Abstract
Recently, prokaryotic DNAs containing unmethylated CpG motifs have been shown to be intrinsically immunostimulatory both in vitro and in vivo, tending to promote Th1-like responses. In contrast, CpG dinucleotides in mammalian DNAs are extensively methylated on cytosines and hence immunologically inert. Since the herpes simplex virus (HSV) genome is unmethylated and G+C rich, we predicted that CpG motifs would be highly prevalent in the HSV genome; hence, we examined the immunostimulatory potential of purified HSV DNA in vitro and in vivo. Mouse splenocyte cultures treated with HSV DNA or HSV-derived oligodeoxyribonucleotides (ODNs) showed strong proliferative responses and production of inflammatory cytokines (gamma interferon [IFN-γ], tumor necrosis factor [TNF], and interleukin-6 [IL-6]) in vitro, whereas splenocytes treated with mammalian CV-1 DNA or non-CpG ODN did not. After immunization with ovalbumin (OVA), only splenocytes from mice immunized with HSV DNA or HSV-ODN as the adjuvants proliferated strongly and produced typical Th1 responses, including CD8+ cytotoxic T-lymphocyte responses, upon restimulation with OVA. Furthermore, HSV-ODN synergized with IFN-γ to induce nitric oxide (NO), IL-6, and TNF production from macrophages. These results demonstrate that HSV DNA and HSV-ODN are immunostimulatory, driving potent Th1 responses both in vitro and in vivo. Considering that HSV DNA has been found to persist in nonneuronal cells, these results fuel speculation that HSV DNA might play a role in pathogenesis, in particular, in diseases like herpes stromal keratitis (HSK) that involve chronic inflammatory responses in the absence of virus or viral antigens.
Historically, DNA has been viewed as immunologically inert. However, numerous recent studies have established that bacterial, but not mammalian, DNAs can activate both innate and adaptive immune responses. This indicates that the vertebrate immune system has evolved to discriminate basic structural differences between invertebrate and mammalian DNAs (27, 62). The motifs that mediate immunostimulation and discrimination of bacterial DNAs have been identified in Escherichia coli DNA as nonmethylated CpG dinucleotides flanked by specific bases (32). CpG dinucleotides are present at 25% of the expected frequency in mammalian DNA, and when they occur, they are invariably methylated on cytosines and usually flanked by bases that constitute immune-neutralizing rather immunostimulatory motifs (6, 31) Nonmethylated CpG DNA induces direct activation of professional antigen-presenting cells (APCs), including dendritic cells, macrophages, and B cells, but not T cells. CpG DNA upregulates expression of major histocompatibility complex (MHC) class II and costimulatory molecules (e.g., B7-1 and B7-2), induces cytokine production by macrophages and DCs, and additionally promotes polyclonal activation of B cells (26, 30, 32), but it does not directly activate T cells (27). Synthetic oligodeoxyribonucleotides (ODNs) containing unmethylated consensus CpG motifs can mimic immunostimulatory bacterial DNAs, and, remarkably, a single nucleotide change as in the case of GpC or methylation of the cytosine within the CpG motif is sufficient to abolish immunostimulatory activity (36, 37). It is now established that innate defense mechanisms are triggered by host reactions to pathogen-associated molecular patterns that distinguish infectious entities from the host itself and additionally discriminate among different invading pathogens (3, 41). Several studies have implicated members of the Toll-like receptor (TLR) family (originally identified in Drosophila) as the receptors for pathogen-associated molecular patterns. Thus, TLR4 and TLR2 react to lipopolysaccharides (LPS) in gram-negative bacteria and peptidoglycans and lipopeptides in gram-positive bacteria, respectively. Hemmi et al. (18) recently demonstrated that cells from TLR9-deficient mice fail to respond to CpG DNA but remain fully responsive to bacterial LPS. In contrast, inactivation of TLR4 blocked responses to LPS but not CpG DNA (22). These results show that unmethylated CpG motifs in bacterial DNAs are yet another molecular pattern for which specific reactions via TLR9 trigger innate immune responses (2, 13). Although human TLR9 also reacts to nonmethylated CpG DNAs, mouse TLR9 does not react to optimal CpG motifs for human TLR9 and vice versa, which suggests that evolutionary divergence between TLR9 molecules determines species-specific reaction to foreign DNAs (16, 58).
Although bacterial DNAs elicit the most potent responses, mammalian cells also react to Saccharomyces cerevisiae, nematode, insect, and viral genomic DNAs but, remarkably, mammalian DNA is relatively inert even when unmethylated (2). To date, there is no evidence for a role of viral CpG-containing genomes in virus infections as most published studies have reported on the adjuvant effects of CpG motifs in viral vectors deployed in DNA vaccination strategies (33). However, considering that TLR4 and CD14 mediate innate responses to respiratory syncytial virus (17) and that vaccinia virus encodes agonists specific for host TLR and IL-1 signaling (7), it would be surprising if TLR9 signaling were not involved in the host response to infection with DNA viruses. We report here that herpesvirus genomic DNAs show considerable variation in the frequencies of immunostimulatory CpG motifs; for example, CpG motifs were profoundly suppressed in the gammaherpesviruses Epstein-Barr virus (EBV) and herpesvirus saimiri (HVS) compared to alphaherpesviruses such as herpes simplex virus (HSV), in which CpG motifs were relatively enriched. Furthermore, we showed that HSV genomic DNA and CpG-containing ODNs derived therefrom are potent immune response activators both in vitro and in vivo. Considering the intrinsic immunostimulatory capacity of HSV DNA and its tendency to persist in a variety of tissues besides latently infected neurons, we speculate that in addition to initiating host innate responses, HSV DNA might be involved in the pathogenesis of HSV disease.
MATERIALS AND METHODS
Mice.
C57BL/6 female mice purchased from Taconic Inc. (Germantown, N.Y.) were maintained in the COH vivarium and used when they were 8 to 10 weeks of age.
Computer-based scans of herpesvirus genomes for stimulatory and inhibitory CpG motifs.
Inhibitory CpG motifs that neutralize stimulation by CpG motifs in cis and trans have recently been identified in the genomes of adenovirus serotypes 2 and 5 but not serotype 12 (31). We used computer-assisted scans of selected herpesvirus and adenovirus genomes to determine frequencies for stimulatory and inhibitory CpG motifs. Published consensus hexamer sequences for stimulatory and inhibitory CpG motifs defined for E. coli and adenovirus DNAs, respectively, were used in this analysis. We derived a CpG index designed to facilitate comparison of immunostimulatory potentials regardless of genome size, G+C content, and overall CpG suppression; this was not done in a prior study with adenoviruses (31). The actual frequencies of stimulatory and inhibitory motifs relative to the respective theoretical frequencies (as determined on the basis of genomic G+C content) were used to calculate a ratio of stimulatory to inhibitory motifs (e.g., for HSV type 1 [HSV-1], 1.049/0.948 = 1.107). This value was multiplied by the total number of CpG motifs found in the genome and then normalized to a 100-kb-sized genome to allow comparisons between different viruses.
DNA and ODNs.
HSV-1 DNA was prepared from virions isolated from CV-1 cells infected with HSV-1 strain F, McKrae, or KOS at low multiplicity of infection of 0.1 PFU/cell. When >90% of the cells showed cytopathic effects (usually by day 3 postinfection), cell cultures were harvested by gentle tapping of the flask to dislodge the cells that were then pelleted by low-speed centrifugation. The culture medium containing released extracellular virions was stored at 4°C, and the cell pellet was washed in ice-cold phosphate-buffered saline (PBS) in a 15-ml conical centrifuge tube. The cells were resuspended by vortexing in cold reticulocyte standard buffer buffer (10 mM Tris-HCl [pH 7.4], 10 mM KCl, 1.5 mM MgCl2) containing 0.5% NP-40, placed on ice for 5 min, and then vortexed again to promote lysis of the plasma membrane while preserving the nuclei intact. This procedure facilitated isolation of cytoplasmic virions relatively free of contamination with cellular DNA. Nuclei were pelleted by centrifugation at 800 × g and 4°C for 10 min, and the supernatant was combined with medium supernatant. The nuclei were washed with cold reticulocyte standard buffer by centrifugation, and the wash supernatant was combined with the medium. Virions in the supernatant medium were pelleted by centrifugation for 1 h at 25,000 rpm in a Ti60 rotor and a Beckman ultracentrifuge. The virus pellet was resuspended in 0.8 ml of 10 mM Tris-HCl (pH 8.4)-10 mM MgCl2. Contaminating cellular nucleic acids were removed by digestion for 2 h at 37°C with DNase I (200 μg/ml) and a mixture of RNase A plus T1. Virion DNA was released by overnight incubation at 37°C with an equal volume of 2× lysis buffer (0.8 M NaCl, 10 mM Tris-HCl [pH 8], 200 mM EDTA, 1% sodium dodecyl sulfate, 200 μg of proteinase K/ml). HSV-1 virion DNA was extracted twice with an equal volume of phenol-chloroform (1:1 [vol/vol]) equilibrated with Tris-HCl (pH 7.5) and twice with chloroform-isoamyl alcohol (24:1) before the DNA was precipitated with a 2.5 volume of 95% ethanol chilled to −20°C. The DNA recovered by centrifugation was rinsed once with 70% ethanol and dissolved in 10 mM Tris-HCl-1 mM EDTA (pH 7.5) (TE buffer) prepared in pyrogen-free glass-distilled water. DNA concentrations were determined spectrophotometrically (optical density at 260 nm of 1 = 50 μg/ml; 260/280 ratio > 1.75 and < 2.0). DNA quality was assessed by agarose gel electrophoresis of intact and BamHI-digested DNA (5 μg) in a 0.8% Tris-acetate-EDTA-agarose gel.
CV-1 cell DNA was prepared similarly except that cellular DNA was extracted from the nuclear pellet and commercially obtained E. coli DNA (Sigma, St. Louis, Mo.) was further purified by phenol-chloroform extraction followed by extraction with Triton X-114 to remove residual contaminating LPS. Unmodified ODNs and ODNs with terminal phosphorothioate (PT) linkages (two at the 5′ end and three at the 3′ end) (PT-ODN) to protect against nuclease degradation were either synthesized at the DNA Synthesis Core Facility or were obtained commercially (MWG Inc., Ebersberg, Germany). The purity of the ODNs was verified by matrix-assisted laser desorption ionization-time of flight (mass spectrometry). All DNAs were tested (using a Limulus amebocyte assay [BioWhittaker, Walkersville, Md.]) for contaminating endotoxins; endotoxin levels were <10 ng/mg for E. coli, CV-1, and HSV-1 DNAs and <1 ng/mg for synthetic ODNs.
In vitro stimulation of mouse spleen cell cultures with CpG DNA.
HSV-1 DNA and ODNs containing consensus CpG motifs derived from the gD and ICP27 genes were tested for their ability to induce proliferation and cytokine production in spleen cell cultures to determine immunostimulatory activity. E. coli DNA and a previously described CpG ODN containing two immunostimulatory CpG motifs were used as positive controls (46). CV-1 DNA and an ODN with a sequence identical to that of the CpG ODN but with an ApC or GpC substituting for CpG were used as negative controls. The sequences of the ODNs are given in Table 1. Spleens were removed from 6- to 8-week-old female C57BL/6 mice (Taconic), and splenocytes prepared from mechanically dissociated spleens (15) were grown in cultures with various concentrations of DNA or ODNs in RPMI 1640 supplemented with 10% fetal bovine serum (FBS), 50 μM 2-mercaptoethanol, 10 mM HEPES, 4 mM glutamine, 10 U of penicillin/ml, and 20 μg of streptomycin/ml (cRPMI-10). Cultures were tested for proliferation after 24 h and for cytokine production by a standard enzyme-linked immunosorbent assay (ELISA) or enzyme-linked immunospot (ELISPOT) assay after 40 h or by reverse transcriptase PCR after 72 h.
TABLE 1.
Oligonucleotides containing immunoregulatory CpG motifs
HSV-derived ODNs were from the indicated HSV genes.
Stimulatory CpG motifs are boxed, and inhibitory CpG motifs (NCCGNN and NNCGRN) are singly underlined. The control ODN (nCpG) is similar to CpG except that the stimulatory motifs have been eliminated (a second control ODN in which the CpG motifs were reversed to GpC at both locations without other sequence changes resulted in responses similar to those of the nCpG ODN [data not shown]). The guanosine run in ICP27 is marked in bold. When 5′- and 3′-terminally modified PT oligonucleotides were used, the backbone linkages indicated in lowercase letters were substituted and the names have been hyphenated with PT (e.g., ICP27-PT). The sequences for the control nCpG and CpG oligonucleotides are from reference 46.
CpG DNA stimulation of macrophage cell cultures.
Peritoneal exudate macrophages were obtained by lavage of the peritoneal cavity of normal C57BL/6 mice with 10 ml of Hanks balanced salt solution. After washing, the cells were adhered in 24-well dishes overnight and nonadherent cells were discarded to give a relatively pure (>95%) culture of macrophages. To obtain bone marrow (BM) macrophages, mice were sacrificed, their femurs were isolated, and BM was flushed out with 3 ml of cRMPI 1640 medium supplemented with 10% low-endotoxin FBS. The cell suspension was dispersed by repeated pipetting and then passed through a cell strainer to remove large cellular aggregates. To obtain macrophage cultures, the cells were plated at a density of 106 cells/well (in a 24-well dish) in cRPMI-10 supplemented with 10% granulocyte-macrophage colony-stimulating factor and 50 μM 2-mercaptoethanol and the cultures were fed with fresh medium every 48 h. On day 8 the cells were used to assess CpG DNA effects. Primary macrophage cultures were CD11b+, CD11c−, and F4/80+, and purity was estimated by FACS analysis to be >92%. The cultures were fed with medium without granulocyte-macrophage colony-stimulating factor but supplemented with 10 U of gamma interferon (IFN-γ)/ml, and the following day CpG ODNs were added to the cultures at 1 to 3 mM final concentration.
The macrophage cell line RAW 264.7 (American Type Culture Collection, Manassas, Va.) was plated at a density of 3.5 × 105 cells/well (in a 48-well dish) in Dulbecco’s minimal essential medium supplemented with 10% low-endotoxin FBS, 4 mM glutamine, and HEPES. The following day, cultures were fed with 550 μl of fresh medium supplemented with CpG ODN (1 to 3 μM) and IFN-γ (5 U/ml) or (as a positive control) purified LPS (10 to 100 ng/ml) (E. coli 0127:B8; Sigma). After 24 h, macrophage culture supernatants were assayed (using a Greiss reaction) for NO and (using a standard ELISA) for TNF and IL-6.
Determination of NO levels in macrophage cultures.
NO levels in culture supernatants were determined (using Greiss reagent: 1% sulfanilamide, 0.1% N-[1-napthyl]ethylenediamine dihydrochloride, 2.1% phosphoric acid) as nitrite concentration levels and quantitated by comparison to a standard curve generated using sodium nitrate (53). Briefly, a 100-μl aliquot of medium from the macrophage cultures was mixed with an equal volume of Greiss reagent. After 5 min at room temperature, absorbance was read at 540 nm. The data presented are averages of results for triplicate cultures ± standard errors of the means and are representative of three to six experiments.
CpG DNA as the adjuvant for immunization of mice with OVA.
Female 8- to 10-week-old C57BL/6 mice (two mice per group) were immunized with ovalbumin (OVA) (50 μg/site) emulsified in mineral oil, namely, incomplete or complete Freund's adjuvant (IFA or CFA, respectively) with or without DNA (50 μg/site) or ODN (25 μg/site). Deeply anesthetized (ketamine-xylazine) mice were injected subcutaneously in the upper flank of each leg with 100 μl of the different OVA formulations (55). All ODN preparations contained <1 ng of endotoxin/mg of DNA, and CV-1 DNAs contained <10 ng of endotoxin/mg of DNA as determined using the Limulus amebocyte assay (BioWhittaker). At 12 days after immunization, mice were sacrificed, the draining lymph nodes (dLN) and spleens were removed, and single-cell suspension cultures were prepared and used for proliferation and cytokine production assays. Serum was collected for determination of antibody levels.
In vitro proliferation and cytokine production by T cells from OVA-immunized mice.
Splenocytes or dLN cells were cultured in 96-well round-bottom plates (5 × 105 cells in 0.2 ml of medium; three to six replicates) with (i) medium alone, (ii) concanavalin A (ConA) at 10 μg/ml, or (iii) OVA at 100 μg/ml. After 72 h, each well was pulsed with 1 μCi of [3H]TdR and tritium incorporation into DNA was measured 16 h later. Results were expressed either as counts per minute of incorporation into DNA or as the stimulation index by comparison to cells with medium alone.
A standard ELISA or ELISPOT assay was used to measure cytokine levels. Cytokine-specific ELISPOT assays were done according to a protocol supplied by Rafi Ahmed (Emory Vaccine Center, Atlanta, Ga.) Briefly, responder spleen cells from immunized C57BL/6 mice (H2b) were cultured with medium alone, ConA, OVA, or the OVA peptide SIINFEKL (to which class I MHC-restricted CD8+ cytotoxic T lymphocyte [CTL] reacts), presented on EL4 (H2b) cells or EMT-6 (H2d) cells at effector-to-target cell ratios of 50:1, 10:1, 2:1, and 0.4:1 in a 96-well Millipore HA filter plate coated with 2 μg of anti-IFN-γ monoclonal antibody (MAB 78; R&D Systems, Minneapolis, Minn.)/ml. Syngeneic spleen cells from nonimmunized mice were added to the cultures to maintain cell density between 5 × 105 and 6 × 105 cells/well, and IL-2 was added to achieve a final concentration of 2 U/ml. Cells were grown in cultures for 40 h to accumulate secreted IFN-γ, the cells were washed off the membrane, and bound IFN-γ was detected using a biotinylated mouse anti-IFN-γ monoclonal antibody (BAF 485; R&D Systems) using streptavidin-horseradish peroxidase and nickel-enhanced 3,3′-diaminobenzidine as the substrate.
RESULTS
Detection of CpG motifs in herpesvirus genomes.
Scans of genomic sequences from HSV-1 and HSV-2 and other selected herpesvirus family members revealed a wide variation in G+C content, with evidence for CpG suppression in some virus families comparable to that of eukaryotic DNAs (Table 2). To gauge the potential for immunostimulatory effects of CpG-containing herpesvirus DNAs, we derived a CpG index that permits comparison of immunostimulatory potentials regardless of genome size, G+C content, and overall CpG suppression. The CpG indices for five human and two animal herpesvirus and two adenovirus serotypes are given in Table 2. Strikingly, there is considerable variation in CpG index values even among members of the same family. Thus, considering members of the alphaherpesvirus family, HSV-1 and HSV-2 have CpG indices of 24 and 25.8, respectively, which predicts that both should be immunostimulatory. Varicella-zoster virus (VZV), with an index of 4.8, should be weakly or possibly nonstimulatory compared to bovine herpes virus type 1 (BHV-1), which had the highest CpG index of 149, ranking its DNA as the most active. The slightly increased total CpG content for the BHV genome compared to those of the VZV and HSV genomes cannot account for the significantly higher BHV-1 CpG index value. Rather, it is the relative skewing toward higher content of stimulatory versus inhibitory motifs in the BHV-1 compared to the VZV and HSV genomes that drives the CpG index higher.
TABLE 2.
Sequence analysis of viral genomes
| Virus | Family | Genomeb
|
CpG motif deviation (%)a
|
CpG indexh | |||||
|---|---|---|---|---|---|---|---|---|---|
| Total CpGe | Consensus motifc
|
||||||||
| Methylated | Size (kb) | G+C (%) | CpG per kbd | Stimulatoryf | Inhibitoryg | ||||
| HHV1 (HSV-1) | Alpha | No | 152.3 | 68.3 | 235.4 | 100.9 | 104.9 | 94.8 | 24.0 |
| HHV2 (HSV-2) | Alpha | No | 154.7 | 70.4 | 262.1 | 105.8 | 106.2 | 96.9 | 25.8 |
| HHV3 (VZV) | Alpha | Noj | 124.9 | 46.0 | 120.2 | 113.5 | 126.8 | 123.3 | 4.8 |
| HHV4 (EBV) | Gamma | Yes | 172.3 | 59.9 | 107.4 | 59.8 | 56.4 | 71.0 | −9.4i |
| HHV5 (HCMV) | Beta | Yes/no | 229.4 | 57.2 | 194.2 | 118.9 | 138.3 | 101.9 | 84.0 |
| HVS | Gamma | Yes | 112.9 | 34.5 | 19.6 | 33.0 | 43.3 | 26.7 | 1.1 |
| BHV-1 | Alpha | No | 135.3 | 72.4 | 311.9 | 118.9 | 134.1 | 94.0 | 148.7 |
| Adenovirus (serotype 5) | No | 35.9 | 55.2 | 134.4 | 88.2 | 88.3 | 80.9 | 8.8 | |
| Adenovirus (serotype 12) | No | 34.1 | 46.5 | 87.9 | 81.2 | 116.6 | 74.4 | 30.1 | |
Deviations in specified motif occurrences relative to those expected based on genomic G+C content.
GenBank accession numbers for genomes analyzed are HSV-1, GI9629378; HSV-2, GI9629267; VZV, GI9625875; EBV, GI9625578; human cytomegalovirus (HCMV), GI9625671; HVS, GI60230; hepatitis A virus type 5 (HAV-5), GI9626187; HAV-12, GI9626621; and BHV-1, GI2653291.
Consensus stimulatory and inhibitory CpG hexamer motifs are based on published analysis (31, 32). They are used in this table as indicators of the general frequencies of stimulatory and inhibitory CpG hexamer motifs in each genome.
Number of CpG hexamer motifs (NNCGNN) occurring in each genome normalized to 100 kb.
Total frequency of CpG hexamer motifs (NNCGNN); expected number based on nucleotide composition of the genome.
Frequency of consensus stimulatory hexamer motifs (RRCGYY).
Frequency of consensus inhibitory hexamer motifs (NCCGNN and NNCGRN).
Calculated from frequencies of stimulatory less inhibitory consensus hexamer motifs as an indicator of stimulation versus inhibition multiplied by total CpG number (normalized to 1 kb) and the overall frequency of CpG (NNCGNN). As an example, the CpG index for HSV-1 is (1.049-0.948) × 235.4 × 1.009 = 24.0.
The EBV CpG index is negative, since the frequency of inhibitory motifs exceeds the frequency of stimulatory motifs.
Presumed nonmethylated based on other alphaherpesviruses.
Similarly, the relatively greater suppression of stimulatory compared to inhibitory motifs in the genomes of the gammaherpesviruses Epstein-Barr virus (EBV) and HVS accounts for their negative and neutral CpG indices, respectively, ranking them as the least stimulatory of the herpesviruses analyzed (Table 2). This view fits with the recent report that besides CpG methylation, the relative ratio of stimulatory to inhibitory sequences in vertebrate DNA is an important factor in accounting for its lack of immunostimulatory activity (52). Previously, Krieg et al. (31) reported that despite comparable frequencies of CpG dinucleotides, DNA from adenovirus serotype 12 was stimulatory while DNA from serotype 5 was nonstimulatory and actually inhibited stimulation by bacterial DNA. Consistent with this result, the CpG index for the nonstimulating serotype 5 is approximately 3.4-fold lower than that for the stimulatory serotype 12 (Table 2), which suggests that the calculated CpG index may have biological relevance.
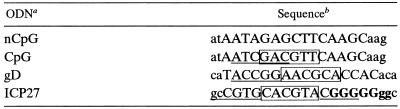
Methylation status of HSV-1, CV-1, and E. coli DNAs.
To determine their methylation statuses, CV-1 (Fig. 1, lanes 1, 2, and 3), HSV (lanes 4, 5, and 6), and E. coli (lanes 7, 8, and 9) DNAs were left undigested (lanes 3, 6, and 9) or digested with the restriction enzymes MspI (lanes 1, 4, and 7) or HpaII (lanes 2, 5, and 8), isoschizomers that are insensitive or sensitive, respectively, to cytosine methylation at the cleavage site (CC↓GG). HpaII does not cleave when the cytosine adjacent to the cleavage site is methylated. The digestion products were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. Figure 1 shows that HSV-1 and E. coli DNA were equally sensitive to digestion with HpaII and MspI, demonstrating that the majority of the CpG dinucleotides were unmethylated. In contrast, CV-1 DNA was largely resistant to digestion with HpaII (compare lanes 2 and 3) but sensitive to digestion with MspI (compare lanes 1 and 3), confirming that (as expected) it is highly methylated. Discreet digestion bands are observed with HSV but not CV-1 DNA because of the difference in genome size (150 kb compared to about 106 kb, respectively).
FIG. 1.
Methylation status of genomic DNAs. Genomic DNA from CV-1 (lanes 1 to 3), HSV-1 (lanes 4 to 6), and E. coli (lanes 7 to 9) were digested with the methylation-insensitive restriction enzyme MspI (lanes 1, 4, and 7) or the methylation-sensitive enzyme HpaII (lanes 2, 5, and 8) or left uncut (lanes 3, 6, and 9). Size markers are given in lanes M.
HSV-1 DNA drives proliferation and cytokine production in spleen cell cultures.
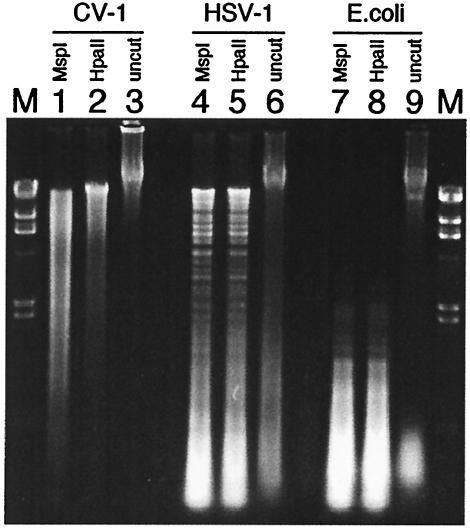
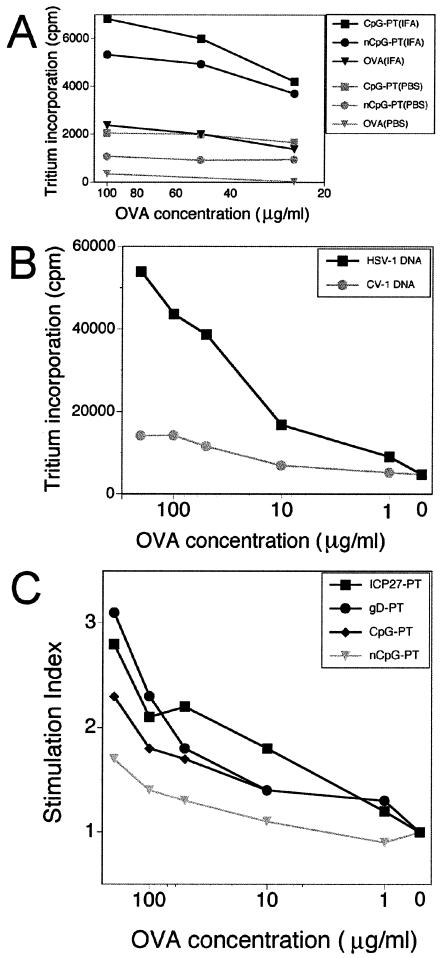
Spleen cells were cultured with ODNs at 10 to 30 μg/ml, and HSV-1, E. coli, and CV-1 DNAs were used at 100 μg/ml. Spleen cells cultured with ConA alone (5 μg/ml) or ConA supplemented with CpG ODN at 30 μg/ml were used as positive controls; negative-control cultures were medium alone or medium supplemented with non-CpG (nCpG) ODN. As shown in Fig. 2A, HSV-1 F strain DNA and gD ODN were as active as E. coli DNA and CpG ODN in stimulating proliferation of naive splenocytes, while CV-1 DNA and nCpG ODN failed to drive proliferation above the background level. The ICP27 ODN was much less active than the gD or CpG ODN and HSV-1 DNA or E. coli DNA in driving proliferation of spleen cells. As expected, ConA was most active in stimulating proliferation, and the addition of CpG ODN slightly augmented the effect. To determine whether the calculated CpG indices shown in Table 2 for different herpesvirus genomes were biologically relevant, we compared proliferation induced by different concentrations of HSV-1, BHV-1, E. coli, and CV-1 DNA. Consistent with its having the highest CpG index, BHV-1 DNA was significantly more potent than HSV-1 (McKrae) and even E. coli DNA at driving spleen cell proliferation (Fig. 2B). CV-1 DNA was inactive at all concentrations and, unexpectedly, so was HSV-1 KOS DNA; whether this was due to differences in the CpG index for KOS compared to those for McKrae and F is unknown, since the KOS genomic sequence is unavailable.
FIG. 2.
In vitro proliferative responses to CpG DNA. (A) Naive splenocytes were cultured in triplicate with various DNAs or ODNs for 24 h; the cultures were then labeled with tritiated thymidine for 18 h before measurement of incorporation of the label into DNA. Negative controls were medium alone, nCpG ODN, and CV-1 DNA; positive controls were E. coli DNA, CpG ODN, and ConA. The number hyphenated to each ODN indicates final concentration (in micrograms per milliliter) in the culture. ConA was used at 5 μg/ml, and CV-1 and HSV-1 DNA were used at 100 μg/ml. Results are representative of results of five experiments. (B) Proliferation in response to treatment with different concentrations of DNA from two different HSV-1 strains and a strain of BHV-1. E. coli DNA served as positive control and CV-1 DNA as negative control. Results are representative of results of two experiments.
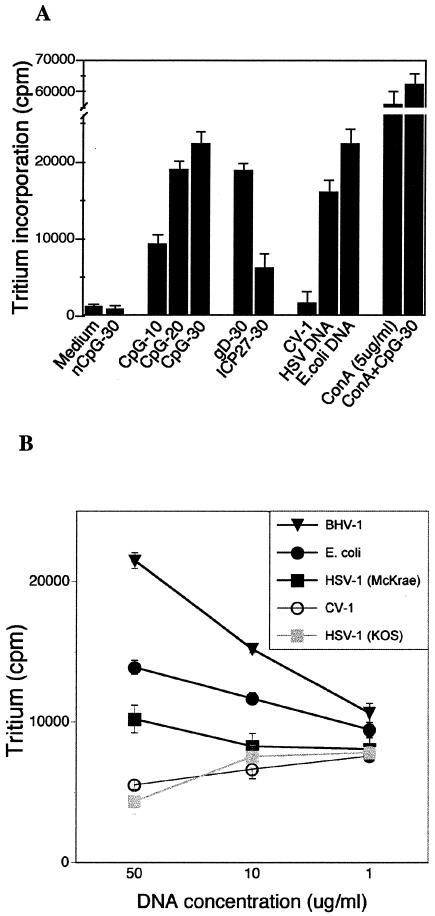
ELISA of culture supernatants and reverse transcription-PCR assay of total spleen cell RNA from the same cultures used as described for Fig. 2 were used to assess cytokine production in splenocyte cultures stimulated with CpG DNA. Figure 3A shows that HSV-1 DNA, E. coli DNA, gD ODN, and CpG ODN cultures all induced significant amounts of IL-6 compared to cultures grown in medium alone. CV-1 DNA and nCpG ODN, which were inactive in driving proliferation, both induced low-level production of IL-6 protein at levels comparable to ConA-treated cultures (Fig. 3A). In contrast, the induction of IFN-γ mRNA and protein by DNA was strictly dependent on the presence of CpG motifs in the DNA, as shown in Fig. 3B and C; neither CV-1 DNA nor nCpG ODN induced IFN-γ mRNA or protein.
FIG. 3.
In vitro cytokine response to CpG DNA. Splenocytes were cultured in the presence of ODNs or DNA for 48 h (which was determined to be the optimal time for cytokine production). Supernatants were analyzed for secreted IL-6 (A) and IFN-γ (B) mRNA transcripts or secreted protein (C). In panel B, the arrow marks the IFN-γ band and the asterisk marks the internal GAPDH control band.
Macrophages respond directly to CpG DNA.
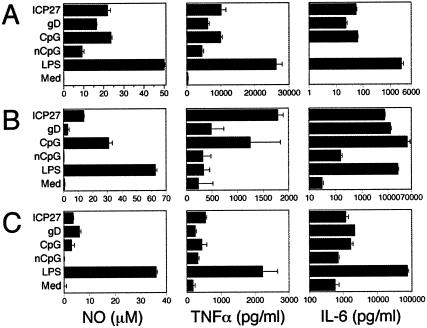
To verify that HSV DNA can activate APCs, as shown for bacterial DNAs and synthetic CpG ODNs (50, 51), production of NO and cytokines was determined for the macrophage cell line RAW 264.7, peritoneal exudate, and BM-derived macrophage cultures treated with HSV CpG ODNs. HSV-derived CpG ODNs synergized with suboptimal levels of IFN-γ (10 U/ml) to activate RAW cells to produce NO and the inflammatory cytokines TNF and IL-6. Representative results are shown in Fig. 4. The patterns of responses to CpG ODNs were similar for all macrophage cells, although overall, lower responses were observed with primary macrophages. Suboptimal levels of IFN-γ alone (10 ng of IFN-γ/ml in medium control as described for Fig. 4) or LPS (0.3 ng/ml) or CpG ODN (data not shown) alone were incapable of activating macrophages to produce significant levels of NO, TNF, or IL-6. Interestingly, the nCpG ODN induced RAW cells to produce significant levels of TNF but no IL-6, which suggests that there may be additional motifs besides CpG motifs in DNA that can activate specific immune cells, as suggested by two recent reports (20, 34) and our unpublished studies. These results confirm that HSV CpG DNA can activate macrophages to produce effector molecules that are normally induced upon encounter with bacterial or viral pathogens or components derived from them. Depletion of macrophages by treatment of HSV-infected mice with cladronate-liposomes results in enhanced mortality, showing that macrophages are important for resistance to HSV (E. Cantin, unpublished results) as previously suggested by others (9, 25). Hence, activation of macrophages by HSV DNA may play a role in the induction of protective innate responses that could also be detrimental if not appropriately regulated.
FIG. 4.
Macrophage responses to CpG ODNs. The RAW 264.7 macrophage cell line (A), primary C57BL/6 BM-derived macrophages (B), and peritoneal exudate macrophages (C) were activated overnight with recombinant IFN-γ (10 U/ml) prior to stimulation with the indicated ODNs at 1 μM or with 100 ng of LPS/ml as the positive control. RAW cells cultured with IFN-γ alone (10 U/ml) served as the negative control (Med). NO levels were determined using the Griess reaction, and cytokine levels were determined using conventional ELISAs (BioSource). Results are representative of four (A and B) or two (C) experiments..
Proliferative responses of spleen cells from mice immunized with HSV-1 DNA as the adjuvant.
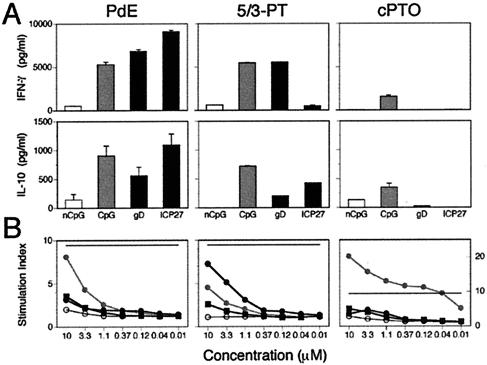
To determine the in vivo immunostimulatory activity for HSV DNA, we used native double-stranded viral DNA that had been mechanically sheared by repeated passage through a 27-gauge needle to reduce its size. For CpG ODNs, it was necessary to utilize ODNs modified to protect against nuclease degradation in vivo. We obtained complete PT ODNs as well as terminally modified ODNs that contained two 5′- and three 3′-terminal PT linkages with the internal central bases connected by phosphodiester linkages (5/3-PT ODN). The rationale for the 5/3-PT ODNs was to avoid perturbation of the CpG motifs. We compared normal ODN to PT-modified ODNs (ODN-PT) for stimulation of proliferation and production of cytokines in naive spleen cell cultures. The results presented in Fig. 5 show that complete PT ODNs behave very differently from PdE and 5/3-PT ODNs in that, overall, they drive weak proliferation and cytokine production. It is also apparent that in vitro proliferation does not correlate strictly with the capacity for cytokine production for modified and unmodified ODNs. Hence, B cells that proliferate respond differently to modified ODNs compared to NK cells and APCs that elaborate cytokines. For example, although gD and ICP27 PdE ODNs drive weak proliferation, they are potent inducers of IFN-γ and IL-10 whereas CpG PdE ODN stimulates both proliferation and cytokine production. These results show that the effects of thiolation of the ODNs are obviously complex and unpredictable. Thus, the ICP27 5/3-PT ODN showed enhanced proliferation and dramatically reduced IFN-γ but only slightly reduced IL-10 production, while for the gD and CpG 5/3-PT ODNs, changes in these responses were less extreme. Complete substitution of the gD and ICP27 ODNs with PT effectively abolished their in vitro activity while it dramatically enhanced proliferation but not cytokine production by the CpG ODN (Fig. 5). Similarly divergent immunological responses have recently been reported for studies examining the responses of spleen cells and macrophages to PT-modified ODNs with distinct CpG motifs (4, 48). Since the 5/3-PT ODN-elicited responses were more similar to those obtained with normal ODNs, they were used for in vivo studies.
FIG. 5.
In vitro proliferative and cytokine responses to phosphodiester and phosphorothioate modified ODNs. PdE, phosphodiester ODN; 5/3-PT, ODN with terminal phosphorothioate linkages; cPTO, completely phosphorothioate ODN. (A) Cytokine production in spleen cell cultures treated with ODNs at 1.1 μM final concentration. Medium-treated samples contained 909 ± 22 pg of IFN-γ/ml and no IL-10. No IL-4 or IL-12 was detected in these samples (not shown). (B) Proliferative response of splenocyte cultures treated with various concentrations of ODN. ODNs were tested in triplicate in 48-h splenocyte cultures that were tritium labeled for the last 24 h. The stimulation index (SI) was calculated as test sample/medium control. nCpG, open circle; CpG, grey circle; gD, black square; ICP27, black circle. The SI for PHA (9.4) is shown as a solid horizontal line in each panel.
Recently, several groups have demonstrated that bacterial and insect DNAs containing unmethylated CpG motifs have potent in vivo adjuvant activity that results from their enhancing effects on APC functionality (21, 54, 60). To demonstrate in vivo immunostimulatory activity, we compared CFA, commonly regarded as the “gold standard,” and HSV-1 DNA or HSV-1-specific ODN as the adjuvants for OVA immunization. An earlier study emphasized that the adjuvant effect of immunostimulatory insect DNA was greater for DNA suspended in mineral oil; soluble insect DNA was ineffective. In contrast, CpG ODNs were shown to significantly enhance priming to antigen suspended in CFA (IFA) compared to antigen in saline, as determined by measuring proliferation and cytokine production in dLN cultures established on day 9 after immunization (55). Having confirmed that soluble CpG ODN was a poor adjuvant (Fig. 6A), we elected to evaluate the adjuvant properties of HSV DNA and ODNs using OVA suspended in IFA. C57BL/6 female mice were immunized in the lower flank of both hind limbs with OVA emulsified in IFA plus total HSV-1 DNA or CV-1 DNA as the adjuvant or with OVA plus CFA as the adjuvant. At 10 days after immunization, the mice were sacrificed and spleen cell proliferation was determined after in vitro restimulation with OVA. As shown in Fig. 6B, spleen cells from mice immunized with HSV-1 DNA proliferated strongly in response to restimulation with OVA, whereas proliferation was much reduced when CV-1 DNA was used as the adjuvant. The gD and ICP27 PT ODN (ODN-PT) also exhibited adjuvant activity when coinjected with OVA emulsified in IFA, while judging by the proliferative response of spleen cells restimulated with OVA, the CpG ODN-PT was somewhat less active (Fig. 6C). Additionally, the CpG, gD, and ICP27 ODN-induced increases in immunoglobulin G2A (IgG2a)/IgG1 ratios followed the pattern of induced proliferation, as shown in Table 3. In agreement with the results of prior studies (55), HSV DNA and the CpG ODN-PTs were more active than CV-1 DNA or the control nCpG ODN-PT in augmenting the weak adjuvant effect of IFA.
FIG. 6.
Memory proliferative response after immunization using CpG DNAs as the adjuvant. Mice were injected in both lower flanks with OVA with or without DNA or ODN-PT as the adjuvant in PBS or IFA. On days 10 to 12, three to four replicate splenocyte cultures containing various concentrations of OVA were used to measure proliferative memory responses. (A) Memory after immunization with OVA alone (triangles), OVA with nCpG-PT (circles), and OVA with CpG-PT (squares) either as solutes in PBS (gray lines) or emulsified in IFA (black lines). (B) Mice were immunized with OVA in IFA with either HSV-1 DNA (squares) or CV-1 DNA (circles). (C) Mice were immunized with OVA in IFA with ICP27-PT (squares), gD-PT (circles), CpG-PT (diamonds), or nCpG-PT (triangles). Stimulation indices are normalized to medium controls for each set of mice (two to three per group). Each panel is representative of two experiments.
TABLE 3.
CpG ODN as adjuvant affects antibody isotope selection
| ODN | Immunizationa
|
OVA-specific antibody titerb
|
||||
|---|---|---|---|---|---|---|
| IFA | CFA | OVA | IgG2a | IgG1 | IgG2a/IgG1 ratio | |
| nCpG | + | − | + | 1:128 | 1:512 | 1:4 |
| CpG | + | − | + | 1:2,048 | 1:1,024 | 2:1 |
| gD | + | − | + | 1:2,048 | 1:1,024 | 2:1 |
| ICP27 | + | − | + | 1:4,096 | 1:1,024 | 4:1 |
| CFA control | NAc | + | + | 1:16,384 | 1:2,048 | 8:1 |
Mice were immunized in the rear footpad with 100 mg of OVA and either ODN, IFA, or CFA as the adjuvant.
On day 12 postimmunization, serum was collected and OVA-specific antibody titers were determined by ELISA. Titers are reported as the highest dilution with an absorbance value greater than the background level.
NA, not applicable.
Cytokine recall responses in mice immunized with HSV-1 DNA.
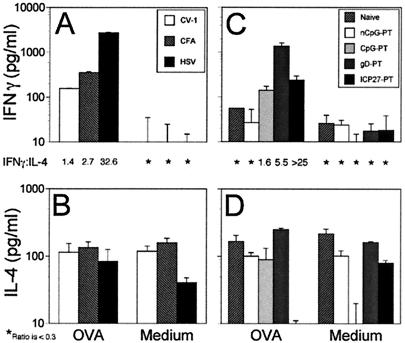
To determine (using HSV CpG DNAs as the adjuvant) the CD4+ T-helper (Th) profile induced by immunization with OVA, dLN were harvested on day 9 after immunization and cytokine production levels were determined by ELISA in culture supernatants obtained 24 h after in vitro restimulation of the dLN cells with OVA. Compared to the results with the CFA adjuvant, HSV-1 DNA induced much higher levels of IFN-γ, while as expected, CV-1 DNA was inactive (Fig. 7A). The gD ODN-PT stimulated IFN-γ production significantly more than the ICP27 or CpG ODN-PTs, both of which induced moderate levels of IFN-γ compared to those obtained after immunization with nCpG-ODN-PT, which failed to induce IFN-γ above background levels (Fig. 7C). Figures 7B and D show that none of the mice immunized with any of the DNA preparations produced IL-4, a characteristic Th2-type cytokine, above background levels. The ratio of IFN-γ to IL-4 shows that HSV DNA and CpG ODNs derived therefrom promoted strong Th1 responses (Fig. 7; ratios given between upper and lower panels).
FIG. 7.
Memory cytokine response after immunization with CpG DNA as the adjuvant. Mice were immunized as described for Fig. 6. On days 10 to 12, splenocytes were cultured in the presence of OVA (200 μg/ml) or medium. Supernatants were collected after 72 h and analyzed by ELISA for IFN-γ and IL-4 levels (BioSource). The ratios of secreted IFN-γ and IL-4 are given between the paired IFN-γ (A and C) and IL-4 (B and D) panels. Asterisks indicate a ratio below 0.3. Results are representative of two experiments.
During Th1 responses, production of IgG2a antibody usually predominates (10, 36); therefore, we determined whether this occurs also in response to immunization with HSV-1 DNA and ODNs as the adjuvants. IgG1 and IgG2a levels were measured by ELISA in sera of mice collected on day 12 after immunization with OVA and various DNA preparations or CFA as the adjuvant. As determined by measuring IgG2a/IgG1 ratios, HSV-1 DNA, E. coli DNA, gD ODN, ICP27 ODN, and CpG ODN preferentially stimulated IgG2a production, whereas nCpG DNAs (CV-1 DNA and nCpG ODN) stimulated OVA-specific IgG1 responses (data not shown). The biased Th1-type cytokine responses and preferential induction of IgG2a production after immunization with HSV-1 DNA and CpG ODN as the adjuvants confirms that CpG motifs in HSV DNA drive biased Th1 responses in vivo, as previously reported for insect and bacterial DNAs (33, 36, 55).
Induction of T-cell responses in mice immunized with HSV-1 DNA.
To examine whether OVA immunization with HSV-1 DNA as the adjuvant drives expansion of antigen-specific T cells in vivo, the frequencies of IFN-γ- and IL-4-producing spleen cells were investigated by ELISPOT assay. When grown in cultures with OVA, spleen cells from mice immunized with HSV-1 DNA produced >5-fold more IFN-γ-specific spot-forming cells (SFC) compared to those from mice immunized with CV-1 DNA as the adjuvant for OVA (478 versus 88 SFC, respectively). No IL-4-specific SFC were produced above background levels (<10 SFC) for any of the immunization groups, and as expected, in vitro activation of spleen cells with phytohemagglutinin produced the greatest number of SFC (data not shown). When splenocytes from OVA-immunized mice were stimulated with MHC-matched EL4 cells (H-2b) versus mismatched EMT-6 cells (H-2d) pulsed with the OVA-specific, MHC class I-restricted CTL peptide epitope SIINFEKL, 158 versus 86 SFC were obtained, respectively.
It has been shown that in vitro methylation of CpG dinucleotides in E. coli and plasmid DNAs or synthetic ODNs blocked their immunostimulatory activity (29, 30). In our studies, digestion of HSV DNA with MspI, which cleaves the CpG motif, blocked its stimulatory activity in vitro, confirming that unmethylated CpG dinucleotides are essential for immunostimulatory activity (data not shown). Although highly unlikely, it is conceivable that the observed immunostimulatory activity of genomic HSV-1 DNA is indirect and due to expression of viral proteins from the injected DNA rather than to activation of immune cells by CpG motifs. To exclude this possibility and confirm the in vivo priming of HSV-specific CD8+ CTL responses (Table 4), splenocyte cultures from mice immunized with OVA plus HSV-1 DNA or CpG ODN as the adjuvant were tested for specific responses to the immunodominant HSV-1 gB CD8+ CTL epitope SSIEFARL (43) and the OVA-specific CD8+ CTL epitope SIINFEKL (5). Table 4 shows that the OVA-specific-CTL epitope (SIINFEKL) induced specific SFC responses, whereas background levels of SFC resulted from restimulation with the gB-specific CTL epitope (SSIEFARL). This result is consistent with HSV-1 DNA not being expressed in vivo, at least at levels sufficient to prime HSV-specific CD8+ T-cell responses. Thus, we conclude that the immunostimulatory activity of HSV-1 DNA and HSV CpG ODN is mediated by CpG motifs and that these drive potent Th1- and Tc1-type responses in vivo. Table 4 shows that the gD-PT and ICP27-PT ODNs are much better at inducing OVA-specific CTL responses than the CpG-PT ODN, a result which is opposite to those obtained by measuring proliferative responses to these ODNs in vitro (Fig. 5). Overall, the results presented here demonstrate that HSV-1 DNA and ODNs are potent adjuvants, being superior to non-CpG DNA or even CFA in priming in vivo CD8+ CTL responses to OVA (Table 4).
TABLE 4.
HSV DNA as adjuvant induces OVA-specific CD8+ CTL
| Adjuvant | No. of CTLs for stimulators pulsed with:
|
||
|---|---|---|---|
| Medium | gB (SSIEFARL) | OVA (SIINFEKL) | |
| CV-1 DNA | 1.0 ± 0.0a | 2.5 ± 0.5 | 17.5 ± 0.5 |
| HSV DNA | 8.5 ± 0.5 | 11.5 ± 2.5 | 78.5 ± 4.0 |
| nCpG-PT | 0.5 ± 0.7 | 2.0 ± 1.4 | 6.0 ± 1.8 |
| CpG-PT | 4.5 ± 2.1 | 6.5 ± 3.5 | 13.0 ± 8.1 |
| gD-PT | 5.5 ± 4.9 | 11.5 ± 4.9 | 56.0 ± 17.0 |
| ICP27-PT | 6.0 ± 2.8 | 7.0 ± 2.8 | 131.5 ± 13.4 |
| CFA | 20.5 ± 7.8 | 22.5 ± 12.0 | 69.5 ± 0.7 |
SFC in IFN-γ ELISPOTs using peptide-pulsed syngeneic splenocytes as stimulators. Medium column has unpulsed stimulators. Numbers are per 106 responder cells from duplicate wells.
DISCUSSION
It is now well established that unmethylated CpG motifs in the genomes of bacteria, insects, and some viruses can directly activate B cells and APCs in vitro and in vivo, resulting in the production of effector molecules that induce potent adaptive Th1-type responses (29, 62). We have shown that in contrast to the results seen for vertebrate DNA like CV-1 DNA, in which cytosines in CpG dinucleotides are extensively methylated, CpG dinucleotides are grossly undermethylated in HSV-1 DNA purified from virions (Fig. 1). By deriving a CpG index on the basis of the relative frequencies of stimulatory and inhibitory motifs that is normalized to a 100-kb genome, it was possible to compare the immunostimulatory potentials of selected herpesvirus and adenovirus genomes independently of genome size, G+C content, and overall CpG suppression. Accordingly, a CpG index of 24 to 25 for the HSV-1 and HSV-2 genomes predicts that both DNAs should be immunostimulatory. In contrast, a CpG index of −9.4 for EBV, the prototypical gammaherpesvirus, predicts this genome should inhibitory rather than stimulatory whereas a CpG index of 149 predicts that BHV-1 genomic DNA should be highly stimulatory (Table 2). Considering that the CpG index for the nonstimulatory serotype 5 adenovirus genome is approximately threefold lower those that for the immunostimulatory HSV-1 and HSV-2 genomes (Table 2), it remains uncertain whether viruses from different families can be directly compared in this manner or whether such comparisons are valid only for viruses within a given family. Another possible shortcoming of this analysis is the limitation to known specific consensus hexameric stimulatory and inhibitory motifs, as there may be other features of DNA sequences that are relevant to their immunostimulatory capacities. Indeed, this is suggested by the fact demethylated mammalian DNA remains relatively immunologically inert and by recent observations that mammalian double-stranded DNA can activate APCs independent of CpG motifs or sequence context (20, 28, 57). In addition, the consensus immunostimulatory CpG motifs used here were defined on the basis of bacterial DNA effects on mouse immune cells; however, recent studies have demonstrated that consensus immunostimulatory motifs for primate immune cells are different (30). Despite these limitations, it appears that the CpG index (as defined for different herpesviruses in Table 2) does have predictive value since BHV-1 genomic DNA was sixfold more active than HSV-1 17+ genomic DNA and unexpectedly even more active than E. coli DNA, the gold standard (Fig. 2B).
The striking suppression of stimulatory CpG motifs in the gammaherpesviruses (exemplified by EBV) (Table 2) suggests a strong selective pressure against these motifs, as previously reported (23). Indeed, the ratio of stimulatory-to-inhibitory motifs is an important determinant of immunostimulatory activity, as recently reported for demethylated mouse DNA (52). It is conceivable that the tropism of these viruses for lymphoid cells, some of which are directly responsive to CpG motifs, provided the evolutionary driving force for suppression of CpG motifs (19). Similar observations for adenoviruses were reported earlier by Kreig and colleagues (31), who observed that DNA from adenovirus type 12 was stimulatory while that from adenovirus types 2 and 5 was not. These authors speculated that differences in biology and, specifically, the propensity of adenovirus 5 to establish persistent infections in lymphocytes (which does not occur with adenovirus 12) might account for suppression of immunostimulatory motifs in adenovirus 5 (31).
Consistent with its CpG index (Table 2), we found HSV-1 DNA to be highly immunostimulatory when tested in splenocyte cultures, driving proliferation and the synthesis of inflammatory cytokines (IFN-γ, TNF, and IL-6), whereas mammalian DNA from CV-1 cells in which the virus was grown was inactive. We also tested two CpG ODNs derived from the HSV-1 gD and ICP-27 (ICP27) genes as well as control CpG and nCpG ODNs that had previously been shown to be immunostimulatory when tested in vivo and in vitro (46). The gD ODN was selected because, like the control CpG ODN that was previously shown to cause inflammation of the lower respiratory tract after intratracheal delivery in mice (46), it contains an optimal stimulatory motif and an overlapping inhibitory motif. The ICP27 ODN, which contains a suboptimal stimulatory motif flanked by two inhibitory motifs (Table 2), was chosen with the expectation that it would be weakly active or nonstimulatory, thus confirming the effect of inhibitory motifs identified in adenovirus DNA (31). These expectations were met when the ODNs were tested for in vitro stimulation of splenocyte cultures: the gD ODN was as active as the control CpG ODN, the ICP27 ODN was significantly less active, and the nCpG ODN was inactive (Fig. 2 and 3). However, the ICP27 ODN was as active as the control CpG ODN and the gD ODN in activating macrophages to produce NO and the inflammatory cytokines, TNF, and IL-6 (Fig. 4). In this regard it is interesting that the ICP27 ODN contains seven consecutive deoxyriboguanosine (dG) residues at the 3′ end that result in spontaneous formation of highly stable structures, probably with a G-quartet conformation, as evidenced by anomalous migration in denaturing polyacrylamide gels (reference 1 and data not shown). Terminal 3′ dG runs in CpG ODNs can exert complex cis-modulatory effects on their activity via a number of mechanisms, including preferential uptake via type A scavenger receptors on APCs (splenic DCs, B cells, and peritoneal macrophages) and enhanced production of cytokines (TNF-α and IL-12) (24, 35, 44). The effects of a dG run vary with the position in the ODN and the chemical constitution of the ODN backbone: a dG run at either the 5′ terminus of a phosphodiester CpG ODN or the 3′ terminus of a PT CpG ODN reduced in vitro cytokine production by APCs (35). Such effects could account for the apparent discrepant activity of the ICP27 ODN relative to those of the gD or CpG ODNs in activation of spleen cells and macrophages. Additionally, we have observed that ODNs comprised of G-rich sequences derived from the HSV genome have an immunostimulatory spectrum distinct from that induced by ODNs with classic CpG motifs (E. Cantin, unpublished results).
To assess the in vivo immunostimulatory activity of HSV-1 DNA and ODNs derived therefrom, we compared immune responses in mice immunized separately with OVA and CFA as the adjuvant to responses in mice immunized with OVA emulsified in IFA and supplemented with HSV-1 or CV-1 DNA or CpG or nCpG ODNs as the adjuvant. 5/3-PT-modified ODNs were used for immunization to provide optimal stability in vivo while permitting retention of internal CpG motifs with a normal phosphodiester backbone. We showed that in vitro, HSV-1-derived and control ODNs modified with terminal PT linkages (5/3-PT ODNs) elicited immunological responses in spleen cell cultures similar to those of their phosphodiester counterparts (Fig. 5). When compared to CFA in in vivo testing, HSV-1 DNA and HSV-1 CpG ODN-PTs were superior adjuvants for eliciting Th1-type responses to OVA. The inability of CFA to augment IFN-γ production by splenocytes from mice immunized with the gD ODN suggests that maximal responses were elicited by the gD ODN. In contrast, the ICP27 ODN induced relatively low levels of IFN-γ yet this same ODN induced the strongest OVA-specific CD8+ CTL responses (Table 4). These seemingly discordant results might have been due to the concerted modulatory effects of flanking inhibitory CpG motifs, a 3′-terminal dG run, and terminal PT linkages in the ICP27 ODN on in vivo responses of different immune cell types (31, 35, 48, 62). Thus, compared to phosphodiester ODN, 10- to 100-fold-lower concentrations of PT-ODN can induce NO production and activate the IL-12 promoter in macrophage cultures and these enhancing effects are attributable to enhanced stability and uptake of PT-ODNs (48). In vitro restimulation of spleen cell cultures from mice immunized with OVA measures predominantly CD4+ T-cell responses (compared to the results of the ELISPOT assay reported in Table 4), where CD8+ T-cell responses were specifically measured. Results with the ICP27-ODN implied that this ODN has different effects on CD4+ and CD8+ T-cell responses. The divergent effects of the ICP27 ODN compared to those of ODNs without dG runs may derive from interaction with other receptors besides TLR9, which transduces CpG signals. The class A macrophage scavenger receptor (MSR-A) that reacts to diverse ligands including polynucleotides having a quadraplex structure is a candidate receptor for the ICP27-ODN (44, 45). Thus, it is of interest that MSR-A−/− mice show enhanced susceptibility to HSV-1 mortality (56).
A central question raised by our results is whether by virtue of being intrinsically highly immunostimulatory in vitro and in vivo, HSV-1 DNA might have a role in pathogenesis. We contend that there is ample indirect evidence to support such speculation and that this evidence warrants further studies to directly address the question. A case in point is that of herpes stromal keratitis (HSK), a blinding immunopathological disease caused by damage inflicted by proinflammatory cytokines (IFN-γ and IL-2) secreted by CD4+ Th1-type cells invading the cornea (59, 61). HSK has several anomalous features, as studied in the mouse ocular model of HSV infection. First, the destructive inflammatory response in the cornea is induced when HSV DNA is the only detectable viral component persisting in the cornea; infectious HSV, viral antigen, or mRNA is undetectable. Second, replication-competent but not replication-incompetent HSV strains are required for causing HSK. And third, HSK can be induced in DO11.10 mice that express an OVA-specific TCR and therefore fail to react to HSV antigens. A model to explain these results postulates that HSK replication exposes cryptic ocular antigens and results in the activation of T cells of unknown specificity that initiate the destructive inflammatory responses, culminating in HSK (11, 12). However, the model does not account for the delayed kinetics of the inflammatory response that is experimentally observed.
We speculate that in mice experimentally inoculated in the cornea with HSV-1, some cells support HSV-1 DNA replication but not the production of infectious progeny virus. Eventually, these abortively infected cells die, possibly due to damage sustained during HSV-1 infection. Macrophages and/or DCs phagocytosing the dead cells would be activated by their cargo of immunostimulatory HSV-1 DNA to produce proinflammatory cytokines (IL-12, IFN-γ, IL-6, and TNF) and chemokines (29). T cells of indeterminate specificity recruited to this cytokine-rich microenvironment would undergo bystander activation, resulting in amplification of the inflammatory response that would culminate in damage to the corneal stroma. Moreover, since DCs activated by CpG DNA are fully competent to present antigen, they might orchestrate autoimmune attack of the cornea by presenting cryptic self-antigens exposed as a consequence of damage to the autoreactive cornea cells that escaped thymic deletion (47). In support of this hypothesis, we cite the observation of Mitchell and colleagues (42) that HSV-1 DNA persisting in the cornea is found closely associated with inflammatory lesions long after infectious HSV-1 has been cleared. Additionally, they reported that despite comparable replication in the eye, HSV-1 strain KOS (compared to strain RE) is a poor inducer of HSK and for some unknown reason its DNA, unlike that of RE, does not persist in the cornea (39).
In this regard, the observation that unlike strain F and McKrae genomic DNAs, KOS DNA failed to drive proliferation of B cells in spleen cell cultures is intriguing and worth following up. The hypothesis that HSV-1 DNA plays a role in the induction of corneal inflammatory responses leading to HSK is attractive, because it provides a framework for rationalizing anomalous features of the disease discussed above. Currently, delivery of HSV-1 ODNs to the corneas of normal mice inoculated with or without a replication-incompetent HSV-1 mutant is being attempted to determine whether this provokes HSK. Our results demonstrating that HSV-1 DNA drives potent CD4+ and CD8+ Th1-type responses in vivo lends credence to this speculation. Indeed, preliminary results show the HSV CpG ODNs but not nCpG ODN delivered to the cornea by intrastromal injection induced a strong angiogenic response (E. Cantin and R. Lausch, unpublished results); this is notable because angiogenesis has been speculated to play a critical role in the development of HSK (63). However, despite the strong early angiogenic response elicited by HSV CpG ODN treatment of mice, HSK failed to develop during the 21-day observation period, even in mice infected with a dose of HSV that fails to induce HSK when given alone, which raises the possibility that induction of angiogenesis and HSK may be unrelated events.
In conclusion, we have shown that HSV-1 DNA is highly immunostimulatory in vitro and in vivo, driving potent Th1-type responses. This property of HSV-1 DNA could be biologically significant and important in pathogenesis; this is evident in the model we propose to explain how HSV-1 DNA might participate in the initiation of an inflammatory response that culminates in HSK. Additionally, Cantin et al. and others (8, 14, 38, 49) previously reported a prolonged inflammatory response in the ganglia of infected mice in the absence of detectable infectious HSV-1 or antigen and attributed this to chronic, low-level production of HSV-1 antigens. However, equally plausible and not exclusive is that HSV-1 DNA persisting in abortively infected cells in the ganglion might contribute to the observed protracted inflammatory response. Activation of APCs for antigen presentation and stimulation of local proinflammatory cytokine production by immunostimulatory viral DNAs warrants consideration as a possible mechanism by which HSV-1 and other viruses might contribute to or exacerbate neuroinflammatory diseases like multiple sclerosis (47). In gene therapy approaches, the immune response-activating properties of the HSV-1 genome might be either beneficial (as when using HSV-1 as a vaccine vector) or deleterious (as in applications involving therapeutic gene delivery to the brain). There is growing interest in the use of γ34.5 null mutants of HSV-1 that fail to replicate or cause encephalitis upon direct inoculation into the brain as oncolytic vectors and as gene transfer vectors for the central nervous system. A recent report that a γ34.5 HSV-1 mutant caused severe inflammation in the brain mediated by nonspecific and specific immune responses suggests the possible involvement of immunostimulatory CpG motifs in the virus genome (40). The demonstration here that HSV DNA is highly immunostimulatory suggests a cautious approach in the application of HSV-1 as a gene therapy vector.
Acknowledgments
We thank Clinton Jones (University of Nebraska-Lincoln) for supplying purified BHV-1 DNA.
This work was supported by Public Health Service grant EY 13061 from the National Eye Institute.
REFERENCES
- 1.Aboul-ela, F., A. I. Murchie, D. G. Norman, and D. M. Lilley. 1994. Solution structure of a parallel-stranded tetraplex formed by d(TG4T) in the presence of sodium ions by nuclear magnetic resonance spectroscopy. J. Mol. Biol. 243:458-471. [DOI] [PubMed] [Google Scholar]
- 2.Aderem, A., and D. A. Hume. 2000. How do you see CG? Cell 103:993-996. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 4.Ballas, Z. K., A. M. Krieg, T. Warren, W. Rasmussen, H. L. Davis, M. Waldschmidt, and G. J. Weiner. 2001. Divergent therapeutic and immunologic effects of oligodeoxynucleotides with distinct CpG motifs. J. Immunol. 167:4878-4886. [DOI] [PubMed] [Google Scholar]
- 5.Bergenthal, A., M. Hofmann, and K. Heeg. 1998. Self-veto mechanism of CD8+ cytotoxic effector T cells. Peptide-induced paralysis affects the peptide-MHC-recognizing cytotoxic T lymphocytes and is independent of Fas/Fas ligand interactions. Eur. J. Immunol. 28:1911-1922. [DOI] [PubMed] [Google Scholar]
- 6.Bird, A. P., M. H. Taggart, R. D. Nicholls, and D. R. Higgs. 1987. Non-methylated CpG-rich islands at the human alpha-globin locus: implications for evolution of the alpha-globin pseudogene. EMBO J. 6:999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantin, E. M., D. R. Hinton, J. Chen, and H. Openshaw. 1995. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J. Virol. 69:4898-4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, H., T. M. Tumpey, H. F. Staats, N. van Rooijen, J. E. Oakes, and R. N. Lausch. 2000. Role of macrophages in restricting herpes simplex virus type 1 growth after ocular infection. Investig. Ophthalmol. Vis. Sci. 41:1402-1409. [PubMed] [Google Scholar]
- 10.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as the adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangappa, S., J. S. Babu, J. Thomas, M. Daheshia, and B. T. Rouse. 1998. Virus-induced immunoinflammatory lesions in the absence of viral antigen recognition. J. Immunol. 161:4289-4300. [PubMed] [Google Scholar]
- 12.Gangappa, S., S. P. Deshpande, and B. T. Rouse. 1999. Bystander activation of CD4+ T cells can represent an exclusive means of immunopathology in a virus infection. Eur. J. Immunol. 29:3674-3682. [DOI] [PubMed] [Google Scholar]
- 13.Hacker, H., R. M. Vabulas, O. Takeuchi, K. Hoshino, S. Akira, and H. Wagner. 2000. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J. Exp. Med. 192:595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halford, W. P., B. M. Gebhardt, and D. J. Carr. 1996. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J. Immunol. 157:3542-3549. [PubMed] [Google Scholar]
- 15.Han, X., P. Lundberg, B. Tanamachi, H. Openshaw, J. Longmate, and E. Cantin. 2001. Gender influences herpes simplex virus type 1 infection in normal and gamma interferon-mutant mice. J. Virol. 75:3048-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann, G., R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164:1617-1624. [DOI] [PubMed] [Google Scholar]
- 17.Haynes, L. M., D. D. Moore, E. A. Kurt-Jones, R. W. Finberg, L. J. Anderson, and R. A. Tripp. 2001. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 75:10730-10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 19.Honess, R. W., U. A. Gompels, B. G. Barrell, M. Craxton, K. R. Cameron, R. Staden, Y. N. Chang, and G. S. Hayward. 1989. Deviations from expected frequencies of CpG dinucleotides in herpesvirus DNAs may be diagnostic of differences in the states of their latent genomes. J. Gen. Virol. 70:837-855. [DOI] [PubMed] [Google Scholar]
- 20.Ishii, K. J., K. Suzuki, C. Coban, F. Takeshita, Y. Itoh, H. Matoba, L. D. Kohn, and D. M. Klinman. 2001. Genomic DNA released by dying cells induces the maturation of APCs. J. Immunol. 167:2602-2607. [DOI] [PubMed] [Google Scholar]
- 21.Jakob, T., P. S. Walker, A. M. Krieg, M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161:3042-3049. [PubMed] [Google Scholar]
- 22.Kaisho, T., and S. Akira. 2001. Dendritic-cell function in Toll-like receptor- and MyD88-knockout mice. Trends Immunol. 22:78-83. [DOI] [PubMed] [Google Scholar]
- 23.Karlin, S., W. Doerfler, and L. R. Cardon. 1994. Why is CpG suppressed in the genomes of virtually all small eukaryotic viruses but not in those of large eukaryotic viruses? J. Virol. 68:2889-2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura, Y., K. Sonehara, E. Kuramoto, T. Makino, S. Yamamoto, T. Yamamoto, T. Kataoka, and T. Tokunaga. 1994. Binding of oligoguanylate to scavenger receptors is required for oligonucleotides to augment NK cell activity and induce IFN. J. Biochem (Tokyo) 116:991-994. [DOI] [PubMed] [Google Scholar]
- 25.Kodukula, P., T. Liu, N. V. Rooijen, M. J. Jager, and R. L. Hendricks. 1999. Macrophage control of herpes simplex virus type 1 replication in the peripheral nervous system. J. Immunol. 162:2895-2905. [PubMed] [Google Scholar]
- 26.Krieg, A. M. 1999. CpG DNA: a novel immunomodulator. Trends Microbiol. 7:64-65. [DOI] [PubMed] [Google Scholar]
- 27.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 28.Krieg, A. M. 1999. Mechanisms and applications of immune stimulatory CpG oligodeoxynucleotides. Biochim. Biophys. Acta 1489:107-116. [DOI] [PubMed] [Google Scholar]
- 29.Krieg, A. M. 2000. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 12:35-43. [DOI] [PubMed] [Google Scholar]
- 30.Krieg, A. M., G. Hartmann, and A. K. Yi. 2000. Mechanism of action of CpG DNA. Curr. Top. Microbiol. Immunol. 247:1-21. [DOI] [PubMed] [Google Scholar]
- 31.Krieg, A. M., T. Wu, R. Weeratna, S. M. Efler, L. Love-Homan, L. Yang, A. K. Yi, D. Short, and H. L. Davis. 1998. Sequence motifs in adenoviral DNA block immune activation by stimulatory CpG motifs. Proc. Natl. Acad. Sci. USA 95:12631-12636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546-549. [DOI] [PubMed] [Google Scholar]
- 33.Krieg, A. M., A. K. Yi, J. Schorr, and H. L. Davis. 1998. The role of CpG dinucleotides in DNA vaccines. Trends Microbiol. 6:23-27. [DOI] [PubMed] [Google Scholar]
- 34.Lang, R., L. Hultner, G. B. Lipford, H. Wagner, and K. Heeg. 1999. Guanosine-rich oligodeoxynucleotides induce proliferation of macrophage progenitors in cultures of murine bone marrow cells. Eur. J. Immunol. 29:3496-3506. [DOI] [PubMed] [Google Scholar]
- 35.Lee, S. W., M. K. Song, K. H. Baek, Y. Park, J. K. Kim, C. H. Lee, H. K. Cheong, C. Cheong, and Y. C. Sung. 2000. Effects of a hexameric deoxyriboguanosine run conjugation into CpG oligodeoxynucleotides on their immunostimulatory potentials. J. Immunol. 165:3631-3639. [DOI] [PubMed] [Google Scholar]
- 36.Lipford, G. B., M. Bauer, C. Blank, R. Reiter, H. Wagner, and K. Heeg. 1997. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur. J. Immunol. 27:2340-2344. [DOI] [PubMed] [Google Scholar]
- 37.Lipford, G. B., K. Heeg, and H. Wagner. 1998. Bacterial DNA as immune cell activator. Trends Microbiol. 6:496-500. [DOI] [PubMed] [Google Scholar]
- 38.Liu, T., Q. Tang, and R. L. Hendricks. 1996. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J. Virol. 70:264-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maggs, D. J., E. Chang, M. P. Nasisse, and W. J. Mitchell. 1998. Persistence of herpes simplex virus type 1 DNA in chronic conjunctival and eyelid lesions of mice. J. Virol. 72:9166-9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McMenamin, M., A. Byrnes, H. Charlton, R. Coffin, D. Latchman, and M. Wood. 1998. A γ34.5 mutant of herpes simplex 1 causes severe inflammation in the brain. Neuroscience 83:1225-1237. [DOI] [PubMed] [Google Scholar]
- 41.Medzhitov, R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135-145. [DOI] [PubMed]
- 42.Mitchell, W. J., P. Gressens, J. R. Martin, and R. DeSanto. 1994. Herpes simplex virus type 1 DNA persistence, progressive disease and transgenic immediate early gene promoter activity in chronic corneal infections in mice. J. Gen. Virol. 75:1201-1210. [DOI] [PubMed] [Google Scholar]
- 43.Nugent, C. T., R. M. Wolcott, R. Chervenak, and S. R. Jennings. 1994. Analysis of the cytolytic T-lymphocyte response to herpes simplex virus type 1 glycoprotein B during primary and secondary infection. J. Virol. 68:7644-7648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearson, A. M., A. Rich, and M. Krieger. 1993. Polynucleotide binding to macrophage scavenger receptors depends on the formation of base-quartet-stabilized four-stranded helices. J. Biol. Chem. 268:3546-3554. [PubMed] [Google Scholar]
- 45.Platt, N., and S. Gordon. 2001. Is the class A macrophage scavenger receptor (SR-A) multifunctional?—the mouse's tale. J. Clin. Investig. 108:649-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz, D. A., T. J. Quinn, P. S. Thorne, S. Sayeed, A. K. Yi, and A. M. Krieg. 1997. CpG motifs in bacterial DNA cause inflammation in the lower respiratory tract. J. Clin. Investig. 100:68-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Segal, B. M., J. T. Chang, and E. M. Shevach. 2000. CpG oligonucleotides are potent adjuvants for the activation of autoreactive encephalitogenic T cells in vivo. J. Immunol. 164:5683-5688. [DOI] [PubMed] [Google Scholar]
- 48.Sester, D. P., S. Naik, S. J. Beasley, D. A. Hume, and K. J. Stacey. 2000. Phosphorothioate backbone modification modulates macrophage activation by CpG DNA. J. Immunol. 165:4165-4173. [DOI] [PubMed] [Google Scholar]
- 49.Shimeld, C., J. L. Whiteland, S. M. Nicholls, E. Grinfeld, D. L. Easty, H. Gao, and T. J. Hill. 1995. Immune cell infiltration and persistence in the mouse trigeminal ganglion after infection of the cornea with herpes simplex virus type 1. J. Neuroimmunol. 61:7-16. [DOI] [PubMed] [Google Scholar]
- 50.Sparwasser, T., T. Miethke, G. Lipford, A. Erdmann, H. Hacker, K. Heeg, and H. Wagner. 1997. Macrophages sense pathogens via DNA motifs: induction of tumor necrosis factor-alpha-mediated shock. Eur. J. Immunol. 27:1671-1679. [DOI] [PubMed] [Google Scholar]
- 51.Stacey, K. J., M. J. Sweet, and D. A. Hume. 1996. Macrophages ingest and are activated by bacterial DNA. J. Immunol. 157:2116-2122. [PubMed] [Google Scholar]
- 52.Stacey, K. J., G. R. Young, F. Clark, D. P. Sester, T. L. Roberts, S. Naik, M. J. Sweet, and D. A. Hume. 2003. The molecular basis for the lack of immunostimulatory activity of vertebrate DNA. J. Immunol. 170:3614-3620. [DOI] [PubMed] [Google Scholar]
- 53.Stuehr, D. J., S. S. Gross, I. Sakuma, R. Levi, and C. F. Nathan. 1989. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J. Exp. Med. 169:1011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun, S., C. Beard, R. Jaenisch, P. Jones, and J. Sprent. 1997. Mitogenicity of DNA from different organisms for murine B cells. J. Immunol. 159:3119-3125. [PubMed] [Google Scholar]
- 55.Sun, S., H. Kishimoto, and J. Sprent. 1998. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T cell responses to specific antigen. J. Exp. Med. 187:1145-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suzuki, H., Y. Kurihara, M. Takeya, N. Kamada, M. Kataoka, K. Jishage, O. Ueda, H. Sakaguchi, T. Higashi, T. Suzuki, Y. Takashima, Y. Kawabe, O. Cynshi, Y. Wada, M. Honda, H. Kurihara, H. Aburatani, T. Doi, A. Matsumoto, S. Azuma, T. Noda, Y. Toyoda, H. Itakura, Y. Yazaki, T. Kodama, et al. 1997. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386:292-296. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki, K., A. Mori, K. J. Ishii, J. Saito, D. S. Singer, D. M. Klinman, P. R. Krause, and L. D. Kohn. 1999. Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc. Natl. Acad. Sci. USA 96:2285-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeshita, F., C. A. Leifer, I. Gursel, K. J. Ishii, S. Takeshita, M. Gursel, and D. M. Klinman. 2001. Cutting edge: role of Toll-like receptor 9 in CpG DNA-induced activation of human cells. J. Immunol. 167:3555-3558. [DOI] [PubMed] [Google Scholar]
- 59.Thomas, J., and B. T. Rouse. 1997. Immunopathogenesis of herpetic ocular disease. Immunol. Res. 16:375-386. [DOI] [PubMed] [Google Scholar]
- 60.Vabulas, R. M., H. Pircher, G. B. Lipford, H. Hacker, and H. Wagner. 2000. CpG-DNA activates in vivo T cell epitope presenting dendritic cells to trigger protective antiviral cytotoxic T cell responses. J. Immunol. 164:2372-2378. [DOI] [PubMed] [Google Scholar]
- 61.Verjans, G. M., L. Remeijer, R. S. van Binnendijk, J. G. Cornelissen, H. J. Volker-Dieben, S. G. Baarsma, and A. D. Osterhaus. 1998. Identification and characterization of herpes simplex virus-specific CD4+ T cells in corneas of herpetic stromal keratitis patients. J Infect. Dis. 177:484-488. [DOI] [PubMed] [Google Scholar]
- 62.Wagner, H. 1999. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv. Immunol. 73:329-368. [DOI] [PubMed] [Google Scholar]
- 63.Zheng, M., S. Deshpande, S. Lee, N. Ferrara, and B. T. Rouse. 2001. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J. Virol. 75:9828-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]