with spectra collected at 35°C showing fewer cross-peaks than spectra acquired at 10°C. Here, we show the change to be the result of a reversible conformational exchange linked to an increase in hydrodynamic radius and secondary structure as the temperature is raised. Combined with analytical ultracentrifugation data showing α-synuclein to be monomeric at both temperatures, we conclude that the poor quality of the 1H-15N HSQC spectra obtained at 35°C is due to conformational fluctuations that occur on the proton chemical shift time scale. Using a truncated variant of α-synuclein, we show the conformational exchange occurs in the first 100 amino acids of the protein. Our data illustrate a key difference between globular and natively disordered proteins. The properties of globular proteins change little with solution conditions until they denature cooperatively, but the properties of natively disordered proteins can vary dramatically with solution conditions.
Keywords: 1H-15N heteronuclear single quantum coherence (HSQC), hydrodynamic radius, natively disordered proteins, Parkinson’s disease, pulsed-field gradient NMR, α-synuclein
Parkinson’s disease affects over one million North Americans, and occurs in ~3% of the population over the age of 65 (Lang and Lozano 1998). Symptoms of rigidity, tremor, and loss of motor function result primarily from the loss of dopaminergic neurons in the substantia nigra (Hirsch 1992). Histologically, the hallmark of Parkinson’s disease is the formation of intracellular protein aggregates called Lewy bodies (Galloway et al. 1992). While proteins such as tau, cytochrome c, and parkin are also found in Lewy bodies, the primary component is the protein, α-synuclein (Spillantini et al. 1997).
α-Synuclein belongs to an enigmatic class called natively disordered proteins, which lack stable tertiary structure in dilute solution. The stable tertiary structure of globular proteins is the scaffolding for their biological functions (Daughdrill et al. 2004). Chemical groups appended to these rigid scaffolds enact the specific and tight binding of ligands for signal transduction and transition states for catalysis. This protein structure–function paradigm is over 70 years old (Mirsky and Pauling 1936; Edsall 1995), but the recent realization that natively disordered proteins function without tertiary structure is adding new excitement to protein chemistry (Daughdrill et al. 2004).
α-Synuclein is a small cytosolic protein commonly found at the presynaptic terminals of dopaminergic neurons, and is implicated in neuronal plasticity and neurotransmitter release (Jakes et al. 1994; George et al. 1995; Wersinger et al. 2003). This 140-amino-acid (14.4 kDa) protein contains three main regions (Maroteaux et al. 1988). The N-terminal region (residues 1–60) contains six imperfect KTKEGV repeats and three disease-associated mutations A30P, E46K, and A53T (Polymeropoulos et al. 1997; Krüger et al. 1998; Zarranz et al. 2004). The middle region (residues 61–95), called the nonamyloid component (NAC), is hydrophobic and flexible. These two regions gain α-helical structure in the presence of synthetic lipid vesicles or sodium dodecyl sulfate (SDS) micelles (Chandra et al. 2003; Bussell and Eliezer 2004). The C-terminal region (residues 95–140) has an excess of negatively charged residues and does not gain stable structure on binding vesicles (Bussell and Eliezer 2004). Recently, the interaction of the C-terminal region with the N terminus and NAC region has been shown to slow aggregation in vitro (Murray et al. 2003; Dedmon et al. 2004; Hoyer et al. 2004; Bertoncini et al. 2005; Li et al. 2005).
α-Synuclein collapses in the presence of 1 M glucose (Morar et al. 2001), and its aggregation accelerates in vitro in stirred solutions crowded with proteins, polysaccharides, or polyethylene glycols (Shtilerman et al. 2002; Uversky et al. 2002; Goers et al. 2003). Additionally, α-synuclein gains secondary structure in the presence of trimethylamine-N-oxide (Uversky et al. 2001b). Lipid vesicles or SDS micelles increase the α-helical content of α-synuclein (Chandra et al. 2003; Bussell and Eliezer 2004; Bisaglia et al. 2005), but decrease its rate of aggregation (Zhu and Fink 2003). Recently, it was shown that the helical content α-synuclein depends on micelle size (Ulmer et al. 2005); lipid-vesicle bound α-synuclein forms one large α-helix comprising the first 100 residues (Eliezer et al. 2001), but the SDS micelle-bound species forms two shorter α-helices connected by a well-ordered 7-amino-acid linker (Ulmer et al. 2005). Here, we explore the effects of the simplest perturbant, temperature, on the conformation of α-synuclein.
Results
Full-length α-synuclein
Figure 1 shows three consecutive 17-h 1H-15N HSQC experiments (total time, 51 h) conducted on a single 1 mM α-synuclein sample. Seventeen hours was selected so the data can be directly compared to our in-cell NMR data (McNulty et al. 2006). The spectra were contoured identically. Figure 1A shows the spectrum acquired at 10°C between hours 0 and 17. Figure 1B shows the spectrum of the same sample at 35°C collected between hours 17 and 34. Figure 1C shows the third experiment conducted between hours 34 and 51 at 10°C. Data for the two variants are located in the supplemental material.
Figure 1.
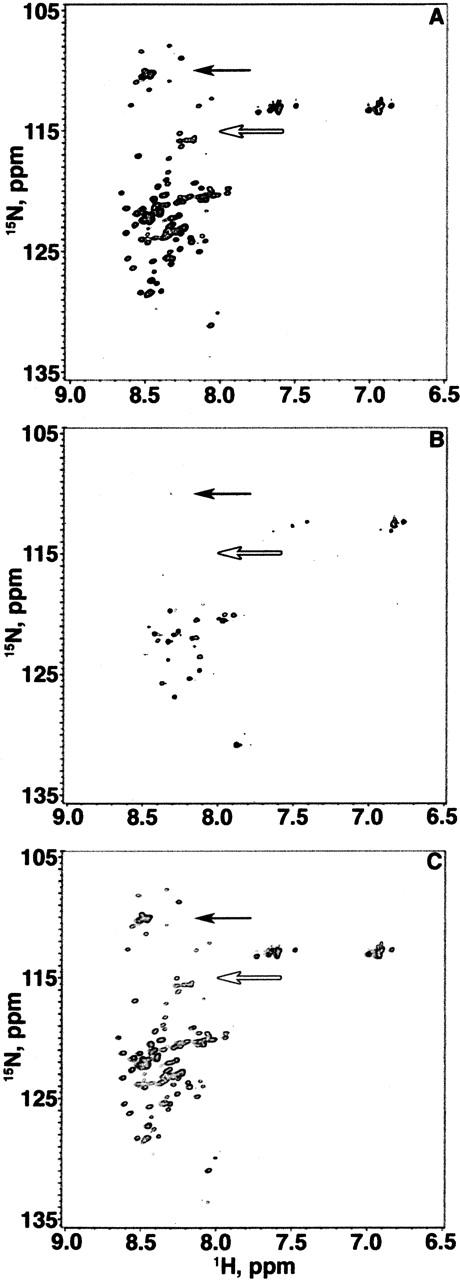
1H-15N HSQC spectra of three consecutive 17-h experiments (total time, 51 h) on a single 1 mM sample of α-synuclein (20 mM sodium phosphate at pH 7.4). The glycine region is indicated by a black arrow and the threonine region is indicated by a white arrow. The first spectrum (A) was acquired between hours 0 and 17 at 10°C. The second spectrum (B) was acquired between hours 17 and 34 at 35°C. The third spectrum (C) was acquired between hours 34 and 51 at 10°C.
The number of cross-peaks observed depends upon temperature. The 10°C spectrum (Fig. 1A) has ~72 backbone cross-peaks. The 35°C spectrum (Fig. 1B) only has ~32 backbone cross-peaks. The long acquisition time would have allowed us to detect even weak cross-peaks had they been present. Although many cross-peaks disappear at 35°C, the cross-peak heights, positions, and volumes of the second 10°C spectrum (Fig. 1C) are equivalent to those for the first 10°C spectrum (Fig. 1A). In summary, 1H-15N HSQC spectra of α-synuclein in dilute solution changes with temperature, and that change is reversible.
The narrow distribution of the cross-peaks in the 1H dimension in Figure 1A suggests α-synuclein is natively disordered or in a PII helix (Lam and Hsu 2003) at 10°C. The spectrum is unlike that of α-synuclein in the presence of SDS micelles, which shows more cross-peak dispersion in the 1H dimension and is indicative of its α-helical structure (Chandra et al. 2003).
The most striking differences between the 10°C and 35°C spectra occur in the regions containing glycine and threonine cross-peaks (8.2–8.6 ppm in the 1H dimension and 107–117 ppm in the 15N dimension). The spectrum shown in Figure 1A is so similar to a previously published assigned spectrum (Eliezer et al. 2001) that we were able to use the assignments directly. Comparing our data with published assignments, the cross-peaks that disappear at 35°C correspond to the first 100 residues of α-synuclein. As temperature increases from 10°C to 25°C (data not shown) to 35°C, cross-peak heights and volumes decrease for the N-terminal 100 residues, but remain constant for the C-terminal 40 residues (data not shown). The loss of N-terminal cross-peaks at 35°C could be caused by temperature induced conformational exchange of monomeric α-synuclein or by aggregation.
To assess the aggregation state, we performed sedimentation equilibrium experiments at the same α-synuclein concentration used for the NMR experiments (Fig. 2). The apparent molecular weight of α-synuclein at both temperatures is consistent with the presence of monomeric α-synuclein (14.4 kDa at 10°C and 13.3 kDa at 35°C). The lower molecular weight for the data in Figure 2B is the result of Donnan nonideality (Roark and Yphantis 1971; Krishnan et al. 2003). We conclude the loss of cross-peaks in the 1H-15N HSQC at 35°C reflects conformational exchange on the time scale of the 1H chemical shifts.
Figure 2.
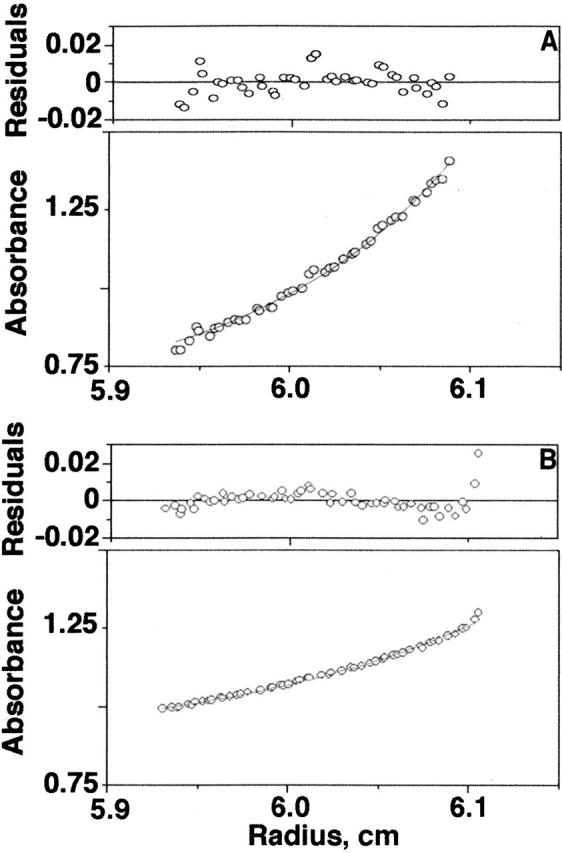
Equilibrium analytical ultracentrifugation data acquired at 293 nm for 1 mM wild-type α-synuclein at 10°C (A) and 35°C (B). Molecular weights of 14.4 kDa and 13.3 kDa were obtained at 10°C and 35°C, respectively.
Pulsed-field gradient studies show the hydrodynamic radius of α-synuclein increases 7.3 ± 1.1 Å, from 23.1 ± 0.6 Å to 31.4 ± 0.9 Å upon increasing the temperature from 10°C to 35°C. Importantly, the radius returns to 23 Å when the temperature is decreased again to 10°C, further confirming the reversibility of the conformational change.
Truncated α-synuclein
We repeated the 1H-15N HSQC experiments presented above with α-synuclein-100, a truncated variant comprising residues 1–100. Figure 3 shows three consecutive 17-h 1H-15N HSQC experiments (total time, 51 h) conducted on a single 500-μM α-synuclein-100 sample. The spectra were contoured identically. Figure 3A shows the spectrum acquired at 10°C. Figure 3B shows the spectrum of the same sample at 35°C. Figure 3C shows the third experiment conducted at 10°C. As in the full-length NMR experiment, the cross-peak heights, positions, and volumes are equivalent in the two 10°C spectra (Fig. 3A,C). Additionally, almost all of the cross-peaks present at 10°C disappear at 35°C, further supporting our conclusion that the conformational exchange occurs in the first 100 amino acids of the full-length protein. In summary, the similarities between the spectra show that the absence of the C-terminal 40 residues does not drastically affect the ensemble of conformations accessible to the first 100 residues.
Figure 3.
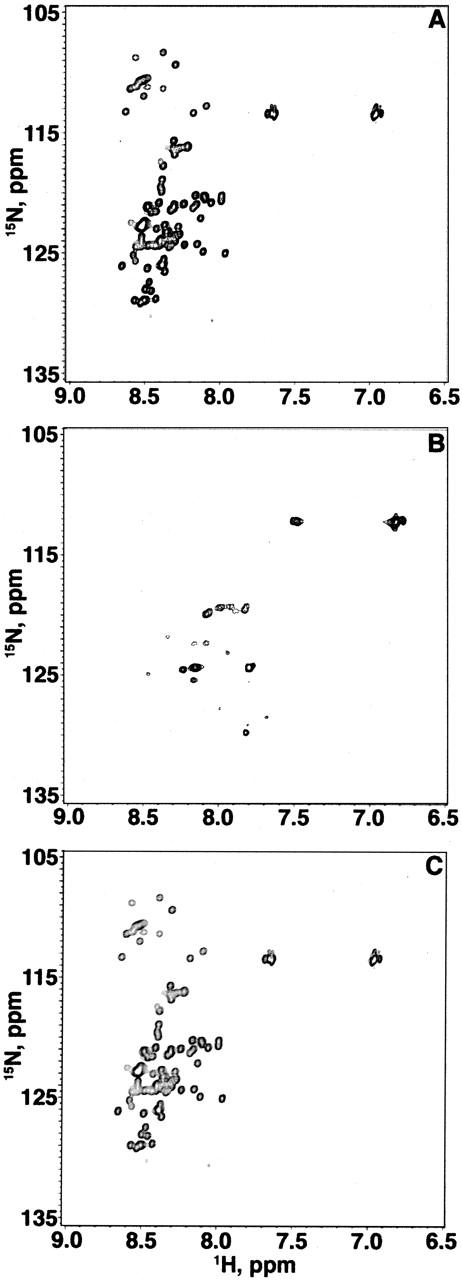
1H-15N HSQC spectra of three consecutive 17-h experiments (total time, 51 h) on a single 1-mM sample of the truncated variant α-synuclein-100 (20 mM sodium phosphate at pH 7.4). The first spectrum (A) was acquired between hours 0 and 17 at 10°C. The second spectrum (B) was acquired between hours 17 and 34 at 35°C. The third spectrum (C) was acquired between hours 34 and 51 at 10°C.
Discussion
We noticed a discrepancy among the published dilute solution 1H-15N HSQC NMR spectra of α-synuclein (Eliezer et al. 2001; Chandra etal. 2003; Bussell and Eliezer 2004). This discrepancy appeared to result from a difference in temperature at which the data were acquired. Specifically, spectra contain more cross-peaks at 10°C (Eliezer et al. 2001; Bussell and Eliezer 2004) than at 25°C (Chandra et al. 2003). To confirm this difference, we performed a three-stage (10°C, 35°C, 10°C) dilute solution experiment on a single sample of α-synuclein. This experiment (Fig. 1) reproduces the spectra of Bussell and Eliezer (Eliezer et al. 2001; Bussell and Eliezer 2004) and Chandra et al. (2003). The identity in cross-peak positions, and volumes for the two 10°C spectra in the 10–35–10°C series (Fig. 1A,C) prove the temperature-induced change is reversible.
What causes this reversible change in the HSQC spectra? Analytical ultracentrifugation data (Fig. 2) show the protein is a monomer at both temperatures, ruling out aggregation as the cause. Using circular dichroism spectropolarimetry (CD), Uversky et al. (2001a) showed that α-synuclein gains secondary structure, most likely α-helix, with increasing temperature. Repeating these experiments (data not shown), we observed the same increase in secondary structure upon increasing the temperature from 10°C to 35°C and that the increase is reversible. Therefore, the change coincides with a gain in secondary structure of monomeric α-synuclein.
Next, we identified the regions of α-synuclein associated with the change in HSQC spectrum at 35°C. As shown by comparing spectra for the full-length protein (Fig. 1) with spectra from truncated α-synuclein (Fig. 3), the cross-peaks that disappear at 35°C are from the N-terminal 100 residues. Spectra acquired at temperatures between 10°C and 35°C exhibit decreased cross-peak volumes for the 100 N-terminal residues, but cross-peak location remains constant (data not shown). Cross-peaks from the C-terminal 40 residues are present at all temperatures studied, and their volume and location are independent of temperature. From these observations, we conclude the region undergoing intermediate exchange at 35°C comprises the N-terminal 100 amino acids, while the last 40 residues remain highly dynamic at all temperatures studied.
This conclusion is also supported by the work of others. The HSQC spectrum obtained at 35°C (Fig. 1B) is similar to that of lipid vesicle associated α-synuclein, in terms of cross-peak number, cross-peak position, and the residues represented—all from the C-terminal 40 amino acids (Eliezer et al. 2001; Dedmon et al. 2002; Bussell and Eliezer 2004). Furthermore, the N-terminal 100 amino acid residues gain α-helical structure when α-synuclein binds to lipid vesicles (Eliezer et al. 2001) and SDS micelles (Chandra et al. 2003).
The disappearance of crosspeaks from the first 100 residues at 35°C could also be caused by increased amide–1H exchange, but several additional observations favor conformational exchange. First, estimates of amide 1H exchange rates with the computer program SPHERE (www.fccc.edu/research/labs/roder/sphere/) show no obvious pattern to suggest much greater exchange in the N-terminal portion of the protein. Second, it is unlikely that the increase in structure at 35°C noted above would lead to increased amide–1H exchange. The reverse is more likely. Third, resonances from the C-terminal end persist at the higher temperature. The C-terminal end of the protein remains unstructured under all conditions tested so far. Lack of structure maximizes amide–1H exchange, yet we see cross-peaks from this region of the protein. Taken together, these observations argue that the absence of cross-peaks is the result of conformational exchange on the chemical shift time-scale, not amide–1H exchange.
From our NMR, AUC, and CD studies, along with the work of others, we conclude that the N-terminal 100 amino acids of α-synuclein undergoes intermediate conformational exchange at 35°C. In other words, the difference in chemical shift between the various conformations within the ensemble at 35°C is approximately equal to the rate of exchange between the conformations. This exchange results in the disappearance of cross-peaks from the N-terminal region for spectra collected at 35°C.
We also characterized the ensembles present at 10°C and 35°C. At 10°C, the limited chemical shift dispersion of cross-peaks in the 1H dimension (Fig. 1A,C) shows that α-synuclein is disordered. The pulsed-field gradient NMR data show the disordered ensemble is collapsed at 10°C. In fact, the hydrodynamic radius at 10°C, 21.3 ± 0.6 Å, is only 2 Å larger than that expected for a native globular protein the size of α-synuclein (Wilkins et al. 1999).
The ensemble expands in hydrodynamic radius as the temperature is increased. The radius increases to 27 Å at 25°C (Morar et al. 2001) and to 31.4 ± 0.9 Å at 35°C. Since it is the N-terminal region that switches to intermediate exchange at 35°C, we conclude that this region plays a key role in the expansion.
In summary, we have shown the N-terminal two-thirds of the natively disordered protein α-synuclein expands and undergoes conformational exchange on the intermediate time scale as the temperature is raised from 10°C to 35°C. The collapsed ensemble present at 10°C is disordered, but the N-terminal two-thirds of the protein expands because it gains α-helical structure upon increasing the temperature to 35°C.
Such a small change in temperature would have an insignificant effect on most globular proteins. Our data highlight the idea that natively disordered proteins are exquisitely sensitive to their environment (Uversky 2003), and the biological significance of what we learn from studies of natively disordered proteins hinges on our choice of solution conditions. Finally, the similarities between the HSQC spectrum of the protein in living cells at 35°C (McNulty et al. 2006) and the spectrum in dilute solution at 10°C suggest that more biologically relevant information about α-synuclein might be obtained by studying the protein in vitro at 10°C.
Materials and methods
Purification of α-synuclein
Escherichia coli BL-21 (DE3-Gold) cells (Stratagene) were transformed with the pT7–7 plasmid containing the α-synuclein gene (supplied by Dr. Peter Lansbury) and spread on Luria broth agar plates with 100 μg/mL ampicillin (LBAmp). A single colony was added to 250 mL of LBAmp, and the culture was grown in a rotary shaker (225 rpm) for 16 h at 37°C.
Fifteen milliliters of the overnight culture were transferred to 250 mL of LBAmp and grown in a rotary shaker (225 rpm) at 37°C to an OD600 nm of 0.4–0.6. Cultures were then centrifuged (Sorvall RC-5B with a SS-34 rotor) at 3000 rpm for 30 min at 4°C. Cell pellets were resuspended in 250 mL of M9 minimal media (Serber et al. 2001) containing 100 μg/mL 15NH4Cl, induced with isopropyl β-D-thiogalactopyranoside to a final concentration of 1 mM and grown in a rotary shaker (225 rpm) at 37°C for 4 h. Cell cultures were centrifuged at 5000 rpm for 30 min at 4°C.
The cell pellet was resuspended in 25 mL of lysis buffer (10 mM Tris at pH 8.0, 1 mM ethylenediaminetetraacetic acid, 1 mM phenylmethanesulfonyl fluoride, 1 mM 1,4-dithio-L-threitol, with a few crystals of RNase, DNase, and lysozyme). The cell lysate was sonicated (Gallenkamp Soniprep 150) continuously for 10 min, boiled for 20 min, and then centrifuged at 15,000 rpm for 30 min at 4°C. The resulting supernatant was again boiled for 20 min and centrifuged at 15,000 rpm for 30 min at 4°C. The final supernatant was subjected to (NH4)2SO4 precipitation (361 g/L) and centrifuged at 15,000 rpm for 30 min at 4°C.
The pellet was resuspended in 20 mM sodium phosphate buffer (pH 7.4), and dialyzed against the same buffer for 5 h at 4°C. The protein was further purified by anion exchange chromatography (ÄKTA FPLC UPC-900, Q-10 column, Amersham Pharmacia Biotech) with a 1-M NaCl linear gradient at 4°C. The fractions containing α-synuclein were pooled and dialyzed against the original buffer for 24 h at 4°C. The dialyzed α-synuclein was concentrated to 1.1 mM in a YM-3 Centricon filter (Millipore, MWCO 3500) at 10°C. The purity of the protein was assessed by using SDS-PAGE with Coomassie brilliant blue staining. Purified α-synuclein was stored at −80°C.
Purification of α-synuclein truncated at residue 100 (α-synuclein-100)
Cells containing 15N-enriched α-synuclein-100 were produced using the methodology described for the full-length protein, but purification differed slightly. The boiling and centrifugation steps were performed only once; subsequent purification steps were performed on ice, and cation exchange chromatography (SP-16/10 column, Amersham Pharmacia Biotech) was performed at 4°C with a 1-M NaCl linear gradient.
Heteronuclear NMR
Samples for dilute solution spectra comprised a 90:10 mixture of pure 1.1 mM α-synuclein solution:D2O in a Shigemi tube. 1H-15N HSQC spectra were acquired at the UNC Biomolecular NMR facility on a Varian 600-MHz NMR spectrometer, equipped with a Triax triple resonance probe for (1H sweep width: 6999 Hz; 15N sweep width: 2100 Hz, 196 transients and 128 increments). Each spectrum required 17 h. For reversibility experiments, three sequential 1H-15N HSQC spectra (196 transients, 128 increments) were acquired on the same sample at 10°C, at 35°C and again at 10°C over the course of 52 h. Data were processed and visualized with NMRPipe and NMRDraw, respectively (Delaglio et al. 1995).
Pulsed-field gradient NMR
Spectra were acquired on the spectrometer described above. To replace exchangeable protons with deuterons, pure 1.1 mM α-synuclein samples were diluted threefold with 100% D2O and concentrated in an Amicon filter (MWCO: 3500) at 4°C. To ensure complete exchange, this process was performed three times more. The final concentration of α-synuclein was 1.1 mM. We used dioxane as the hydrodynamic radius standard (Wilkins et al. 1999), at a final concentration of 1 mM. Three sequential pulsed-field gradient experiments were conducted as previously described (Morar et al. 2001) on a single sample at 10°C, at 35°C and again at 10°C with a few alterations. The recycle delay was 2 s, there were 256 scans and each experiment took 6 h. Once the radius change was shown to be reversible, additional experiments were conducted at either 10°C or 35°C to increase the number of replicates used to obtain the mean and standard error. Data were processed and visualized with Varian’s VNMRJ (Version 1.1C).
Analytical ultracentrifugation (AUC)
Sedimentation equilibrium experiments were carried out at the UNC Macromolecular Interactions Facility on a Beckman XL-I ultracentrifuge. The samples were spun at 20,000 rpm for 20 h. Absorbance was monitored at 293 nm every 2 h. Separate experiments were conducted at 10°C and 35°C. α-Synuclein samples ranged in concentration from 0.2 mM to 1 mM. Equilibrium was assumed to have been reached when two consecutive scans gave identical absorption profiles. The absorbance offset was determined by the meniscus depletion method after a 6-h overspeed run at 45,000 rpm.
Site-directed mutagenesis
The A30P and A53T mutants were generated with a Stratagene site-directed mutagenesis kit and the following primers: A30P-FWD 5′-GGGTGTGGCAGAAGCACCAGCAAAGACAAAA GAGGG-3′; A30P-REV 5′-CCCTCTTTTGTCTTTCCTGGT GCTTCTGCCACACCC-3′; A53T-FWD 5′-GTGGTGCATG GTGTGACAACAGTGGCTGAGAAGACC-3′; A53T-REV 5′-GGTCTTCTCAGCCACTGTTGTCACACCATGCACC AC-3′.
The sequences of the mutants were verified at the UNC-CH Genome Analysis facility with the primer: 5′-GGGAGACCAC AACGGTTTCCCTCTAG-3′.
A truncated variant of α-synuclein, comprising amino acids 1–100 (α-synuclein-100), was generated by inserting a premature stop codon with a Stratagene site-directed mutagenesis kit by using the following primers: Syn100-FWD 5′-TTGTC AAAAAGGACCAGTAGGGCAAGAATGAAGAAGG-3′; Syn 100- REV 5′-CCTTCTTCATTCTTGCCCTACTGGTCCTT TTTGACAA-3′. The sequence of the truncated variant was verified as described above.
Electronic supplemental material
Figures 4 and 5 show the 1H-15N HSQC spectra of the A30P and A53T variants collected at 10°C, 35°C, and again at 10°C. The figures show how the two disease-related variants exhibit the same temperature-induced behavior as the wild-type protein.
Acknowledgments
We thank Wolfgang Hoyer for useful discussions regarding purification of the truncated variant; Peter Lansbury (Harvard Medical School) for the α-synuclein construct; and Elizabeth Pielak, Matthew Redinbo, and members of the Pielak lab for comments on the manuscript. The NSF (MCB0212939) and a G.A.A.N.N. Fellowship supported this work.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051867106.
Supplemental material: see www.proteinscience.org
References
- Bertoncini, C.W., Jung, Y.S., Fernandez, C.O., Hoyer, W., Greisinger, C., Jovin, T.M., and Zweckstetter, M. 2005. Release of long range tertiary interactions potentiates aggregation of natively unstructured α-synuclein. Proc. Natl. Acad. Sci. 102: 1430–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisaglia, M., Tessari, I., Pinato, L., Bellanda, M., Giraudo, S., Fasano, M., Bergantino, L., and Mammi, S. 2005. A topological model of the interaction between α-synuclein and sodium dodecyl sulfate micelles. Biochemistry 44: 329–339. [DOI] [PubMed] [Google Scholar]
- Bussell Jr., R. and Eliezer, D. 2004. Effects of Parkinson’s disease linked mutations on the structure of lipid associated α-synuclein. Biochemistry 43: 4810–4818. [DOI] [PubMed] [Google Scholar]
- Chandra, S., Chen, X., Rizo, J., Jahn, R., and Sudhof, T.C. 2003. A broken α-helix in folded α-synuclein. J. Biol. Chem. 278: 15313– 15318. [DOI] [PubMed] [Google Scholar]
- Daughdrill, G.W., Pielak, G.J., Uversky, V.N., Cortese, M.S., and Dunker, A.K. 2004. Natively disordered proteins. In The protein folding handbook (ed. T. Kiefhaber), pp. 275–357. Wiley-VCH, Weinheim.
- Dedmon, M.M., Patel, C.N., Young, G.B., and Pielak, G.J. 2002. FlgM gains structure in living cells. Proc. Natl. Acad. Sci. 99: 12681– 12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedmon, M.M., Lindorff-Larsen, K., Christodoulou, J., Vendruscolo, M., and Dobson, C.M. 2004. Mapping long-range interactions in α-synuclein using spin-label NMR and ensemble molecular dynamics simulations. J. Am. Chem. Soc. 127: 476–477. [DOI] [PubMed] [Google Scholar]
- Delaglio, F., Grzeseik, S., Vuister, G.W., Zhu, G., and Pfeifer, J. 1995. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6: 277–293. [DOI] [PubMed] [Google Scholar]
- Edsall, J.T. 1995. Hsien Wu and the first theory of protein denaturation (1931). Adv. Protein Chem. 46: 1–5. [PubMed] [Google Scholar]
- Eliezer, D., Kutluay, E., Bussell Jr., R., and Browne, G. 2001. Conformational properties of α-synuclein in its free and lipid associated states. J. Mol. Biol. 307: 1061–1073. [DOI] [PubMed] [Google Scholar]
- Galloway, P.G., Mulvihill, P., and Perry, G. 1992. Filaments of Lewy bodies contain insoluble cytoskeletal elements. Am. J. Pathol. 140: 809–822. [PMC free article] [PubMed] [Google Scholar]
- George, J.M., Jin, H., Woods, W.S., and Clayton, D.P. 1995. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron 15: 361–372. [DOI] [PubMed] [Google Scholar]
- Goers, J., Uversky, V.N., and Fink, A.L. 2003. Polycation-induced oligomerization and accelerated fibrillation of human α-synuclein in vitro. Protein Sci. 12: 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, E.C. 1992. Why are nigral catecholaminergic neurons more vulnerable than other cells in Parkinson’s disease? Ann. Neurol. 32: S88– SS93. [DOI] [PubMed] [Google Scholar]
- Hoyer, W., Cherny, D., Subramaniam, V., and Jovin, T.M. 2004. Impact of the acidic C-terminal region comprising amino acids 109–140 on α synuclein aggregation in vitro. Biochemistry 43: 16233–16242. [DOI] [PubMed] [Google Scholar]
- Jakes, R., Spillantini, M.G., and Goedert, M. 1994. Identification of two distinct synucleins from human brain. FEBS Lett. 345: 27–32. [DOI] [PubMed] [Google Scholar]
- Krishnan, S., Chi, E.Y., Wood, S.J., Kendrick, B.S., Li, C., Garzon-Rodriguez, W., Wypych, J., Randolph, T.W., Narhi, L.O., Biere, A.L., et al. 2003. Oxidative dimer formation is the critical rate-limiting step for Parkinson’s disease. Biochemistry 42: 829–837. [DOI] [PubMed] [Google Scholar]
- Krüger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Eppelen, J.T., Schols, L., and Reiss, O. 1998. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat. Genet. 18: 106–108. [DOI] [PubMed] [Google Scholar]
- Lam, S.K. and Hsu, V.L. 2003. NMR identification of left-handed polyproline type II helicies. Biopolymers 69: 270–281. [DOI] [PubMed] [Google Scholar]
- Lang, A.E. and Lozano, A.M. 1998. Parkinson’s disease. Second of two parts. N. Engl. J. Med. 339: 1130–1143. [DOI] [PubMed] [Google Scholar]
- Li, W., West, N., Colla, E., Pletnikova, O., Troncoso, J.C., Marsh, L., Dawson, T.M., Jakala, P., Hartmann, T., Price, D.L., et al. 2005. Aggregation promoting C-terminal truncation of α-synuclein is a normal cellular process and is enhanced by the familial Parkinson’s disease-linked mutations. Proc. Natl. Acad. Sci. 102: 2162–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux, L., Campanelli, J.T., and Scheller, R.H. 1988. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 8: 2804–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty, B.C., Young, G.B., and Pielak, G.J. 2006. Macromolecular crowding in the Escherichia coli periplasm maintains α-synuclein disorder. J. Mol. Biol. (in press). [DOI] [PubMed]
- Mirsky, A.E. and Pauling, L. 1936. On the structure of native, denatured and coagulated proteins. Proc. Nat. Acad. Sci. 22: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morar, A.S., Olteanu, A., Young, G.B., and Pielak, G.J. 2001. Solvent-induced collapse of α-synuclein and acid-denatured cytochrome c. Protein Sci. 10: 2195–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, I.V., Giasson, B.I., Quinn, S.M., Koppaka, V., Axelsen, P.H., Ischiropoulos, H., Trojanowski, J.Q., and Lee, V.M. 2003. Role of α-synuclein carboxy terminus on fibril formation in vitro. Biochemistry 42: 8530–8540. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos, M.H., Lavedan, C., Leroy, E., Ide, S.E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., et al. 1997. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276: 2045–2047. [DOI] [PubMed] [Google Scholar]
- Roark, D.E. and Yphantis, D.A. 1971. Equilibrium centrifugation of nonideal systems. The Donnan effect in self-associating systems. Biochemistry 10: 3241–3249. [DOI] [PubMed] [Google Scholar]
- Serber, Z., Ledwidge, R., Miller, S.M., and Dötsch, V. 2001. Evaluation of parameters critical to observing proteins inside living Escherichia coli by in-cell NMR spectroscopy. J. Am. Chem. Soc. 123: 8895– 8901. [DOI] [PubMed] [Google Scholar]
- Shtilerman, M.D., Ding, T.T., and Lansbury Jr., P.T. 2002. Molecular crowding accelerates fibrillization of α-synuclein: Could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry 41: 3855–3860. [DOI] [PubMed] [Google Scholar]
- Spillantini, M.G., Schmidt, M.L., Lee, V.M.Y., Trojanowski, J.Q., Jakes, R., and Goedert, M. 1997. α-Synuclein in Lewy bodies. Nature 388: 839–840. [DOI] [PubMed] [Google Scholar]
- Ulmer, T.S., Bax, A., Cole, N.B., and Nussbaum, R.L. 2005. Structure and dynamics of micelle-bound human α-synuclein. J. Biol. Chem. 280: 9595–9603. [DOI] [PubMed] [Google Scholar]
- Uversky, V.N. 2003. A protein-chameleon: Conformational plasticity of α-synuclein, a disordered protein involved in neurodegenerative disorders. J. Biomol. Struct. Dyn. 21: 211–234. [DOI] [PubMed] [Google Scholar]
- Uversky, V.N., Li, J., and Fink, A.L. 2001a. Evidence for a partially folded intermediate in α-synuclein fibril formation. J. Biol. Chem. 276: 10737– 10744. [DOI] [PubMed] [Google Scholar]
- ———. 2001b. Trimethylamine-N-oxide induced folding of α-synuclein. FEBS Lett. 509: 31–35. [DOI] [PubMed] [Google Scholar]
- Uversky, V.N., Cooper, E., Bower, K.S., Li, J., and Fink, A.L. 2002. Accelerated α-synuclein fibrillation in crowded milieu. FEBS Lett. 515: 99–103. [DOI] [PubMed] [Google Scholar]
- Wersinger, C., Prou, D., Vernier, P., Niznik, H.B., and Sidhu, A. 2003. Mutations in the lipid-binding domain of α-synuclein confer overlapping, yet distinct, functional properties in the regulation of dopamine transporter activity. Mol. Cell. Neurosci. 24: 91– 105. [DOI] [PubMed] [Google Scholar]
- Wilkins, D.K., Grimshaw, S.B., Receveur, V., Dobson, C.M., Jones, J.A., and Smith, L.J. 1999. Hydrodynamic radii of native and denatured proteins measured by pulsed field gradient NMR techniques. Biochemistry 38: 16424–16431. [DOI] [PubMed] [Google Scholar]
- Zarranz, J.J., Alegre, J., Gomez-Esteban, J.C., Lezcano, E., Ros, R., Ampuero, I., Vidal, L., Hoenicka, J., Rodriguez, O., Atares, B., et al. 2004. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55: 164–173. [DOI] [PubMed] [Google Scholar]
- Zhu, M. and Fink, A.L. 2003. Lipid binding inhibits α-synuclein fibril formation. J. Biol. Chem. 278: 16873–16877. [DOI] [PubMed] [Google Scholar]


