Abstract
CpsA, CpsB, CpsC, and CpsD are part of a tyrosine phosphorylation regulatory system involved in modulation of capsule synthesis in Streptococcus pneumoniae and many other gram-positive and gram-negative bacteria. Using an immunoblotting technique, we observed distinct laddering patterns of S. pneumoniae capsular polysaccharides of various serotypes and found that transfer of the polymer from the membrane to the cell wall was independent of size. Deletion of cps2A, cps2B, cps2C, or cps2D in the serotype 2 strain D39 did not affect the ability to transfer capsule to the cell wall. Deletion of cps2C or cps2D, which encode two domains of an autophosphorylating tyrosine kinase, resulted in the production of only short-chain polymers. The function of Cps2A is unknown, and the polymer laddering pattern of the cps2A deletion mutants appeared similar to that of the parent, although the total amount of capsule was decreased. Loss of Cps2B, a tyrosine phosphatase and a kinase inhibitor, resulted in an increase in capsule amount and a normal ladder pattern. However, Cps2B mutants exhibited reduced virulence following intravenous inoculation of mice and were unable to colonize the nasopharynx, suggesting a diminished capacity to sense or respond to these environments. In D39 and its isogenic mutants, the amounts of capsule and tyrosine-phosphorylated Cps2D (Cps2D∼P) correlated directly. In contrast, restoration of type 2 capsule production followed by deletion of cps2B in Rx1, a laboratory passaged D39 derivative containing multiple uncharacterized mutations, resulted in decreased capsule amounts but no alteration in Cps2D∼P levels. Thus, a factor outside the capsule locus, which is either missing or defective in the Rx1 background, is important in the control of capsule synthesis.
Streptococcus pneumoniae is a gram-positive pathogen that is a leading cause of otitis media, pneumonia, and meningitis. Production of a polysaccharide capsule is essential to S. pneumoniae virulence, and 90 structurally and serologically distinct forms have been recognized (6, 30, 34, 35). The capsule allows the organism to evade host defenses by preventing complement receptors from engaging surface-bound complement (14, 64, 65) and, to a lesser extent, by reducing the amount of complement deposited (1). The type 2 capsular polysaccharide repeat unit is a branched hexasaccharide consisting of →4)-β-d-Glcp-(1→3)-α-l-Rhap-(1→3)-α-l-Rhap-(1→3)-β-l-Rhap-(1→ with a disaccharide side chain of α-d-GlcpUA-(1→6)-α-d-Glcp linked α-(1→2) from the first rhamnose in the backbone to the side chain glucose (38). The genetic locus that encodes the enzymes required to produce the type 2 capsule is approximately 18 kb in length and is predicted to comprise a single operon (36). The organization of the type 2 locus is similar to that found in all S. pneumoniae isolates, consisting of a central region unique to each capsule type (i.e., type specific) flanked upstream and downstream by regions common to all capsule types (4, 20, 22, 31).
Production of most S. pneumoniae capsular polysaccharides, including type 2, is expected to occur via the formation of a lipid-linked repeat unit that is synthesized on the intracellular face of the membrane, exported to the cell surface, and polymerized (40, 63). At some point during polymerization, the capsule is covalently linked to the cell wall (57). Aspects of this mechanism occur in the synthesis of capsules from other streptococci, staphylococci, and many gram-negative bacteria. In type III Streptococcus agalactiae (group B streptococcus), the capsule is linked to the N-acetylglucosamine of the peptidoglycan backbone via a phosphodiester bond and an oligosaccharide linker (19). One common finding among these diverse bacteria is the presence of homologous sequences in the capsule loci. In the S. pneumoniae type 2 locus, these sequences are represented by cps2A, cps2B, cps2C, and cps2D. Cps2A has homology to LytR, a transcriptional attenuator in Bacillus subtilis (31, 43), and to CpsIaA in S. agalactiae, which has been linked to transcriptional regulation of capsule gene expression in that system (17). Cps2B, Cps2C, and Cps2D are part of a phosphoregulatory system that is involved in modulation of capsule production (10, 50). Cps2C and Cps2D represent the transmembrane activation domain and cytosolic ATPase domain, respectively, of an autophosphorylating tyrosine kinase. The Cps2C and Cps2D homologues in Escherichia coli, and a number of other gram-negative encapsulated bacteria, are expressed as a single protein (29, 37, 59, 66). A possible role for CpsC and CpsD in the regulation of capsular polysaccharide chain length is suggested by their homology with ExoP from Sinorhizobium meliloti. ExoP, a protein similar to Wzccps from Escherichia coli K30, is an autophosphorylating tyrosine kinase that is involved in modulation of the overall molecular size of the exopolysaccharide succinoglycan (EPS I) (8, 9, 27, 54). The final common protein, Cps2B, is both a novel phosphotyrosine phosphatase that modulates Cps2D phosphorylation and a kinase inhibitor that may prevent the initial phosphorylation of Cps2D (10). Deletion of cpsB in an S. pneumoniae strain expressing the type 19F capsule resulted in the reduction of manganese-dependent cellular phosphatase activity (49).
Previous work using the S. pneumoniae serotype 19F capsule expressed in the laboratory strain Rx1 (Rx1-19F) suggested that phosphorylated CpsD was a negative regulator of capsule production (50). In contrast, Weiser et al. observed a positive correlation between capsule production and CpsD phosphorylation in S. pneumoniae clinical isolates (62). The hypothesis that phosphorylation of CpsD is a positive modulator of capsule synthesis parallels data obtained with its homologue Wzccps in the E. coli K30 system (66). In the present study, we used an immunoblotting technique to analyze the molecular size of S. pneumoniae capsule in the type 2 parent D39 and its isogenic derivatives containing deletions of the common genes. Our results suggest that the phosphoregulatory system may interact with factors outside the capsule locus to positively affect capsule production.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. The Cps2A, Cps2C, and Cps2D mutants contain internal deletions comprising 50, 85, and 95% of the respective genes (3, 10). Construction of the Cps2B mutants is described below. S. pneumoniae strains were grown in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) (Difco) or on blood agar base no. 2 (Difco) containing 3% defibrinated sheep blood (BAP) (Colorado Serum Company). E. coli DH5αF′ was grown and maintained in L broth (10 g of tryptone, 5 g of yeast extract, 5 g of NaCl, and 1 g of glucose per liter) or L agar (L broth with the addition of 15 g of Bacto agar per liter). Where appropriate, media were supplemented with erythromycin (Em) (0.3 μg/ml for S. pneumoniae or 300 μg/ml for E. coli) or ampicillin (Ap) (100 μg/ml).
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| S. pneumoniae D39 derivatives | ||
| AM1000 | Δcps2A-H, type 2 Cps− | 44 |
| D39 | Type 2 parent strain, Cps+ | 7 |
| KA1501 | Δcps2A, reduced type 2 Cps | 3 |
| KA1502 | Δcps2A, reduced type 2 Cps | 3 |
| MB512 | Δcps2D, highly reduced type 2 Cps | 10 |
| MB513 | Δcps2D, highly reduced type 2 Cps | 10 |
| MB516 | Δcps2C, highly reduced type 2 Cps | 10 |
| MB517 | Δcps2C, highly reduced type 2 Cps | 10 |
| MB526 | Δcps2B, type 2 Cps+ | This study |
| MB527 | Δcps2B, type 2 Cps+ | This study |
| S. pneumoniae Rx1 derivatives | ||
| MB532a | D39 × Rx1, type 2 Cps+ | This study |
| MB532 | MB532a × Rx1, type 2 Cps+ | This study |
| MB533 | MB532 (Δcps2B), reduced type 2 Cps | This study |
| MB536 | MB532 (Δcps2B), reduced type 2 Cps | This study |
| Other S. pneumoniae strains | ||
| TIGR4 | Type 4 capsule strain | 58 |
| BG7428 | Type 9 capsule strain | 61 |
| BG5668 | Type 14 capsule strain | 45 |
| BG7371 | Type 14 capsule strain | 11 |
| DBL5 | Type 5 capsule strain | 69 |
| DBL1 | Type 6A capsule strain | 12 |
| L-1 | Type 19F capsule strain | This study |
| WU2 | Type 3 capsule strain | 13 |
| E. coli | ||
| DH5αF′ | F′φ80dlacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ−thi-1 gyrA96 re1A1 | Life Technologies |
| Plasmids | ||
| pGEM-T Easy | PCR cloning vector, Apr | Promega |
| pJY4164 | Lacks origin of replication for S. pneumoniae, Emr | 70 |
| pKA173 | pJY4164 derivative containing PCR fragments from the amplification of D39 chromosomal DNA by the primer pairs Cps2-A4-Cps2-A5 and Cps2-B4-Cps2-C1; used to delete cps2B | 3 |
Fractionation of S. pneumoniae.
S. pneumoniae cultures were grown to 3 × 108 CFU/ml, and the cells were sedimented at 20,000 × g for 15 min at 4°C. The pellets were washed once in phosphate-buffered saline (PBS) (140 mM NaCl, 3 mM KCl, 5 mM Na2HPO4, 2 mM KH2PO4 [pH 7.4]), sedimented at 20,000 × g for 15 min at 4°C, and resuspended in protoplast buffer (PPB) (20% sucrose, 50 mM MgSO4, 50 mM Tris [pH 7.4]) at 1/100 of the original culture volume. Forty units of mutanolysin (Sigma) were added to each milliliter of these extracts, which were then incubated at room temperature overnight. Under these conditions, the S. pneumoniae autolysin (LytA) is also active (72). After incubation, formation of protoplasts was confirmed by using light microscopy. A portion of the reaction mixture was saved, and the remainder was sedimented at 20,000 × g for 10 min at 4°C. The supernatant containing the cell wall fraction was filtered (0.22-μm-pore-size syringe filter [Millipore]) to remove any residual protoplasts or whole cells. The sedimented protoplasts were washed once in PPB and then resuspended in PPB to a volume equal to that of the cell wall extract. The cell wall and protoplast fractions were stored at −80°C. Cross-contamination of the fractions was determined as described in the text. The amounts of protoplast and cell wall samples tested for tyrosine-phosphorylated Cps2D (Cps2D∼P) were equivalent to that used below for whole cells.
Immunoblot analyses.
Capsular polysaccharides were detected in cell wall and protoplast fractions by using immunoblotting. Each sample (20 μl) was combined with 10 μl of buffer B1 (50 mM EDTA, 0.5% Tween 20, 0.5% Triton X-100, 50 mM Tris [pH 8] [Qiagen]) and 2 μl of Qiaprotease (20 μg/μl [Qiagen]). Fractions prepared from the encapsulated parent strains and the D39 Cps2B mutants were diluted (5 μl of sample and 15 μl of PPB) before use. The samples were incubated at 37°C for 30 min, after which 10 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was added, and samples were heated for 8 min at 100°C. SDS-polyacrylamide gels containing between 8 and 14% polyacrylamide were run until the bromphenol blue dye front reached the bottom of the gel. Gels were transferred onto nitrocellulose membranes (Micron Separations Inc.) using a semidry transfer apparatus (Bio-Rad), and the membranes were blocked for 1 h at room temperature in 5% nonfat dried milk in PBST (PBS with 0.05% Tween 20). Detection of capsule was performed using a 1:1,000 dilution of a polyclonal antiserum against the capsular serotype being detected (Statens SerumInstitut) that had been absorbed against the type 2 capsule negative strain AM1000. For analysis of C-polysaccharide, cultures were treated as described under “Fractionation of S. pneumoniae” above and either the entire mutanolysin-treated samples (total cells) or the cell wall fractions were separated by SDS-PAGE. A 1:5,000 dilution of polyclonal antiserum against C-polysaccharide (Statens SerumInstitut) was used for detection. After overnight incubation at 4°C with the primary antisera, blots were washed three times for 5 min with PBST and then incubated for 45 min with goat α-rabbit immunoglobulin conjugated to biotin (Southern Biotechnology Associates) diluted 1:1,000 and incubated along with streptavidin conjugated to alkaline phosphatase (SAP; Southern Biotechnology Associates) diluted 1:2,500. The membranes were washed twice for 5 min in PBST and developed by using 5-bromo-4-chloro-3-indolyl-phosphate and nitroblue tetrazolium (0.25 and 0.05 mg/ml, respectively) in 1 M Tris (pH 8.8).
For the analysis of Cps2D and tyrosine phosphorylated Cps2D (Cps2D∼P), cells were harvested (A600 = 0.45; containing ∼5 × 108 CFU/ml), normalized to culture A600, concentrated 1:50, and boiled in SDS-PAGE loading buffer for 8 min. Proteins were separated by SDS-14% polyacrylamide gel electrophoresis. For Cps2D blots, 24 μl of lysate were used for the Δcps2C, Δcps2D, and Cps− mutants, whereas 8 μl was used for all others. For the Cps2D∼P blots, one-half of these amounts were used. The proteins were then transferred as above onto nitrocellulose membranes and blocked for 1 h at room temperature in 3% Blot Qualified bovine serum albumin (Promega) in Tris-buffered saline (TBS) (100 mM Tris [pH 7.4], 0.9% NaCl) with 0.05% Tween 20 (TBST) for Cps2D∼P detection and in 5% nonfat dried milk in PBST for Cps2D detection. For detection of Cps2D∼P, blots were incubated for 1 h at room temperature with a 1:15,000 dilution of monoclonal antibody against phosphotyrosine conjugated to horseradish peroxidase (α-pTyr) (PT-66-HRP; Sigma) in TBST. Reactive bands were visualized by using the Supersignal substrate (Pierce) for HRP detection. Photometric illumination from the HRP-labeled blots was detected by using X-OMAT film (Kodak). Cps2D was detected by using the rabbit polyclonal α-CpsD serum described by Weiser et al. (62) and development as described above for capsule and C-polysaccharide blots. Protein molecular weights were determined by comparison to the SeeBlue Plus2 prestained molecular-weight marker (Invitrogen).
Construction and characterization of cps2B deletion mutants.
cps2B deletions were generated in S. pneumoniae D39 by using previously described techniques (34). Briefly, PCR fragments flanking the desired deletion were generated by using D39 chromosomal DNA as a template and the primer pairs Cps2-A4-Cps2-A5 and Cps2-B4-Cps2-C1. The sequences of these primers and their location within the published type 2 capsule sequence (36) are shown in Table 2. The two resulting PCR products (one for each primer pair) were cloned into pGEM-T Easy (Promega) and maintained in DH5αF′. Each PCR fragment was excised by digestion with EcoRI (contained within the multiple cloning site of pGEM-T Easy). The upstream and downstream fragments were subcloned together into the S. pneumoniae suicide vector pJY4164 (70), creating pKA173 (Δcps2B). The presence of the correct insert and orientation was confirmed by restriction enzyme digestion and sequencing. pKA173 was transformed into competent D39 (34), reactions were plated on BAP without selection, and patches obtained from single colonies were pooled to facilitate PCR screening by using primers Cps2-A4 and Cps2-C1. Deletion of the region between nucleotides 2896 and 3584, which includes the removal of the translational start site for Cps2B (ATG) and all but the C-terminal 18 amino acids, was confirmed by Southern blotting and sequencing of the entire transferred region. S. pneumoniae derivatives with deletions in cps2B (MB526, MB527) were isolated from two independent transformations of D39 with pKA173.
TABLE 2.
Primers used in this study
| Primera | Sequence | Descriptionb |
|---|---|---|
| Cps2-A4 (+) | GGTCGCAACCAACAAAAGG | cps2A2584-2602 |
| Cps2-A5 (−) | CCATCACATCCTGTATAGC | cps2A2896-2878 |
| Cps2-B4 (+) | GCTCAGGAACTTTTTATAGAC | cps2B3584-3604 |
| Cps2-C1 (−) | GTCAGTTGGTACAGTCACTTG | cps2C4005-3985 |
| Cps2-D1 (−) | CCGACAGGAGCTGTATCTAC | cps2D4820-4801 |
| Cps2-D3 (+) | CTCACAGGCAAAATTGGATTTTG | cps2D4368-4390 |
| Cps2-G1 (+) | GGTCTTTCTGACAACGAGTATC | cps2G9078-9089 |
| Cps2-I3 (−) | CCACCTGAATTTGTCCCAATAAC | cps2I11907-11884 |
| LDH-F (+) | GTCGGTGATGGTGCTGTAGGTTCATC | ldh164-189 |
| LDH-R (−) | GTCGATGTTAGCGTGTGACCAAACAG | ldh710-687 |
Forward and reverse primers are represented by plus (+) and minus (−), respectively.
To analyze the effect of deleting cps2B on type 2 capsular molecular size in the Rx1 background, an Rx1 derivative that produced type 2 capsule was generated by transformation of Rx1 with a crude cellular lysate of D39 (71). The transformation was plated on BAP without selection, and colonies were screened for the smooth morphology typical of type 2 strains. Production of type 2 capsule was confirmed by the Quellung reaction (53), and the presence of the type 2 capsule locus was confirmed by PCR using primers inside the common region of the type 2 capsule locus (Cps2-A4-Cps2-C1) and surrounding the putative type 2 polymerase, cps2H (Cps2-G1-Cps2-I3). This strain was backcrossed once, and the confirmation steps were repeated to yield MB532 (Rx1 type 2). Two independent cps2B deletion mutants (MB533 and MB536) were constructed in MB532 by using pKA173, as described above for D39.
Indirect enzyme-linked immunosorbent assays (ELISAs), performed as previously described (10), were used to quantify type 2 capsule production. The polyclonal antiserum to the type 2 capsule (Statens SerumInstitut) was absorbed against the capsule-negative AM1000 prior to use. The integrity of the capsule was examined in indirect ELISAs using intact cells and a polyclonal antiserum against a serotype 19 S. pneumoniae (typing serum; Statens SerumInstitut), as previously described (1, 34). Because the type 19 capsule is poorly immunogenic, the majority of the antibodies in this antiserum are directed towards noncapsular surface antigens (34). The indirect ELISAs provide results comparable to those obtained when cells in suspension are used (1). The results from the ELISAs were analyzed by using Student's t test.
Bacteria were prepared for electron microscopy essentially as described by Kolkman (41). Briefly, cells from 10-ml cultures were pelleted, washed three times in PBS, suspended in 1 ml of fixative (4CF-1G) (1% glutaraldehyde, 4% formaldehyde, 67.5 mM NaOH, 84 mM NaH2PO4 [pH 7.2]), and incubated at 4°C for 30 min. Following centrifugation, the fixation step was repeated, and the cells were washed in 1 ml of phosphate buffer (135 mM NaH2PO4 [pH 7.2], 105 mM NaOH, 3.7% formaldehyde), embedded in agarose and sectioned. Samples were viewed at a magnification of ×50,000. By this method, encapsulated bacteria exhibit a distinct extracellular substance that is not present on isogenic nonencapsulated mutants (data not shown) (3, 41).
Analysis of cps2D transcripts.
RNA was isolated from 25 ml of S. pneumoniae cultures by a hot acid phenol procedure, as previously described (26), and RNA concentrations were determined by UV spectrophotometry. The level of transcript was determined by using a slot-blot procedure, as previously described (2, 5). RNA was denatured in 3 volumes of a solution containing 500 μl of formamide, 162 μl of 12.3 M (37%) formaldehyde, and 100 μl of MOPS [3-(N-morpholino)-propanesulfonic acid] buffer (0.2 M MOPS [pH 7.0], 0.5 M sodium acetate, and 0.01 M EDTA) for 15 min at 65°C. Denatured RNA (5 μg and 0.5 μg for each sample) was blotted onto nylon membranes, which were then UV-cross-linked (Stratalinker; Stratagene) and prehybridized for 3 h in high SDS hybridization buffer (7% SDS, 50% formamide, 5× SSC [3 M NaCl, 0.3 M sodium citrate {pH 7}, 2% blocking reagent {32}, 50 mM sodium phosphate {pH 7.0}, and 0.1% N-laurylsarcosine) at 42°C. The RNA was hybridized overnight at 42°C with a denatured (100°C, 10 min) digoxigenin-labeled (Roche) PCR product obtained by using primers Cps2-D1 and Cps2-D3 (Table 2) and added directly to the prehybridization solution. Following hybridization, membranes were washed twice at room temperature with 2× SSC containing 0.1% SDS and twice with 0.5× SSC containing 0.1% SDS for 15 min at 65°C. Blots were developed by using the Genius system (Roche). The amount of transcript was quantitated by densitometry using ImageJ software (http://rsb.info.nih.gov/ij) and normalized to lactate dehydrogenase (ldh) transcript levels as an internal control for each sample. The ldh probe was obtained by using primers LDH-F and LDH-R (Table 2).
Mouse infections.
Female BALB/cByJ mice, 8 to 12 weeks old, were used for systemic infections and colonization studies, performed essentially as described previously (34, 44). S. pneumoniae cultures were grown in THY to approximately 3 × 108 CFU/ml and diluted in lactated Ringer's solution. For intraperitoneal (i.p.) infections, D39 and MB526 were inoculated at doses of 8 × 105 CFU and MB527 was inoculated at a dose of 106 CFU (inocula were determined by plating). For intravenous (i.v.) infections, mice were inoculated with 2 × 107 CFU of the parent D39 and 5 × 107 CFU of each mutant. As noted in the text, these doses are 20- to 50-fold above the respective 50% lethal doses (unpublished data). All mice were monitored for 21 days postinfection. For blood clearance studies, mice infected i.v. were bled retro-orbitally at the times indicated in the text. The numbers of bacteria were determined by plating on BAP. For colonization studies, mice were inoculated intranasally with 1.5 × 109 CFU and sacrificed after 7 days to determine the number of bacteria colonizing the nasopharyngeal cavity, as previously described (44). Statistical comparisons where performed by using Fisher's exact test for survival analyses or Student's t test for comparison of the numbers of bacteria recovered from nasal washes or blood.
RESULTS
Linkage of capsule to the cell wall is not dependent on chain length.
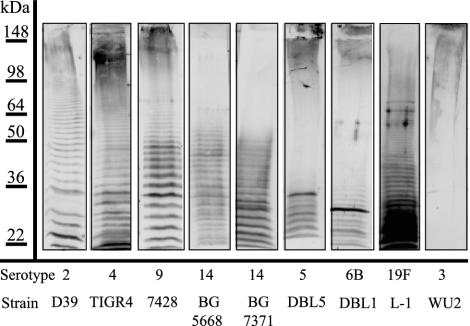
Using SDS-PAGE and immunoblotting with type-specific antisera, we were able to distinguish laddering patterns for the capsular polysaccharides from strains representing a number of different S. pneumoniae serotypes (Fig. 1). The distributions of polymer sizes varied among the isolates examined, a result that could reflect underlying differences in the control of their syntheses or differences in their recognition by the polyclonal antisera used in the detection. A range of polymer sizes was seen for most of the serotypes (Fig. 1). The type 3 polysaccharide, however, was predominantly of very high molecular size that did not enter the resolving gel, and a ladder pattern was not observed (Fig. 1 and data not shown). Unlike the other capsule types examined, the type 3 capsule is synthesized by a processive mechanism in which repeat units are not formed (16), and polysaccharide that is released from the membrane into the surrounding cell wall and environment (24, 33) is not covalently attached to the peptidoglycan (57).
FIG. 1.
Immunoblot analysis of cell wall fractions of S. pneumoniae strains of different capsular serotypes. Cell wall-associated capsule was fractionated by SDS-8% PAGE and, after transfer to nitrocellulose, was reacted with polyclonal antiserum specific to each serotype, as indicated at the bottom of the figure. Protein molecular mass standards (in kilodaltons) were used to standardize each gel to run length and do not indicate actual polymer sizes.
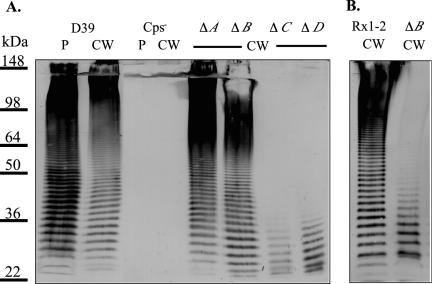
Analysis of the polysaccharide laddering patterns from protoplast and cell wall fractions revealed similar size distributions, indicating that transfer of maturing capsule from the membrane to the cell wall is not dependent on polymer size. The fractionation for the type 2 strain D39 is shown in Fig. 2A, and we obtained similar results for strains of other serotypes (data not shown). To assess the level of cross-contamination between the D39 cell wall and protoplast fractions, each was analyzed for β-galactosidase activity and for the presence of tyrosine-phosphorylated Cps2D (Cps2D∼P). The S. pneumoniae β-galactosidase is a surface-localized protein that contains an LPXTG-motif (73), which is expected to result in anchoring of the protein to peptidoglycan and localization of the majority of β-galactosidase activity with the cell wall fraction (52). In enzyme assays performed essentially as described by Miller (46), approximately 85 and 15% of the β-galactosidase activity was detected in the cell wall and protoplast fractions, respectively. β-Galactosidase activity in the protoplast fraction represents protein that is still membrane anchored and yet to be transferred to the cell wall (52), as well as protein that is anchored to peptidoglycan that survived enzymatic digestion. Contamination of the protoplast fraction with undigested peptidoglycan and associated capsule was thus minimal. Similarly, all of the Cps2D∼P detectable by immunoblotting was present in the protoplast fraction, indicating that only minimal lysis and contamination of the cell wall fraction with cytoplasmic contents or membrane fragments had occurred. In the cell wall fraction, only low-molecular-size teichoic acid (C-polysaccharide) was detectable, indicating that the majority of the released peptidoglycan had been completely digested (data not shown).
FIG. 2.
Immunoblot analysis of type 2 derivatives. (A) Protoplast-associated (P) or cell wall-associated (CW) capsule from D39, AM1000 (Cps−), KA1501 (ΔA), MB526 (ΔB), MB516 (ΔC), or MB512 (ΔD) was separated by SDS-8% PAGE and analyzed by immunoblotting using polyclonal antiserum against type 2 capsule. AM1000, KA1501, MB512, and MB516 contain fourfold more sample than D39 and MB526. Identical results were obtained for two independent isolates of each mutant. (B) Cell wall-associated (CW) capsule of the Rx1-type 2 derivative MB532 (Rx1-2) and its cps2B deletion mutant MB533 (ΔB) separated by SDS-10% PAGE and analyzed by immunoblotting using polyclonal antiserum against type 2 capsule. Identical results were obtained with the independently derived Rx1-Δcps2B mutant MB536 (data not shown).
Polymer size and capsule amounts are altered by deletions in the common genes.
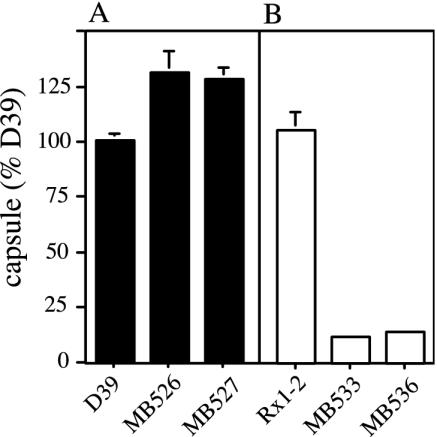
The effects of deletion mutations in cps2A, B, C, and D on polymer size and distribution were examined by using the immunoblotting technique described above. Although no capsule is detectable by ELISA on the Cps2C and Cps2D mutants (10), low-molecular-size products were observed for both by this technique (Fig. 2A). The size distributions for the Cps2A mutants, which exhibit a 50% reduction in total capsule (3) (K. Ambrose and J. Yother, unpublished data), and for the Cps2B mutants appeared similar to those observed for the D39 parent (Fig. 2A). Analysis of the Cps2B mutants MB526 and MB527 using intact cells in ELISAs revealed an approximate 30% increase in capsule amount, compared to the D39 parent (Fig. 3A). For each of the mutants, the polymer size distribution was the same in both protoplast and cell wall fractions, indicating that none of the mutations affected the ability to link the polysaccharide to the cell wall (data not shown). None of the mutants exhibited differences in the distribution or amount of teichoic acid (C-polysaccharide, data not shown).
FIG. 3.
Capsule production by Cps2B mutants. Capsule amounts were determined by indirect ELISAs and are expressed relative to D39. (A) D39 and its cps2B deletion derivatives MB526 and MB527. (B) The Rx1-type 2 derivative MB532 (Rx1-2) and its cps2B deletion mutants MB533 and MB536. The P values obtained by comparison to D39 (n = 6) were <0.05 for MB526 (n = 6), <0.005 for MB527 (n = 5), and <0.0005 for MB533 (n = 4) and MB536 (n = 4). MB532 (n = 4) was not different from D39.
Cps2B mutants have different phenotypes in the D39 and Rx1 backgrounds.
The alterations in total capsule amounts resulting from the cps2A, cps2C, and cps2D deletions in D39 are similar to those described for mutations in the S. pneumoniae type 19F homologues of these genes (50). However, the highly encapsulated phenotype of the Cps2B mutants in the D39 background was contrary to the reduced capsular phenotype (10-fold reduction) previously reported by Morona et al., following deletion of cpsB from the type 19F capsule locus (50). The underlying differences between the earlier study and our work are the capsular serotype and the background of the strain used to construct the deletion mutation. Morona et al. used the S. pneumoniae strain Rx1 into which the type 19F capsule locus had been transformed (47). Rx1 is a highly passaged derivative of D39 that was originally isolated as a nonencapsulated variant and later transformed to type 3 encapsulation. A spontaneous mutant producing low levels of type 3 capsule was subsequently isolated and used to derive strains that exhibited highly increased transformation efficiencies, ultimately resulting in Rx1 (21, 56).
We constructed a type 2 capsule-producing Rx1 derivative by transforming Rx1 with cellular lysate from D39. We then generated two independent cps2B deletion mutants in this background, as described in Materials and Methods. The amount of capsule produced by the Rx1 type 2 derivative was similar to that of D39, but the Rx1-Δcps2B mutants produced approximately 13% of this level (Fig. 3B). This reduced capsule phenotype was similar to that seen for the Rx1-Δcps19fB mutant constructed by Morona et al. (50) but unlike what we observed for cps2B deletions in D39 (Fig. 3A). The Rx1-Δcps2B mutants were reduced in high-molecular-weight capsular polysaccharide (Fig. 2B), similar to the D39-Δcps2C and D39-Δcps2D mutants but unlike the D39-Δcps2B mutants (Fig. 2A). Also, unlike the D39-Δcps2C and D39-Δcps2D mutants, a ladder extending to the full range of the gel for the Rx1-Δcps2B mutants was seen, albeit at a severely reduced level (Fig. 2). This long-chain polymer may account for the reactivity of the Rx1-Δcps2B mutants when whole cells and type 2 antiserum are used in an ELISA, whereas the D39-Δcps2C and D39-Δcps2D mutants were not reactive (10).
Levels of Cps2D∼P are positively correlated with levels of capsule in the D39 background.
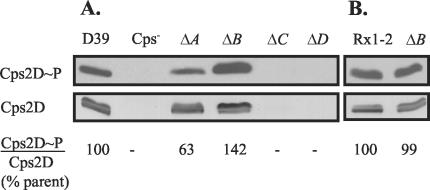
Comparison of the levels of Cps2D and Cps2D∼P in D39 and its isogenic derivatives revealed decreased levels of Cps2D phosphorylation following the loss of Cps2A but increased levels in Cps2B mutants (Fig. 4A), mirroring the changes observed for capsule production. In contrast, the ratio of Cps2D∼P and Cps2D in the Rx1-type 2 Cps2B mutant was unchanged from its parent (Fig. 4B). In D39, deletion of cps2C resulted in near-complete loss of Cps2D (Fig. 4A). This effect was not due to the loss of cps2D transcripts, as these levels were essentially unchanged from those observed in the parent (data not shown). The similar levels of Cps2D in the parent strains and in the cps2A and cps2B deletion mutants make it unlikely that the phenotypes of these strains are related to polarity of the mutations on other capsule genes, all of which are located downstream of cps2D in an apparent operon (36).
FIG. 4.
Comparison of Cps2D protein and tyrosine phosphorylation levels in type 2 capsule mutants. In the upper panel, tyrosine phosphorylation of Cps2D (Cps2D∼P) was detected by using Western immunoblotting with a mouse monoclonal antibody against phosphotyrosine clone PT-66 conjugated to horseradish peroxidase. In the lower panel, Cps2D was detected by using Western immunoblotting and a CpsD-specific polyclonal antiserum. This antiserum recognizes both phosphorylated and nonphosphorylated forms of Cps2D (62), accounting for the doublet seen in the Cps2D blot. For both blots, the Cps− (AM1000), ΔC, and ΔD lanes contained threefold more sample than the D39, ΔA, ΔB, Rx1-2, and Rx1-2 ΔB lanes. The 22- to 32-kDa size range is shown. Comparable results were obtained for two independent isolates of each mutant. Densitometry using ImageJ software (http://rsb.info.nih.gov/ij) was used to determine the intensity of each band. The ratios of the Cps2D∼P and Cps2D intensities for each mutant were normalized to that for D39 or Rx1-2 to obtain the percentage of parent value (% parent value) shown.
Cps2B mutants are reduced in virulence.
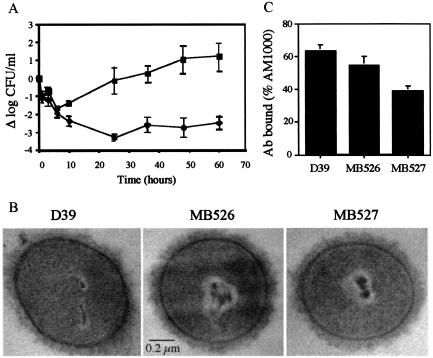
Although the Cps2B mutants produced increased amounts of capsule and a distribution of chain lengths similar to that of the parent D39, they exhibited significant reductions in their ability to colonize the nasopharynx and to cause systemic infections in mice following i.v. inoculation (Table 3). The mice infected with the Cps2B mutants did appear ill, however, exhibiting reduced movement and ruffled fur prior to recovery. Blood clearance assays showed that they harbored low levels of bacteria for several days (Fig. 5A). In contrast to these results, virulence following i.p. inoculation was not altered by the cps2B mutation (Table 3).
TABLE 3.
Nasopharyngeal colonization and systemic virulence of D39 and Cps2B mutants in BALB/cByJ mice
| S. pneumoniae strain | No. of mice colonized or alive/total no. infected (route of infection)a
|
||
|---|---|---|---|
| Colonized (i.n.) | Alive (i.v.) | Alive (i.p.) | |
| D39 | 7/7b | 1/7c | 2/7 |
| Δcps2Bd | 2/14e | 14/14e | 0/14f |
Mice were inoculated with doses 20- to 50-fold (i.v.) or 20-fold (i.p.) above the 50% lethal dose. i.n., intranasal infection.
One mouse succumbed to infection.
The median time to death was 70 h.
Combined data for the two independent mutants MB526 and MB527.
P ≤ 0.001 when compared to D39.
Not significantly different from D39. The median times to death for the D39- and Δcps2B infected mice (20 and 18.5 h, respectively) also were not different.
FIG. 5.
Analysis of Cps2B mutants of D39. (A) Blood clearance following i.v. inoculation. The numbers of bacteria were significantly different (P ≤ 0.005) at all time points after 10 h. At 84 h, the mice inoculated with the Δcps2B mutants had no bacteria remaining, and three of the mice inoculated with D39 had died. ▪, D39 (n = 7); ⧫, Δcps2B mutants (data combined for MB526 and MB527; n = 10). (B) Electron micrographs. The extracellular substance surrounding D39 and the Cps2B mutants was not present on a nonencapsulated derivative (not shown). Samples were viewed at a magnification of ×50,000. (C) Binding of polyclonal antiserum reactive with S. pneumoniae surface antigens. Results are expressed relative to the value for nonencapsulated AM1000, which is taken as 100%. Reductions in binding occur as a result of blocking of the surface by the capsule. For each strain, n = 3. MB527 was significantly different from D39 (P < 0.05).
Further examinations of the capsules produced by Cps2B mutants grown under laboratory culture conditions failed to reveal specific defects that could account for the reduced virulence phenotype. On blood agar medium, colonies of the mutants appeared similar to those of the parent, and microscopic examination using the Quellung reaction revealed capsules that were normal in appearance. Electron microscopy demonstrated a distinct and similar surface layer in D39 and the Cps2B mutants (Fig. 5B), which was not present in nonencapsulated derivatives (not shown). To examine the integrity of the capsule, the reactivity of the Cps2B mutants with polyclonal antiserum against noncapsule surface antigens was examined. Access of antibodies to surface antigens is limited by intact capsules but can be increased by alterations in capsule amount or composition, paralleling the accessibility to complement (C3b) bound to the cell wall (1, 34). Consistent with their capsule phenotypes, the Cps2B mutants effectively reduced access to underlying surface antigens (Fig. 5C).
DISCUSSION
In this study, we used an immunoblotting technique to examine the molecular sizes of capsular polysaccharides localized to the membrane and cell wall of S. pneumoniae. We observed no differences in the size distributions of the capsule in the protoplast (membrane-associated) and cell wall fractions, and both low- and high-molecular-size polymers were transferred to the cell wall. This result strongly suggests that the mechanism of capsule transfer to the cell wall is not based upon the degree of polymerization. The enzymes and mechanisms involved in the attachment of capsules to bacterial surfaces have not been elucidated, but most capsules produced by gram-negative bacteria are thought to be linked to the outer membrane by a lipid moiety (28, 42), whereas the capsules of the gram-positive staphylococci and streptococci are usually covalently linked to the cell wall (18, 19, 25, 57, 67, 68). In S. pneumoniae, covalent attachment occurs with most serotypes, with the exception of type 3 (57), which is synthesized by a processive mechanism that does not involve the formation of repeat units (16). Control of capsule synthesis also likely occurs by a different mechanism, as the common genes in the type 3 locus are mutated and not transcribed (4, 15) and no tyrosine-phosphorylated proteins have been detected in type 3 strains (50; M. H. Bender and J. Yother, unpublished data).
Although cps2C and cps2D deletion mutants of the type 2 D39 produce levels of surface polymer that are undetectable by ELISA (10), immunoblotting demonstrated the presence of short chains, indicating that only a severe reduction in capsule size and not an elimination of polymerization had occurred. This result is consistent with a role for Cps2C and Cps2D in chain length modulation and is similar to observations made for the CpsC and CpsD homologues ExoP in S. meliloti, Wzccps in E. coli, and CpsC and CpsD from S. agalactiae (8, 9, 17, 23). In S. meliloti, ExoP mutants produce reduced amounts of succinoglycan, with lower-molecular-weight species being increased relative to higher-molecular-weight species (8, 9). cpsC and cpsD deletion mutants of S. agalactiae exhibited only 50% reductions in chain length size but greater than 90% reductions in the total amount of polysaccharide (17). Wzccps mutants of E. coli also produced reduced levels of high-molecular-weight K30 capsular antigen (23). Deletion of cps2C in S. pneumoniae resulted in near-complete loss of Cps2D, although cps2D transcript levels were essentially unchanged. This result could reflect decreased stability of Cps2D in the absence of its cognate transmembrane domain or, on a broader level, could suggest the requirement for these proteins in a complex that is functionally altered when one member is missing.
Due to their apparent effects on the degree of polymerization, CpsC and CpsD and their homologues have been termed “polysaccharide co-polymerases” and classified in families PCP2a and PCP2b based on predicted structural similarities and functions (51). Their roles in polysaccharide polymerization in both gram-positive and gram-negative bacteria have been linked to their autophosphorylating tyrosine kinase activity (50, 54, 60, 66). In the S. meliloti succinoglycan and E. coli K30 capsule systems, phosphorylation is essential for production of wild-type levels of high-molecular-weight polymer (54, 55, 66). Our results with type 2 capsule in S. pneumoniae D39 likewise reveal a positive correlation between phosphorylation and capsule synthesis. The differences in results obtained in our system and in that described by Morona et al. for the type 19F S. pneumoniae capsule appear to relate in part to the expression of the latter in the Rx1 background and in part to a misinterpretation of the Rx1-19F data. The conclusions suggesting negative regulation of capsule synthesis by phosphorylation of CpsD were based on the observation of an increase in phosphorylation and a decrease in capsule following deletion of cpsB, along with the elimination of phosphorylation and an increase in colony mucoidy following mutation of the tyrosine phosphorylation sites of CpsD (50). We also observed a high level of phosphorylation and a reduction in the amount of type 2 capsule in Rx1 following deletion of cps2B, in contrast to the phenotype of the D39 cps2B deletion mutant. Thus, an additional factor located outside the capsule locus, and missing or defective in Rx1, appears to play an important role in regulating capsule production, possibly through a direct or indirect interaction with CpsB.
The increased colony mucoidy observed by Morona et al. occurred in mutants containing site-specific mutations that eliminated the tyrosine phosphorylation sites and, consequently, phosphorylation of the type 19F CpsD. The mucoidy was originally interpreted to reflect an increase in capsule, although no such increase could be demonstrated (50). While the present study was under review, Morona et al. published a second study characterizing the effect of mutation of the tyrosine residues on encapsulation and CpsD phosphorylation (48). Although the authors again conclude that tyrosine phosphorylation of CpsD negatively regulates capsule production, the immunoassays and chemical analyses presented there show that the increased mucoidy is not due to an increase in capsule. Although the basis for the mucoidy was not determined, the strains, in fact, produced less capsule and less phosphorylated CpsD than the parent Rx1-19F. In addition, mutants that produced C-terminally truncated CpsD proteins also exhibited less phosphorylation and less capsule production than the parent (48). These results are thus in agreement with a positive correlation between phosphorylation and capsule production. How, or if, the factor missing in Rx1 interfaces with the phosphotyrosine system, as well as a specific role for the latter in regulation, is at present unknown. Indeed, it is not yet clear whether phosphorylation of CpsD has a direct effect on capsule production or merely correlates with that phenotype. Because D39 is the virulent parent from which Rx1 was derived, it seems likely that a positive correlation between tyrosine phosphorylation and capsule production reflects the wild-type scenario, as was observed by Weiser et al. in the examination of clinical S. pneumoniae isolates of different serotypes (62). Taken together, the results indicate that CpsC and CpsD are required for the production of high-molecular-weight capsule and that tyrosine phosphorylation of CpsD is required to produce elevated amounts of capsule.
CpsB is a phosphotyrosine phosphatase and a tyrosine kinase inhibitor (10) that is important for phosphatase activity in S. pneumoniae (49; Bender and Yother, unpublished data). The loss of Cps2B in D39 resulted in an increase in tyrosine phosphorylation of Cps2D, the only S. pneumoniae protein demonstrated to be modified in this way. Despite the increase in capsule amount and the apparently normal display and function of capsule in the Cps2B mutants, the activity of Cps2B was critical for survival of D39 during systemic infections and colonization. Possibly, the regulation of capsule or other factors that may be controlled through Cps2B activity is altered in these mutants in the animal environment. Other proteins, either through their own autokinase activity or as a result of the transphosphorylation activity of Cps2D (10) may be phosphorylated and subject to Cps2B control in different environments. Modulation of capsule amounts in response to environmental conditions, as has been noted with carbon dioxide levels, the transition between opaque and transparent phase variants, and the reduced amounts of capsule sufficient for nasopharyngeal colonization suggest that capsule production is regulated in a manner that may be dependent on the site of infection (39, 44, 62).
The specific role that CpsA plays in the modulation of capsule synthesis remains unclear. Due to its homology to LytR, a transcriptional attenuator of autolysin expression in B. subtilis (43), CpsA is frequently referred to as a transcriptional regulator of capsule production in S. pneumoniae (31, 48-50). However, no data have yet been presented to support this role. Deletion of the homologous cpsIaA in S. agalactiae resulted in reduced levels of cpsIaD transcripts, as determined by reverse transcription-PCR analysis, but the underlying mechanism involved was not determined (17). Following deletion of cps2A in S. pneumoniae D39, we observed no significant changes in the level of Cps2D; however, a decrease in Cps2D phosphorylation and encapsulation occurred. These data suggest that CpsA modulation may involve mechanisms other than, or in addition to, transcriptional control.
To date, no direct role for any of the common proteins in controlling capsule production has been demonstrated. Understanding the mechanisms through which tyrosine phosphorylation and factors encoded both within and outside the capsule locus interface is central to developing an integrated picture of capsule synthesis and its modulation.
Acknowledgments
We thank Karita Ambrose for the construction of pKA173 and the cps2A mutants, David Briles for S. pneumoniae clinical isolates, and Jeffrey Weiser for the CpsD antiserum.
This work was supported by Public Health Service grants GM53017, AI28457, T32 GM08111, and T32 HL07553 from the National Institutes of Health.
REFERENCES
- 1.Abeyta, M., G. G. Hardy, and J. Yother. 2003. Genetic alteration of capsule type but not PspA type affects accessibility to surface-bound complement and non-capsular surface antigens in Streptococcus pneumoniae. Infect. Immun. 71:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alwine, J. C., D. J. Kemp, and G. R. Stark. 1977. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl. Acad. Sci. USA 74:5350-5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambrose, K. D. 2001. Global effects of alterations in capsule production in Streptococcus pneumoniae. University of Alabama at Birmingham, Birmingham.
- 4.Arrecubieta, C., E. Garcia, and R. Lopez. 1995. Sequence and transcriptional analysis of a DNA region involved in the production of capsular polysaccharide in Streptococcus pneumoniae type 3. Gene 167:1-7. [DOI] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology, vol. 1. John Wiley and Sons, Inc., Boston, Mass.
- 6.Avery, O. T., and R. Dubos. 1931. The protective action of a specific enzyme against type 3 pneumococcus infection in mice. J. Exp. Med. 54:73-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avery, O. T., C. M. MacLeod, and M. McCarty. 1944. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J. Exp. Med. 79:137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker, A., K. Niehaus, and A. Puhler. 1995. Low-molecular-weight succinoglycan is predominantly produced by Rhizobium meliloti strains carrying a mutated ExoP protein characterized by a periplasmic N-terminal domain and a missing C-terminal domain. Mol. Microbiol. 16:191-203. [DOI] [PubMed] [Google Scholar]
- 9.Becker, A., and A. Puhler. 1998. Specific amino acid substitutions in the proline-rich motif of the Rhizobium meliloti ExoP protein result in enhanced production of low-molecular-weight succinoglycan at the expense of high-molecular-weight succinoglycan. J. Bacteriol. 180:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bender, M. H., and J. Yother. 2001. CpsB is a modulator of capsule-associated tyrosine kinase activity in Streptococcus pneumoniae. J. Biol. Chem. 276:47966-47974. [DOI] [PubMed] [Google Scholar]
- 11.Briles, D. E., M. J. Crain, B. M. Gray, C. Forman, and J. Yother. 1992. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect. Immun. 60:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briles, D. E., C. Forman, and M. Crain. 1992. Mouse antibody to phosphocholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect. Immun. 60:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, E. J. 1985. Interaction of gram-positive microorganisms with complement. Curr. Top. Microbiol. Immunol. 121:159-187. [DOI] [PubMed] [Google Scholar]
- 15.Caimano, M. J., G. G. Hardy, and J. Yother. 1998. Capsule genetics in Streptococcus pneumoniae and a possible role for transposition in the generation of the type 3 locus. Microb. Drug Resist. 4:11-23. [DOI] [PubMed] [Google Scholar]
- 16.Cartee, R. T., W. T. Forsee, J. S. Schutzbach, and J. Yother. 2000. Mechanism of type 3 capsular polysaccharide synthesis in Streptococcus pneumoniae. J. Biol. Chem. 275:3907-3914. [DOI] [PubMed] [Google Scholar]
- 17.Cieslewicz, M. J., D. L. Kasper, Y. Wang, and M. R. Wessels. 2001. Functional analysis in type Ia group B Streptococcus of a cluster of genes involved in extracellular polysaccharide production by diverse species of streptococci. J. Biol. Chem. 276:139-146. [DOI] [PubMed] [Google Scholar]
- 18.De Cueninck, B. J., G. D. Shockman, and R. M. Swenson. 1982. Group B, type III streptococcal cell wall: composition and structural aspects revealed through endo-N-acetylmuramidase-catalyzed hydrolysis. Infect. Immun. 35:572-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng, L., D. L. Kasper, T. P. Krick, and M. R. Wessels. 2000. Characterization of the linkage between the type III capsular polysaccharide and the bacterial cell wall of Group B Streptococcus. J. Biol. Chem. 275:7497-7504. [DOI] [PubMed] [Google Scholar]
- 20.Dillard, J. P., M. Caimano, T. Kelly, and J. Yother. 1995. Capsules and cassettes: genetic organization of the capsule locus of Streptococcus pneumoniae. Dev. Biol. Stand. 85:261-265. [PubMed] [Google Scholar]
- 21.Dillard, J. P., M. W. Vandersea, and J. Yother. 1995. Characterization of the cassette containing genes for type 3 capsular polysaccharide biosynthesis in Streptococcus pneumoniae. J. Exp. Med. 181:973-983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dillard, J. P., and J. Yother. 1994. Genetic and molecular characterization of capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 3. Mol. Microbiol. 12:959-972. [DOI] [PubMed] [Google Scholar]
- 23.Drummelsmith, J., and C. Whitfield. 1999. Gene products required for surface expression of the capsular form of the group 1 K antigen in Escherichia coli (O9a:K30). Mol. Microbiol. 31:1321-1332. [DOI] [PubMed] [Google Scholar]
- 24.Forsee, W. T., R. T. Cartee, and J. Yother. 2000. Biosynthesis of type 3 capsular polysaccharide in Streptococcus pneumoniae. Enzymatic chain release by an abortive translocation process. J. Biol. Chem. 275:25972-25978. [DOI] [PubMed] [Google Scholar]
- 25.Fournier, J. M., W. F. Vann, and W. W. Karakawa. 1984. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect. Immun. 45:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgellis, D., S. Arvidson, and A. von Gabain. 1992. Decay of ompA mRNA and processing of 9S RNA are immediately affected by shifts in growth rate, but in opposite manners. J. Bacteriol. 174:5382-5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez, J. E., C. E. Semino, L. X. Wang, L. E. Castellano-Torres, and G. C. Walker. 1998. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 95:13477-13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gotschlich, E. C., B. A. Fraser, O. Nishimura, J. B. Robbins, and T. Y. Liu. 1981. Lipid on capsular polysaccharides of gram-negative bacteria. J. Biol. Chem. 256:8915-8921. [PubMed] [Google Scholar]
- 29.Grangeasse, C., P. Doublet, E. Vaganay, C. Vincent, G. Deleage, B. Duclos, and A. J. Cozzone. 1997. Characterization of a bacterial gene encoding an autophosphorylating protein tyrosine kinase. Gene 204:259-265. [DOI] [PubMed] [Google Scholar]
- 30.Griffith, F. 1928. The significance of pneumococcal types. J. Hygiene 27:113-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guidolin, A., J. K. Morona, R. Morona, D. Hansman, and J. C. Paton. 1994. Nucleotide sequence analysis of genes essential for capsular polysaccharide biosynthesis in Streptococcus pneumoniae type 19F. Infect. Immun. 62:5384-5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hakansson, A., H. Roche, S. Mirza, L. S. McDaniel, A. Brooks-Walter, and D. E. Briles. 2001. Characterization of binding of human lactoferrin to pneumococcal surface protein A. Infect. Immun. 69:3372-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardy, G. G., M. J. Caimano, and J. Yother. 2000. Capsule biosynthesis and basic metabolism in Streptococcus pneumoniae are linked through the cellular phosphoglucomutase. J. Bacteriol. 182:1854-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy, G. G., A. D. Magee, C. L. Ventura, M. J. Caimano, and J. Yother. 2001. Essential role for cellular phosphoglucomutase in virulence of type 3 Streptococcus pneumoniae. Infect. Immun. 69:2309-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henrichsen, J. 1995. Six newly recognized types of Streptococcus pneumoniae. J. Clin. Microbiol. 33:2759-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iannelli, F., B. J. Pearce, and G. Pozzi. 1999. The type 2 capsule locus of Streptococcus pneumoniae. J. Bacteriol. 181:2652-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ilan, O., Y. Bloch, G. Frankel, H. Ullrich, K. Geider, and I. Rosenshine. 1999. Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J. 18:3241-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jansson, P. E., B. Lindberg, M. Anderson, U. Lindquist, and J. Henrichsen. 1988. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 2, a reinvestigation. Carbohydr. Res. 182:111-117. [DOI] [PubMed] [Google Scholar]
- 39.Kim, J. O., and J. N. Weiser. 1998. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J. Infect. Dis. 177:368-377. [DOI] [PubMed] [Google Scholar]
- 40.Kolkman, M. A., W. Wakarchuk, P. J. Nuijten, and B. A. van der Zeijst. 1997. Capsular polysaccharide synthesis in Streptococcus pneumoniae serotype 14: molecular analysis of the complete cps locus and identification of genes encoding glycosyltransferases required for the biosynthesis of the tetrasaccharide subunit. Mol. Microbiol. 26:197-208. [DOI] [PubMed] [Google Scholar]
- 41.Kolkman, M. A. B. 1997. Capsular polysaccharide synthesis in Streptococcus pneumoniae. University of Utrecht, Utrecht, The Netherlands.
- 42.Kuo, J. S., V. W. Doelling, J. F. Graveline, and D. W. McCoy. 1985. Evidence for covalent attachment of phospholipid to the capsular polysaccharide of Haemophilus influenzae type b. J. Bacteriol. 163:769-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazarevic, V., P. Margot, B. Soldo, and D. Karamata. 1992. Sequencing and analysis of the Bacillus subtilis lytRABC divergon: a regulatory unit encompassing the structural genes of the N-acetylmuramoyl-L-alanine amidase and its modifier. J. Gen. Microbiol. 138:1949-1961. [DOI] [PubMed] [Google Scholar]
- 44.Magee, A. D., and J. Yother. 2001. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 69:3755-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McDaniel, L. S., D. O. McDaniel, S. K. Hollingshead, and D. E. Briles. 1998. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect. Immun. 66:4748-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller, J. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 47.Morona, J. K., A. Guidolin, R. Morona, D. Hansman, and J. C. Paton. 1994. Isolation, characterization, and nucleotide sequence of IS1202, an insertion sequence of Streptococcus pneumoniae. J. Bacteriol. 176:4437-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morona, J. K., R. Morona, D. C. Miller, and J. C. Paton. 2003. Mutational analysis of the carboxy-terminal (YGX)4 repeat domain of CpsD, an autophosphorylating tyrosine kinase required for capsule biosynthesis in Streptococcus pneumoniae. J. Bacteriol. 185:3009-3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morona, J. K., R. Morona, D. C. Miller, and J. C. Paton. 2002. Streptococcus pneumoniae capsule biosynthesis protein CpsB is a novel manganese-dependent phosphotyrosine-protein phosphatase. J. Bacteriol. 184:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morona, J. K., J. C. Paton, D. C. Miller, and R. Morona. 2000. Tyrosine phosphorylation of CpsD negatively regulates capsular polysaccharide biosynthesis in Streptococcus pneumoniae. Mol. Microbiol. 35:1431-1442. [DOI] [PubMed] [Google Scholar]
- 51.Morona, R., L. Van Den Bosch, and C. Daniels. 2000. Evaluation of Wzz/MPA1/MPA2 proteins based on the presence of coiled-coil regions. Microbiology 146:1-4. [DOI] [PubMed] [Google Scholar]
- 52.Navarre, W. W., and O. Schneewind. 1999. Surface proteins of gram-positive bacteria and the mechanism of their targeting to the cell wall envelope. Microbiol. Mol. Biol. Rev. 63:174-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neufeld, F. 1902. Ueber die Agglutination der Pneumokokken und uber die Theorieen der Agglutination. Z. Hyg. Infektionskr. 40:54-72. [Google Scholar]
- 54.Niemeyer, D., and A. Becker. 2001. The molecular weight distribution of succinoglycan produced by Sinorhizobium meliloti is influenced by specific tyrosine phosphorylation and ATPase activity of the cytoplasmic domain of the ExoP protein. J. Bacteriol. 183:5163-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paiment, A., J. Hocking, and C. Whitfield. 2002. Impact of phosphorylation of specific residues in the tyrosine autokinase, Wzc, on its activity in assembly of group 1 capsules in Escherichia coli. J. Bacteriol. 184:6437-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shoemaker, N. B., and W. R. Guild. 1974. Destruction of low efficiency markers is a slow process occurring at a heteroduplex stage of transformation. Mol. Gen. Genet. 128:283-290. [DOI] [PubMed] [Google Scholar]
- 57.Sorensen, U. B., J. Henrichsen, H. C. Chen, and S. C. Szu. 1990. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb. Pathog. 8:325-334. [DOI] [PubMed] [Google Scholar]
- 58.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 59.Vincent, C., P. Doublet, C. Grangeasse, E. Vaganay, A. J. Cozzone, and B. Duclos. 1999. Cells of Escherichia coli contain a protein-tyrosine kinase, Wzc, and a phosphotyrosine-protein phosphatase, Wzb. J. Bacteriol. 181:3472-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vincent, C., B. Duclos, C. Grangeasse, E. Vaganay, M. Riberty, A. J. Cozzone, and P. Doublet. 2000. Relationship between exopolysaccharide production and protein-tyrosine phosphorylation in gram-negative bacteria. J. Mol. Biol. 304:311-321. [DOI] [PubMed] [Google Scholar]
- 61.Waltman, W. D., B. M. Gray, C. Svanborg, R. Facklam, and D. E. Briles. 1991. Epidemiologic studies of group 9 pneumococci in terms of protein type and 9N versus 9V capsular type. J. Infect. Dis. 163:812-818. [DOI] [PubMed] [Google Scholar]
- 62.Weiser, J. N., D. Bae, H. Epino, S. B. Gordon, M. Kapoor, L. A. Zenewicz, and M. Shchepetov. 2001. Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect. Immun. 69:5430-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly, and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 64.Winkelstein, J. A. 1981. The role of complement in the host's defense against Streptococcus pneumoniae. Rev. Infect. Dis. 3:289-298. [DOI] [PubMed] [Google Scholar]
- 65.Wood, W. B., and M. R. Smith. 1949. Inhibition of surface phagocytosis by capsular “slime layer” of pneumococcus type III. J. Exp. Med. 90:85-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wugeditsch, T., A. Paiment, J. Hocking, J. Drummelsmith, C. Forrester, and C. Whitfield. 2001. Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J. Biol. Chem. 26:2361-2371. [DOI] [PubMed] [Google Scholar]
- 67.Yeung, M. K., and S. J. Mattingly. 1983. Biosynthesis of cell wall peptidoglycan and polysaccharide antigens by protoplasts of type III group B Streptococcus. J. Bacteriol. 154:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeung, M. K., and S. J. Mattingly. 1983. Isolation and characterization of type III group B streptococcal mutants defective in biosynthesis of the type-specific antigen. Infect. Immun. 42:141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yother, J., C. Forman, B. M. Gray, and D. E. Briles. 1982. Protection of mice from infection with Streptococcus pneumoniae by anti-phosphocholine antibody. Infect. Immun. 36:184-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yother, J., G. L. Handsome, and D. E. Briles. 1992. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning of the pspA gene. J. Bacteriol. 174:610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yother, J., L. S. McDaniel, and D. E. Briles. 1986. Transformation of encapsulated Streptococcus pneumoniae. J. Bacteriol. 168:1463-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yother, J., and J. M. White. 1994. Novel surface attachment mechanism of the Streptococcus pneumoniae protein PspA. J. Bacteriol. 176:2976-2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zahner, D., and R. Hakenbeck. 2000. The Streptococcus pneumoniae beta-galactosidase is a surface protein. J. Bacteriol. 182:5919-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]