Abstract
Over the course of thousands of generations of growth in a glucose-limited environment, 3 of 12 experimental populations of Escherichia coli spontaneously and independently evolved greatly increased mutation rates. In two of the populations, the mutations responsible for this increased mutation rate lie in the same region of the mismatch repair gene mutL. In this region, a 6-bp repeat is present in three copies in the gene of the wild-type ancestor of the experimental populations but is present in four copies in one of the experimental populations and two copies in the other. These in-frame mutations either add or delete the amino acid sequence LA in the MutL protein. We determined that the replacement of the wild-type sequence with either of these mutations was sufficient to increase the mutation rate of the wild-type strain to a level comparable to that of the mutator strains. Complementation of strains bearing the mutator mutations with wild-type copies of either mutL or the mismatch repair gene uvrD rescued the wild-type mutation rate. The position of the mutator mutations—in the region of MutL known as the ATP lid—suggests a possible deficiency in MutL's ATPase activity as the cause of the mutator phenotype. The similarity of the two mutator mutations (despite the independent evolutionary histories of the populations that gave rise to them) leads to a discussion of the potential adaptive role of DNA repeats.
Mutation is a process under the control of complex systems of cellular machinery. The components of these systems are encoded by genes, and mutations in these genes can thus alter the type or rate of future mutations. In particular, mutators are mutations that impair the fidelity of DNA replication or the efficacy of DNA repair, resulting in an increase in the mutation rate. In an evolving population, mutators arise through spontaneous mutation and potentially present a target for selection for adjustments of the mutation rate of the population (reviewed in reference 48). While long-term selection is generally predicted to favor decreased mutation rates and thus select against the fixation of mutator mutations (17, 18, 20, 21, 28), there is some theoretical evidence that frequent bouts of strong selection, such as those experienced by pathogenic microbes, might result in long-term selection for increased mutation rates in asexual populations (16, 21, 25, 56). Over shorter time scales, mutator alleles can become transiently common, and can even rise to fixation, in asexual populations by hitchhiking with one or more linked beneficial mutations (36, 45, 49, 52).
Experimental evolution studies with microbial populations present particularly good opportunities to observe the change in frequency of mutator alleles (6, 7, 12, 32, 35, 36, 45, 49). For example, work in a variety of experimental systems has established that when an increase in the frequency of mutators does occur it is exclusively as a result of hitchhiking with linked beneficial mutations and not as a result of direct selection on the mutator allele itself (7, 32, 36, 45). A particularly well-studied system employs 12 replicate populations of Escherichia coli which have evolved in a constant environment for over 20,000 generations (22, 24). Three of the populations independently fixed mutators in the first 10,000 generations of evolution and retained these high mutation rates for thousands of subsequent generations (45, 49). There is no evidence that any long-term fitness advantage accrued to any of the mutator strains after the mutator had been fixed (8, 9). Since it seems that the mutator phenotype provides no advantage in terms of an increased rate of adaptation subsequent to its fixation and since a high mutation rate is certainly expected to carry a potent fitness penalty in the form of an increased supply of deleterious mutations, the following question is raised: why does the mutator phenotype persist for thousands of generations in these populations in the face of its likely selective cost?
A possible solution to the problem of the persistence of mutators in these populations is that the mutator phenotype might not be easily revertible; for example, the original mutator phenotype might have resulted from the deletion of genes coding for an integral part of the DNA repair system. Initial attempts to determine the precise mutations responsible for the mutator phenotype in these populations (49) implicated the methyl-directed mismatch repair (MMR) system, the major component of postreplication DNA error checking and repair in E. coli (19). Of the three mutator populations, the responsible mutation in one (designated the Ara+3 mutator population) was determined to consist of the insertion of a single base pair in the MMR gene mutS (45); such a mutation is, in principle, readily revertible. However, the exact nature of the mutator mutations from the other two populations (Ara−2 and Ara−4) has to this point remained unconfirmed.
Here we report the surprising finding that despite the completely independent evolutionary histories of all of the experimental populations, the mutator mutations from populations Ara−2 and Ara−4 are very similar: each is the result of an alteration in the copy number of the same 6-bp repeat in the MMR gene mutL. Previous work (45) had demonstrated the presence of these repeat alterations in the mutator populations but did not address the possibility that they were responsible for the mutator phenotype. In this work, we show experimentally that these sequence alterations are sufficient to produce a greatly increased mutation rate. In addition to casting serious doubt on the hypothesis that these mutators have managed to persist simply by virtue of being nonrevertible, these results are intriguing for several other reasons. First, these particular mutations are unusual in that they are rescued by complementation with wild-type copies of either mutL or uvrD, another component of the MMR system. Second, the repeat within which both of the mutations occur lies in a region of mutL that forms the lid of the ATP-binding pocket of the MutL protein. The ATP lid has been pointed out as possibly playing an important role in determining the function of members of the GHKL superfamily of proteins to which MutL belongs (10). To our knowledge, no other similar mutations which alter the length of the ATP lid (and thus, presumably, its conformation) have been described for mutL or any of its homologs. Finally, the fact that both mutations are independent alterations of the same repeat draws attention to the possible role of short repeats as mutational hotspots in loci involved in DNA replication and repair (44).
MATERIALS AND METHODS
Plasmids and strains.
The plasmids and strains used in the study are listed in Table 1.
TABLE 1.
Plasmids and strains used in this study
| Plasmid or strain | Genotype and/or description | Reference or source |
|---|---|---|
| Plasmids | ||
| pGT236 | uvrD+ Ampr Camr Tetr | 53 |
| pGW1842 | mutL+ Ampr Tetr | 40 |
| Strains | ||
| REL606 | ara thi T6r Strr | 23 |
| PS17 | REL606, Ara−2 population 10,000 generation clone; mut | 49 |
| PS19 | REL606, Ara−4 population 10,000 generation clone; mut | 49 |
| PS2348 | REL606, mutL from PS17 | This work |
| PS2350 | REL606, mutL from PS19 | This work |
| PS2353 | PS2348, pGT236 | This work |
| PS2355 | PS2350, pGT236 | This work |
| PS2358 | PS2348, pGW1842 | This work |
| PS2360 | PS2350, pGW1842 | This work |
Experimental system.
The experimental system used in the study has been described previously (reviewed in reference 22). Briefly, an Ara− clone of E. coli strain B (REL606) was used to found 12 replicate populations: Ara−1 through Ara−6 (founded from REL606) and Ara+1 through Ara+6 (founded from REL607, a spontaneous Ara+ revertant of REL606). These populations were propagated independently by daily 100-fold dilutions in 10 ml of minimal glucose medium, a transfer schedule which resulted in approximately seven generations of growth per day. The experiment is still continuing and the strains have experienced over 20,000 generations of growth in these conditions. Each population was regularly sampled and frozen for archival purposes, first at 100-generation intervals (for the first 2,000 generations) and then every 500 generations thereafter. Mutation rates at several loci were measured in clones taken from each population at 10,000 generations; the clones from populations Ara+3, Ara−2, and Ara−4 were found to be mutators (49). The rates of mutation of these mutators to several different phenotypes were tested; the magnitude of the mutator effect was found to be consistent at approximately 100-fold higher than that of the wild-type strain (49). All tests of mutation rate described in the present paper were performed using selection with the antibiotic nalidixic acid.
Spot plating.
Since the magnitude of the mutator effect was so large, qualitative differences in mutation rate could be easily determined using a spot plate assay, as described previously (45). Four replicate cultures of each strain were grown overnight in minimal medium; the next day, 100 μl of each culture was spotted onto Luria-Bertani (LB) agar supplemented with 20 μg of nalidixic acid/ml. Expected distributions of the number of resistant colonies for wild-type and mutator strains were determined via computer simulation on the basis of an algorithm describing the distribution of mutants in a growing culture (31). Results from a particular assay were compared to the results of the simulation for classification of the experimental strains as mutator or wild type. In all cases described here, the results of a spot-plating assay were only considered definitive when they could classify the tested strain with more than 99.9% certainty.
Fluctuation tests.
For more exact measurements of mutation rate, fluctuation tests were performed. The fluctuation test is a classical technique (30) which employs one of a variety of methods to transform an observed distribution of mutant counts into a precise estimate of the mutation rate. In the tests performed here, a number of replicate liquid cultures for each strain were grown to maximum density in an appropriate volume of minimal medium. The entire culture was then plated onto LB agar supplemented with 20 μg of nalidixic acid/ml, and the number of resistant mutant colonies visible after 48 h was recorded for each plate. This distribution of mutant counts was analyzed with a computer program developed by one of the authors which implements a maximum-likelihood estimation technique (51). Statistical-confidence limits for estimates produced with this technique were determined using a maximum-likelihood estimation theory, which agrees closely with the method suggested by Stewart (51).
Plasmid construction.
Complete sequences of the mutL gene were obtained via PCR as described previously (45). The total size of the PCR product was 2.35 kb, which included the 1.85-kb mutL sequence as well as the surrounding noncoding sequence. Amplifications from PS17, the Ara−2 10,000-generation clone, and PS19, the Ara−4 10,000-generation clone, were cloned into pGem T-Easy vector (Promega, Madison, Wis.) and transformed into chemically competent JM109 cells. Plasmid DNA was isolated using QIAprep Miniprep kits (Qiagen, Valencia, Calif.), and the NotI fragment containing the PCR product was isolated via gel extraction. This fragment was ligated (using T4 DNA ligase [Promega]) into the NotI sites of plasmid pKO3 (29), a suicide vector designed for allele replacement via homologous recombination. The pKO3-mutL ligation construct was transformed via electroporation into XL-1 Blue cells and then isolated via a Miniprep procedure. Intact plasmid was transformed into chemically competent REL606.
Gene replacement.
Selection for gene replacement was as described previously (29); integration of the plasmid was selected for by growth on LB agar supplemented with 20 μg of chloramphenicol/ml at 43°C, and excision and curing of the plasmid were selected for by growth on LB agar supplemented with 5% (wt/vol) sucrose. The nature of this procedure is such that a substantial proportion of the clones obtained at the final step retain their original genotype rather than inserting the target sequence, so all clones must be screened for the proper genotype. A total of 20 clones (10 with each plasmid) that had been put through this procedure were tested by spot plating. Sequencing of strains displaying an increased mutation rate to test for proper integration was performed at the Nucleic Acid/Protein Core Research Facility at the Children's Hospital of Pennsylvania.
Complementation testing.
Strains constructed as described above were transformed with plasmids carrying fully functional, wild-type copies of uvrD (pGT26) (53) and mutL (pGW1842) (40). Techniques for measurement of mutation rates were unchanged except that media were supplemented with 100 μg of ampicillin/ml and growth times were extended to compensate for the cost to the strains of carrying the plasmids.
RESULTS
Sequence of the mutL mutations.
The 6-bp repeat CTGGCG, beginning at position 213 of the E. coli B mutL gene, is present in three copies in REL606, the ancestor of the experimental strains (Fig. 1). In previous work (45), alterations in the copy number of the repeat were found in two experimental populations of E. coli which had evolved a mutator phenotype. In population Ara−2, fixation of this mutator phenotype took place approximately between generations 1500 and 3500; an additional copy of the repeat was found in mutator isolates from the Ara−2 population both at the beginning of this time period and at the end. In population Ara−4, which fixed its mutator roughly between generations 6500 and 9000, the copy number of the repeat in mutator samples from both the beginning and the end of this time period was reduced to two.
FIG. 1.
Alignment of a portion of the mutL sequence of the experimental system's wild-type ancestor, REL606, with the mutations found in the populations Ara−2 and Ara−4. Also shown is the amino acid sequence of the corresponding section of the MutL protein. The numbering given is for the REL606 nucleic acid sequence.
The region of mutL in which the CTGGCG repeat occurs codes for the amino acid sequence LALALA in REL606, beginning at position 68 of the MutL protein. As a result of the mutations described above, this sequence is lengthened to LALALALA in Ara−2 and shortened to LALA in Ara−4. This region of MutL comprises the C-terminal end of α-helix B, according to the labeling scheme of Ban and Yang (3); other attempts at structural identification instead place at least part of this region in the disordered loop connecting two α-helices (for an example, see reference 33). In either case, the repeat region lies at the N-terminal end of the relatively disordered structure that forms a lid over the ATP-binding pocket of MutL and that is thus referred to as the ATP lid (3).
For this work, sequencing of the entire mutL gene was carried out for PS17 and PS19, the Ara−2 and Ara−4 isolates from 10,000 generations, to determine whether any subsequent mutations in mutL might have accumulated. Only one additional coding difference from the REL606 ancestor was found to have arisen at 10,000 generations: Ara−4 substituted valine for alanine at amino acid position 606 (608 in the wild-type protein) at the extreme C-terminal end of the MutL protein. No differences in DNA sequence between REL606 and either of the 10,000-generation isolates were observed in the region extending 200 bp upstream of the beginning of mutL or 300 bp downstream of the end.
Confirmation of the mutator mutation.
To analyze the precise effects of the mutL sequence alterations in an isogenic background with a low, wild-type mutation rate, the putative mutator mutL sequences from PS17 and PS19 were inserted into REL606. The technique used to introduce the mutator sequences was developed on the basis of homologous recombination between the target strain and a suicide vector bearing the sequence to be inserted (29); this ensures that no unwanted flanking material is introduced along with the mutation of interest, as occurs with transduction-based methods. To increase the success rate of the replacement technique as well as eliminate the remote possibility of introducing a nonhomologous sequence into mutL, the fragments which were inserted into the suicide vector included long stretches of a homologous sequence extending beyond the boundaries of the mutL gene both up- and downstream of the target mutations.
Spot plating (see Materials and Methods) was used to verify that the mutL sequence had been replaced successfully; several of the clones were classified as mutators. Sequencing of these mutator clones verified that the REL606 mutL repeat sequence had been cleanly replaced by the appropriate mutation, verifying that the mutL repeat alterations were sufficient to produce a mutator phenotype. As a control, a clone that had been classified as wild type by spot plating was sequenced and was confirmed to have retained the REL606 sequence. Since all successful clones produced by this technique are isogenic, further results described below were all carried out using only two of the mutator clones, PS2348 (Ara−2) and PS2350 (Ara−4).
Measurement of mutation rates.
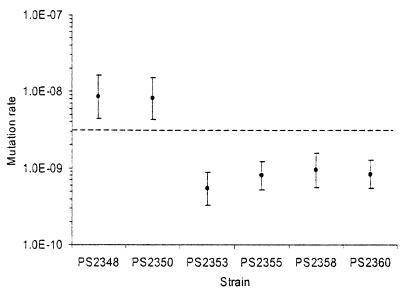
After spot plating had been used to confirm the mutator status of PS2348 and PS2350, measurements of their mutation rates were obtained using fluctuation tests (see Materials and Methods). REL606 mutates from nalidixic acid sensitivity to resistance at a rate of approximately 10−10 per replication, while the rates for the evolved Ara−2 and Ara−4 mutators are roughly 100 times as high (49). Tests of the rate of mutation to nalidixic acid resistance performed with 10-fold replication yielded a mutation rate of 8.51 × 10−9 per replication for PS2348 and 8.11 × 10−9 per replication for PS2350 (Fig. 2), rates consistent with a mutator phenotype. There is presently no reliable and well-tested method for statistical comparison of fluctuation test results, and it is therefore not possible to rigorously test these figures for similarity with those obtained for the original Ara−2 and Ara−4 mutators. Instead, we note that 95% confidence intervals calculated as described above (see Materials and Methods) for PS2348 and PS2350 comfortably overlap previously obtained estimates for PS17 and PS19 (49). A fuller treatment of the relationship between the mutation rates measured here and those measured previously is included in the Discussion section.
FIG. 2.
Rate of mutation to nalidixic acid resistance determined by fluctuation test. The mutation rate is shown on a log scale. Strains PS2348 and PS2350 exhibited mutation rates consistent (as defined in Results) with those measured previously for PS17 and PS19, the strains from which the mutL mutations were obtained. The four strains derived from PS2348 and PS2350 and complemented with uvrD+ (PS2353 and PS2355) or mutL+ (PS2358 and PS2360) all showed drastically reduced mutation rates. Error bars represent 95% confidence intervals calculated as described in Materials and Methods. The dashed line represents the upper 95% confidence interval calculated previously for REL606, the wild-type ancestor of PS2348 and PS2350 (49); this line was used as the cutoff to determine whether complementation had restored the wild-type mutation rate.
Complementation by mutL+ and uvrD+.
Previous work (49) has shown that the high mutation rate of PS17 and PS19 is partially or fully restored to the wild-type level by transformation with a high-copy plasmid expressing a wild-type copy of either mutL or uvrD but not any other member of the MMR system (including mutS and mutH) or other well-known mutator genes (including mutT, dnaE, and dnaQ). Phenotypic assays and sequence analysis have provided no evidence for any deficiency in uvrD itself in these strains (45). To determine whether uvrD's ability to rescue the wild-type phenotype is in fact linked directly to the mutL sequence alterations, we transformed PS2348 and PS2350 with plasmids bearing mutL and uvrD. Fluctuation tests for nalidixic acid resistance were performed on these plasmid-bearing strains with eight replicates per strain; the results are shown in Fig. 2.
For all four plasmid-bearing strains, a large and consistent decrease in mutation rate was observed relative to those of PS2348 and PS2350. In every case, the mutation rate estimate of the complemented strain fell below the upper 95% confidence interval of REL606; this is the standard that was used previously, in the absence of robust statistical procedures, to indicate rescue of the wild-type rate by complementation (49). Thus, it is clear that the mutL mutations described above are sufficient to create a mutator phenotype which can be rescued by either mutL+ or uvrD+.
DISCUSSION
In an experimental system consisting of 12 replicate populations of E. coli evolving independently in a constant environment, 3 of the populations spontaneously fixed mutators in the first 10,000 generations of evolution (49). The mutation responsible for the mutator phenotype in one of the populations, Ara+3, has been determined to be an insertion in the MMR gene mutS (45). In this work, we show experimentally that the mutator mutations in the other two populations, Ara−2 and Ara−4, are alterations in copy number of the same 6-bp repeat in the MMR gene mutL; in Ara−2, an additional copy is added, while in Ara−4, a copy of the repeat is deleted. The introduction of either of these mutations into a wild-type strain is sufficient to increase the mutation rate to a level comparable to that of the mutators. The wild-type, low mutation rate can be restored in strains carrying these mutator mutations by complementation with wild-type copies of either mutL or uvrD.
Origin versus persistence in evolving populations.
Experimental studies have shown that when mutators fix in evolving asexual populations, they do so not as the result of direct selection on the mutator phenotype but instead only by hitchhiking with linked beneficial mutations (36, 45). By virtue of their increased mutation rates, mutators are more likely than wild-type individuals to acquire these beneficial mutations; thus, they stand an increased chance of hitchhiking to high frequency. This relationship is illustrated by experimental manipulations which boost the proportion of mutators in bacterial populations; a mutator subpopulation only a fraction of the size of the wild-type subpopulation increases in frequency at the expense of the wild type (7, 12, 57). The importance of hitchhiking to the evolution of mutation rates is also borne out by theory, which indicates that indirect selection can be a powerful force for the fixation of mutator alleles in asexual populations exposed to strong selection (39, 52, 54).
While this combined body of experimental and theoretical work has made it clear that mutators of large effect can be fixed in rapidly evolving asexual populations, such short-term selection is likely to increase the mutation rate to a level higher than the optimum for long-term evolution (reviewed in reference 48). The increased load of deleterious mutations that a high mutation rate brings with it represents a potent selective disadvantage, one which should in the long run outweigh the advantage that comes from an increased supply of beneficial mutations. This disparity becomes especially pronounced in populations in which the initial selective pressure that resulted in fixation of the mutator alleles in the first place is relaxed and the rate or magnitude of beneficial mutation decreases; when this happens, the mutator allele retains its full cost while losing some of its advantage.
It is thus somewhat difficult to explain why the mutators described here continued to persist for thousands of generations after their initial fixation and out to at least 20,000 generations from the start of the experiment (A. C. Shaver and P. D. Sniegowski, unpublished data). In the experimental populations used in this study, the bulk of the increase in fitness occurred within the first 3,000 generations; subsequently, adaptation seems to have slowed almost to a halt as, presumably, those beneficial mutations most likely to arise or those of largest fitness effect had already fixed and only very unlikely or ones of very minor effect were left (24). If wild-type revertants could arise on the mutator background in these highly evolved populations, they would no longer suffer the increased deleterious load of the mutators and would therefore be predicted eventually to increase in frequency. The data presented here and in previous work (45) are important because they show that the simple answer—that the nature of the mutator mutations is such that they simply cannot revert—can be ruled out for all three populations. Thus, some other explanation must be found for the persistence of these mutators.
There are three possible reasons why wild-type revertants have failed to increase in frequency in these populations. First, the rate of adaptation in the experimental populations, even after thousands of generations of evolution, may remain high enough for the mutator phenotype to retain its advantage over the wild type. Second, the magnitude of selection in favor of wild-type revertants on the basis of their decreased genetic loads may be too small to have an effect over the time scale surveyed. Finally, it is possible that further mutations might have accumulated in the mutator populations which make it impossible for wild-type revertants to arise, although this possibility is not supported by data from the 10,000-generation isolates (49).
Of possible relevance to the question of the persistence of mutators in these experimental populations is the observation that while the mutation rates observed for the two mutator strains constructed on the ancestral background were broadly consistent with previously reported measurements (49) for 10,000-generation mutator isolates from the same populations, the mutation rates of the constructed strains do seem to have been lower than those of the 10,000-generation strains. As mentioned in Results, the absence of reliable procedures for statistical hypothesis testing with mutation rate estimates makes it impossible at this time to rule out experimental error as an explanation for the apparent decrease. However, the possible further evolution of mutation rates in the time between the first appearance of the mutators and 10,000 generations might also explain the difference. The search for such long-term but relatively slight changes in mutation rates subsequent to mutator fixation is the subject of further work currently in progress.
Relevance to structural studies of MutL.
The protein MutL is a member of the GHKL superfamily of ATPases and kinases; this grouping is defined by its members' unique ATP-binding pocket, which is covered in the ATP-bound conformation by a loop structure known as the ATP lid (10). The structures and positions of the ATP lid seem to differ greatly between GHKL members, and there is ample evidence that the lid plays a potentially important role in determining the activity of the ATP-binding site (2, 5, 10, 33).
Mutations have previously been located in or near the ATP lid region of MutL or its homologs (1, 37, 41-43, 46, 47). In almost every case, these are missense mutations rather than insertions or deletions; an exception is a 1-codon deletion in human MLH1 (41). To our knowledge, no other mutations have been described which alter the length and therefore presumably the conformation of the ATP lid.
Complementation by uvrD.
MutL's crucial role in DNA mismatch repair is to mediate between the detection of a base pair mismatch by MutS and the nicking, unwinding, excision, and recopying of the mismatched region by other repair proteins. In E. coli, MutL has been shown to interact physically with MutS (13); the endonuclease MutH (3, 15); and DNA helicase II, the gene product of uvrD (14, 58); MutL also binds DNA, particularly single-stranded DNA (2, 4). Most of these interactions are dependent on MutL's ATPase activity, which has been shown to be specifically required for mismatch repair both in E. coli (50) and in Saccharomyces cerevisiae (55). MutL's position at the center of a network of interacting repair proteins makes it conceivable that the effect of specific mutL mutations might be rescued by complementation with other MMR genes. In fact, some mutL mutators in E. coli seem to be partially or fully complemented by mutS+ or mutH+ (1). However, we are aware of no previously reported mutL mutator which is rescued by complementation with uvrD+ (for an example, see reference 38).
The mutators described in this work showed an equivalent decrease in mutation rate when complemented with either mutL+ or uvrD+. Direct interaction with DNA helicase II, the uvrD gene product, occurs in the C-terminal region of MutL (14), while the mutations described here were located at the extreme N terminus of the MutL protein, indicating that impairment of ATPase function was more likely to be the cause of the mutator phenotype than specific deficiencies in MutL-DNA helicase II interaction. Since ATPase function is important for many of MutL's protein-protein interactions, it is striking that only uvrD was able to rescue the mutator phenotype.
Repeats and local hypermutability.
As described above, the general cost of an increased mutation rate stems from the greater load of deleterious mutations suffered by a mutator. However, by increasing the mutability of only certain regions of DNA where the potential for beneficial mutation is high, some of the cost of a high mutation rate can be avoided while the possible payoff is maximized. Such local hypermutability is displayed by contingency loci, specific areas in the genomes of pathogenic microorganisms which are involved in the interaction with the host's immune system and are thus exposed to constantly shifting selection (11, 34). A high rate of mutation is consistently desirable at these loci, since it assists the pathogen population in outpacing the immune response of the host and so mechanisms which elevate the mutation rate in such regions while preserving a low mutation rate in the rest of the genome are selected for. These mechanisms often involve DNA repeats, since repeats increase mutability both as a result of slipped-strand mispairing (26, 27) and by presenting a target for homologous recombination.
Although it is certainly true that DNA repeats can be targets of selection in specific instances, such as the contingency loci described above, it is less clear whether they play a role of more general adaptive significance. It has been suggested (44) that short repeats in genes involved in DNA repair or stress response are the targets of selection to increase variability in times of stress via slipped-strand mispairing. The mutations described here lend support to the more limited hypothesis that alterations in such short repeats make up a disproportionately large segment of the potential pool of mutators which can hitchhike to high frequency. While a sample of three populations is certainly not large enough to permit broad conclusions, the fact that two of the mutator mutations arose independently from the same 18-bp region is unlikely to be a coincidence. However, it is not necessary to conclude from these results that the repeated sequence in this region was targeted by selection to increase the region's mutability.
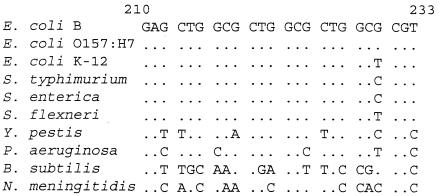
Any potential selection that would act to increase the mutability of the mutL locus would have no immediate effect but to increase the chance of creating a mutL mutator, which in turn would have no positive selective effect but to increase the rate of beneficial mutations at other loci. This “third-order” selection is, by its very nature, extremely weak—much weaker, for example, than the direct selective advantage provided by the pathogenic contingency loci described above. An analysis of the repeat region of mutL in a variety of bacterial species (Fig. 3) shows that there is little evidence for the selective maintenance of the repetitive nature of this region. Even in other strains of E. coli and in very closely related taxa the copy number of the repeat is decreased, and in more distantly related bacterial species any trace of the repeated sequence disappears completely.
FIG. 3.
Alignment of the mutL repeat sequence in the experimental E. coli B strain (REL606) with homologous sequences from mutL genes of other bacterial species. Positions at which the sequence of another species or strain is identical to that of E. coli B are indicated by periods. Only E. coli O157:H7 shares complete sequence identity with E. coli B within this stretch. E. coli K-12, the two Salmonella species, and Shigella flexneri all contain only two complete repeats. The sequences of more distantly related bacterial species, including many not shown here, were found to contain no trace of any repeated elements in this region. The E. coli B sequence obtained from REL606 and all other sequences shown here are available from GenBank. The numbering given is for the E. coli B nucleic acid sequence.
While a role for higher-order selection in the similarity of the mutations described in this study cannot be ruled out, an alternative worthy of consideration is that the repeat in mutL is the product of genetic drift and its apparently increased rate of mutation is simply an accidental result. The uncertainty in this case is an example of the difficulty in studying complex phenomena such as the evolution of mutation rates, in which the causes of a selective event cannot always be inferred easily from its consequences (48). Fortunately, the experimental system described here presents a unique opportunity for future research to address the question of whether such higher-order selection can operate in evolving populations.
Acknowledgments
This research was supported by a grant to P.D.S. from the National Science Foundation (DEB 9981518). A.C.S. was supported by a predoctoral fellowship from the Howard Hughes Medical Institute.
We thank R. E. Lenski for generous access to his strains, G. M. Church for providing us with the pKO3 plasmid, and two anonymous reviewers for helpful comments. Computer code written by P. J. Gerrish served as the basis for an early version of the fluctuation test analysis program used in this work.
REFERENCES
- 1.Aronshtam, A., and M. G. Marinus. 1996. Dominant negative mutator mutations in the mutL gene of Escherichia coli. Nucleic Acids Res. 24:2498-2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ban, C., M. Junop, and W. Yang. 1999. Transformation of MutL by ATP binding and hydrolysis: a switch in DNA mismatch repair. Cell 97:85-97. [DOI] [PubMed] [Google Scholar]
- 3.Ban, C., and W. Yang. 1998. Crystal structure and ATPase activity of MutL: implications for DNA repair and mutagenesis. Cell 95:541-552. [DOI] [PubMed] [Google Scholar]
- 4.Bende, S. M., and R. H. Grafström. 1991. The DNA binding properties of the MutL protein isolated from Escherichia coli. Nucleic Acids Res. 19:1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilwes, A. M., C. M. Quezada, L. R. Croal, B. R. Crane, and M. I. Simon. 2001. Nucleotide binding by the histidine kinase CheA. Nat. Struct. Biol. 8:353-360. [DOI] [PubMed] [Google Scholar]
- 6.Boe, L., M. Danielsen, S. Knudsen, J. B. Petersen, J. Maymann, and P. R. Jensen. 2000. The frequency of mutators in populations of Escherichia coli. Mut. Res. 338:47-55. [DOI] [PubMed] [Google Scholar]
- 7.Chao, L., and E. C. Cox. 1983. Competition between high and low mutating strains of Escherichia coli. Evolution 37:125-134. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, V. S., and R. E. Lenski. 2000. The population genetics of ecological specialization in evolving Escherichia coli populations. Nature 407:736-739. [DOI] [PubMed] [Google Scholar]
- 9.de Visser, J. A. G. M., C. W. Zeyl, P. J. Gerrish, J. L. Blanchard, and R. E. Lenski. 1999. Diminishing returns from mutation supply rate in asexual populations. Science 283:404-406. [DOI] [PubMed] [Google Scholar]
- 10.Dutta, R., and M. Inouye. 2000. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 25:24-28. [DOI] [PubMed] [Google Scholar]
- 11.Field, D., M. O. Magnasco, E. R. Moxon, D. Metzgar, M. M. Tanaka, C. Wills, and D. S. Thaler. 1999. Contingency loci, mutator alleles, and their interactions. Ann. N. Y. Acad. Sci. 870:378-382. [DOI] [PubMed] [Google Scholar]
- 12.Giraud, A., I. Matic, O. Tenaillon, A. Clara, M. Radman, M. Fons, and F. Taddei. 2001. Costs and benefits of high mutation rates: adaptive evolution of bacteria in the mouse gut. Science 291:2606-2608. [DOI] [PubMed] [Google Scholar]
- 13.Grilley, M., K. M. Welsh, S.-S. Su, and P. Modrich. 1989. Isolation and characterization of the Escherichia coli mutL gene product. J. Biol. Chem. 264:1000-1004. [PubMed] [Google Scholar]
- 14.Hall, M. C., J. R. Jordan, and S. W. Matson. 1998. Evidence for a physical interaction between the Escherichia coli methyl-directed mismatch repair proteins MutL and UvrD. EMBO J. 17:1535-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall, M. C., and S. W. Matson. 1999. The Escherichia coli MutL protein physically interacts with MutH and stimulates the MutH-associated endonuclease activity. J. Biol. Chem. 274:1306-1312. [DOI] [PubMed] [Google Scholar]
- 16.Ishii, K., H. Matsuda, Y. Iwasa, and A. Sasaki. 1989. Evolutionary stable mutation rate in a periodically changing environment. Genetics 121:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura, M. 1967. On the evolutionary adjustment of spontaneous mutation rates. Genet. Res. 9:23-34. [Google Scholar]
- 18.Kondrashov, A. 1995. Modifiers of mutation-selection balance: general approach and the evolution of mutation rates. Genet. Res. 6:53-70. [Google Scholar]
- 19.Kornberg, A., and T. A. Baker. 1992. DNA replication, 2nd ed. W. H. Freeman and Co., New York, N.Y.
- 20.Leigh, E. G. 1970. Natural selection and mutability. Am. Nat. 104:301-305. [Google Scholar]
- 21.Leigh, E. G. 1973. The evolution of mutation rates. Genetics 73(Suppl.):1-18. [PubMed] [Google Scholar]
- 22.Lenski, R. E., J. A. Mongold, P. D. Sniegowski, M. Travisano, F. Vasi, P. J. Gerrish, and T. M. Schmidt. 1998. Evolution of competitive fitness in experimental populations of E. coli: what makes one genotype a better competitor than another? Antonie Leeuwenhoek 73:35-47. [DOI] [PubMed] [Google Scholar]
- 23.Lenski, R. E., M. R. Rose, S. C. Simpson, and S. C. Tadler. 1991. Long-term experimental evolution in E. coli. I. Adaptation and divergence during 2000 generations. Am. Nat. 138:1315-1341. [Google Scholar]
- 24.Lenski, R. E., and M. Travisano. 1994. Dynamics of adaptation and diversification: a 10,000-generation experiment with bacterial populations. Proc. Natl. Acad. Sci. USA 91:6808-6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levins, R. 1967. Theory of fitness in a heterogeneous environment. VI. The adaptive significance of mutation. Genetics 56:163-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levinson, G., and G. Gutman. 1987. High frequencies of short frameshifts in poly-CA/TG tandem repeats borne by bacteriophage M13 in Escherichia coli K-12. Nucleic Acids Res. 15:5323-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levinson, G., and G. Gutman. 1987. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol. Biol. Evol. 4:203-221. [DOI] [PubMed] [Google Scholar]
- 28.Liberman, U., and M. Feldman. 1986. Modifiers of mutation rate: a general reduction principle. Theor. Popul. Biol. 30:125-142. [DOI] [PubMed] [Google Scholar]
- 29.Link, A. J., D. Phillips, and G. M. Church. 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179:6228-6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luria, S. E., and M. Delbrück. 1943. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma, W., G. vH. Sandri, and S. Sarkar. 1992. Analysis of the Luria-Delbrück distribution using discrete convolution powers. J. Appl. Prob. 29:255-267. [Google Scholar]
- 32.Mao, E. F., L. Lane, J. Lee, and J. H. Miller. 1997. Proliferation of mutators in a cell population. J. Bacteriol. 179:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marina, A., C. Mott, A. Auyzenberg, W. A. Hendrickson, and C. D. Waldburger. 2001. Structural and mutation analysis of the PhoQ histidine kinase catalytic domain. J. Biol. Chem. 276:41182-41190. [DOI] [PubMed] [Google Scholar]
- 34.Moxon, E. R., P. B. Rainey, M. A. Nowak, and R. E. Lenski. 1994. Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr. Biol. 4:24-33. [DOI] [PubMed] [Google Scholar]
- 35.Notley-McRobb, L., and T. Ferenci. 2000. Experimental analysis of molecular events during mutational periodic selections in bacterial evolution. Genetics 156:1493-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Notley-McRobb, L., S. Seeto, and T. Ferenci. 2002. Enrichment and elimination of mutY mutators in Escherichia coli populations. Genetics 162:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nyström-Lahti, M., C. Perrera, M. Räschle, E. Panyushkina-Seiler, G. Marra, A. Curci, B. Quaresima, F. Costanzo, M. D'Urso, S. Venuta, and J. Jiricny. 2002. Functional analysis of MLH1 mutations linked to hereditary nonpolyposis colon cancer. Genes Chromosomes Cancer 33:160-167. [PubMed] [Google Scholar]
- 38.Oliver, A., F. Baquero, and J. Blázquez. 2002. The mismatch repair system (mutS, mutL and uvrD genes) in Pseudomonas aeruginosa: molecular characterization of naturally occurring mutants. Mol. Microbiol. 43:1641-1650. [DOI] [PubMed] [Google Scholar]
- 39.Painter, P. R. 1975. Mutator genes and selection for the mutation rate in bacteria. Genetics 79:649-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pang, P. P., A. S. Lundberg, and G. C. Walker. 1985. Identification and characterization of the mutL and mutS gene products of Salmonella typhimurium LT2. J. Bacteriol. 163:1007-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raevaara, T. E., T. Timoharju, K. E. Lönnqvist, R. Kariola, M. Steinhoff, R. M. W. Hofstra, E. Mangold, Y. J. Vos, and M. Nyström-Lahti. 2002. Description and functional analysis of a novel in frame mutation linked to hereditary non-polyposis colorectal cancer. J. Med. Genet. 39:747-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Räschle, M., P. Dufner, G. Marra, and J. Jiricny. 2002. Mutations within the hMLH1 and hPMS2 subunits of the human MutLα mismatch repair factor affect its ATPase activity, but not its ability to interact with hMutSα. J. Biol. Chem. 277:21810-21820. [DOI] [PubMed] [Google Scholar]
- 43.Richardson, A. R., and I. Stojiljkovic. 2001. Mismatch repair and the regulation of phase variation in Neisseria meningitidis. Mol. Microbiol. 40:645-655. [DOI] [PubMed] [Google Scholar]
- 44.Rocha, E. P. C., I. Matic, and F. Taddei. 2002. Over-representation of repeats in stress response genes: a strategy to increase versatility under stressful conditions? Nucleic Acids Res. 30:1886-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaver, A. C., P. D. Dombrowski, J. Y. Sweeney, T. Treis, R. M. Zappala, and P. D. Sniegowski. 2002. Fitness evolution and the rise of mutator alleles in experimental Escherichia coli populations. Genetics 162:557-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimodaira, H., N. Filosi, H. Shibata, T. Suzuki, P. Radice, R. Kanamaru, S. H. Friend, R. D. Kolodner, and C. Ishioka. 1998. Functional analysis of human MLH1 mutations in Saccharomyces cerevisiae. Nat. Genet. 19:384-389. [DOI] [PubMed] [Google Scholar]
- 47.Sia, E. A., M. Dominska, L. Stefanovic, and T. D. Petes. 2001. Isolation and characterization of point mutations in mismatch repair genes that destabilize microsatellites in yeast. Mol. Cell. Biol. 21:8157-8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sniegowski, P. D., P. J. Gerrish, T. Johnson, and A. Shaver. 2000. The evolution of mutation rates: separating causes from consequences. Bioessays 22:1057-1066. [DOI] [PubMed] [Google Scholar]
- 49.Sniegowski, P. D., P. J. Gerrish, and R. E. Lenski. 1997. Evolution of high mutation rates in experimental populations of E. coli. Nature 387:703-705. [DOI] [PubMed] [Google Scholar]
- 50.Spampinato, C., and P. Modrich. 2000. The MutL ATPase is required for mismatch repair. J. Biol. Chem. 275:9863-9869. [DOI] [PubMed] [Google Scholar]
- 51.Stewart, F. M. 1994. Fluctuation tests: how reliable are the estimates of mutation rates? Genetics 137:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taddei, F., M. Radman, J. Maynard Smith, B. Toupance, P. H. Gouyon, and B. Godelle. 1997. Role of mutator alleles in adaptive evolution. Nature 387:700-702. [DOI] [PubMed] [Google Scholar]
- 53.Taucher-Scholz, G., and H. Hoffman-Berling. 1983. Identification of the gene for DNA helicase II of Escherichia coli. Eur. J. Biochem. 137:573-580. [DOI] [PubMed] [Google Scholar]
- 54.Tenaillon, O., B. Toupance, H. Le Nagard, F. Taddei, and B. Godelle. 1999. Mutators, population size, adaptive landscape and the adaptation of asexual populations. Genetics 152:485-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tran, P. T., and R. M. Liskay. 2000. Functional studies on the candidate ATPase domains of Saccharomyces cerevisiae MutLα. Mol. Cell. Biol. 20:6390-6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Travis, J. M. J., and E. R. Travis. 2002. Mutator dynamics in fluctuating environments. Proc. R. Soc. Lond. B 269:591-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tröbner, W., and R. Piechocki. 1984. Competition between isogenic mutS and mut+ populations of Escherichia coli K12 in continuously growing cultures. Mol. Gen. Genet. 198:175-176. [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi, M., V. Dao, and P. Modrich. 1998. MutS and MutL activate DNA helicase II in a mismatch-dependent manner. J. Biol. Chem. 273:9197-9201. [DOI] [PubMed] [Google Scholar]