Abstract
E1 deletion adenoviral vectors of the human serotype 5 (AdHu5) and the chimpanzee serotype 68 (AdC68) expressing the rabies virus glycoprotein (rab.gp) were tested for induction of transgene product-specific antibodies upon intranasal or oral immunization of newborn mice. Both vectors induced antibodies to rabies virus that could be detected in serum and mucosal secretions. Serum rabies virus-neutralizing antibody titers sufficed to protect neonatally vaccinated mice against a subsequent challenge with rabies virus. The efficacy of the AdHu5rab.gp vector given orally to newborn mice born to AdHu5-immune dams was not impaired by maternally transferred antibodies to the vaccine carrier.
Vaccines remain the most efficacious medical intervention to reduce mortality and morbidity due to viral infections. Although more than 400 distinct viruses can cause symptomatic infections in humans, prophylactic vaccines are available for fewer than 20 of these pathogens. The threat of viral infections is increasing due to emerging viruses that with modern modes of transportation and the resulting highly mobile populations in developed countries spread rapidly from their places of origin. Also, there is a heightened threat of terroristic release of viruses with potentially increased virulence due to molecular engineering. Both situations necessitate the generation of additional vaccines which can be distributed rapidly to large segments of a susceptible population for protection against viral pathogens.
Traditionally, vaccines were developed by inactivation or attenuation of pathogens. Advances in molecular biology now allow for the generation of recombinant subunit vaccines based on different carriers, which have a strong impact on the magnitude and the type of the immune response to the vaccine antigen. The type of vaccine vehicle also imposes constraints on the potential routes of vaccine delivery. Most currently used vaccines are applied systemically. In less developed countries in general and in developed countries in states of emergency, the more convenient route of oral vaccination would be highly desirable to allow for less expansive and more expeditious vaccine distribution.
Adenoviral (Ad) recombinants of the human serotype 5 (AdHu5) have outstanding efficacy as vaccine carriers in experimental animals (8, 14, 20, 21, 22, 32) and are now undergoing clinical trials (12). Intranasal application of such vaccines has been tested (5, 27) and was shown to induce antibody responses at mucosal surfaces, the most common port of entry for most viral pathogens. Replication-defective or replication-competent Ad recombinants of human or porcine serotypes have been demonstrated to induce cellular and humoral immunity to the target antigen upon oral or enteric administration (7, 9, 18). Epicutaneous application through dermal patches has been tried with some, albeit limited, success (11, 19). Taken together, these data attest to the versatility of the vaccination routes suitable for Ad recombinant vaccine vehicles (23).
AdHu5, the most commonly used vector for preclinical vaccine studies, is a ubiquitous pathogen, and circulating serotype-specific neutralizing antibodies found in up to 45% of the adult U.S. population interfere with the efficacy of systemically delivered Ad vaccines based on the homologous serotype (3, 13, 15, 16, 28). To circumvent this interference, an alternative vector system based on an Ad that originated from the lymph nodes of a chimpanzee was developed. E1 deletion recombinants derived from this virus, designated chimpanzee serotype 68 (AdC68), induce a transgene product-specific response in rodents upon systemic or intranasal application, and this response is not impacted by preexisting immunity to common human serotypes of Ad (28). These studies were conducted with a mouse rabies virus model that is considered an appropriate model for human rabies vaccines. Current vaccine lots are de facto analyzed by a so-called National Institutes of Health potency test in rodents (4) before their release for use in humans. Rabies virus is a simple RNA virus that encodes only five antigens. Of those, the glycoprotein is the sole target of virus-neutralizing antibodies (VNAs), which provide protection against viral challenge (29).
Here we used this model system to address whether oral delivery of E1 deletion Ad vectors of the human serotype 5 and the chimpanzee serotype 68 expressing the glycoprotein of the fixed Evelyn Rokitniki Abelseth (ERA) strain of rabies virus stimulates systemic and mucosal antibody responses and protection against severe rabies virus challenge. We furthermore analyzed whether the transgene product-specific humoral immune response to oral Ad vaccination is impaired by preexisting antibodies to the vaccine carrier and whether such a response can be boosted by a second dose of the homologous vaccine carrier given per os. Our data confirm that at appropriately high vaccine doses, E1 deletion AdHu5 and AdC68 recombinants induce both systemic and mucosal transgene product-specific antibody responses. Responses to oral vaccination are not strongly impaired by preexisting immunity to the vaccine carrier. In agreement with this observation, homologous oral primer-booster regimens were found to be highly effective in enhancing mucosal and systemic transgene product-specific antibody titers.
MATERIALS AND METHODS
Mice.
Female inbred C57BL/6 mice and outbred ICR mice were used at 6 to 12 weeks of age.
Cell lines.
BHK-21 and 293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics.
Viruses.
The AdHu5rab.gp recombinant, an E1 deletion Ad recombinant of the human serotype 5 expressing the glycoprotein of the ERA strain of rabies virus, has been described previously (1). Generation of the more recently developed E1 deletion AdC68rab.gp vaccine expressing the same transgene product in a simian Ad vector has been reported previously (3, 28). Viral recombinants as well as wild-type AdHu5 were propagated and titrated on 293 cells. Dosing of the recombinant Ad vectors was in terms of numbers of PFU. Dosing of wild-type Ad was in terms of numbers of virus particles (vp) so that induction of antibodies against the Ad antigens by defective vp was taken into account. The ERA strain of rabies virus was grown on BHK-21 cells, purified by gradient centrifugation, and inactivated by treatment with beta-propiolactone (BPL) (25). The protein content of the inactivated virus (ERA-BPL) was determined and adjusted to 1 mg/ml. The rabies CVS-11 strain used for challenge was propagated and titrated on BHK-21 cells (26).
Immunization and challenge of mice.
Mice were immunized once or twice with various doses (given in numbers of PFU) of the AdHu5 or AdC68 constructs given per os or intramuscularly (i.m.). It had been shown previously that mice immunized with an Ad recombinant expressing a viral antigen not derived from rabies virus failed to induce rabies virus-specific antibodies (28). This control was thus not included in the described set of experiments. It had also been reported previously that oral immunization with 106 PFU of the AdHu5rab.gp vaccine failed to induce titers of antibodies to rabies virus in sera. We modified the previously described vaccination procedure by applying the vaccines with a feeding tube to ensure swallowing rather than inhalation or spillage of the vaccine. Furthermore, we diluted the vaccine in a buffered salt solution rather than in saline. Mice were immunized with wild-type AdHu5 given intranasally or i.m. Mice were challenged with 10 mean lethal doses of the CVS-11 strain of rabies virus injected directly into the brain. Experiments were conducted two to five times with groups of five to eight mice to ensure reproducibility.
Preparation of samples.
Blood was harvested by retro-orbital puncture. Sera were prepared and heat inactivated at 56°C for 30 min. Sera were tested for rabies virus neutralization starting at a 1:5 dilution and for neutralization of AdHu5 starting at a 1:20 dilution. They were analyzed by enzyme-linked immunosorbent assay (ELISA) starting with a 1:200 dilution. Antibody isotypes were tested with a 1:800 dilution of sera. Vaginal lavage fluid was harvested by rinsing the vaginal cavity three times with 50 μl of saline (final volume of 150 μl). The sample was centrifuged at 5,000 rpm for 5 min to remove debris. Vaginal lavage fluid was titrated starting at a dilution of 1:2; antibody isotypes were determined with a 1:8 dilution (30). Feces were collected and suspended at 50 mg/ml in PBS containing 1% NaN3. After 1-h incubation at room temperature, samples were vortexed and debris was removed by centrifugation at 14,000 rpm in an Eppendorf centrifuge (27). Samples were tested for antibody titers starting at a 1:2 dilution and for isotypes at a 1:5 dilution.
Spleens, cervical and mesenteric lymph nodes, and Peyer's patches were harvested 18 to 72 h after oral immunization.
ELISA.
Sera, vaginal lavage fluids, and fecal suspensions were tested on rabies virus-coated plates as described previously (30). Briefly, round-bottom microtiter plate wells were coated overnight with 0.2 μg of ERA-BPL virus or purified AdHu5 diluted in 100 μl of coating buffer (15 mM Na2CO3, 35 mM NaHCO3, and 3 mM Na2N, pH 9.6). The next day, plates were treated for 24 h with phosphate-buffered saline (PBS) containing 3% bovine serum albumin. The following day, plates were washed two times with 150 μl of PBS for 24 h, dried, and kept at −20°C. Sera were serially diluted in PBS containing 3% bovine serum albumin. The different dilutions of sera were incubated in duplicate at 100 μl per well on the ERA-BPL-coated plates for 1 h at 4°C. Fecal suspensions and vaginal lavage fluids were incubated overnight. Plates were washed five times with PBS and treated with an alkaline phosphatase-conjugated goat anti-mouse immunoglobulin (Ig) for 1 h at 4°C. Plates were washed and incubated for 20 min with the substrate (10 mg of d-nitrophenyl phosphate disodium dissolved in 10 ml of 1 mM MgCl2-3 mM NaN3-0.9 M diethanolamine [pH 9.8]). Plates were then read in an automated ELISA reader at 405 nm. Isotypes of antibodies to rabies virus were tested on ERA-BPL-coated plates with the Calbiochem isotyping kit, which has comparable sensitivities for different antibody isotypes (23).
Virus neutralization assay.
Sera were tested for neutralization of CVS-11 virus, which is antigenically closely related to the ERA virus, as described previously (24). Sera were tested for neutralization of AdHu5 by a plaque reduction assay as described previously (3).
Reverse transcription-PCR.
Mice were sacrificed, and lymphoid tissues were harvested and disrupted by a polytron probe in a solution of Tri-reagent (Molecular Research Center [MRC], Cincinnati, Ohio). RNA was isolated from individual samples as described by the manufacturer. Briefly, 100 μl of F solution (1-bromo-3-chloropropane) (MRC) was added to every sample. The aqueous phase was transferred to fresh tubes, and RNA was precipitated by isopropanol, washed with 70% ethanol, and resuspended in diethyl pyrocarbonate-treated water (Ambion, Inc., Houston, Tex.). DNA was removed by treatment with DNase (Ambion, Inc.) for 30 min at 37°C. DNase was removed with the DNase removal kit (Ambion, Inc.). cDNA was synthesized from RNA samples with Moloney murine leukemia virus reverse transcriptase (Life Technologies, Inc., Rockville, Md.). Samples were amplified for the synthesis of cDNA for the rabies virus glycoprotein and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by using the following primers: rabies glycoprotein forward primer, 5′-AAA GCA TTT CCG CCC AAC AC-3′; rabies glycoprotein reverse primer, 5′-GGT TAC TGG AGC AGT AGG TAG A; GAPDH forward primer, 5′-GGT GAA GGT CGG TGT GAA CGG ATT T-3′; and GAPDH reverse primer, 5′-AAT GCC AAA GTT GTC ATG GAT GAC C-3′. PCR conditions for all genes consisted of initial denaturation at 94°C for 5 min followed by 40 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 2 min, and extension at 72°C for 3 min. The amplicons were separated by electrophoresis on a 1% agarose-ethidium bromide gel against molecular weight standards. The gels were analyzed with a FluorImager SI (Vistra Fluorescence).
RESULTS
E1 deletion Ad vectors of human and simian serotypes induce transgene product-specific antibodies in sera upon oral application.
The rabies virus glycoprotein was used to test for the induction of antibodies by Ad recombinants based on the human serotype 5 (AdHu5rab.gp) or the chimpanzee serotype 68 (AdC68rab.gp). It had been shown previously that upon subcutaneous (s.c.) or i.m. immunization both vaccines stimulate antibodies to rabies virus, although serum antibody titers were markedly higher upon vaccination with the AdHu5rab.gp vector. Upon intranasal immunization, both vaccines induced more comparable titers of rabies virus-specific antibodies in sera (28). This may in part reflect differences in levels of transgene product expression, which in most albeit not all cell types is higher upon infection with AdHu5 vectors (28). In this study, we orally immunized outbred ICR and inbred C57BL/6 mice with escalating doses of the AdHu5rab.gp and AdC68rab.gp vectors. The latter strain of mice generally mounts less vigorous B-cell responses to the rabies virus glycoprotein than mice of other strains, such as ICR and C3H/He mice (unpublished observation). Mice of either strain developed titers of antibodies to rabies virus at doses of or above 2 × 106 PFU (Fig. 1A). Oral immunization was not as effective as i.m. vaccination, as shown in Fig. 1B for the AdHu5rab.gp vector applied at high (107 PFU) and low (105 PFU) doses to groups of ICR mice.
FIG. 1.
Oral immunization with Ad recombinants expressing rabies virus glycoprotein results in rabies virus-specific serum antibodies and protection against rabies virus challenge. (A) Groups of eight ICR or C57BL/6 mice were immunized orally with 2 × 107 (open symbols), 2 × 106 (closed symbols), or 2 × 105 (half-closed symbols) PFU of AdHu5rab.gp (left panels) or AdC68rab.gp (right panels). Mice were bled 21 days later, and sera were tested for antibodies to rabies virus in comparison to sera from naïve mice (X) by an ELISA. Data show means ± standard deviations for pools of sera tested in duplicate. (B) Groups of five ICR mice were immunized either per os (closed squares) or i.m. (open squares) with 107 (left) or 105 (right) PFU of AdHu5rab.gp. Serum antibody titers were tested 4 weeks later. X, sera from naïve ICR mice. (C) The graph shows data generated with sera from groups of eight ICR mice immunized orally with 2 × 104 to 2 × 107 PFU of AdHu5rab.gp (striped bars) or AdC68rab.gp (solid bars). Sera were tested 3 weeks after vaccination for neutralizing antibodies to rabies virus. Data are expressed as international units determined by comparison to a World Health Organization reference serum. Normal mouse sera tested in parallel had titers below 0.5 IU. (D) The graph shows survival of the same groups of mice whose results are shown in Fig. 1C upon intracerebral challenge with 10 mean lethal doses of rabies virus. Striped bars, AdHu5rab.gp; solid bars, AdC68rab.gp. (E) Isotypes of serum antibodies to rabies virus from ICR and C57BL/6 mice immunized orally 3 weeks previously with 2 × 107 PFU of AdHu5rab.gp (striped bars) or AdC68rab.gp (solid bars) or with nothing (speckled bars) were tested by an ELISA. Data show results for pooled sera tested in duplicate at a 1:200 dilution.
To ensure that oral immunization resulted in rabies virus-specific VNAs, which are crucial for protection against virus infection (29), sera from AdHu5rab.gp- or AdC68rab.gp-vaccinated ICR mice were tested for neutralization of rabies virus. Both vaccines induced serum VNA responses to rabies virus upon oral application (Fig. 1C) and, correspondingly, protective immunity to rabies virus challenge given directly to the central nervous system (Fig. 1D). VNA titers and protective immunity, unlike titers tested by ELISA, showed a dose response curve for both vaccines. Upon systemic immunization with recombinant vaccines, titers detected by ELISA correlate with those determined by neutralization assays (28, 31, 32), indicating that most antibodies elicited against the vector-encoded viral protein are directed against epitopes expressed on correctly folded protein and that these antibodies possess neutralizing activity. Upon oral immunization, this correlation was less rigorous, suggesting that the B-cell response targeted in part unfolded or partially degraded rabies virus glycoprotein, resulting in a high fraction of nonneutralizing antibodies that are detected by the ELISA only. Although both vaccines were less efficacious upon oral immunization than upon systemic immunization, complete protection could be achieved with either vaccine upon oral application of 2 × 107 PFU of the vectors.
It was described previously that upon s.c. immunization, the AdHu5rab.gp vector induced a mixed Th1-Th2 response, with a IgG2a/IgG1 ratio of ∼2, while the AdC68rab.gp vector favored stimulation of a Th1 response (IgG2a/IgG1 ratio of ∼10) (28). This difference in isotype distributions of the transgene product-specific antibodies was seen neither upon intranasal immunization, as reported earlier (28), nor upon oral vaccine application, as shown in Fig. 1E.
Upon i.m. injection of the Ad recombinants, lymph nodes draining the injection sites rapidly acquire, within less than 24 h, transgene product-expressing cells with morphological and phenotypic characteristics of mature dendritic cells. These cells presumably become infected at an immature stage at the site of inoculation and then upon maturation migrate to lymphatic tissues, where they present the antigen to naïve T cells (unpublished data). To determine which lymphatic tissues became infiltrated by recombinant Ad-infected migratory cells and thus were likely to participate in induction of an immune response upon oral application of the Ad vectors, mice were fed 108 PFU of AdHu5rab.gp or AdC68rab.gp. Lymph nodes (cervical and mesenteric) and Peyer's patches were harvested 18, 48, or 72 h later, and RNA was isolated, reverse transcribed, and amplified for rabies virus glycoprotein- and GAPDH-specific cDNA by PCR. Rabies virus-specific amplicons could be detected in all of the lymph nodes at at leastone of the time points, indicating that the vaccines had been taken up within the oral cavity as well as within the intestine (data not shown).
E1 deletion Ad vaccines induce mucosal antibody responses upon oral application.
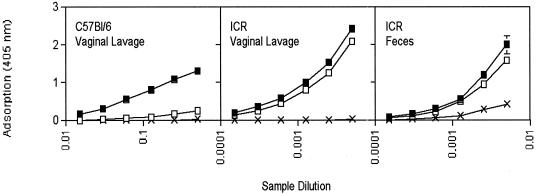
Mucosal immunization, such as through the oral or respiratory route, favors induction of antibodies secreted at mucosal surfaces. This observation was analyzed further with ICR mice fed either the AdHu5rab.gp or the AdC68rab.gp vaccine. Titers of antibodies to rabies achieved with either vaccine at the vaginal mucosae and in fecal suspensions were comparable in orally vaccinated outbred ICR mice (Fig. 2). C57BL/6 mice generated antibodies in vaginal secretions upon oral vaccination with the AdHu5rab.gp vaccine. In contrast, oral application of the AdC68rab.gp vaccine at all doses tested (data shown only for the highest vaccine dose) induced only low levels of mucosal antibodies in C57BL/6 mice, although these mice developed substantial serum antibody titers with the AdC68rab.gp vaccine.
FIG. 2.
Oral immunization with Ad recombinants induces mucosal antibodies to the transgene product. Vaginal lavage fluids and fecal suspensions from ICR or C57BL/6 mice vaccinated with 2 × 107 PFU of AdHu5rab.gp (closed symbols) or AdC68rab.gp (open symbols) vector or with nothing (X) were tested by ELISA as described in the legend to Fig. 1A. The dilution is a relative sample dilution of the vaginal lavage fluids and the fecal suspensions as detailed in Materials and Methods.
Oral vaccination overcomes interference by preexisting neutralizing antibodies to the Ad vaccine carrier.
It had been shown previously that in mice preexposed to AdHu5, the serum antibody response to the rabies virus glycoprotein presented by the AdHu5rab.gp vaccine given systemically was strongly reduced while the antibody response to the AdC68rab.gp vaccine was not impaired (28). To test for an effect of preexisting immunity to the vaccine carrier on the humoral response elicited by oral vaccination, mice were immunized with replication-competent (in its natural host) AdHu5 given at 5 × 1011 vp i.m. or at a lower dose of 5 × 1010 vp intranasally, the natural route of infection of humans by this virus. Titers of antibodies to the AdHu5 vector in sera, tested 4 weeks later by a neutralization assay starting with a 1:20 dilution of sera, were ∼1:160 in the i.m. vaccinated group, which is comparable to titers commonly found in human adults. Intranasally vaccinated mice had neutralizing antibody titers below 1:20, although antibodies to Ad could readily be detected by ELISA (data not shown). AdHu5-immune as well as naïve mice were subsequently vaccinated with the AdHu5rab.gp vector given either per os or i.m. Mice were bled 2 weeks later, and titers of antibodies to rabies virus in sera were determined by a neutralization assay. As shown in Fig. 3A, the antibody response to i.m. vaccination with the AdHu5rab.gp vector, which induced a potent response in naïve ICR mice, was completely abolished in mice preexposed by i.m. inoculation with AdHu5 and decreased from 490 to 160 IU upon intranasal preexposure. It has been demonstrated previously that even very low levels of neutralizing antibodies strongly inhibit gene transfer by E1 deletion AdHu5 vectors (10), which supports our findings that mice with titers of less than 1:20 still showed a reduction in the transgene product-specific antibody response upon i.m. application of the AdHu5rab.gp vector. Titers of antibodies to rabies virus generated upon oral immunization were overall lower in naïve mice than those achieved by i.m. immunization, as shown in Fig. 1. In mice preimmunized systemically with AdHu5 prior to per os vaccination with the AdHu5rab.gp vector, titers of VNAs to rabies virus were identical to those elicited in mice that had not been preexposed to the vaccine. Preexposure through the airways caused an increase in VNA titers elicited by oral vaccination. Overall, these data suggest that the efficacy of oral immunization is relatively unaffected by preexisting neutralizing antibodies to the vaccine carrier. Part of the experiment was repeated using different doses of the AdHu5rab.gp vector to test whether the efficacy of low vaccine doses could be inhibited by preexisting antibodies to the vaccine carrier. Intranasal preexposure was chosen for these experiments as this regimen is better suited to induce mucosal antibodies of the IgA isotype (30). Intranasally preexposed and naïve mice were vaccinated orally with the AdHu5rab.gp vector. Two groups of mice were vaccinated with an intermediate dose of the AdC68rab.gp vector. Preexposure to AdHu5 had no effect on the transgene product-specific antibody titers in sera (Fig. 3B) or at vaginal surfaces (Fig. 3C) induced by the two higher doses (2 × 106 and 2 × 107 PFU per mouse) of the AdHu5rab.gp vector or the one intermediate dose (2 × 106 PFU) of the AdC68rab.gp vaccine. The serum and vaginal antibody responses to the lowest dose (2 × 105 PFU per mouse) of the AdHu5rab.gp vaccine were marginally reduced in AdHu5-preexposed mice, suggesting that low-dose oral immunization may be affected by mucosal preexposure to AdHu5. Interestingly, the vaginal response to the highest dose of the AdHu5rab.gp vector as well as to the intermediate dose of the AdC68rab.gp vector was slightly increased in preimmune mice, suggesting a potential benefit from the AdHu5 preexposure on the transgene product-specific mucosal B-cell response to the vaccine antigen. The VNA response, as observed in the dose titration experiments whose results are shown in Fig. 1, showed no strict correlation with the serum antibody titers determined by ELISA. Again, VNA titers were not strongly reduced in AdHu5-preexposed mice vaccinated with the high or intermediate dose of AdHu5rab.gp or AdC68rab.gp; the response to the intermediate dose of AdHu5rab.gp was slightly increased by ∼2-fold, which is within the range of assay variability. The isotype profiles of serum and vaginal antibodies to rabies virus were not affected in AdHu5rab.gp vector-fed mice preexposed to AdHu5 through the airways (Fig. 3E). The isotype profile of vaginal antibodies to rabies virus elicited by oral application of the AdC68rab.gp vaccine was shifted towards IgA in AdHu5-preimmune mice, suggestive of an effect of AdHu5-specific T helper cells on the vaccine-induced B-cell response.
FIG. 3.
The effect of preexposure to AdHu5 on the transgene product-specific antibody response to immunization with Ad recombinants expressing the rabies virus glycoprotein. (A) Groups of five ICR mice were immunized i.m. with 5 × 1011 vp of AdHu5 given i.m. or 5 × 1010 vp given intranasally. Two different doses were chosen for technical reasons to accommodate the limited volume associated with intranasal immunization. Four weeks later, AdHu5-immune mice and groups of naïve mice were vaccinated i.m. (left) or per os (right) with 2 × 106 PFU of AdHu5rab.gp. Serum VNA titers were tested 2 weeks later. (B) Groups of five ICR mice were immunized intranasally with AdHu5. Four weeks later, AdHu5-exposed mice (closed symbols) as well as age-matched naïve mice (open symbols) were vaccinated orally with 2 × 107, 2 × 106, or 2 ×105 PFU of AdHu5rab.gp or 2 × 106 PFU of AdC68rab.gp. Titers of antibodies to rabies virus in sera were tested 3 weeks later. X, normal mouse serum. (C) The same groups of mice were tested for titers of antibodies to rabies virus in vaginal lavage fluids. Closed symbols, preexposed, vaccinated mice; open symbols, unexposed, vaccinated mice; X, naïve mice. (D) Sera from the same groups of mice were tested for VNA titers. Closed bars, preexposed, vaccinated mice; open bars, unexposed, vaccinated mice. VNA titers in sera from naïve mice were below the level of detectability (data not shown). (E) The antibody isotype profiles of sera or vaginal lavage fluids of naïve (open bars) or AdHu5-preexposed (closed bars) mice vaccinated 3 weeks previously per os with 2 × 107 PFU of AdHu5rab.gp or AdC68rab.gp vector were determined by ELISA. Speckled bars, naïve control mice.
Oral booster immunization enhances the antibody response to the transgene product of Ad vectors.
To test whether a second dose of an Ad vector of the rabies virus glycoprotein enhanced antibody titers with oral immunization, groups of ICR mice were vaccinated with an intermediate dose of 2 × 106 PFU of either AdC68rab.gp or AdHu5rab.gp. Mice were given oral booster immunizations 4 weeks later with the homologous or the heterologous carrier at the same dose used for priming. Serum antibody responses to the rabies virus glycoprotein were analyzed 2 and 12 weeks later. As shown in Fig. 4A, the oral booster immunization enhanced serum antibody responses at both time points tested. The AdHu5rab.gp-primed group responded to booster immunization with the homologous or heterologous vaccine carrier with similar increases in rabies virus-specific antibody titers, as shown by ELISA. VNA titers suggested an advantage for the homologous booster immunization (Fig. 4B). In AdC68rab.gp-primed mice, booster immunization with the heterologous AdHu5rab.gp vector resulted in slightly higher rabies virus-specific antibody titers, as determined by ELISA and a neutralization assay, than those achieved with a second dose of the AdC68rab.gp vector. In either combination, priming with the AdHu5rab.gp vector elicited higher titers of antibodies than priming with the AdC68rab.gp vector. These results were compared with those of systemic primer-booster regimens in which mice were immunized with a low dose (105 PFU) of either AdHu5rab.gp or AdC68rab.gp vector. Mice received booster immunizations 2 months later with the same dose of either the homologous or the heterologous vaccine carrier. Control groups did not receive the second dose of vaccine. Titers of antibodies to rabies virus in sera analyzed 2 weeks after the booster immunization showed high VNA titers of 1:100 IU upon a single immunization with the AdHu5rab.gp vector. A second immunization with either the AdHu5rab.gp or the AdC68rab.gp vector failed to increase these titers. The AdC68rab.gp vector, on the other hand, induced at these low doses only modest VNA titers, which failed to increase upon booster immunization with the homologous construct, indicating that neutralizing antibodies to the vaccine carrier impaired uptake of the second vaccine dose. Booster immunization of AdC68rab.gp-immune mice with the AdHu5rab.gp vector, on the other hand, dramatically increased titers of VNAs to rabies virus (Fig. 4C). These data again demonstrate the high susceptibility of systemic Ad vector immunization to interference by neutralizing antibodies to the vaccine carrier. The same groups of orally vaccinated mice for whom results are shown in Fig. 4A and B were analyzed for isotypes of rabies virus-specific antibodies in vaginal lavage fluids. An unexpected difference in the effects of homologous versus heterologous primer-booster vaccination became apparent (Fig. 4D). Upon priming, vaginal lavage fluids from mice fed the AdHu5rab.gp or the AdC68rab.gp vector contained antibodies to rabies virus that by 2 weeks after vaccination were mainly those of the IgA isotype. Upon booster immunization of AdHu5rab.gp-primed mice with either the AdHu5rab.gp or the AdC68rab.gp vector, mice developed a pronounced IgG2a response within 2 weeks that exceeded the IgA response. Two months after booster immunization, the composition of rabies virus-specific antibodies in vaginal lavage fluids of mice vaccinated twice per os with AdHu5rab.gp reversed to a preponderance of the IgA isotype. In contrast, in AdHu5rab.gp-primed mice booster immunized with the AdC68rab.gp vector, vaginal antibodies to rabies virus remained dominated by antibodies of the IgG2a isotype, although levels of IgA antibodies were also substantial. AdC68rab.gp-primed mice showed low levels of IgA in their vaginal lavage fluids 2 weeks after booster immunization with either the homologous or the heterologous vaccine carrier, and levels of IgG antibodies were marginal. After 2 months, mice developed a pronounced IgG2a response that exceeded the IgA response. This was especially noticeable in mice vaccinated twice with the AdC68rab.gp vector (Fig. 4D). To further assess the apparent preference of the two viral vectors to differentially induce mucosal IgA versus IgG2a antibodies to the transgene product, mice were vaccinated in follow-up experiments with an increased dose of either vaccine given at 2 × 107 PFU per os. One month later, mice received booster immunizations with the same dose of the homologous vectors, and titers and isotypes of antibodies to rabies in vaginal lavage fluids were tested 2 months later. As shown in Fig. 4E, AdHu5rab.gp-vaccinated mice developed high levels of IgA antibodies to rabies virus while this isotype was virtually absent in vaginal lavage fluids from mice immunized twice with the AdC68rab.gp vector, which instead contained predominantly rabies virus-specific antibodies of the IgG2a isotype. Figure 4F summarizes the ratios of rabies virus-specific antibodies of the IgA and IgG2a isotypes in vaginal lavage fluids from the different groups of mice receiving primer-booster immunization with the Ad vaccines and stresses the preferential induction of mucosal IgA by the AdHu5 vector and the dominant stimulation of mucosal IgG2a by the AdC68 vector.
FIG. 4.
Transgene product-specific antibody response upon homologous or heterologous primer-booster immunization with Ad recombinants. (A) Groups of five ICR mice were vaccinated per os with 2 × 106 PFU of AdHu5rab.gp or AdC68rab.gp. Mice were bled 2 weeks later. Mice were given booster immunizations 4 weeks after vaccination with 2 × 106 PFU of the homologous virus (closed symbols), i.e., AdHu5rab.gp-immune mice were given AdHu5rab.gp and AdC68rab.gp-immune mice were given AdC68rab.gp, or the heterologous virus (half-closed symbols), i.e., AdHu5rab.gp-immune mice were given AdC68rab.gp and AdC68rab.gp-immune mice were given AdHu5rab.gp. Sera were tested 2 weeks and 3 months after the booster immunizations in parallel with sera harvested after the first immunization (open symbols) and normal mouse serum controls (X). 1std, first dose. (B) The same set of mice whose results are shown in Fig. 1A were tested 2 weeks after priming (−2) and 2 and 12 weeks after booster immunization for VNAs to rabies virus. Solid bars, homologous primer-booster immunization; striped bars, heterologous primer-booster immunization. (C) Groups of five ICR mice were vaccinated i.m. with 105 PFU of AdC68 and then received booster immunizations i.m. with either 105 PFU of the same vector or 105 PFU of the AdHu5rab.gp vector. One group did not receive booster immunizations. Mice were bled 4 weeks later, and titers of VNAs to rabies virus were determined by comparison of sera from vaccinated ICR mice to sera from unvaccinated ICR mice (control). Data are expressed in international units, determined by comparison with a World Health Organization reference serum. (D) Vaginal lavage fluids from mice immunized per os with 2 × 106 PFU of AdHu5rab.gp (left panels) or AdC68rab.gp (right panels) and then given booster immunizations with the same dose of the homologous or heterologous construct 4 weeks later as described for panel A were tested for the isotype profile of antibodies to rabies virus. The upper panels show the profiles 2 weeks after priming with AdHu5rab.gp (solid bars) or AdC68rab.gp (striped bars), the middle panels show the isotype profiles 2 weeks after booster immunization, and the lower panels show isotypes 6 weeks thereafter. Levels of vaginal lavage fluid antibodies from control mice are shown with speckled bars. (E) Groups of mice were vaccinated per os with 2 × 107 PFU of AdHu5rab.gp (closed bars) or AdC68rab.gp (open bars). They received booster immunizations 4 weeks later with the same dose of the homologous construct given orally. Antibody titers in vaginal lavage fluids and antibody isotypes were tested 2 months later. Speckled bars, control vaginal lavage fluid. (F) The graph summarizes data from Fig. 4B and C, showing the ratio of IgA antibodies to IgG2a antibodies to rabies virus in vaginal lavage fluids of mice that had been immunized twice orally with the recombinant vaccines. (G) Fecal suspensions of mice immunized 4 weeks earlier with 2 × 107 (solid bars) or 2 × 106 (striped bars) PFU of AdHu5rab.gp virus were tested for isotypes of antibodies to Ad in comparison to control samples (speckled bars).
The unexpected efficacy of homologous oral primer-booster vaccination with the AdHu5 vector was compatible with the observed lack of interference of preexposure to wild-type AdHu5 in the transgene product-specific antibody response to oral AdHu5rab.gp vaccination. These results may suggest that upon oral immunization intestinal production of antibodies to the vaccine carrier is either low or short-lived and that preexposure or priming through the intranasal or oral route thus fails to affect uptake of the same vaccine carrier given several weeks later per os. It had been shown previously that intranasal immunization with the AdHu5 vector resulted in sustained titers of antibodies to the antigens of the vaccine carrier in fecal suspensions (27). Here we extended these studies to mice vaccinated orally 1 month previously with 2 × 107 or 2 × 106 PFU of the AdHu5rab.gp vaccine. Again, mice had readily detectable titers of antibodies to the antigens of AdHu5 in fecal suspensions (data not shown), and the antibodies were mainly of the IgA isotype (Fig. 4G).
DISCUSSION
Rabies virus, a simple RNA virus, is well defined and the correlates of immune protection, i.e., neutralizing antibodies against the viral glycoprotein, are known (21). Animal models, including those based on rodents, are considered valid for preclinical vaccine testing. This viral model has thus been commonly chosen for use in preclinical trials with novel vaccine carriers or adjuvants that aim to induce neutralizing antibody responses to the vaccine antigen (27, 28, 31, 32). To mimic the genetic diversity of the human population, most of our studies, including the experiments presented here, have used outbred ICR mice in addition to better-characterized inbred strains of mice.
In the past, we have focused our efforts on E1 deletion Ad recombinants of the human serotype 5. Vaccine studies demonstrated that E1 deletion Ad recombinants induce, even if given at moderate doses, superb B- and CD8+-T-cell responses in experimental animals (8, 14, 20, 21, 22, 32). The immune responses to the transgene product far surpass those achieved with other types of subunit vaccines, such as vaccinia virus recombinants and genetic vaccines (30, 32). In the rabies model, full and long-lasting protection against a severe challenge with rabies virus can be readily induced with a single moderate s.c. or i.m. dose of the AdHu5rab.gp vaccine, while in contrast, the currently used vaccines require three doses or more. The high immunogenicity of Ad recombinants relates in part to the noncytopathic nature of such E1 deletion viruses, which results in sustained antigen expression (32). In addition, Ads transduce dendritic cells (33), the main and potentially the only cells able to present antigen to a naïve immune system. Although E1 deletion Ad recombinants of human serotypes have yielded highly promising results as vaccines in adult and neonatal rodents, primates, and canines (8, 14, 20, 21, 22, 32), the preexisting immunity of humans was shown to interfere with the efficacy of systemically applied Ad vaccines (13, 15, 28). The AdC68 vector was therefore developed and was shown to circumvent interference due to preexposure to common human serotypes of Ad (28).
Here we tested the AdHu5 and AdC68 recombinants expressing the rabies virus glycoprotein for the induction of serum and mucosal antibody responses to rabies virus upon oral immunization. Vaccine carriers that achieve protective immune responses upon oral immunization are needed for several reasons. Vaccines that can be given through the oral route are highly desirable for developing countries where lack of skilled medical personnel and insufficient resources cause logistic problems for mass vaccination by injection. Repeated use of inefficiently sterilized needles can lead to inadvertent spread of other human pathogens, such as human immunodeficiency virus type 1, thus negating the benefit of vaccination (9). In developed countries facing an increased risk of purposely released pathogens (6), oral vaccines would allow for far more rapid mass vaccination than could possibly be achieved with vaccines applied by injection or by propulsion devices. In addition, mucosal vaccination, such as intranasal or oral vaccination, favors the induction of antibodies secreted at mucosal surfaces (27), which are the most common ports of entry for many pathogens, including those that spread through aerosols or sexual contact. Intranasal vaccination is cumbersome and difficult to dose. Oral vaccination, on the other hand, has been proven highly successful by the poliovirus eradication campaign (17), in which millions of children were treated with the live attenuated polio vaccine dropped onto lumps of sugar.
It was previously reported that oral vaccination with a low dose of an AdHu5 recombinant diluted in saline failed to induce an antibody response to the transgene product. Upon increase of the vaccine dose and modification of the oral immunization protocol, seroconversion was achieved with oral application, as had been demonstrated by others with similar Ad vaccine constructs (7, 18). As previously reported for systemic immunization, upon oral administration the AdHu5rab.gp vaccine outperformed the AdC68rab.gp construct in eliciting VNA titers and protective immunity to challenge with rabies virus. Oral immunization with either of the two Ad vectors was less efficacious than systemic immunization, and higher oral than systemic doses of vaccine were needed to induce protection against a severe intracerebral challenge with rabies virus. Notwithstanding, considering the potency of Ad vaccines given systemically, the doses needed for fully efficacious oral immunization were still moderate at 2 × 107 PFU per mouse. In addition to serum antibodies, mice vaccinated orally with either of the Ad vaccine carriers developed mucosal antibodies to rabies virus that could be detected in vaginal lavage fluids and in fecal suspensions. In outbred mice, both Ad vaccines induced serum antibodies of IgG1, IgG2a, and IgG2b isotypes indicative of a mixed Th1-Th2 response.
The AdC68 vector was developed to overcome preexisting serotype-specific neutralizing antibodies to AdHu5 that have been shown to interfere with the efficacy of the homologous vaccine carrier given systemically (28) and that could only in part be circumvented by increasing the vaccine dose. The serum and mucosal (i.e., vaginal) antibody responses to oral immunization with the AdHu5rab.gp vaccine were notably resistant to interference by preexisting vector-specific neutralizing antibodies. A similar finding had been reported previously for mucosal vaccination with a poxvirus vector that was not affected by systemic preexposure to the vaccine carrier (1). The authors concluded that systemic preimmunization failed to result in the mucosal immune responses needed to interfere with the mucosally delivered vaccine. Using either an i.m. preexposure protocol, which resulted in high systemic titers of antibodies to Ad, or a mucosal, i.e., intranasal, immunization regimen, we obtained similar results. Nevertheless, we feel that lack of mucosally secreted antibodies does not explain our results. Upon systemic as well as intranasal Ad immunization, antibodies to Ad are secreted at the mucosal surfaces of the intestinal and genital tracts, as reported previously (27), and we thus expected neutralization of the vaccine and reduction of the transgene product-specific antibody response. Lack of interference may reflect a number of pathways. Although we could detect IgA antibodies to Ad antigens and to the transgene product in fecal suspensions of mucosally immunized mice (27), we could not establish formally that fecal antibodies were indeed neutralizing like those detected in sera. Titers of antibodies at the oral and gastrointestinal mucosae may not have sufficed to impact uptake of the vaccine and consequently the immune response. Our finding that some interference was observed at the lowest dose (2 × 105 PFU) of the AdHu5rab.gp vector supports the latter explanation. The volume of the gut lumen and the large surface areas of the oral cavity and the intestine covered by epithelial cells that express the coxsackie Ad receptor used by both AdHu5 and AdC68 (2) may prevent efficient neutralization of the vaccine. Alternatively—and this is a hypothesis that awaits experimental confirmation—distinct immune mechanisms induced by the initial AdHu5 exposure with opposing effects on the immunogenicity of the vaccine may have contributed to the apparent lack of interference at the higher doses of the AdHu5rab.gp vaccine. Preexposure to AdHu5 not only induces serotype-specific neutralizing antibodies but also nonneutralizing antibodies and presumably CD4+ T helper cells that are in part cross-reactive with different Ad serotypes. This B- and T-cell cross-reactivity can be anticipated considering the high degree of conservation between different Ad serotypes of human and chimpanzee origins. T help may be more limited at the oral and gastrointestinal mucosal induction sites than in lymph nodes draining the inoculation sites of systemically or intranasally applied Ad vectors. Ad-specific T helper cells induced by immunization through the respiratory tract may thus benefit the transgene product-specific B-cell response to oral vaccination with an AdHu5 recombinant vaccine and thereby mask the effect of interference by Ad vector-specific neutralizing antibodies. Although this pathway is speculative, the assumption that T help induced by preexposure influences the mucosal antibody response to the vaccine antigen is supported by the observed increase in rabies virus-specific vaginal antibody titers and the shift of isotypes in AdHu5-preexposed, AdC68rab.gp-vaccinated mice. Isotype switching is controlled by T helper cells through cytokines, with interleukin-5, a Th2 cytokine, promoting IgA production and interleukin-4, a Th2 cytokine, inhibiting IgG2a production, which is counterbalanced by gamma interferon, a Th1 cytokine. Alternative pathways, such as effects of nonneutralizing Ad-specific antibodies on antigen presentation, cannot be excluded but are unlikely, as they should also have affected the antibody isotype profile of mice exposed to AdHu5 prior to vaccination with the AdHu5rab.gp vector. Primer-booster experiments using combinations of the Ad vaccine carriers given orally showed no advantage of heterologous over homologous boosters. Instead, maximal VNA titers were induced by repeated homologous immunization with the AdHu5rab.gp vector. Considering the lack of interference by preexisting vector-neutralizing antibodies in the transgene product-specific antibody response to oral vaccination, this result was predictable, as was the inefficiency of systemic homologous primer-booster immunization. Oral homologous versus heterologous booster immunizations with the two Ad vaccine carriers had a distinct effect on the isotypes of vaginal antibodies, as was also observed upon preexposure to AdHu5. Heterologous primer-booster vaccination, regardless of the sequence of the vaccine carriers, resulted in a balanced ratio of IgA antibodies to IgG2a antibodies (IgA/IgG2a ratio of ∼0.8). Double immunization with the AdHu5rab.gp vector, on the other hand, favored induction of vaginal IgA antibodies over IgG2a antibodies to rabies virus (IgA/IgG2a ratio of >2), while repeated oral application of the AdC68rab.gp vector strongly favored induction of vaginal IgG2a responses over IgA responses (IgA/IgG2a ratio of >0.3). This again implies that T helper cells induced by the original vaccine influenced the types of the T helper and B-cell responses to the second vaccine.
The finding that the efficacy of orally delivered Ad-based vaccines is not impaired by preexisting Ad serotype-specific antibodies has implications for further development of such vaccines for a human target population. The findings indicate that one of the major drawbacks of recombinant vaccines based on Ad delivery systems, that is, the concomitant induction of carrier-specific neutralizing antibodies that reduce the efficacy of homologous booster immunizations for the same or a different transgene product, does not apply to oral delivery. Preexposure can modify the isotype profile of mucosal antibodies. This effect may be complex in humans that encounter natural infections with a multitude of different replicating Ad serotypes, which may vary in their preferential inductions of different T helper cell subsets. The one disadvantage of oral delivery of Ad recombinants, that is, the need for increased vaccine doses to achieve protective immunity, may at least in part be overcome by the use of more sophisticated delivery systems in larger target species.
Acknowledgments
We thank C. Cole for preparation of the manuscript.
This work was funded by grants from NIH/NIAID.
REFERENCES
- 1.Belyakov, I. M., B. Moss, W. Strober, and J. A. Berzofsky. 1999. Mucosal vaccination overcomes the barrier to recombinant vaccinia immunization caused by pre-existing poxvirus immunity. Proc. Natl. Acad. Sci. USA 96:4512-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen, C. J., Z. Q. Xiang, G. P. Gao, H. C. J. Ertl, J. M. Wilson, and J. M. Bergelson. 2002. Chimpanzee adenovirus CV-68 adapted as a gene delivery vector interacts with the coxsackievirus and adenovirus receptor. J. Gen. Virol. 83:151-155. [DOI] [PubMed] [Google Scholar]
- 3.Farina, S. F., G. P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, H. C. J. Ertl, and J. M. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald, E. A, M. Gallagher, W. S. Hunter, and E. B. Seligmann, Jr. 1978. Use of the antibody assay in immunized mice for the determination of rabies vaccine potency. Dev. Biol. Stand. 40:183-186. [PubMed] [Google Scholar]
- 5.Gogev, S., N. Vanderheijden, M. Lemaire, F. Schynts, J. D'Offay, I. Deprez, M. Adam, M. Eloit, and E. Thiry. 2002. Induction of protective immunity to bovine herpesvirus type 1 in cattle by intranasal administration of replication-defective human adenovirus type 5 expressing glycoprotein gC or gD. Vaccine 20:1451-1465. [DOI] [PubMed] [Google Scholar]
- 6.Gostin, L. O., J. W. Sapsin, S. P. Teret, S. Burris, J. S. Mair, J. G. Hodge, Jr., and J. S. Vernick. 2002. The Model State Emergency Health Powers Act: planning for and response to bioterrorism and naturally occurring infectious diseases. JAMA 288:622-628. [DOI] [PubMed] [Google Scholar]
- 7.Hammond, J. M., E. S. Jansen, C. J. Morrissy, M. M. Williamson, A. L. Hodgson, and M. A. Johnson. 2001. Oral and sub-cutaneous vaccination of commercial pigs with a recombinant porcine adenovirus expressing the classical swine fever virus gp55 gene. Arch. Virol. 146:1787-1793. [DOI] [PubMed] [Google Scholar]
- 8.He, Z., A. P. Wlazlo, D. W. Kowalczyk, J. Cheng, Z. Q. Xiang, W. Giles-Davis, and H. C. J. Ertl. 2000. Viral recombinant vaccines to the E6 and E7 antigens of HPV-16. Virology 270:146-161. [DOI] [PubMed] [Google Scholar]
- 9.Jodar, L., P. Duclos, J. B. Milstien, E. Griffiths, M. T. Aguado, and C. J. Clements. 2001. Ensuring vaccine safety in immunization programmes-a W. H. O. perspective. Vaccine 19:1594-1605. [DOI] [PubMed] [Google Scholar]
- 10.Kuriyama, S., K. Tominaga, M. Kikukawa, T. Nakatani, H. Tsuinoue, M. Yamazaki, S. Nagao, Y. Toyokawa, A. Mitoro, and H. Fukui. 1998. Inhibitory effects of human sera on adenovirus-mediated gene transfer into rat liver. Anticancer Res. 18:2345-2352. [PubMed] [Google Scholar]
- 11.Lees, C. Y., D. J. Briggs, X. Wu, R. D. Davis, S. M. Moore, C. Gordon, Z. Q. Xiang, H. C. J. Ertl, C. C. de Tang, and Z. F. Fu. 2002. Induction of protective immunity by topic application of a recombinant adenovirus expressing rabies virus glycoprotein. Vet. Microbiol. 85:295-303. [DOI] [PubMed] [Google Scholar]
- 12.Mincheff, M., I. Altankova, S. Zoubak, S. Tchakarov, C. Botev, S. Petrov, E. Krusteva, G. Kurteva, P. Kurtev, V. Dimitrov, M. Ilieva, G. Georgiev, T. Lissitchkov, I. Chernozemski, and H. T. Meryman. 2001. In vivo transfection and/or cross-priming of dendritic cells following DNA and adenoviral immunizations for immunotherapy of cancer—changes in peripheral mononuclear subsets and intracellular IL-4 and IFN-gamma lymphokine profile. Crit. Rev. Oncol. Hematol. 39:125-132. [DOI] [PubMed] [Google Scholar]
- 13.Moffatt, S., J. Hays, H. HogenEsch, and S. K. Mittal. 2000. Circumvention of vector-specific neutralizing antibody response by alternating use of human and non-human adenoviruses: implications in gene therapy. Virology 272:159-167. [DOI] [PubMed] [Google Scholar]
- 14.Moraes, M. P., G. A. Mayr, P. W. Mason, and M. J. Grubman. 2002. Early protection against homologous challenge after a single dose of replication-defective human adenovirus 5 expressing capsid protein of foot and mouth disease virus (FMDV) strain A24. Vaccine 20:1631-1639. [DOI] [PubMed] [Google Scholar]
- 15.Mutwiri, G., C. Bateman, M. E. Baca-Estrada, M. Snider, and P. Griebel. 2000. Induction of immune responses in newborn lambs following enteric immunization with a human adenovirus vaccine vector. Vaccine 19:1284-1293. [DOI] [PubMed] [Google Scholar]
- 16.Papp, Z., L. A. Babiuk, and M. E. Baca-Estrada. 1999. The effect of pre-existing adenovirus-specific immunity on immune responses induced by recombinant adenovirus expressing glycoprotein D of bovine herpesvirus type 1. Vaccine 17:933-943. [DOI] [PubMed] [Google Scholar]
- 17.Sabin, A. B. 1965. Oral poliovirus vaccine. History of its development and prospects for eradication of poliomyelitis. JAMA 194:872-876. [DOI] [PubMed] [Google Scholar]
- 18.Sharpe, S., A. Fooks, J. Lee, K. Hayes, C. Clegge, and M. Cranage. 2002. Single oral immunization with replication deficient recombinant adenovirus elicits long-lived transgene-specific cellular and humoral immune responses. Virology 293:210-216. [DOI] [PubMed] [Google Scholar]
- 19.Shi, Z., M. Zeng, G. Yang, F. Siegel, L. J. Cain, K. R. van Kampen, C. A. Elmets, and D.-C. C. Tang. 2001. Protection against tetanus by needle-free inoculation of adenovirus-vectored nasal and epicutaneous vaccines. J. Virol. 75:11474-11482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 22.Tims, T., D. J. Briggs, R. D. Davis, S. M. Moore, Z. Q. Xiang, H. C. J. Ertl, and Z. F. Fu. 2000. Adult dogs receiving a rabies booster dose with a recombinant adenovirus expressing rabies virus glycoprotein develop high titers of neutralizing antibodies. Vaccine 18:2804-2807. [DOI] [PubMed] [Google Scholar]
- 23.Vos, A., A. Neubert, E. Pommerening, T. Muller, L. Dohner, L. Neubert, and K. Hughes. 2001. Immunogenicity of an E1-deleted human adenovirus against rabies by different routes of administration. J. Gen. Virol. 82:2191-2197. [DOI] [PubMed] [Google Scholar]
- 24.Wiktor, T. J. 1973. Laboratory techniques in rabies: tissue culture methods. WHO Monogr. Ser. 23:101-123. [PubMed] [Google Scholar]
- 25.Wiktor, T. J., P. Atanasiu, M. Bahmanyar, K. Boegel, J. H. Cox, A. M. Diaz, E. A. Fitzgerald, E. Kuwert, R. Netter, M. Selimov, G. Turner, and G. van Steenis. 1978. Comparison studies on potency tests for rabies vaccines. Dev. Biol. Stand. 40:171-178. [PubMed] [Google Scholar]
- 26.Wiktor, T. J., R. I. Macfarlan, K. J. Reagan, B. Dietzschold. P. J. Curtis, W. H. Wunner, M. P. Kieny, R. Lathe, J. P. Lecocq, and M. Mackett. 1984. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc. Natl. Acad. Sci. USA 81:7194-7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiang, Z. Q., and H. C. J. Ertl. 1999. Induction of mucosal immunity with a replication defective adenoviral recombinant. Vaccine 17:2003-2008. [DOI] [PubMed] [Google Scholar]
- 28.Xiang, Z. Q., G. P. Gao, A. Reyes-Sandoval, C. J. Cohen, Y. Li, J. M. Bergelson, J. M. Wilson, and H. C. J. Ertl. 2002. Novel, chimpanzee serotype 68-based adenoviral vaccine carrier for induction of antibodies to a transgene product. J. Virol. 76:2667-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang, Z. Q., B. B. Knowles, J. W. McCarrick, and H. C. J. Ertl. 1995. Immune effector mechanisms required for protection to rabies virus. Virology 214:398-404. [DOI] [PubMed] [Google Scholar]
- 30.Xiang, Z. Q., S. Pasquini, and H. C. J. Ertl. 1999. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J. Immunol. 162:6716-6723. [PubMed] [Google Scholar]
- 31.Xiang, Z. Q., S. Spitalnik, M. Tran, W. H. Wunner, J. Cheng, and H. C. J. Ertl. 1994. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology 199:132-140. [DOI] [PubMed] [Google Scholar]
- 32.Xiang, Z. Q., Y. Yang, J. M. Wilson, and H. C. J. Ertl. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219:220-227. [DOI] [PubMed] [Google Scholar]
- 33.Zhong, L., A. Granelli-Piperno, Y. Choi, and R. M. Steinman. 1999. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur. J. Immunol. 29:964-972. [DOI] [PubMed] [Google Scholar]