Abstract
Varicella-zoster virus (VZV) results in a lifelong latent infection in human sensory and cranial nerve ganglia after primary infection. VZV open reading frame 47 (ORF47) and ORF66 encode protein kinases that phosphorylate several viral proteins, including VZV glycoprotein gE and ORF32, ORF62, and ORF63 proteins. Here we show that the ORF47 protein kinase also phosphorylates gI. While ORF47 is essential for virus replication in human T cells and skin, we found the gene to be dispensable for establishment of latent infection in dorsal root ganglia of rodents. ORF66 protein is expressed during latency. Rodents infected with VZV unable to express ORF66 developed latent infection at a rate similar to that for the parental virus. ORF63 transcripts, a hallmark of VZV latency, were also detected in similar numbers of animals infected with the ORF47 and ORF66 mutants and with the parental virus. VZV mutants unable to express four of the six genes that do not have herpes simplex virus (HSV) homologs (ORFs 1, 13, 32, 57) were also unimpaired for establishment of latency. While a truncated HSV VP16 mutant was previously reported to be unable to establish latency in a mouse model, we found that VZV with a deletion of ORF10, the homolog of HSV VP16, was dispensable for establishment of latency. Thus, seven genes, including one expressed during latency, are dispensable for establishing latent VZV infection.
Primary infection with varicella-zoster virus (VZV) causes chicken pox, and the virus disseminates throughout the body. Infection of the nervous system during primary infection results in establishment of virus latency in sensory ganglia. The same virus can reactivate years later to cause herpes zoster. Although both VZV and herpes simplex virus (HSV) establish latency in sensory neurons, several aspects of latent infection differ for each virus. VZV usually reactivates once, if at all, during the lifetime of immunocompetent persons, whereas HSV often reactivates multiple times. The latency-associated transcripts are the only viral mRNAs abundantly expressed during latency of HSV, and no viral proteins are expressed. In contrast, during VZV latency, multiple genes are expressed. Transcripts from open reading frames (ORFs) 4, 21, 29, 62, 63, and 66 have been demonstrated in trigeminal or dorsal root ganglia latently infected with VZV from humans (9-13, 15, 22, 31) and from rats (2, 21, 37). ORF63 transcripts are the most abundant VZV mRNAs expressed during latency (12). ORF63 protein has also been detected during latency by several laboratories (16, 27, 29), and other proteins, including the ORF21, ORF29, ORF62, and ORF66 proteins, have been reported in single studies to be expressed during latency (11, 27).
A number of VZV genes are dispensable in cell culture (8). Several of these genes have important roles in viral pathogenesis. VZV encodes two protein kinases, ORF47 and ORF66 proteins, that are dispensable for virus replication in melanoma cells and fibroblasts (18, 19, 33). ORF47 protein phosphorylates the VZV ORF62 and ORF63 immediate-early proteins, gE, and ORF47 and ORF32 proteins (18, 23, 24, 35). ORF47 is required for infection of human lymphocytes and skin (33, 41). ORF66 is important for VZV replication in lymphocytes but is dispensable for growth in skin (33, 41).
Other nonessential genes may be important in the pathogenesis of VZV infection. ORF10 encodes a virion-associated transactivator, the homolog of HSV VP16, which is essential for replication of HSV. Although ORF10 shares functions similar to those of VP16 as a transactivator, it is dispensable in cell culture (6, 34). VZV has six genes, ORFs 1, 2, 13, 32, 57, and S/L, which do not have homologs in HSV and which are dispensable for growth of the virus in cell culture (5, 7, 14, 20, 35, 39).
We have found that inoculation of cotton rats intramuscularly with VZV results in establishment of latent infection in the dorsal root ganglia. These latently infected ganglia usually express ORF63 transcripts but rarely express ORF40 transcripts, the latter of which are usually not associated with latency (39). These results are similar to what has been observed with latently VZV-infected human and rat ganglia (21, 22). Reactivation, with recovery of infectious VZV, has not been documented with the rat or cotton rat model.
Using the cotton rat model, we showed that VZV ORF2 (39), ORF17 (38), ORF21 (45), and ORF61 (40) are dispensable for latency. Here we show that the VZV protein kinases (ORF47 and ORF66), the VZV homolog of HSV VP16 (ORF10), and most of the VZV genes not conserved with HSV (ORF1, 13, 32, and 57) are dispensable for latent infection. We also show that ORF47 protein phosphorylates VZV gI.
MATERIALS AND METHODS
Cells and viruses.
Human melanoma cells, a gift from Charles Grose, were used for preparation of virus stocks. Recombinant viruses were derived from cosmids corresponding to the vaccine Oka strain of VZV (5). VZV mutants unable to express ORFs 1, 10, 13, 32, 47, 57, and 66 have been described previously (5, 6, 7, 14, 18, 19, 35).
Immunoprecipitations.
VZV-infected or uninfected cells were radiolabeled with [35S]methionine or [33P]- or [32P]orthophosphate acid and lysed, and supernatants were incubated with murine monoclonal antibody to VZV gI (Biodesign International, Saco, Maine) or gE (Chemicon, Temecula, Calif.). Immune complexes were precipitated with protein G-Sepharose, washed, and fractionated on sodium dodecyl sulfate-polyacrylamide gels, and autoradiography was performed.
Animal experiments.
Animal experiments were performed as described previously (39). Seven-week-old male or female cotton rats were anesthetized and inoculated intramuscularly with 3 × 105 PFU of VZV-infected melanoma cells at six sites on each side of the thoracic and lumber spine. Some animals were inoculated with uninfected melanoma cells, while other animals received VZV recombinant Oka (ROka)-infected melanoma cells that had been heat inactivated. The animals were sacrificed one month after inoculation, and thoracic and lumbar dorsal root ganglia were removed. DNA was obtained from pooled left dorsal root ganglia of mock- or VZV-infected animals by using a Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.). RNA was obtained from pooled right dorsal root ganglia by using Trizol (Invitrogen, Carlsbad, Calif.). The RNA was treated with RNase-free DNase I at 37°C, followed by incubation at 65°C to inactivate the DNase I. cDNA was obtained by incubating RNA with Superscript II reverse transcriptase (Invitrogen) and oligo(dT)12-18 (Invitrogen) at 42°C.
Dorsal root ganglia DNA (500 ng), cDNA, or serial dilutions of cosmid NotIA or MstIIA in 500 ng of DNA from ganglia of uninfected animals was amplified by PCR with ORF21 or ORF63 primers as described previously (39).
Southern blots.
PCR products from ganglia were fractionated on agarose gels, transferred to nylon membranes, and hybridized to [32P]dCTP radiolabeled probes. ORF21 and ORF63 probes were prepared as reported previously (39). The radioactive signal on the blot was quantified by using a phosphorimager. The copy numbers of VZV DNA in 500 ng of genomic DNA of cotton rats were estimated with standard curves, which were constructed based on the amount of PCR products obtained from serial dilutions of cosmids NotIA and MstIIA for VZV ORF21 and ORF63, respectively, mixed with 500 ng of ganglion DNA from uninfected animals (39, 45).
Statistical analyses.
Student's t tests and nonparametric analyses (Mann-Whitney U test and chi-square test) were performed to determine if differences in the frequencies of positive samples and mean copy numbers of VZV DNA among the groups were significant.
RESULTS
ORF47 protein phosphorylates gI.
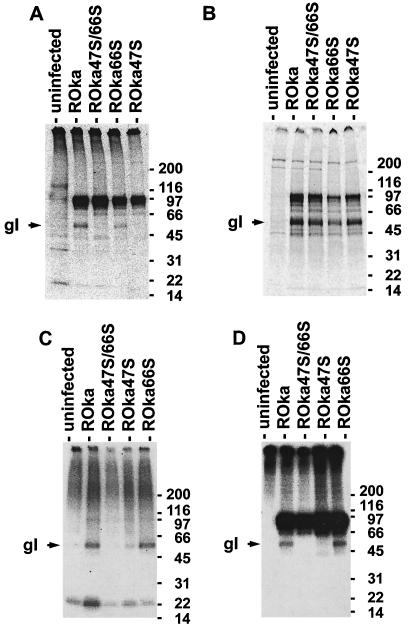
VZV encodes two protein kinases, ORF47 and ORF66 proteins. ORF47 protein phosphorylates gE, ORF32, ORF47, ORF62, and ORF63 proteins (23, 24, 35), while ORF66 protein phosphorylates ORF62 protein (18). To determine if either ORF47 or ORF66 protein could phosphorylate gI, melanoma cells were infected with parental ROka VZV or recombinant VZV unable to express either or both of the protein kinases (ROka47S, ROka66S, or ROka47S/66S). The infected cells were radiolabeled with [35S]methionine or [32P]orthophosphate and immunoprecipitated with antibody to gE.
gE was detected as bands of 67 to 98 kDa in cells infected with parental VZV and each of the mutants (Fig. 1). Since gE forms a complex with gI, antibody to gE coimmunoprecipitated a band corresponding to mature gI (58 kDa) in cells infected with parental VZV (ROka) that were radiolabeled with [33P]orthophosphate (Fig. 1A) or [35S]methionine (Fig. 1B). gI was immunoprecipitated from radiolabeled ROka66S-infected cells with antibody to gE. In contrast, while gI was detected in ROka47S- or ROka47S/66S-infected cells that were radiolabeled with [35S]methionine, gI was not detected in ROka47S- or ROka47S/66S-infected cells that were radiolabeled with [33P]orthophosphate and immunoprecipitated with antibody to gE.
FIG. 1.
Immunoprecipitation of gE and gI from VZV-infected cells. Cells infected with parental VZV (ROka) or with ORF47 or ORF66 mutant viruses (ROka47S, ROka66S, or ROka47S/66S) were radiolabeled with [33P]orthophosphate (A), [35S]methionine (B), or [32P]orthophosphate (C and D). Cells were immunoprecipitated with monoclonal antibody to gE (A, B, and D) or gI (C). The numbers to the right of the blots indicate molecular masses (in kilodaltons). gE is 67 to 98 kDa, and gI is 58 kDa (indicated by arrowheads).
To confirm these results, immunoprecipitation was performed in VZV-infected cells radiolabeled with [32P]orthophosphate by using monoclonal antibody to gI. While gI was readily detected in cells infected with ROka or ROka66S, gI was barely detectable in cells infected with ROka47S or ROka47S/66S (Fig. 1C). As a control, immunoprecipitation was performed from another aliquot of the [32P]orthophosphate-labeled lysate by using antibody to gE. gE was immunoprecipitated from lysates of cells infected with the parental and each of the mutant viruses (Fig. 1D). Thus, ORF47 is primarily responsible for phosphorylation of gI in VZV-infected cells.
VZV mutants unable to express ORF47 or ORF66 are not required for establishment of latency.
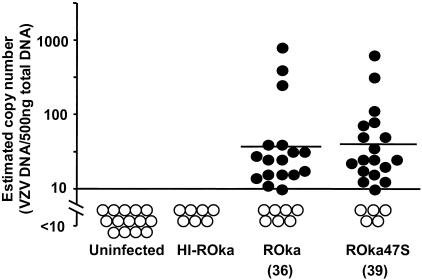
VZV ORF47 protein kinase is essential for replication of VZV in human lymphocytes and skin (33, 41). To determine whether VZV unable to express ORF47 might also be impaired in establishing latent infection, cotton rats were inoculated with cells containing VZV ROka or ROka47S and one month later DNA was isolated from pooled thoracic and lumbar dorsal root ganglia. PCR assay followed by Southern blotting showed positive signals (≥10 copies of VZV DNA/500 ng of ganglion DNA) in ganglia from 17 of 24 ROka-infected and 18 of 23 ROka47S-infected cotton rats (Fig. 2). As a control, DNA extracted from ganglia of uninoculated cotton rats or from animals inoculated with uninfected cells or heat-inactivated ROka-infected cells showed no detectable VZV DNA. The geometric mean number of VZV copies from PCR-positive ganglia of animals infected with ROka47S (39 copies) was similar to that for animals infected with ROka (36 copies).
FIG. 2.
Infection of cotton rats with cells containing VZV unable to express ORF47 or with parental virus results in similar VZV DNA copy numbers in dorsal root ganglia. Geometric mean copy numbers for animals considered positive (•) are shown. Results for animals with copy numbers below the threshold (10 copies of VZV DNA per 500 ng of ganglia DNA) for reliable detection are indicated by open circles (○). The horizontal bars indicate the geometric mean copy numbers per 500 ng of ganglion DNA for animals who tested positive by PCR. Animals inoculated with cells containing heat-inactivated ROka (HI-ROka) and those receiving either uninfected cells or those not inoculated (Uninfected) had no detectable viral DNA in their ganglia.
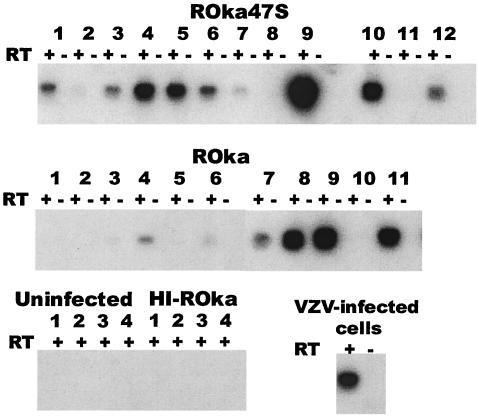
To verify that animals with latent VZV DNA also expressed VZV transcripts, RNA was isolated from dorsal root ganglia of cotton rats and reverse transcription was performed, followed by amplification of the cDNA by PCR by using ORF63 primers and Southern blotting. ORF63 transcripts were present in ganglia from 9 of 12 VZV ROka47S-infected and 6 of 11 ROka-infected cotton rats, a difference that was not significant (Fig. 3).
FIG. 3.
Infection of cotton rats with cells containing ROka or ROka47S results in similar numbers of animals with ORF63 transcripts in dorsal root ganglia. RNA was isolated from dorsal root ganglia, cDNA was amplified, and PCR was performed, followed by Southern blotting for ORF63. cDNA was prepared in the presence (+) or absence (−) of reverse transcriptase (RT). ORF63 transcripts were detected in animals 1, 3, 4, 5, 6, 7, 9, 10, and 12 infected with VZV ROka47S and animals 4, 6, 7, 8, 9, and 11 infected with ROka. ORF63 RNA was present in VZV-infected cells. Animals inoculated with uninfected cells or cells containing heat-inactivated ROka (HI-ROka) have no detectable ORF63 transcripts.
To further confirm that the animals were latently infected with VZV and not undergoing a persistent infection involving other organs, DNA was isolated from the brains and lungs of cotton rats that had been infected with VZV ROka for 1 month. PCR assays followed by Southern blotting showed no positive signals in the lungs of 9 of 9 animals (<10 copies of VZV DNA/500 ng of lung DNA) and a positive signal in the brain of only 1 of 9 animals (79 copies of VZV DNA/500 ng of brain DNA for the positive animal). These results indicate that the animals were latently infected with VZV rather than having a persistent systemic infection with the virus.
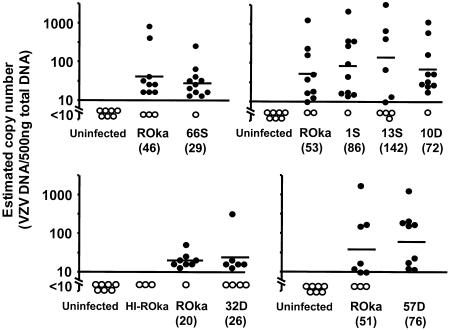
VZV ORF66 transcripts and protein have been detected during latency in human ganglia, suggesting that they are important for latency (11). To determine if ORF66 is required for establishment of latency, cotton rats were inoculated with VZV unable to express ORF66, and VZV DNA was amplified from dorsal root ganglia 1 month later. Similar numbers of animals from both groups had latent viral DNA (Fig. 3), and the geometric mean VZV copy number from ganglia containing VZV DNA was similar for animals infected with VZV ROka (46 copies) and for those infected with VZV ROka66S (29 copies). ORF63 transcripts were detected in ganglia from five of five animals infected with VZV ROka and four of five animals infected with ROka66S (Table 1).
TABLE 1.
Frequency of ORF63 transcripts in VZV-infected cotton rats
| Virus | No. of animals with VZV ORF63 transcripts
|
||||
|---|---|---|---|---|---|
| Expt
| |||||
| 1 | 2 | 3 | 4 | 5 | |
| ROka | 6/11 | 5/5 | 4/5 | 5/5 | 3/5 |
| ROka1S | 4/5 | ||||
| ROka10D | 3/5 | ||||
| ROka13S | 5/5 | ||||
| ROka32D | 4/5 | ||||
| ROka47S | 9/12 | ||||
| ROka57D | 4/5 | ||||
| ROka66S | 4/5 | ||||
VZVs unable to express ORFs 1, 10, 13, 32, or 57 are not impaired in their ability to establish latent infection.
VZV ORFs 1, 13, 32, and 57 do not have homologs in HSV. Since herpesvirus mutants unable to express these proteins have not been tested for establishment of latency in animals, we inoculated cotton rats with each of the VZV mutants and determined if they were impaired for establishment of latency. While ORF10 has a homolog in HSV (VP16), these two proteins have different activities, since the HSV protein is essential for virus replication while the VZV protein is dispensable (6). Therefore, we also tested a VZV mutant with ORF10 deleted for latency.
The frequency and the mean geometric copy numbers of positive ganglia from animals infected with mutants unable to express ORFs 1, 10, 13, 32, or 57 were not significantly different from those for the parental virus (P > 0.05 for each mutant tested versus ROka) (Fig. 4). In addition, ORF63 RNA transcripts were detected in similar numbers of animals inoculated with the mutants as those for animals infected with the parental virus (Table 1) (P > 0.05). No VZV transcripts were detected in animals inoculated with uninfected cells or heat-inactivated ROka-infected cells.
FIG. 4.
Infection of animals with cells containing VZV mutants or with parental VZV. Geometric mean copy numbers for animals considered positive (•) or below the threshold for reliable detection (○) for VZV DNA are shown. The horizontal bars indicate the geometric mean copy numbers for animals who tested positive by PCR. Each panel represents a separate experiment. HI-ROka indicates animals inoculated with heat-inactived ROka-infected cells. Uninfected indicates animals that were either inoculated with uninfected cells or not inoculated.
DISCUSSION
We explored the role of VZV genes that are critical for replication in certain cell types or that lack homologs in other human herpesviruses and found that none of them altered the ability of the virus to establish latency. The VZV ORF47 protein kinase is dispensable for establishment of latent infection. Human T cells infected with VZV unable to express ORF47 showed production of an immediate-early protein (ORF62) but rarely expressed a late protein (gE) (41). Thus, while ORF47 protein is not required for viral entry into lymphocytes, it is critical for replication. Furthermore, VZV unable to express ORF47 is impaired by approximately 1,000-fold for spread from lymphocytes to melanoma cells. In addition, the ORF47 mutant cannot replicate in human fetal skin cells (33). During primary infection, VZV is postulated to infect cells in the mucosa of the respiratory tract and produce a viremia by infecting lymphocytes. Virus is then transferred from lymphocytes to other tissues in the body, including sensory nerve and cranial nerve ganglia, to establish a latent infection. Thus, while we detected latency in animals inoculated intramuscularly along the spine with the ORF47 mutant, it is likely that latency could not occur during natural infection with the ORF47 mutant, since the virus is blocked for replication in T cells and for transfer of virus from T cells to other cells.
During vaccination, VZV is inoculated subcutaneously and may spread to the nervous system directly in the absence of viremia or infection of skin cells. The observation that zoster occurs at the site of inoculation (17) suggests that the virus can migrate directly to the ganglia and back and implies that viremia is not required for infection of the nervous system. The finding that VZV unable to express ORF47 is able to establish a latent infection similar to that for parental virus after intramuscular inoculation of rodents implies that this virus might be able to establish latency if administered subcutaneously, as with vaccination. However, its inability to replicate in skin and lymphocytes would likely reduce its ability to subsequently cause disease.
We found that the ORF47 protein phosphorylates gI. gI is also phosphorylated by a cyclin-dependent kinase (46). While gI is not required for growth in most cell lines in vitro (4, 30), it is essential for virus replication in human fetal lymphocytes and skin (32). Since ORF47 phosphorylates gI, the phosphorylation of the glycoprotein may be critical for its role in growth in lymphocytes and skin. Phosphorylation of gI may enhance its interactions with gE. gE and gI form heterodimers, and gI is required for the normal trafficking of gE in the cell. Since gE is likely required for VZV replication (30; J. Cohen, unpublished data), phosphorylation of gI by ORF47 may play a role in efficient replication for certain cell types.
We have shown that the VZV ORF66 protein kinase is dispensable for establishment of latent infection. ORF66 transcripts and protein are expressed during latency in human ganglia (11). ORF66 phosphorylates ORF62 protein and inhibits its nuclear localization (26). Lungu et al. (27) showed that ORF62 protein was present in neurons during latency but was sequestered in the cytoplasm. They postulated that the exclusion of immediate-early ORF62 protein and other viral proteins from the nucleus might prevent additional virus gene expression and replication, thereby allowing the virus to maintain a latent infection. Therefore, the presence of ORF66 protein in latently infected neurons might maintain ORF62 protein in a phosphorylated form and prevent its nuclear localization in neurons. However, the ability of VZV unable to express ORF66 to establish latency implies that phosphorylation of ORF62 and its exclusion from the nucleus might not be required for latency. ORF66 is also important for packaging ORF62 protein into virions (25). The observation that ORF66 is not required for latency suggests that the presence of ORF62 in virions may also be dispensable for establishing a latent infection.
VZV ORF10 was not required for establishment of latency. ORF10 is the homolog of HSV VP16, and both proteins function as virion-associated transactivators (34). While VP16 is essential for replication of HSV, ORF10 is dispensable for replication of VZV (6). Similarly, while HSV with a carboxy-terminal-truncated VP16 mutant was unable to establish a latent infection in mice after corneal inoculation (42), ORF10 was dispensable for establishment of latency. Therefore, VZV ORF10 and HSV VP16 have different activities during both replication and establishment of latency.
VZV ORFs 1, 13, 32, and 57 were dispensable for establishment of latency. ORFs 1, 13, 32, and 57 do not have homologs in HSV and are dispensable for replication of VZV in vitro and for infection of human T cells (41). ORFs 1, 32, and 57 have homologs in equine herpesvirus (43, 44), and ORF13, the viral thymidylate synthetase, has a homolog in human herpesvirus 8 (36) and herpesvirus saimiri (1). Equine and gammaherpesviruses with mutations in these genes have not been tested for impairment of latency.
Our results indicate that a number of VZV genes are dispensable for establishment of latency in the rodent, including one gene, ORF66, which is expressed during latency. It is likely that some of these genes would be required for VZV to disseminate to the ganglia if infection had been tested by the natural, respiratory route, rather than by intramuscular injection. Several of these genes may also be necessary for other aspects of latency, such as maintenance or reactivation. Additional models will be needed to study VZV reactivation. Recent developments using in vitro models of VZV latency and reactivation (3) or using the simian varicella virus animal model (28) may be helpful in determining the roles of these proteins in other aspects of latent infection.
Acknowledgments
Hitoshi Sato was the recipient of a Japan Society for the Promotion of Science (JSPS) fellowship in biomedical and behavioral research at the National Institutes of Health.
We thank Stephen Straus for reviewing the manuscript.
REFERENCES
- 1.Albrecht, J. C., J. Nicholas, D. Biller, K. R. Cameron, B. Biesinger, C. Newman, S. Wittmann, M. A. Craxton, H. Coleman, B. Fleckenstein, and R. W. Honess. 1992. Primary structure of the herpesvirus saimiri genome. J. Virol. 66:5047-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunell, P. A., L. C. Ren, J. I. Cohen, and S. E. Straus. 1999. Viral gene expression in rat trigeminal ganglia following neonatal infection with varicella-zoster virus. J. Med. Virol. 58:286-290. [DOI] [PubMed] [Google Scholar]
- 3.Chen, J., A. Gershon, Z. S. Li, O. Lungu, and M. D. Gershon. 2003. Latent and lytic infection of isolated guinea pig enteric ganglia by varicella zoster virus. J. Med. Virol. 70(Suppl. 1):S71-S78. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, J. I., and H. Nguyen. 1997. Varicella-zoster virus glycoprotein I is essential for growth of virus in Vero cells. J. Virol. 71:6913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen, J. I., and K. E. Seidel. 1993. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc. Natl. Acad. Sci. USA 90:7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, J. I., and K. E. Seidel. 1994. Varicella-zoster virus (VZV) open reading frame 10 protein, the homolog of the essential herpes simplex virus protein VP16, is dispensable for VZV replication in vitro. J. Virol. 68:7850-7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, J. I., and K. E. Seidel. 1995. Varicella-zoster virus open reading frame 1 encodes a membrane protein that is dispensable for growth of VZV in vitro. Virology 206:835-842. [DOI] [PubMed] [Google Scholar]
- 8.Cohen, J. I., and S. E. Straus. 2001. Varicella-zoster virus and its replication, p. 2702-2730. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott, Williams, & Wilkins, Philadelphia, Pa.
- 9.Cohrs, R. J., M. Barbour, and D. H. Gilden. 1996. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 70:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohrs, R. J., M. B. Barbour, R. Mahalingam, M. Wellish, and D. H. Gilden. 1995. Varicella-zoster virus (VZV) transcription during latency in human ganglia: prevalence of VZV gene 21 transcripts in latently infected human ganglia. J. Virol. 69:2674-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohrs, R. J., D. H. Gilden, P. R. Kinchington, E. Grinfeld, and P. G. E. Kennedy. 2003. Varicella-zoster virus gene 66 transcription and translation in latently infected human ganglia. J. Virol. 77:6660-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohrs, R. J., J. Randall, J. Smith, D. H. Gilden, C. Dabrowski, H. van Der Keyl, and R. Tal-Singer. 2000. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J. Virol. 74:11464-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohrs, R. J., K. Srock, M. B. Barbour, G. Owens, R. Mahalingam, M. E. Devlin, M. Wellish, and D. H. Gilden. 1994. Varicella-zoster virus (VZV) transcription during latency in human ganglia: construction of a cDNA library from latently infected human trigeminal ganglia and detection of a VZV transcript. J. Virol. 68:7900-7908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox, E., S. Reddy, I. Iofin, and J. I. Cohen. 1998. Varicella-zoster virus ORF57, unlike its pseudorabies virus UL3.5 homolog, is dispensable for viral replication in cell culture. Virology 250:205-209. [DOI] [PubMed] [Google Scholar]
- 15.Croen, K. D., J. M. Ostrove, L. J. Dragovic, and S. E. Straus. 1988. Patterns of gene expression and sites of latency in human nerve ganglia are different for varicella-zoster and herpes simplex viruses. Proc. Natl. Acad. Sci. USA 85:9773-9777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Debrus, S., C. Sadzot-Delvaux, A. F. Nikkels, J. Piette, and B. Rentier. 1995. Varicella-zoster virus gene 63 encodes an immediate-early protein that is abundantly expressed during latency. J. Virol. 69:3240-3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy, I., A. A. Gershon, S. P. Steinberg, and P. LaRussa. 1991. The incidence of zoster after immunization with live attenuated varicella vaccine—a study in children with leukemia. N. Engl. J. Med. 325:1545-1550. [DOI] [PubMed] [Google Scholar]
- 18.Heineman, T. C., and J. I. Cohen. 1995. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J. Virol. 69:7367-7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heineman, T. C., K. E. Seidel, and J. I. Cohen. 1996. The varicella-zoster virus ORF66 protein induces kinase activity and is dispensable for viral replication. J. Virol. 70:7312-7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemble, G. W., P. Annunziato, O. Lungu, R. E. Winter, T. A. Cha, S. J. Silverstein, and R. R. Spaete. 2000. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J. Virol. 74:11311-11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy, P. G., E. Grinfeld, S. Bontems, and C. Sadzot-Delvaux. 2001. Varicella-zoster virus gene expression in latently infected rat dorsal root ganglia. Virology 289:218-223. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy, P. G., E. Grinfeld, and J. W. Gow. 1999. Latent varicella-zoster virus in human dorsal root ganglia. Virology 258:451-454. [DOI] [PubMed] [Google Scholar]
- 23.Kenyon, T. K., J. I. Cohen, and C. Grose. 2002. Phosphorylation by the varicella-zoster virus ORF47 protein serine kinase determines whether endocytosed viral gE traffics to the trans-Golgi network or recycles to the cell membrane. J. Virol. 76:10980-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenyon, T. K., J. Lynch, J. Hay, W. Ruyechan, and C. Grose. 2001. Varicella-zoster virus ORF47 protein serine kinase: characterization of a cloned, biologically active phosphotransferase and two viral substrates, ORF62 and ORF63. J. Virol. 75:8854-8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinchington, P. R., K. Fite, A. Seman, and S. E. Turse. 2001. Virion association of IE62, the varicella-zoster virus (VZV) major transcriptional regulatory protein, requires expression of the VZV open reading frame 66 protein kinase. J. Virol. 75:9106-9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinchington, P. R., and S. E. Turse. 2000. Nuclear accumulation of IE62, the varicella-zoster virus (VZV) major transcriptional protein, is inhibited by phosphorylation mediated by the VZV open reading frame 66 protein kinase. J. Virol. 74:2265-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lungu, O., C. A. Panagiotidis, P. W. Annunziato, A. A. Gershon, and S. J. Silverstein. 1998. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc. Natl. Acad. Sci. USA 95:7080-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahalingam, R., V. Traina-Dorge, M. Wellish, J. Smith, and D. H. Gilden. 2002. Naturally acquired simian varicella infection in African green monkeys. J. Virol. 76:8548-8550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahalingam, R., M. Wellish, R. Cohrs, S. Debrus, J. Piette, B. Rentier, and D. H. Gilden. 1996. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc. Natl. Acad. Sci. USA 93:2122-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallory, S., M. Sommer, and A. M. Arvin. 1997. Mutational analysis of the role of glycoprotein I in varicella-zoster virus replication and its effects on glycoprotein E conformation and trafficking. J. Virol. 71:8279-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier, J. L., R. P. Holman, K. D. Croen, J. E. Smialek, and S. E. Straus. 1993. Varicella-zoster virus transcription in human trigeminal ganglia. Virology 193:193-200. [DOI] [PubMed] [Google Scholar]
- 32.Moffat, J., H. Ito, M. Sommer, S. Taylor, and A. M. Arvin. 2002. Glycoprotein I of varicella-zoster virus is required for viral replication in skin and T cells. J. Virol. 76:8468-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffat, J. F., L. Zerboni, M. H. Sommer, T. C. Heineman, J. I. Cohen, H. Kaneshima, and A. M. Arvin. 1998. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc. Natl. Acad. Sci. USA 95:11969-11974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriuchi, H., M. Moriuchi, R. Picchyangkura, S. J. Triezenberg, S. E. Straus, and J. I. Cohen. 1995. Hydrophobic cluster analysis predicts an amino-terminal domain of varicella-zoster virus ORF10 required for transcriptional activation. Proc. Natl. Acad. Sci. USA 92:9333-9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy, S. M., E. Cox, I. Iofin, W. Soong, and J. I. Cohen. 1998. Varicella-zoster virus (VZV) ORF32 encodes a phosphoprotein that is posttranslationally modified by the VZV ORF47 protein kinase. J. Virol. 72:8083-8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sadzot-Delvaux, C., S. Debrus, A. Nikkels, J. Piette, and B. Rentier. 1995. Varicella-zoster virus latency in the adult rat is a useful model for human latent infection. Neurology 45(Suppl. 8):S18-S20. [DOI] [PubMed] [Google Scholar]
- 38.Sato, H., L. D. Callanan, L. Pesnicak, T. Krogmann, and J. I. Cohen. 2002. Varicella-zoster virus (VZV) ORF17 protein induces RNA cleavage and is critical for replication of VZV at 37°C but not 33°C. J. Virol. 76:11012-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato, H., L. Pesnicak, and J. I. Cohen. 2002. Varicella-zoster virus open reading frame 2 encodes a membrane phosphoprotein that is dispensable for viral replication and for establishment of latency. J. Virol. 76:3575-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato, H., L. Pesnicak, and J. I. Cohen. 2003. Use of a rodent model to show that varicella-zoster virus ORF61 is dispensable for establishment of latency. J. Med. Virol. 70(Suppl. 1):S79-S81. [DOI] [PubMed] [Google Scholar]
- 41.Soong, W., J. C. Schultz, A. C. Patera, M. H. Sommer, and J. I. Cohen. 2000. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J. Virol. 74:1864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tal-Singer, R., R. Pichyangkura, E. Chung, T. M. Lasner, B. P. Randazzo, J. Q. Trojanowski, N. W. Fraser, and S. J. Triezenberg. 1999. The transcriptional activation domain of VP16 is required for efficient infection and establishment of latency by HSV-1 in the murine peripheral and central nervous systems. Virology 259:20-33. [DOI] [PubMed] [Google Scholar]
- 43.Telford, E. A., M. S. Watson, K. McBride, and A. J. Davison. 1992. The DNA sequence of equine herpesvirus-1. Virology 189:304-316. [DOI] [PubMed] [Google Scholar]
- 44.Telford, E. A., M. S. Watson, J. Perry, A. A. Cullinane, and A. J. Davison. 1998. The DNA sequence of equine herpesvirus-4. J. Gen. Virol. 79:1197-1203. [DOI] [PubMed] [Google Scholar]
- 45.Xia, D., S. Srinivas, H. Sato, L. Pesnicak, S. E. Straus, and J. I. Cohen. 2003. Varicella-zoster virus open reading frame 21, which is expressed during latency, is essential for virus replication but dispensable for establishment of latency. J. Virol. 77:1211-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye, M., K. M. Duus, J. Peng, D. H. Price, and C. Grose. 1999. Varicella-zoster virus Fc receptor component gI is phosphorylated on its endodomain by a cyclin-dependent kinase. J. Virol. 73:1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]