Abstract
The plant Mentzelia pumila (family Loasaceae) has leaves and stems densely covered with tiny hooked trichomes. The structures entrap and kill insects and therefore are most probably protective. But they are also maladaptive in that they incapacitate a coccinellid beetle (Hippodamia convergens) that preys upon an aphid enemy (Macrosiphum mentzeliae) of the plant. The adaptive benefit provided by the trichomes is evidently offset by a cost.
Keywords: evolutionary benefit, evolutionary cost, Mentzelia pumila, Coccinellidae, Aphididae
The leaves and stems of many plants are beset with small hairs, hooks, spines, or scales (1, 2). These epidermal elaborations, or trichomes, which can impart on a plant a characteristic pubescent appearance or abrasive “feel,” generally are believed to be defensive. Indeed, a number of insect herbivores, including aphids, leaf beetles, leafhoppers, and caterpillars, have been shown to be physically deterred by trichomes (2–5). In the course of field studies undertaken by two of us (T.E. and M.E.) in southern Arizona, we came across a plant, Mentzelia pumila (family Loasaceae), that seems to reap both benefit and harm from its possession of trichomes. It benefits because its trichomes are broadly incapacitating to insects, and it is harmed because among the insects incapacitated is a coccinellid beetle that preys on an aphid enemy of the plant.
The observations we made are not nearly as extensive as we would have liked. They are presented here because we do not anticipate having future occasion to study the plant. Specifically, we provide a description of M. pumila’s trichomes, and data on how these structures affect insects generally and a coccinellid beetle in particular.
MATERIALS AND METHODS
The Plant (M. pumila).
This plant is a multibranched herb, typically 30–60 cm in height, with lanceolate toothed leaves and yellow flowers (Fig. 1A). Its range extends from Wyoming southward to Texas, Arizona, and Mexico (6). Like other members of its genus, it bears a dense covering of tiny trichomes, which render its leaves and stems characteristically sandpaper-like to the touch. We made our observations in May 1991, on stands of the plant that we located in Cochise County, AZ, northward of Douglas, along highway U.S. 80 and in the environs of Portal.
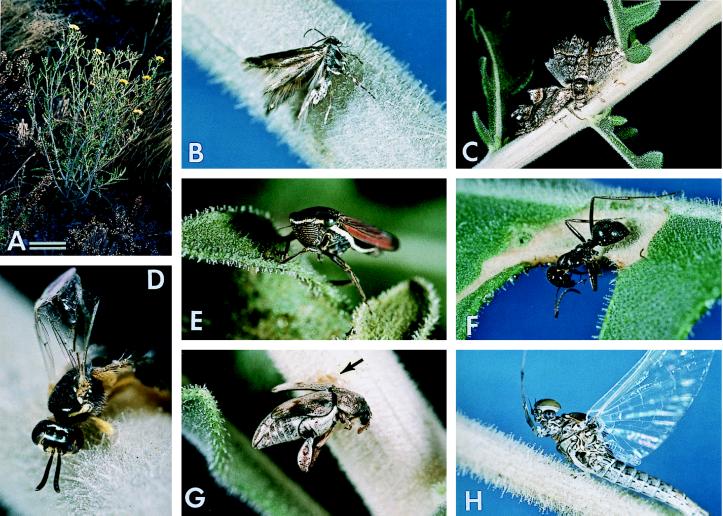
Figure 1.
(A) M. pumila (bar = 10 cm). (B–H) Various insects entrapped on M. pumila: (B and C) moths; (D) andrenid bee; (E) leafhopper; (F) ant; (G) bruchid beetle (arrow denotes clotted blood); and (H) mayfly. More detailed identifications are given in text. (Magnifications: B, ×7.5; C, ×1.5; D, ×10; E and F, ×6; G, ×5; and H, ×5.5.)
Microscopy.
Fresh material was photographed with a Wild M400 photomicroscope. Items viewed with a scanning electronmicroscope (pieces of M. pumila leaves and stems; insects stuck to the plant) were prepared by being dehydrated in ethanol, critical-point dried, and gold-coated (7).
Insects Found Entrapped on M. pumila.
These were identified to species or genus where possible, otherwise mostly to family.
The Aphid (Macrosiphum mentzeliae).
Two species of aphid, M. mentzeliae and Pleotrichophorus wasatchii, have been reported from Mentzelia plants (8). We found only the former feeding on M. pumila at our study sites.
The Coccinellid Beetle (Hippodamia convergens).
This was by far the dominant coccinellid on M. pumila at our field sites. The species occurs throughout the United States (9).
Tests with H. convergens.
To obtain some measure of the susceptibility of this coccinellid to entrapment on M. pumila, a group of larvae (n = 9, third and fourth instar, field-collected on M. pumila) and another of adults (n = 15, also field-collected on M. pumila), each was released into a small plastic chamber (approximately 110 cm2 floor surface, 2 cm height) packed loosely with pieces of M. pumila leaves. The two chambers were checked at intervals of 10 min over the course of an hour, to take counts of the number of larvae and adults that had become entrapped by trichomes.
A separate test was performed with another group of larvae (n = 18, first and second instar, raised from two egg clusters found on M. pumila, and fed on M. mentzeliae) placed in a similar chamber, and checked daily over a period of 3 days, to obtain counts of the number of larvae that died as a consequence of entrapment.
RESULTS
Field Observations.
What first drew our attention to M. pumila was that the plants seemed invariably to have numbers of dead insects stuck to them. The insects were present on leaves and stems, were of various sizes and kinds, and all seemed to be held in place by the trichomes of the plant. Some of the entrapped insects were still live when spotted, indicating that death came slowly to those caught, and that survival was still an option for those able to pull loose.
Most trapped insects were of types that had no obligatory trophic relationship to the plant. The moths and mayfly depicted in Fig. 1 B, C, and H are examples. Such insects simply might have been caught when incidentally landing on the plant or when colliding with the plant in flight. The leafhopper, bruchid beetle, and flea beetle, shown in Fig. 1 E and G, and Fig. 2E, all of which are jumping insects, could have been trapped while leaping.
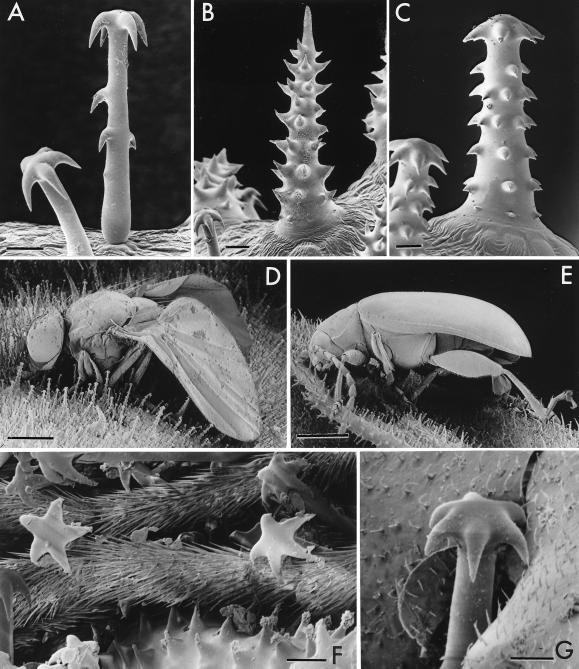
Figure 2.
(A–C) M. pumila trichomes: type 1 (A), type 2 (B), and type 3 (C). (D) Agromyzid fly and (E) chrysomelid (“flea”) beetle entrapped on M. pumila. (F) Closeup of antennae of an entrapped sciarid fly; the antenna in lower half of picture is wedged between two trichomes of type 1 and one trichome of type 2. (G) Trichome of type 1 that has cut into the membranous hindwing of an entrapped H. convergens. (Bars: A–C, F, and G, 20 μm; D and E, 0.5 mm.)
Interestingly, we found the aphid, M. mentzeliae (Fig. 3 A and B), to be ubiquitous on M. pumila (a leaf-by-leaf check of 24 individual plants from two sites revealed all to be infested). The aphid tended to occur in small colonies, restricted mostly to single leaves. Remarkably, the aphid seemed to be invulnerable to the trichomes of the plant. In dozens of colonies that we examined with a stereomicroscope, we found not a single trapped aphid.
Figure 3.
(A) Colony of the aphid, M. mentzeliae. (B) Single aphid, closeup. (C–I) The coccinellid, H. convergens, on M. pumila: (C) egg cluster (arrow denotes site where egg has been injured by trichomes); (D) entrapped larva (dead); (E) pupa (live), amidst colony of M. mentzeliae; (F) entrapped adult, struggling to free itself (left hindleg is being restrained by a single trichome, hooked to tarsus); (G–I) three adults that were entrapped and died (arrows denote clotted blood.) (Magnifications: A, E, and G–I, ×4; B, ×15; C, ×20, and D and F, ×4.5.)
Also of interest was the wide occurrence of H. convergens on the plant. We noted presence of both larvae and adults of this beetle (Fig. 3 D and F–I), the latter outnumbering the former by far. Not surprisingly, given that coccinellids are traditional enemies of aphids (3, 9, 10), we found both adults and larvae of H. convergens foraging in the midst of M. mentzeliae colonies (both stages of the beetle readily fed on M. mentzeliae offered to them in captivity). Occasionally, we also spotted egg clusters (Fig. 3C) and pupae (Fig. 3E) of H. convergens on M. pumila, always in the vicinity of M. mentzeliae colonies.
To obtain some measure of the insect-killing capacity of M. pumila, we took a visual count of the total number of insects (exclusive of coccinellids) found entrapped (dead or struggling) on 12 specimens of the plant (the plants were from a single site, and of size comparable to that of the plant shown in Fig. 1A). The count, which easily could have missed the smallest insects, amounted to 73 individuals. Insects identified included the following:
Ephemeroptera: undet. sp. (Fig. 1H);
Hemiptera: Miridae, Neurocolpus arizonensis;
Homoptera: Cicadellidae, Oncometopia lateralis (Fig. 1E);
Coleoptera: Cleridae, Phyllobaenus discoideus; Melyridae, Tanaops mimus, T. coelestinus, and Collops sp. vittatus group; Bostrichidae, Xylobiops sp. pb. sextuberculatus; Bruchidae, Algarobius prosopis (Fig. 1G); Chrysomelidae, Alticinae (“flea beetle”), undet. sp. (Fig. 2E);
Lepidoptera: Geometridae, Anacamptodes dataria (Fig. 1C); Gracillariidae, undet. sp. (Fig. 1B);
Diptera: Bombyliidae, Villa sp.; Sepsidae, undet. sp.; Sciaridae, undet. sp. (Fig. 2F); Calliphoridae, undet. sp.; Anthomyiidae, undet. sp.; Agromyzidae, undet. sp. (Fig. 2D);
Hymenoptera: Braconidae, undet. sp.; Formicidae, undet. sp. (Fig. 1F); Halictidae, undet. sp.; Andrenidae, Perdita sp. (Fig. 1D); Colletidae, Colletes sp.
A separate count that we made of H. convergens on another set of M. pumila plants (23 plants, of same size and from same site as those of the previous sample) but in which we tallied (separately for larvae and adults) whether the individuals were moving freely on the plant when spotted, or were entrapped and struggling, or entrapped and dead, gave the breakdown shown in Table 1. Excluded from the table are three egg clusters and two pupae of H. convergens that we also located on the plants.
Table 1.
Status of H. convergens larvae and adults, detected on 23 M. pumila plants in the wild
| Larvae, n = 15
|
Adults n = 100
|
||||
|---|---|---|---|---|---|
| No. free | No. entrapped
|
No. free | No. entrapped
|
||
| Live | Dead | Live | Dead | ||
| 11 | 3 | 1 | 82 | 16 | 2 |
The Trichomes.
Observation of leaf and stem surfaces of M. pumila with a stereomicroscope showed the trichomes to be minute, closely spaced, and of more than one type. Prodding with a needle showed them also to be rigid and not readily detached. Scanning electron microscopy revealed their detailed structure. One type of trichome (Fig. 2A, type 1), the most numerous, bears a characteristic crown of recurved barbs at the tip, as well as occasional such barbs along its shaft. At its base, this type is sharply tapered, indicating that it might be tiltable. A second type (Fig. 2B, type 2) is stouter, conical in shape, terminally pointed, and also barbed (but more densely so, and with the barbs curved toward the trichome tip). The third type (Fig. 2C, type 3) resembles the second in shape, but bears recurved barbs at the tip like the first, as well as recurved barbs along its length. We found the first type to be variable in length (and occasionally entirely barb-free along its shaft), and as a rule shorter than the other two types (note scale bars in Fig. 2 A–C).
One easily can envision how the three types of trichomes could operate in combination to immobilize an insect. Trichomes of type 1 and 3 could serve primarily as grappling devices, whereas those of type 2 could function both to restrain and perforate the insect. We obtained ample evidence for the first of these actions. Insects that we found entrapped live on M. pumila and watched with a stereomicroscope as they struggled to free themselves always were noted to be restrained by numbers of trichomes of type 1 that had hooked onto their legs, mouthparts, antennae, a combination of these appendages, or even the wings. Specimens that we found dead on the plant were similarly held in place by trichomes of type 1, as were specimens that we examined close-up with the scanning electron microscope (Fig. 2 D–G). A single trichome of type 1 could suffice to restrain a leg of a live insect (Fig. 3F).
Although we did not find direct visual evidence for the piercing action of the type 2 trichome, we did note that trapped insects can sustain injury as a consequence of getting caught. On repeated occasions we noted clotted residues of blood (hemolymph) beside insects that had died or were dying on the plant (arrows, Figs. 1G and 3 H and I). There even was evidence that trichomes of type 1 could tear into an insect with their sharp barbs (Fig. 2G).
Tests with H. convergens.
It was clear from the 1-hr tests (Table 2) that both larvae and adults of the beetle are subject to entrapment when ambulatory on M. pumila. At most time transects that observations were made at least some individuals of either stage were noted to be caught. For larvae, the fraction stuck at any time was on average 15%, and for adults it was 12%. Individuals did not necessarily remain stuck for long, because the larval and adult composition of the counts varied from count to count. For both larvae and adults, therefore, exposure to M. pumila initially could lead to no more than a series of entrapment-and-escape episodes.
Table 2.
Incidence of entrapment, monitored at 10-min intervals, among larval and adult H. convergens, confined for 1 hr with M. pumila leaves
| Larvae, n = 9*
|
Adults, n = 15
|
|
|---|---|---|
| Time (min) | No. entrapped (live) | No. entrapped (live) |
| 10 | 1 | 1 |
| 20 | 0 | 3 |
| 30 | 0 | 1 |
| 40 | 2 | 1 |
| 50 | 2 | 3 |
| 60 | 3 | 2 |
Larvae and adults were tested in separate chambers, each as a group.
Third and fourth instar.
Prolonged exposure, on the other hand, can lead to death. Virtually half (44%) of the larvae that we caged for 3 days with M. pumila leaves succumbed to entrapment (Table 3).
Table 3.
Cumulative total in number of fatalities among a group of larvae of H. convergens confined for 3 days in a chamber with M. pumila leaves
| Larvae, n = 18*
| |
|---|---|
| Time, hr | No. entrapped, dead |
| 24 | 2 |
| 48 | 7 |
| 72 | 8 |
First and second instar.
DISCUSSION
M. pumila is a veritable killer plant. Its trichomes are potentially lethal to any number of insects. Although one can only speculate about which natural enemies of M. pumila nowadays might be deterred by the devices, there can be little question that over evolutionary time the trichomes must have prevented many an insect herbivore from becoming trophically dependent on the plant.
It is not unusual in nature for adaptive barriers to be breached, so it should come as no surprise that there should be insects, such as M. mentzeliae, capable of feeding on M. pumila. We are mystified as to how this aphid manages to avoid entrapment by the trichomes. Our only explanation is that by walking slowly, which it does, and by having fine-tipped legs (Fig. 3B), it is able somehow to “tiptoe through the thorns.”
Equally familiar is the notion that evolutionary specialization entails cost. It is typical for aphid colonies to draw coccinellid predators, and obviously beneficial to a plant to be “deloused” by coccinellids. In M. pumila, however, the trichomes, although primarily defensive, are secondarily detrimental to the plant, in that they are harmful to the plant’s coccinellid “allies.”
The degree to which coccinellids are incapacitated by the plant is substantial. Our test results predict that somewhere in the order of 10–20% of H. convergens larvae and adults present on M. pumila at any one time should be in a state of fresh entrapment (that is, caught but still alive). The actual values derived from our field observations of M. pumila, 20% for live entrapped larvae and 16% for live entrapped adults, are in line with this prediction.
Coccinellids are prodigious feeders. Larvae and adults may consume, respectively, upward of 30 and 100 aphids per day (11). There can be little doubt, therefore, that by hampering coccinellids and cutting down on their effective predatory intake, the trichomes negatively impact the plant.
Larvae of H. convergens were present on M. pumila in far lesser numbers than the adults of the beetle. This finding could reflect several causes, including low survivability of the larvae, reduced oviposition on the plant (do the trichomes discourage egg laying?), and decreased egg viability. There were, in fact, far fewer H. convergens eggs on M. pumila than might have been predicted from the abundance of adult beetles on the plant, and such eggs as did occur, bore occasional evidence of having been punctured by trichomes (Fig. 3C).
In one possible way the trichomes could mitigate the harm inflicted by the aphid on M. pumila. Aphids frequently are tended (and defended) by ants. We found ants to be entirely absent from the aphid colonies and only rarely present elsewhere on the plant. One ant that we did find was noted to be entrapped by trichomes (Fig. 1F). The trichomes might well be generally deterrent to ants, and indirectly harmful, therefore, to the aphids.
Interestingly, among the insects we found dead on M. pumila there were several individuals of an andrenid bee, Perdita sp. (Fig. 1D), of a genus known to include pollinators of Mentzelia (12, 13). Evidently, the trichomes could be a hazard to any number of insects beneficial to the plant.
Finally, one cannot help but wonder whether by killing insects M. pumila might not derive subtle nutritional benefit. Insects trapped on the plant, once dead, inevitably must decay, and one could imagine the nitrogenous products of this decay leaching into the ground and becoming available to the plant as fertilizer. The nutritional supplement might be meager, but perhaps under deficient soil conditions sufficient to “make a difference.” Another plant that also kills insects, but by use of a sticky exudate instead of trichomes, might similarly benefit (14). The notion is testable.
Acknowledgments
Terry Griswold, Jonathan Mawdsley, and J. G. Franclemont provided some of the insect identifications. Mark Deyrup and Jerrold Meinwald commented on the manuscript. M.E. and T.E. thank our hostess in Portal, AZ, Mrs. Mary Willy, for numerous kindnesses. Voucher specimens of H. convergens and Perdita sp. are deposited in the Cornell University Insect Collection. This study was supported by Grant AI02908 from the National Institutes of Health.
References
- 1.Rodriguez E, Healey P L, Mehta I. Biology and Chemistry of Plant Trichomes. New York: Plenum; 1984. [Google Scholar]
- 2.Levin D A. Q Rev Biol. 1973;48:3–15. [Google Scholar]
- 3.Dixon A F G. Aphid Ecology. New York: Chapman and Hall; 1985. [Google Scholar]
- 4.Stipanovic R D. In: Plant Resistance to Insects. Hedin P A, editor. Am. Chem. Soc., Washington, DC: ACS Symposium Series 208; 1983. pp. 69–100. [Google Scholar]
- 5.Gilbert L E. Science. 1971;172:585–586. doi: 10.1126/science.172.3983.585. [DOI] [PubMed] [Google Scholar]
- 6.Steere W C. The Flowers of the United States. Vol. 4. New York: McGraw–Hill; 1970. p. 188. [Google Scholar]
- 7.Dawes C J. Biological Techniques for Transmission and Scanning Electron Microscopy. Burlington, VT: Ladd Research Industries; 1979. [Google Scholar]
- 8.Palmer M A. Aphids of the Rocky Mountain Region. Denver: Hirschfeld; 1952. [Google Scholar]
- 9.Gordon R D. J N Y Entomol Soc. 1985;93:1–912. [Google Scholar]
- 10.Hagen K S. Annu Rev Entomol. 1962;7:289–326. [Google Scholar]
- 11.Hodek I. Ecology of Aphidophagous Insects. The Hague: Dr. W. Junk; 1966. [Google Scholar]
- 12.Timberlake P H. Univ Calif Publ Entomol. 1962;28:1–124. [Google Scholar]
- 13.Griswold T L, Parker F D. Pan-Pacific Entomologist. 1988;64:43–52. [Google Scholar]
- 14.Eisner T, Aneshansley D J. Annals Entomol Soc Am. 1983;76:295–298. [Google Scholar]