Abstract
In almost all animal species, immature oocytes are arrested naturally in the first meiotic prophase, with a large nucleus called the germinal vesicle. A number of previous studies showed that both activation of maturation/M phase-promoting factor (MPF) (assayed by semiquantitative cytological methods) and some other maturational events occur essentially normally in enucleated oocytes from many amphibian species and mice. Hence, for nearly three decades, it has generally been believed that nuclear material is dispensable for MPF activation and the meiotic cell cycle in vertebrate oocytes. Here, we have challenged this view by examining the histone H1 kinase activities and the molecular forms of MPF in experimentally manipulated Xenopus oocytes. We show that oocytes injected with nuclear material undergo much more rapid MPF activation and maturation than uninjected control oocytes. Conversely, enucleated oocytes, unlike nucleated counterparts, undergo only weak MPF activation in meiosis I and no detectable MPF reactivation in meiosis II, the latter accompanying inhibitory tyrosine phosphorylation of cdc2 kinase, the catalytic subunit of MPF. These results argue strongly that nuclear material is indispensable for the meiotic cell cycle, particularly MPF reactivation (or cdc2 tyrosine dephosphorylation) on entry into meiosis II, in Xenopus oocytes. The classical and general view may thus need reconsideration.
Meiotic maturation of oocytes, or oocyte maturation, is a final step of oogenesis and is a prerequisite process for the immature oocyte to become fertilizable (1). In almost all species, immature oocytes are arrested naturally in the first meiotic prophase (prophase I) or the late G2 phase, and resume meiosis usually in response to hormonal stimuli (2, 3). The maturation stimuli induce universally activation of maturation/M phase-promoting factor (MPF), a key G2/M regulator in eukaryotic cells that consists of cdc2 kinase and cyclin B (4, 5). The activated MPF then induces germinal vesicle breakdown (GVBD), chromosome condensation, and spindle formation in meiosis I (1); MPF is then transiently inactivated and reactivated again to induce meiosis II (6, 7). In vertebrates, oocyte meiosis is arrested again at the second meiotic metaphase (metaphase II) before fertilization, by a cytoplasmic factor called cytostatic factor (8), whose essential component is known to be Mos (9).
The germinal vesicle, a huge nucleus in the immature oocyte, undergoes GVBD soon after MPF activation, and its material (or nucleoplasm) mixes with the cytoplasm (or ooplasm) of maturing oocytes (10, 11). Because the meiosis I/meiosis II transition or “interkinesis” that follows GVBD accompanies no nucleus reformation (1, 12), germinal vesicle (or nuclear) material remains distributed in the cytoplasm throughout the course of post-GVBD maturation (10). The distributed nuclear material is required for sperm chromatin decondensation and cleavage in fertilized eggs (13, 14); the nuclear factor required for the chromatin decondensation is identified as nucleoplasmin (15). Besides nucleoplasmin, the germinal vesicle contains a number of proteins, such as histones and DNA polymerases, that are used immediately after fertilization (16).
In starfish oocytes, nuclear material is required for the activation of MPF upon maturation (17, 18). In vertebrate species, however, nuclear material has long been thought to be dispensable for MPF activation and maturation in oocytes. Thus, a number of classical studies showed that enucleated amphibian oocytes and anucleate mouse oocyte fragments undergo MPF activation soon after release from prophase I arrest, as semiquantitatively evidenced by their cytoplasmic activities to induce GVBD in recipient immature oocytes (8, 19–23) or to induce chromosome condensation in anucleate fragments-fused blastomeres (24, 25). Moreover, enucleated amphibian oocytes well after prophase I release are long known to show both surface changes (20, 22, 23) and a cortical response (to activation stimuli) (8, 26, 27) that are normally indication of the completion of meiotic maturation in amphibian oocytes (1). Hence, for nearly three decades, it has generally been believed that nuclear material is dispensable for MPF activation and the meiotic cell cycle in vertebrate oocytes, particularly amphibian oocytes (see refs. 1, 5, 28–30). Despite the numerous cytological studies (8, 13, 14, 19–27), however, the progression of the meiotic cell cycle in enucleated vertebrate oocytes has not so far been examined at all at the molecular level.
In the present study, we have reevaluated the role of nuclear material in the meiotic cell cycle of the amphibian Xenopus oocytes, by examining the histone H1 kinase activities and the molecular forms of MPF in experimentally manipulated oocytes. To our surprise, our results strongly indicate that the germinal vesicle or the nucleus contains a factor or factors that play important roles in the meiotic cell cycle, particularly MPF reactivation or entry into meiosis II, in Xenopus oocytes. Based on the present results, we raise a view on the meiotic control in vertebrate oocytes, claiming reconsideration of the above-mentioned general view.
MATERIALS AND METHODS
Preparation and Culture of Oocytes.
Stage VI Xenopus oocytes were defolliculated either manually or by collagenase treatment, microinjected, treated with progesterone (to induce maturation), and cultured in modified Barth’s solution at 20–22°C, as described (7, 9).
Nucleocytoplasmic Mixing.
The artificial nucleocytoplasmic mixing in the oocyte was performed in two ways. (i) A glass needle pipette was inserted into the germinal vesicle of the oocyte, and clear sap (or nuclear material) was sucked up into the pipette and then injected back into the oocyte, which caused nuclear membrane disruption and the resulting nucleocytoplasmic mixing; this physical mixing was repeated ≈10 times, with the needle pipette being kept inserted into the oocyte, until the clear sap was no longer observable. As a control of this experiment, a glass needle pipette was inserted into the vegetal half of the oocyte, and the cytoplasm alone was mixed. (ii) Nuclear material from one oocyte was transferred to the cytoplasm of another oocyte as follows. A small puncture was made at the animal pole of the donor oocyte using a fine syringe needle (31); then, nearly whole nuclear material was sucked up (with a glass needle pipette) from the emerging nucleus and was immediately injected into (and mixed a few times with) the cytoplasm of another oocyte (this injection was made at the equator of the recipient oocyte, to avoid injuring the nucleus of the recipient). As a control of this nuclear material transfer, an equivalent volume of cytoplasm taken from one oocyte was transferred to the cytoplasm of another oocyte. Oocytes that did not tolerate these manipulations were discarded.
Enucleation.
Oocytes were enucleated in half-strength modified Barth’s solution and healed for 20 min in a healing buffer, exactly as described (30, 31). Only completely healed oocytes were transferred to normal-strength modified Barth’s solution, and, 2 hr after the enucleation, they were treated with progesterone. Enucleated oocytes that showed any sign of deterioration during culture or maturation were all discarded.
In some cases, nuclear material alone was removed from immature oocytes as follows. A smaller puncture (than that for enucleation) was first made on the animal pole, and then an even smaller puncture was made, using a glass needle, on the surface membrane of the nucleus, a portion of which was just emerging from the animal pole. Nuclear material alone was then immediately squeezed out by using forceps, and the oocyte (which retained the nuclear membrane and the material surrounding it) was healed as above. As a control of enucleation or nuclear material removal, nucleated oocytes were punctured at their equators and then healed.
When both the nucleus and the enucleated oocyte (or cytoplasm) from maturing oocytes were to be analyzed directly without healing (see Fig. 1), enucleation was performed in Ca2+-free modified Barth’s solution to avoid inactivation of MPF, which would otherwise occur by the external Ca2+. This procedure was not necessary, however, for enucleation of immature oocytes that were to be analyzed after healing and cultivation.
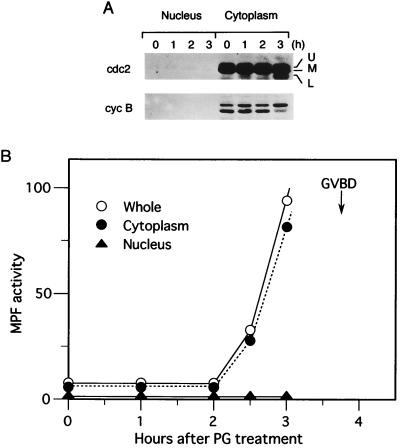
Figure 1.
Cytoplasmic occurrence of MPF activation before GVBD. (A) Oocytes were treated with progesterone and, at the indicated times, were manually separated into the nucleus and the cytoplasm. The isolated nucleus and cytoplasm, each equivalent to one oocyte, were subjected to Western blot analysis for either cdc2 kinase (cdc2) or cyclin B2 (cyc B) (see the legend of Fig. 4, for explanation of the multiple bands of cdc2 and cyclin B2). 50% of the oocytes underwent GVBD 3 hr and 45 min after the progesterone treatment. (B) Either the whole oocyte (○), cytoplasm (•), or nucleus (▴), each obtained as above, was subjected to histone H1 kinase assays of MPF. On the ordinate, MPF activity (or H1 kinase activity) is represented in an arbitrary unit.
Measurements of MPF Activity.
MPF activity in oocytes was assayed in two ways, either cytologically or biochemically. In the cytological way, oocytes to be tested were homogenized in an extraction buffer (EB) (1 μl per oocyte) on ice (6) and briefly centrifuged (the EB buffer contained: 80 mM β-glycerophosphate/20 mM EGTA/15 mM MgCl2/0.1 mM DTT/20 μM leupeptin/10 μM pepstatin/2 mM PMSF/10 μg/ml aprotinin/1 mM Na3VO4/1 mM NaF, pH 7.5). The supernatant (or cytoplasmic extracts) obtained was diluted or not with EB buffer and then injected into about twenty immature oocytes (at 40 nl per oocyte); 4 hr after the injection, the recipient oocytes were scored for the percentage GVBD, which represents classical MPF activity of the donor oocyte (6, 8). In the biochemical way for assaying MPF activity, cytoplasmic extracts as described above were subjected to in vitro histone H1 kinase assays in the presence of protein kinase A inhibitor PKI, essentially as described (7). Under these conditions, most (>85%) of the H1 kinase activities in the extracts were due to the activities of cdc2/cyclin B complexes (data not shown).
Western Blot Analysis.
Western blot analysis of oocyte extracts was performed as described (7), by using either anti-PSTAIRE antibody (for cdc2 kinase; ref. 32) or anti-Xenopus cyclin B2 antibody (raised against sheep; a gift from J. Maller, Howard Hughes Medical Institute, University of Colorado School of Medicine, Denver, CO). For the better separation of a Tyr-15 (and Thr-14)-phosphorylated form of cdc2 from other forms (see Fig. 4), samples were run on a 12% polyacrylamide gel that was made in 0.1% SDS/0.375 M Tris buffered to pH 9.0, a pH value slightly higher than the usual one (8.8).
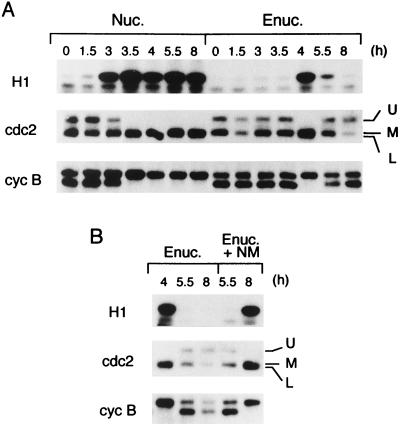
Figure 4.
Molecular forms of cdc2 kinase and cyclin B2 in enucleated oocytes. (A) Either control nucleated oocytes (Nuc.) or enucleated oocytes (Enuc.) were treated with progesterone, and at the indicated times, their extracts were subjected to either H1 kinase assays of MPF (H1) or Western blot analysis for cdc2 kinase (cdc2) or cyclin B2 (cyc B). In cdc2, the upper band (U) corresponds to a Tyr-15 (and Thr-14)-phosphorylated form, the lower band (L) to a Tyr-15 (and Thr-14)-dephosphorylated form, and the middle band (M) to either a Thr-14-phosphorylated or a Tyr-15-phosphorylated form; the former two are also phosphorylated on Thr-161 and are associated with cyclin B (7, 33, 37, 38; unpublished data). [The relative mobilities of the upper, middle, and lower bands in this figure are different from those of previously published ones (33, 37, 38) and those in Fig. 1A, because of the special conditions of SDS/PAGE employed here; see Materials and Methods.] In cyclin B2, the upper band and the lower band correspond to a phosphorylated form and an unphosphorylated form, respectively; the upper band is associated with the lower form of cdc2, forming active MPF (35–37). (B) Enucleated oocytes 4 hr after progesterone treatment were injected (Enuc. + NM) or not (Enuc.) with nuclear material (NM), and at the indicated times (after progesterone treatment), their extracts were processed as in (A). In both A and B, 50% GVBD in control nucleated oocytes occurred 3.5 hr after progesterone treatment.
RESULTS
Cytoplasmic Occurrence of MPF Activation Before GVBD.
In Xenopus, progesterone induces meiotic maturation of prophase I (or G2)-arrested immature oocytes by activating MPF, and the activated MPF soon induces breakdown (GVBD) of the large nucleus or germinal vesicle. By classical, semiquantitative cytoplasmic transfer experiments (8, 19, 20), the initial MPF activation prior to GVBD has been suggested to occur principally in the cytoplasm of progesterone-treated oocytes. First, we reexamined this notion in biochemical ways, by using a manually isolated nucleus and the cytoplasm of progesterone-treated Xenopus oocytes. Western blot analyses of cdc2 kinase and cyclin B2, two MPF components, revealed that almost all (>95%) of them were localized in the cytoplasm of maturing oocytes prior to GVBD (the oocytes underwent GVBD about 3.5 hr after progesterone treatment) (Fig. 1A). Consistent with these results, MPF activity, measured by histone H1 kinase assays, increased exclusively in the cytoplasm before GVBD (Fig. 1B). Thus, these results confirm biochemically the classical notion that MPF activation prior to GVBD is essentially cytoplasmic (8, 19, 20). [Although activated MPF may enter the nucleus just before GVBD, as previously shown in starfish oocytes (33), this likely possibility could not be tested because the nucleus just before GVBD was too fragile to isolate.]
Promotion of Cytoplasmic MPF Activation by the Nucleocytoplasmic Mixing.
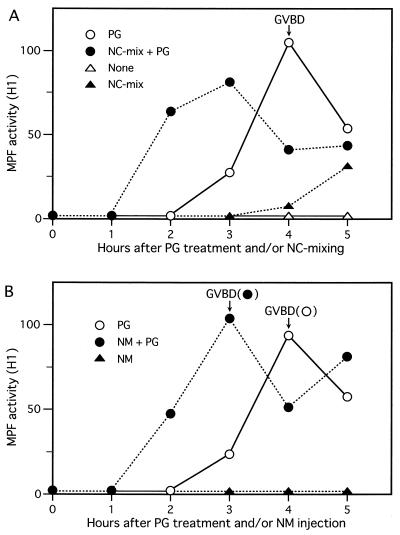
We then addressed the question of whether nuclear material has any activity that can affect cytoplasmic MPF activation in progesterone-treated oocytes. For this, first we physically mixed (within the oocyte) nuclear material and the cytoplasm by using a glass needle pipette (see Materials and Methods) and, after progesterone treatment, measured MPF activity in the oocytes by H1 kinase assays. In these oocytes, MPF activation occurred much earlier (by ≈1.5 h) than that in control oocytes (in which the cytoplasm alone was mixed), which occurred ≈3 hr after progesterone treatment. In addition and interestingly, even without progesterone treatment MPF activation occurred, albeit very slowly, after the artificial nucleocytoplasmic mixing (Fig. 2A). These results were very reproducible (in five independent experiments), if the mechanical nucleocytoplasmic mixing was extensively done. To confirm these interesting results, next we sucked up nuclear material (or contents) from an immature oocyte by using a glass needle pipette, and injected it into the cytoplasm of another oocyte that retained an intact nucleus (see Materials and Methods). After progesterone treatment, the injected oocytes underwent both MPF activation and GVBD about 1 hr faster than control oocytes (into which cytoplasm alone was injected) (Fig. 2B). (In this case, without progesterone treatment, MPF activation did not occur appreciably at least until 5 hr after the injection, presumably because of reentry of part of the injected nuclear material into the intact nucleus.) These results were also very reproducible (in six independent experiments), given the successful transfer of whole nuclear material. Thus, very intriguingly, the nucleus or the germinal vesicle does seem to contain a factor or factors that, upon mixing with the cytoplasm, can promote cytoplasmic MPF activation and maturation in progesterone-treated Xenopus oocytes (although, even without progesterone treatment, the nuclear factor seems to have a weak activity to induce MPF activation).
Figure 2.
Promotion of cytoplasmic MPF activation by the artificial nucleocytoplasmic mixing. (A) Oocytes were subjected to the physical nucleocytoplasmic (NC) mixing (see Materials and Methods), and subsequently treated (•) or not (▴) with progesterone (PG); as controls, oocytes with the cytoplasmic mixing alone were treated (○) or not (▵) with progesterone. At the indicated times, three oocytes each were sampled and an aliquot of the oocyte extracts (equivalent to 0.4 oocyte) was subjected to an H1 kinase assay of MPF. The time of 50% GVBD in control progesterone-treated oocytes is indicated. MPF activity is represented in an arbitrary unit. (B) Oocytes that received injection of nuclear material (NM) from another oocyte (see Materials and Methods) were treated (•) or not (▴) with progesterone; as a control, oocytes injected with the cytoplasm were treated with progesterone (○). They were then processed as described above. The times of 50% GVBD for the respective groups of oocytes (•, ○) are also indicated.
Requirement for Nuclear Material in MPF (Re)activation During Maturation.
If the above results are physiologically relevant to MPF activation during normal maturation, then they would contradict, at least in part, the classical and general view that nuclear material is dispensable for MPF activation and the meiotic cell cycle in (vertebrate) oocytes (see refs. 1, 5, 28–30). We therefore challenged this view by using enucleated Xenopus oocytes, as previously performed (8, 19, 22, 23) (as a control of this experiment, we used nucleated oocytes that received a small puncture at their equators; see Materials and Methods). First, we measured MPF activity in progesterone-treated, enucleated oocytes by classical cytoplasmic transfer (6, 8), i.e., by assaying the activity of cytoplasmic extracts (from enucleated oocytes) to induce GVBD in recipient immature oocytes. When measured around the time of GVBD (or within 30 min after GVBD in control nucleated oocytes), MPF activity in enucleated oocytes, like that in control oocytes, was strong enough to induce nearly 100% GVBD in recipient oocytes, as reported (8, 19), but upon a 3-fold dilution of the extracts, it turned out to be significantly lower than that in control oocytes (Fig. 3A). On the other hand, very surprisingly, when measured well after meiosis I (or 4 hr after GVBD), MPF activity was not detected at all in enucleated oocytes (even with undiluted extracts), contrasting sharply with the very high MPF activity in control nucleated oocytes.
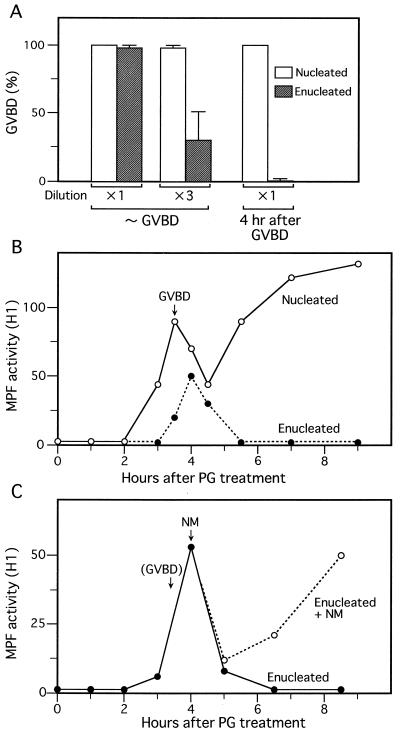
Figure 3.
Requirement for nuclear material in MPF activation during meiotic maturation of oocytes. (A) Either control nucleated oocytes (□) or enucleated oocytes (▨) were treated with progesterone; their cytoplasmic extracts, prepared either within 30 min after GVBD or at 4 hr after GVBD, were undiluted or 3-fold diluted and then injected into 20 immature oocytes to assay MPF activities (represented by % GVBD in the recipient oocytes). (B) Control nucleated oocytes (○) or enucleated oocytes (•) were treated with progesterone (PG), and their extracts were subjected to H1 kinase assays of MPF at the indicated times. The time of 50% GVBD in control nucleated oocytes is indicated. MPF activity (or H1 kinase activity) is represented in an arbitrary unit. (C) Enucleated oocytes (•) and those enucleated oocytes (○) that received injection of nuclear material (NM) at the indicated time were subjected to H1 kinase assays of MPF. The time of 50% GVBD in control nucleated oocytes is indicated in parentheses.
Measurement of MPF activity by cytoplasmic transfer is only semiquantitative because the GVBD response of the recipient oocyte occurs in an all-or-none fashion, depending on dilutions of the transferred cytoplasm (6, 30). Therefore, we next measured MPF activity in the oocytes by much more quantitative H1 kinase assays, throughout the course of maturation. In enucleated oocytes, MPF activity increased after the progesterone treatment, but significantly more weakly (by 30–50%) and slightly more slowly (by ≈30 min) than that in control nucleated oocytes (Fig. 3B). The MPF activity in enucleated oocytes then decreased sharply, like that in control oocytes, most certainly reflecting the completion of meiosis I (6). After this decrease, however, MPF activity in enucleated oocytes did not reincrease at all at least for 6–7 hr, whereas that in control nucleated oocytes reincreased sharply and remained high thereafter, representing entry into meiosis II and arrest at metaphase II (7). The lack of MPF reactivation after meiosis I (as well as the weak MPF activation in meiosis I) in enucleated oocytes was very reproducible (in 16 out of 18 independent experiments, the remaining two showing only partial MPF reactivation after meiosis I), and was consistent with the result with cytoplasmic transfer as described above. We obtained essentially similar results with oocytes from which nuclear material alone, instead of the whole nucleus, had been removed (not shown but see Materials and Methods), strongly supporting the idea that removal of nuclear material, but not material surrounding the nucleus, was responsible for the lack of MPF reactivation in enucleated oocytes. To confirm this idea, we injected enucleated oocytes with nuclear material when their MPF activity was peaking in meiosis I, and later assayed H1 kinase activity of MPF. Results revealed that MPF activity was restored considerably after its transient decrease, or after meiosis I (Fig. 3C). Thus, it seems almost certain that the failure of enucleated oocytes to reactivate MPF after meiosis I was due largely to the lack of nuclear material. Taken together, the present results argue strongly that nuclear material not only contributes significantly to the rapid and full MPF activation in meiosis I, but also is essential for MPF reactivation or entry into meiosis II in Xenopus oocytes.
Requirement for Nuclear Material in Cdc2 Tyr-15 Dephosphorylation on Entry into Meiosis II.
To assess the role of nuclear material in MPF reactivation or entry into meiosis II, we examined the molecular forms of cdc2 kinase and cyclin B2, two MPF components, in enucleated oocytes. In immature Xenopus oocytes, cdc2 is phosphorylated on Tyr-15 (and Thr-14) and is associated mainly with an unphosphorylated form of cyclin B2, forming inactive MPF called pre-MPF (34–36). Upon MPF activation in meiosis I, cdc2 and cyclin B2 in enucleated oocytes—like those in control nucleated oocytes—underwent characteristic electrophoretic-mobility shifts (Fig. 4A), most certainly due to Tyr-15 (and Thr-14) dephosphorylation and serine phosphorylation, respectively (see refs. 7, 33, 36, 37; see also the legend to Fig. 4A). After meiosis I, however, their phosphorylation/dephosphorylation states in enucleated oocytes were both reversed to those before MPF activation or in immature oocytes, whereas those in control nucleated oocytes remained the same. Thus somewhat surprisingly (see refs. 5 and 36), even in enucleated oocytes after meiosis I (in which MPF activity was lacking), cyclin B2 was present at considerable levels, and MPF appeared to exist as a complex of cdc2 and cyclin B2, but with inhibitory Tyr-15 (and Thr-14) phosphorylation of cdc2, just as in immature oocytes [the upper band in Fig. 4A is the Tyr-15 (and Thr-14)-phosphorylated, inactive form of cdc2; for details, see the legend]. To test whether inhibitory Tyr-15 phosphorylation in enucleated oocytes was due to the lack of nuclear material, we injected nuclear material into enucleated oocytes, as we did previously (see Fig. 3C). Results revealed that these oocytes restored cdc2 Tyr-15 dephosphorylation, as well as cyclin B2 phosphorylation and MPF reactivation, after meiosis I (Fig. 4B). Thus, the present results strongly suggest that nuclear material is required for cdc2 Tyr-15 (and Thr-14) dephosphorylation (and hence MPF reactivation) on entry into meiosis II. In a preliminary experiment, we observed that cdc25, a specific phosphatase of cdc2 Thr-14/Tyr-15 (5), is mostly cytoplasmic in immature oocytes (data not shown), suggesting that cdc25 cannot be the nuclear factor required for cdc2 Tyr-15 (and Thr-14) dephosphorylation.
DISCUSSION
Reevaluation of the General View.
Our results differ considerably from the longstanding and general view that nuclear material is dispensable for MPF activation and the meiotic cell cycle in vertebrate oocytes (see refs. 1, 5, 28–30). Originally, this general view was built on a number of classical, cytological observations that both enucleated amphibian oocytes and anucleate mouse oocyte fragments undergo MPF activation shortly after release from prophase I arrest (8, 19–25), and that “mature” enucleated oocytes (from many amphibian species) show surface changes and a cortical response (to activation stimuli) that are typical of completely mature oocytes arrested at metaphase II (20, 22, 23, 26, 27). Looking into the classical studies (on amphibian oocytes), however, it seems clear that most of the previous workers examined only the initial MPF activation in meiosis I, but not MPF reactivation in meiosis II, in enucleated oocytes, probably because they were unaware of the oscillation in MPF activity during maturation, which was found only in 1984 (6); instead, they took the cortical changes (in mature enucleated oocytes) as indication of the completion of “meiotic” maturation (see refs. 8, 19, 20, 22, 23, 26, 27). As far as the initial MPF activation or entry into meiosis I is concerned, our results agree basically to the view, although a significant nuclear contribution to the initial MPF activation seems to be obvious (Fig. 3 A and B) (see also refs. 22, 23). However, as for MPF reactivation or entry into meiosis II, our results clearly contradict the view: although we certainly observed a cortical response to activation stimuli of enucleated oocytes after meiosis I (not shown), we were unable to detect any MPF activity in such oocytes (Fig. 3). [Actually, we observed significant levels of MPF activity in enucleated oocytes very long (>14 hr) after meiosis I (not shown), as reported (30); however, this is most likely to be due to a (slow) resynthesis of nuclear material that was removed by enucleation (30, 39).] Thus, it seems very likely that while nuclear material is dispensable for surface changes such as cortical maturity, it is indispensable for MPF reactivation or entry into meiosis II, in (amphibian) oocytes. In this regard, we note that, unlike normal counterparts arrested at metaphase II, mature enucleated oocytes from both amphibians and mice cannot stably condense exogenously introduced chromatin (25, 40), which probably reflects a lack of (stable) MPF in such oocytes (25).
In starfish oocytes, MPF activation cannot be detected in enucleated oocytes, when assayed by cytoplasmic transfer (17, 18); however, the H1 kinase activity of MPF oscillates essentially normally in enucleated oocytes (41). This apparent discrepancy may question the integrity of identity between the cytologically defined and the biochemically defined MPFs (42). In Xenopus oocytes, however, both the transferable activity and the H1 kinase activity of MPF were equally affected by enucleation (Fig. 3 A and B), enabling rather straightforward conclusion of our results.
Importance of GVBD for Entry into Meiosis II.
Our nucleocytoplasmic mixing (Fig. 2) and enucleation experiments (Fig. 3) disclose that the germinal vesicle contains a factor or factors that play important roles in MPF activation in Xenopus oocytes. (i) The nuclear factor(s) seems to contribute significantly to the initial MPF activation in meiosis I (Fig. 3A and B). As this initial MPF activation is essentially cytoplasmic and precedes GVBD (Fig. 1), at least part of the nuclear factor must be distributed to the cytoplasm even before (complete) GVBD, perhaps via the progesterone signaling pathway (Fig. 5). (Indeed, part of several nuclear proteins has been shown to be distributed to the cytoplasm well before the completion of GVBD; refs. 45, 46). Thus, it is tempting to speculate that in the immature oocyte, sequestration of the nuclear factor from the cytoplasm may have a role to prevent premature MPF activation, which would otherwise occur even without progesterone treatment (as shown in Fig. 2A).
Figure 5.
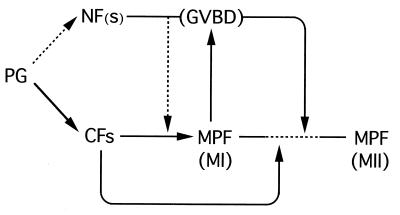
Model of the role for the nuclear factor(s) in the meiotic cell cycle of Xenopus oocytes. Progesterone (PG) induces activation or inactivation of many cytoplasmic factors (CFs), such as cdc25 and Mos (5, 43, 44), which alone can (in principle) induce cytoplasmic MPF activation in meiosis I (MI); normally, however, this cytoplasmic MPF activation seems to be promoted by some nuclear factor(s) (NF), at least part of which would be distributed to the cytoplasm even before GVBD, presumably via the progesterone signaling pathway. The activated MPF then induces GVBD, upon which all of the nuclear factors should be distributed to the cytoplasm and probably functions, in an absolutely required manner, for MPF reactivation or entry into meiosis II (MII), presumably by acting on some cytoplasmic factor(s). The mechanisms of the MI/MII transition are largely unknown (5–7, 47) as indicated by the dashed line, but GVBD and the resulting cytoplasmic distribution of NF now seem to be prerequisite to the transition. In the figure, solid arrows denote either established or very likely pathways, while dashed arrows show likely or possible pathways.
(ii) More importantly, some nuclear factor(s)—presumably the same factor as the above—apparently functions, but in an absolutely-required manner, for MPF reactivation and entry into meiosis II. As readily inferred from the restoration experiments (Fig. 3C), its function is probably ensured only via the complete breakdown of the germinal vesicle, upon which all of the nuclear factor should be distributed to the cytoplasm and interact with some cytoplasmic factor(s), to drive the oocyte (which underwent GVBD) to enter meiosis II (Fig. 5). These considerations are particularly important because the mechanisms of entry into meiosis II in oocytes have long been elusive (5–7, 47), and because the importance of GVBD (and hence of nuclear material) in meiotic maturation has long been neglected or, at the best, underestimated (1, 28, 29). It seems worth emphasizing, therefore, that GVBD, which invariably occurs shortly before meiosis II, is not simply a manifestation of maturation but is a prerequisite event for the oocyte to enter meiosis II (Fig. 5).
Involvement of the Nuclear Factor(s) in Cdc2 Tyr-15 Dephosphorylation.
We do not know at present how the nuclear factor(s) functions for MPF activation, particularly that for entry into meiosis II, in Xenopus oocytes. However, our results show that in enucleated oocytes after meiosis I, MPF exists as a complex of cdc2 kinase and cyclin B2, but with inhibitory Tyr-15 (and Thr-14) phosphorylation of cdc2 kinase, just as in G2-arrested immature oocytes (Fig. 4A), and that injection of nuclear material back into the enucleated oocytes can restore cdc2 Tyr-15 dephosphorylation and MPF reactivation (Fig. 4B). Thus, it seems likely that the nuclear factor functions somehow for cdc2 Tyr-15 dephosphorylation (or for its maintenance), to cause activation (or stabilization) of MPF. [The nuclear factor could also function for cdc2 Tyr-15 dephosphorylation in the initial MPF activation in meiosis I, but not in an absolutely required manner (Figs. 4, 5).] In vertebrate oocytes, many cell cycle regulators, such as cdc25, wee1, and Mos, are involved directly or indirectly in cdc2 Tyr-15 (and Thr-14) dephosphorylation (5, 43, 44). In Xenopus, however, both cdc25 (a cdc2 Thr-14/Tyr-15 phosphatase; ref. 5) and Mos (an indirect activator or stabilizer of MPF; refs. 43, 44) are mostly cytoplasmic in oocytes before GVBD, and cdc25 is not activated in enucleated oocytes after meiosis I (unpublished data; see also Results). Thus, some factor(s) other than cdc25 and Mos must be the nuclear factor required for cdc2 Tyr-15 (and Thr-14) dephosphorylation (a good candidate would be Plx1, which is a direct activator of cdc25; ref. 48). Identification of the nuclear factor will be essential to understand the longstanding problems of the meiotic control in oocytes (5, 29).
In summary, we have shown that nuclear material is indispensable for the meiotic cell cycle, particularly entry into meiosis II, in Xenopus oocytes. The general view that the meiotic control in (vertebrate) oocytes depends entirely on cytoplasmic factors may therefore need reconsideration (Fig. 5). The nuclear factor(s) required for the meiotic cell cycle remains to be identified.
Acknowledgments
We thank J. Maller for his generous gift of anti-Xenopus cyclin B2 antibody and M. Yamashita for anti-PSTAIRE antibody. We also thank K. Ohsumi, T. Kishimoto, N. Nakajo, and N. Furuno for discussions and M. Egashira for her editing the manuscript. This work was supported in part by Grants-in-aid for Scientific Research from the Ministry of Education, Science and Culture of Japan, and in part by the grant from The Mitsubishi Foundation.
ABBREVIATIONS
- GVBD
germinal vesicle breakdown
- MPF
maturation/M phase-promoting factor
References
- 1.Masui Y, Clarke H J. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- 2.Masui Y. In: Biology of Fertilization. Metz C B, Monroy A, editors. Vol. 1. Orlando, FL: Academic; 1985. pp. 189–219. [Google Scholar]
- 3.Sagata N. Trends Cell Biol. 1996;6:22–28. doi: 10.1016/0962-8924(96)81034-8. [DOI] [PubMed] [Google Scholar]
- 4.Nurse P. Nature (London) 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- 5.Murray A, Hunt T. The Cell Cycle: An Introduction. New York: Freeman; 1993. [Google Scholar]
- 6.Gerhart J, Wu M, Kirschner M. J Cell Biol. 1984;98:1247–1255. doi: 10.1083/jcb.98.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furuno N, Nishizawa M, Okazaki K, Tanaka H, Iwashita J, Nakajo N, Ogawa Y, Sagata N. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masui Y, Markert C L. J Exp Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 9.Sagata N, Watanabe N, Vande Woude G F, Ikawa Y. Nature (London) 1989;342:512–518. doi: 10.1038/342512a0. [DOI] [PubMed] [Google Scholar]
- 10.Dreyer C. In: Developmental Biology: A Comprehensive Synthesis. Diberardino M A, Etkin L D, editors. Vol. 6. New York: Plenum; 1989. pp. 31–57. [Google Scholar]
- 11.Hausen P, Riebesell M. The Early Development of Xenopus Laevis: An Atlas of the Histology. Berlin: Springer; 1991. [Google Scholar]
- 12.Gard D L. Dev Biol. 1992;151:516–530. doi: 10.1016/0012-1606(92)90190-r. [DOI] [PubMed] [Google Scholar]
- 13.Katagiri C, Moriya M. Dev Biol. 1976;50:235–241. doi: 10.1016/0012-1606(76)90080-4. [DOI] [PubMed] [Google Scholar]
- 14.Balakier H, Tarkowski A K. Exp Cell Res. 1980;128:79–85. doi: 10.1016/0014-4827(80)90389-4. [DOI] [PubMed] [Google Scholar]
- 15.Laskey R A, Mills A D, Philpott A, Leno G H, Dilworth S M, Dignwall C. Phil Trans R Soc London B. 1993;339:263–269. doi: 10.1098/rstb.1993.0024. [DOI] [PubMed] [Google Scholar]
- 16.Davidson E H. Gene Activity in Early Development. Orlando, FL: Academic; 1986. [Google Scholar]
- 17.Kishimoto T, Hirai S, Kanatani H. Dev Biol. 1981;81:177–181. doi: 10.1016/0012-1606(81)90360-2. [DOI] [PubMed] [Google Scholar]
- 18.Picard A, Dorée M. Dev Biol. 1984;104:357–365. doi: 10.1016/0012-1606(84)90091-5. [DOI] [PubMed] [Google Scholar]
- 19.Schorderet-Slatkine S, Drury K C. Cell Differ. 1973;2:247–254. doi: 10.1016/0045-6039(73)90013-4. [DOI] [PubMed] [Google Scholar]
- 20.Reynhout J K, Smith L D. Dev Biol. 1974;38:394–400. doi: 10.1016/0012-1606(74)90016-5. [DOI] [PubMed] [Google Scholar]
- 21.Masui Y, Meyerhof P G, Ziegler D H. J Steroid Biochem. 1979;11:715–722. doi: 10.1016/0022-4731(79)90005-0. [DOI] [PubMed] [Google Scholar]
- 22.Skoblina M N, Pivnitsky K K, Kondratieva O T. Cell Differ. 1984;14:153–157. doi: 10.1016/0045-6039(84)90040-x. [DOI] [PubMed] [Google Scholar]
- 23.Gautier J. Dev Biol. 1987;123:483–486. [Google Scholar]
- 24.Balakier H, Czolowska R. Exp Cell Res. 1977;110:466–469. doi: 10.1016/0014-4827(77)90314-7. [DOI] [PubMed] [Google Scholar]
- 25.Balakier H, Masui Y. Dev Biol. 1986;113:155–159. doi: 10.1016/0012-1606(86)90118-1. [DOI] [PubMed] [Google Scholar]
- 26.Skoblina M N. Exp Cell Res. 1969;55:142–144. doi: 10.1016/0014-4827(69)90472-8. [DOI] [PubMed] [Google Scholar]
- 27.Smith L D, Ecker R E. Dev Biol. 1969;19:281–309. doi: 10.1016/0012-1606(69)90065-7. [DOI] [PubMed] [Google Scholar]
- 28.Schuetz A W. In: Developmental Biology: A Comprehensive Synthesis. Browder L W, editor. Vol. 1. New York: Plenum; 1985. pp. 3–83. [Google Scholar]
- 29.Masui Y, Shibuya E K. In: Molecular Regulation of Nuclear Events in Mitosis and Meiosis. Schlegel R A, Halleck M S, Rao P N, editors. Orlando, FL: Academic; 1987. pp. 1–42. [Google Scholar]
- 30.Dabauvalle M C, Dorée M, Bravo R, Karsenti E. Cell. 1988;52:525–533. doi: 10.1016/0092-8674(88)90465-5. [DOI] [PubMed] [Google Scholar]
- 31.Ford C C, Gurdon J B. J Embryol Exp Morph. 1977;37:203–209. [PubMed] [Google Scholar]
- 32.Yamashita M, Yoshikuni M, Hirai T, Fukada S, Nagahama Y. Dev Growth Differ. 1991;33:617–624. doi: 10.1111/j.1440-169X.1991.00617.x. [DOI] [PubMed] [Google Scholar]
- 33.Ookata K, Hisanaga S, Okano T, Tachibana K, Kishimoto T. EMBO J. 1992;11:1763–1772. doi: 10.1002/j.1460-2075.1992.tb05228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrell J E, Wu M, Gerhart J C, Martin G S. Mol Cell Biol. 1991;11:1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautier J, Maller J L. EMBO J. 1991;10:177–182. doi: 10.1002/j.1460-2075.1991.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobayashi H, Minshull J, Ford C, Golsteyn R, Poon R, Hunt T. J Cell Biol. 1991;114:755–765. doi: 10.1083/jcb.114.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorca T, Labbé J C, Devault A, Fesquet D, Capony J P, Cavadore J C, Le Bouffant F, Dorée M. EMBO J. 1992;11:2381–2390. doi: 10.1002/j.1460-2075.1992.tb05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Solomon M J, Lee T, Kirschner M W. Mol Biol Cell. 1992;3:13–27. doi: 10.1091/mbc.3.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldherr C M, Paine P L, Hodges P. Exp Cell Res. 1988;179:527–534. doi: 10.1016/0014-4827(88)90290-x. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler D, Masui Y. J Cell Biol. 1976;68:620–628. doi: 10.1083/jcb.68.3.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picard A, Labbe J C, Dorée M. Dev Biol. 1988;128:129–135. doi: 10.1016/0012-1606(88)90274-6. [DOI] [PubMed] [Google Scholar]
- 42.Okumura E, Sekiai T, Hisanaga S, Tachibana K, Kishimoto T. J Cell Biol. 1996;132:125–135. doi: 10.1083/jcb.132.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matten W T, Vande Woude G F. Semin Dev Biol. 1994;5:173–181. [Google Scholar]
- 44.Sagata N. BioEssays. 1997;19:13–21. doi: 10.1002/bies.950190105. [DOI] [PubMed] [Google Scholar]
- 45.Hausen, P., Wang, Y. H., Dreyer, C. & Stick, R. (1985) J. Embryol. Exp. Morphol. 89 Suppl., 17–34. [PubMed]
- 46.Miller M, Reddy B A, Kloc M, Li X X, Dreyer C, Etkin L D. Development (Cambridge, UK) 1991;113:569–575. doi: 10.1242/dev.113.2.569. [DOI] [PubMed] [Google Scholar]
- 47.Furuno N, Ogawa Y, Iwashita J, Nakajo N, Sagata N. EMBO J. 1997;16:3860–3865. doi: 10.1093/emboj/16.13.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumagai A, Dunphy W G. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]