Abstract
Fur is a well-known iron-responsive repressor of gene transcription, which is used by many bacteria to respond to the low-iron environment that pathogens encounter during infection. The fur gene in Neisseria meningitidis has been described as an essential gene that may regulate a broad array of genes. We succeeded in obtaining an N. meningitidis mutant with the fur gene knocked out and used it to undertake studies of fur-mediated iron regulation. We show that expression of both Fur and the transferrin binding protein Tbp2 is iron regulated and demonstrate that this regulation is Fur mediated for the Tbp2 protein. Footprinting analysis revealed that Fur binds to two distinct sites upstream of its coding region with different affinities and that these binding sites overlap two promoters that differentially control transcription of the fur gene in response to iron. The presence of two independently regulated fur promoters may allow meningococcus to fine-tune expression of this regulator controlling iron homeostasis, possibly during infection.
Iron is an essential element for almost all living organisms. The human body as a host provides an environment of iron limitation, as iron is complexed to carrier molecules and therefore not readily available. As a consequence, pathogenic bacteria have developed high-affinity iron uptake systems by which they may scavenge iron in vivo. The human pathogen Neisseria meningitidis is a common colonizer of the nasopharynx, and in a small percentage of carriers, meningococcus can cross the epithelial barrier to enter the bloodstream, causing septicemia, and then further cross the blood-brain barrier, causing meningitis. The genome sequence of N. meningitidis suggests that this bacterium possesses several iron-scavenging strategies(32). Although N. meningitidis does not produce siderophores, it possesses outer membrane receptors that have been postulated to scavenge the iron-loaded siderophores secreted by other bacteria colonizing the nasopharyngeal tract (5). Once inside the host, the organism must compete for iron with host iron proteins, and meningococcus possesses receptors for transferrin, lactoferrin, and hemoglobin (24).
The importance of iron for meningococcal pathogenesis is well documented: treatment with inorganic iron enhances N. meningitidis infection in mice (4, 18), and strains with mutations in iron uptake systems are attenuated in animal models (13, 29, 31). The ability of meningococcus to acquire iron has been shown to play an important role in promoting the survival of the organism within the host, both in its ability to replicate within epithelial cells (15) and in its in vivo survival in the bloodstream (29). Given the location of iron receptors on cell surfaces, their role in pathogenicity, and often their interstrain sequence conservation, these types of proteins have been under study as possible candidates for vaccines against meningococcal infection (1, 2, 17).
Iron overload results in toxicity; therefore, iron uptake is tightly regulated and, in many bacteria, this regulation is mediated by the ferric uptake regulator (Fur) protein (8). The Fur protein senses cellular iron concentrations and acts as a transcriptional repressor by binding to sequences in the promoters of iron-regulated genes and blocking the entry of RNA polymerase, thus inhibiting initiation of RNA transcription. A 19-bp consensus Fur binding site (Fur box) has been elucidated, and Fur proteins from different bacteria have been shown to bind this sequence (20, 38). Classically, Fur-regulated promoters are repressed under high-iron conditions. However, it has recently been demonstrated that in Helicobacter pylori, Fur regulates iron-repressed and iron-activated promoters (7). Due to Fur's involvement in the regulation of activities as varied as the acid tolerance response, the oxidative stress response, metabolic pathways, and virulence factors, it has been proposed to be a global regulator in response to environmental iron concentration (8).
The fur gene of N. meningitidis has been cloned and was shown to be capable of regulating Escherichia coli iron-regulated promoters (33). Furthermore, sequences resembling those that encode the Fur box have been identified in meningococcal iron-regulated genes (16, 21, 22, 29, 35) as well as in those of gonococci (25). One of the major limitations in the research on the role of Fur in N. meningitidis has been the inability to make a fur null mutant. Unsuccessful attempts to isolate insertional null mutants of both N. meningitidis (33) and Neisseria gonorrhoeae (34) have been reported; however, a fur mutant containing a point mutation in the N. gonorrhoeae gene was subsequently isolated by manganese selection (34).
In the present study, we report the construction of a fur null mutant, which suggests that this gene is not essential in Neisseria spp., and present initial studies of Fur-mediated iron regulation showing autoregulation of the fur gene and demonstrating that the iron-dependent expression of the transferrin binding protein Tbp2 is Fur mediated.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. N. meningitidis strains were routinely cultured in GC-based (Difco) agar medium containing Kellogg's supplement I (12) at 37°C in a 5% CO2-95% air atmosphere at 95% humidity. Strains were stocked in 10% skim milk and stored at −80°C. Each bacterial manipulation was started from an overnight culture of a frozen stock. For liquid cultures, N. meningitidis strains were grown overnight on solid medium, diluted in phosphate-buffered saline (PBS) to an optical density at 600 nm (OD600) of 1, and inoculated at a 1:100 dilution into GC broth supplemented with Kellogg's supplement I, 12.5 μM Fe(NO3)3, and, when required, erythromycin and kanamycin added to achieve final concentrations of 5 and 100 μg/ml, respectively. For transformation by naturally competent N. meningitidis, four to five colonies of a freshly grown overnight culture were resuspended in 20 μl of PBS, spotted onto GC medium plates to which 5 to 10 μg of linearized plasmid DNA was added, allowed to dry, and incubated for 6 to 8 h at 37°C. Transformants were then selected on plates containing erythromycin (5 μg/ml) and kanamycin (150 μg/ml), and single colonies were restreaked on selective media for further analysis. Single colonies were diluted in 50 μl of PBS, placed in a boiling water bath for 5 min, and centrifuged in a bench top centrifuge for 5 min at 8,000 × g. One microliter of the sample was used as the template for PCR analysis. E. coli cultures were grown in Luria-Bertani medium, and when required, ampicillin and kanamycin were added to achieve final concentrations of 100 and 25 μg/ml, respectively.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5-α | supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 10 |
| BL21(DE3) | hsdS gal (λcIts857 ind-1 Sam7 nin-5 lacUV5-T7 gene 1) | 30 |
| N. meningitidis strains | ||
| MC58 | Clinical isolate; sequenced strain | 32 |
| MC-Fko | fur mutant; derivative of MC58 in which the fur gene is replaced by a kanamycin cassette; fur Kmr | This study |
| MC-Fko-C | Complemented fur mutant; derivative of MC-Fko in which the fur gene under the control of its own promoter was inserted along with an erythromycin cassette in the noncoding region between the NMB1074 and NMB1075 genes; fur+ Kmr Eryr | This study |
| MC-furlacZ | Derivative of MC58 containing 317 bp consisting of the 5′ end of the fur gene and the entire upstream region fused to a promoterless lacZ gene chromosomally located between the NMB1074 and NMB1075-genes; Eryr | This study |
| MC-smpAlacZ | Derivative of MC58 containing 277 bp consisting of the 5′ end of the smpA gene and the entire upstream intergenic region fused to a promoterless lacZ gene chromosomally located between the NMB1074 and NMB1075 genes; Eryr | This study |
| Plasmids | ||
| pET15b | Expression plasmid for expression of recombinant proteins with the N-terminal His tag and thrombin site for removal of the tag | Invitrogen |
| pGem3Z | Cloning vector | Promega |
| pGemT | Cloning vector | Promega |
| pILL600 | Plasmid containing the kanamycin cassette from Campylobacter coli | 14 |
| pSL1190 | Cloning vector | Pharmacia |
| pCMVβ | Plasmid containing the lacZ gene of E. coli | Clontech |
| pET15furB | Derivative of pET15b containing a 435-bp NdeI-BamHI fragment of the fur coding region obtained by PCR on MC58 DNA using primers Fmb-F and Fmb-R | This study |
| pGemFkoB:Km | pGem3Z derivative containing a 587-bp EcoRI-BamHI region upstream of the fur gene obtained by PCR with primers FkoB-1 and FkoB-2, a 1.4-kb BamHI fragment of the kanamycin gene from plasmid pILL600, and a 465-bp BamHI-PstI region downstream of the fur gene obtained by PCR with primers FkoB-3 and FkoB-4; this plasmid was selected because it contains the kanamycin cassette oriented in the same direction as that of fur gene transcription | This study |
| pSLFur-C1 | pSL1190 derivative containing a 510-bp SpeI-XhoI PCR fragment of the NMB1074 locus with primers Fla-UP-L and Fla-UP-R, a 1.1-kb XhoI-PstI PCR fragment of the erm gene obtained with primers Eryt-DO and Eryt-UP, a 658-bp NsiI-BamHI PCR fragment of the fur promoter and coding region obtained with primers Fur-N and Fmb-R, and a 909-bp BamHI-XmaI PCR fragment of the NMB1075 locus obtained with primers Fla-DO-L and Fla-DO-R | This study |
| pSL-furlacZ | pSL1190 derivative containing a 510-bp SpeI-XhoI PCR fragment of the NMB1074 locus obtained with primers Fla-UP-L and Fla-UP-R, a 1.1-kb XhoI-PstI PCR fragment of the ermAM gene obtained with primers Eryt-DO and Eryt-UP, a 317-bp NsiI-SphI fragment of the fur promoter region amplified with primers Fur-N and Fur-P2, a 3.4-kb SmaI-BamHI fragment carrying the lacZ gene from plasmid pCMVβ (Clontech), and a 909-bp BamHI-XmaI PCR fragment of the NMB1075 locus obtained with primers Fla-DO-L and Fla-DO-R | This study |
| pSL-smpAlacZ | pSL-furlacZ derivative in which the fur promoter region was replaced with a 277-bp NsiI-SphI PCR fragment of the smpA promoter region with primers Upf-N2 and Upf-S | This study |
| pGemT-Fur | Derivative of pGemT containing the fur promoter region cloned as a 322-bp PCR product with primers Fur-P1 and Fur-P2 | This study |
DNA techniques.
DNA manipulations were carried out routinely as described by Sambrook et al. (23). Small- and large-scale plasmid DNA preparations were carried out with a QIAprep Spin Mini kit and Plasmid Midi kit (QIAGEN, Inc.) according to the manufacturer's instructions. DNA fragments or PCR products for cloning purposes were purified from agarose gels with a QiaEx DNA purification kit (QIAGEN, Inc.). PCR was performed in a Perkin-Elmer 2400 thermal cycler with Platinum Taq polymerase (Invitrogen). One microliter of each reaction mixture contained 10 to 50 ng of chromosomal DNA or 1 μl of bacterial sample (see above), 100 pmol of the required primers, and a 200 μM concentration of each deoxynucleotide in a volume of 100 μl of 1× PCR buffer containing MgCl2 (New England Biolabs, Inc.). After the initial denaturing step at 95°C for 5 min, 30 cycles of denaturing at 95°C, annealing at the temperatures appropriate for the specific primers, and elongation at 72°C were carried out. DNA fragments were sequenced according to the dideoxy-chain termination method by using [α-32P]dATP (Amersham) and a T7 sequencing kit (Pharmacia).
Construction and complementation of a fur mutant of N. meningitidis.
In order to generate an N. meningitidis fur mutant in which the fur gene is deleted and replaced by allelic exchange with a kanamycin gene orientated similarly and lacking transcriptional terminators, the MC58 strain was transformed with plasmid pGemFkoB:Km (Table 1). Kanamycin-resistant colonies were selected and checked by PCR for correct insertion by a double-homologous-recombination event. Primer pairs internal (FkoB-1-FkoB-4) and external (FkoB-5-FkoB-6) to the recombination sites as well as internal to the fur gene (Fmb-F-Fmb-R) were used to check transformants, and those with the correct PCR profile were further checked by Western blot analysis. We generated one MC58 isogenic fur mutant, MC-Fko. Complementation of the MC-Fko fur mutant was achieved by insertion of the fur locus, complete with promoter and full coding region, into a noncoding chromosomal location between the two converging open reading frames (ORFs) NMB1074 and NMB1075, flanked on both sides with transcriptional terminators. For complementation by allelic replacement, the MC-Fko fur mutant was transformed with the pSLFur-C1 plasmid (Table 1). Transformants were selected on erythromycin and checked by PCR, and complementation of the fur mutant strain was verified by Western blot analysis.
Construction of chromosomally located transcriptional lacZ fusions.
To generate transcriptional lacZ fusions of the promoters under study at a chromosomal location between the two converging ORFs NMB1074 and NMB1075, flanked on both sides with transcriptional terminators, plasmids pSL-furlacZ and pSL-smpAlacZ for allelic exchange in N. meningitidis strains were constructed (Table 1). The erm erythromycin resistance gene (36) was used as a selection marker. These plasmids were then transformed into MC58, and transformants were selected on erythromycin and verified by PCR with primer pairs (Fla-UP-C-Ery-DO-C and Fla-DO-C2-Lac-DO-C); the resultant strains are listed in Table 1.
Expression and purification of the Fur protein.
Plasmid pET15furB (Table 1) was transformed into the E. coli strain BL21(DE3). From an overnight culture of the BL21(DE3)(pET15furB) strain, 200 ml of Luria-Bertani medium was inoculated and grown to an OD600 of 0.5, and expression of the recombinant Fur protein containing an N-terminal histidine tag was induced by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and further incubation for 3 h. The protein was purified from the harvested cells by Ni-nitrilotriacetic acid (QIAGEN) affinity chromatography under nondenaturing conditions according to the manufacturer's instructions. The purified protein preparation was then diluted to 1 μg/μl and dialyzed overnight in PBS at 4°C. To remove the His tag, the dialyzed protein was then digested at a concentration of 0.5 μg/μl with thrombin (10 U/μg protein; Pharmacia/Amersham) at room temperature for 4 h, and the thrombin was then deactivated by incubation with 1 mM of phenylmethylsulfonyl fluoride at 37°C for 15 min. The digested His tag was removed by twice dialyzing the protein preparation against 1 liter of PBS at 4°C in a 6,000- to 8,000-molecular-weight-cutoff dialysis tube (Membrane Filtration Products, Inc.). The protein preparation was then dialyzed against storage buffer (20 mM Tris [pH 7.9], 50 mM NaCl, 10 mM MgCl2, 0.01% NP-40, 1 mM dithiothreitol, 50% glycerol). The purity of the protein was estimated to be 99% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The concentration of the protein in this preparation was determined by using the Bradford colorimetric assay (Bio-Rad), and the protein was aliquoted and stored at −80°C.
Generation of anti-Fur antiserum and Western blot analysis.
To prepare anti-Fur antiserum, 20 μg of purified protein was used to immunize 6-week-old CD1 female mice (Charles River Laboratories), and four mice were used. The protein was given intraperitoneally, together with complete Freund's adjuvant for the first dose and incomplete Freund's adjuvant for the second (day 21) and third (day 35) booster doses. Bleed-out samples were taken on day 49 and used in Western blot analysis. Colonies from freshly grown overnight plate cultures were diluted in 4 ml of PBS until an OD600 of 1.0 was reached. One milliliter was then pelleted at 8,000 × g and resuspended in 100 μl of SDS-PAGE loading buffer, and 10 μl of each total protein sample was separated on an SDS-15% polyacrylamide gel and transferred onto a nitrocellulose filter by standard methods (23). Filters were blocked for 1 h at room temperature by agitation in blocking solution (3% skim milk and 0.1% Triton X-100 in PBS) and incubated for 1 h more with a 1:1,000 dilution of the Fur protein serum in blocking solution. After being washed, the filters were incubated in a 1:2,000 dilution of peroxidase-conjugated anti-mouse immunoglobulin (Dako) in blocking solution for 1 h and the resulting signal was detected with the Supersignal West Pico chemiluminescent substrate (Pierce).
DNase I footprinting.
Probe preparation and DNase I footprinting were carried out as previously described (6, 7) except for the following variations. The plasmid pGemT-Fur was 5′-end labeled with [γ-32P]ATP (5,000 Ci/mmol; Amersham) at its BamHI site and separated from the vector by PAGE after digestion with EcoRI, thereby producing a probe labeled at one extremity only. Binding reactions were performed in binding buffer consisting of 20 mM Tris-HCl (pH 7.9), 50 mM KCl, 10 mM MgCl2, 0.01% NP-40, 100 μM MnCl2, and 10% glycerol containing 1 μg of sonicated salmon sperm DNA as nonspecific competitor DNA. DNase I digestion was carried out by the addition of 1 μl of DNase I (0.02 U/μl) in binding buffer containing 5 mM CaCl2 for precisely 1 min at room temperature. As a molecular weight marker, a G+A sequence reaction (19) was performed for each DNA probe and run in parallel to the corresponding footprinting reactions.
RNA preparation and primer extension analysis.
N. meningitidis strains were grown in liquid culture to logarithmic phase and then split in three and harvested immediately or after a 15-min treatment of iron limitation (addition of 100 μM 2,2′-dipyridyl; Sigma). To harvest cells, cultures were placed first on ice for 5 min and then centrifuged at 5,000 × g in a bench top centrifuge at 4°C. RNA was extracted from the pelleted cells as previously described (28). In each case primer extension was performed as previously reported (7). To ensure correct mapping of the promoter, the sequencing reaction was carried out with a T7 sequencing kit (U.S. Biochemical Corp.) by using the same primer that was used in the primer extension reactions and the plasmid consisting of the relevant cloned promoter.
S1 nuclease mapping.
Radioactively labeled DNA probes for quantitative S1 nuclease mapping of each promoter were prepared. A Fur probe, consisting of a 533-bp NsiI-EcoRI fragment labeled at the EcoRI site was prepared as follows. The pSLFur-C1 plasmid was digested with EcoRI, and the ends were dephosphorylated with calf intestinal phosphatase (New England Biolabs). The 5.4-kb vector backbone was then purified from the internal EcoRI fragments by extraction from an agarose gel. Approximately 2 pmol of the purified 5.4-kb fragment was labeled with T4 polynucleotide kinase and 4 pmol of [γ-32P]ATP (5,000 Ci/mmol; Amersham) and digested with NsiI, and the 533-bp NsiI-EcoRI probe was purified from a 5% preparative polyacrylamide gel. For the Smp probe, a 325-bp fragment was amplified by PCR with the Us1-Us2 primer pair (Table 2). After purification of the fragment from an agarose gel, 2 pmol of the PCR product was labeled at both extremities with T4 polynucleotide kinase and 4 pmol of [γ-32P]ATP. One labeled extremity was removed by digestion with BamHI, a site for which is incorporated into the Us2 primer, and the resultant Smp probe labeled at one end was extracted from a preparative polyacrylamide gel. Probes extracted from polyacrylamide gels were first eluted overnight in 3 ml of elution buffer (10 mM Tris-HCl [pH 8], 1 mM EDTA, 300 mM Na acetate [pH 5.2], 0.2% SDS) at 37°C with shaking, phenol-chloroform extracted, ethanol precipitated, and resuspended in 100 μl of Tris-EDTA. Approximately 20 fmol of labeled probe was coprecipitated with 15 μg of total RNA and resuspended in 20 μl of hybridization buffer (80% formamide, 60 mM Tris-HCl [pH 7.5], 400 mM NaCl, 0.4 mM EDTA). The mixture was overlaid with 5 μl of paraffin oil, denatured at 100°C for 3 min, and then incubated at an annealing temperature (Tm) calculated on the basis of the following formula: Tm = 81.5 + 0.5(%G+C) + 16.6(natural log of Na concentration) − 0.6(%formamide). After 4 to 16 h of hybridization, 180 μl of ice-cold S1 buffer (33 mM Na acetate [pH 5.2], 5 mM ZnSO4, 250 mM NaCl) and 100 U of S1 nuclease (Invitrogen) were added and S1 nuclease digestion was carried out for 30 min at 37°C. Samples were then extracted once with phenol-chloroform, precipitated with ethanol, resuspended in 5 μl of sequencing loading buffer (23), and subjected to urea-6% PAGE. Quantification of the signals from the digested probes was performed by using a PhosphorImager and ImageQuant software (Molecular Dynamics).
TABLE 2.
Primers used in this study
| Primer | Sequencea | Siteb |
|---|---|---|
| Eryt-UP | GCAAACTgcAGAGTGTGTTGATAG | PstI |
| Eryt-DO | CCGTAGGCGCTcGaGACCTCTTTAGCTTCTTG | XhoI |
| Ery-DO-C | CAGGTTACTAAAGGGAATGGAG | |
| Fla-UP-L | GGTTCCGTACTAgTTGTACTGTCTGC | SpeI |
| Fla-UP-R | aatttaactcgagCCACCAATCCCACACCACCCTTACC | XhoI |
| Fla-DO-L | ATAAATGTAAAGGaTCCGTTTCATAGCTAAGG | BamHI |
| Fla-DO-R | CGCCGTCAACCCgGgTGCCGAGCTGGAAAAAGAGC | XmaI |
| Fla-UP-C | CTGAAGCAAAGTCGGAAAACGCCGGC | |
| Fla-DO-C2 | CTCGAAACCGGTTCTGACGG | |
| FurP-1 | cggatgaattcTCACGGAAATGCCTTTCTGTGC | EcoRI |
| FurP-2 | attcagggatccCTTCCGCATGCGTCTCGAAC | BamHI |
| Fmb-F | cggatccatATGGAAAAATTCAACAATATTGCAC | NdeI |
| Fmb-R | attcagggatccTTAACGTTTGCCCTTGGCCTG | BamHI |
| Fur-N | attcaggatgcatTCACGGAAATGCCTTTCTGTGC | NsiI |
| FkoB-1 | attcaggaattcGAGCGGTGTCATGTGTGTTCC | EcoRI |
| FkoB-2 | attcagggatccGACGTTATAATACGCAATTTCGGCC | BamHI |
| FkoB-3 | attcagggatccCCGGACGGTTTGTTGTTCAGAC | BamHI |
| FkoB-4 | attcagctgcagGAATGCGCGTACCCCATTTCG | PstI |
| FkoB-5 | CGGGATGGTTGTTGACGGC | |
| FkoB-6 | CGTTTCACCGCTTTCATCGGG | |
| Lac-DO-C | CGCTACCATTACCAGTTGGTCTGG | |
| LacZ-PE | TAGCAGGCTCTTTCGATCC | |
| Upf-N2 | attcagatgcatGGTAACCTTCAGACCGCTGTC | NsiI |
| Upf-S | attcaggcatgcTCACGGAAATGCCTTTCTGTGC | SphI |
| Us1 | CCAGCGGTCGGTATGGAATGCG | |
| Us2 | CGATGCGCGGACAGGGCggatccCGCTTAATG | BamHI |
Capital letters indicate N. meningitidis-derived sequences, italicized capital letters indicate E. coli-derived sequences, lowercase letters indicate sequences added for cloning purposes, and underlined letters indicate recognition sites.
Restriction enzyme sites added for cloning purposes.
RESULTS
Construction and complementation of a fur null mutant.
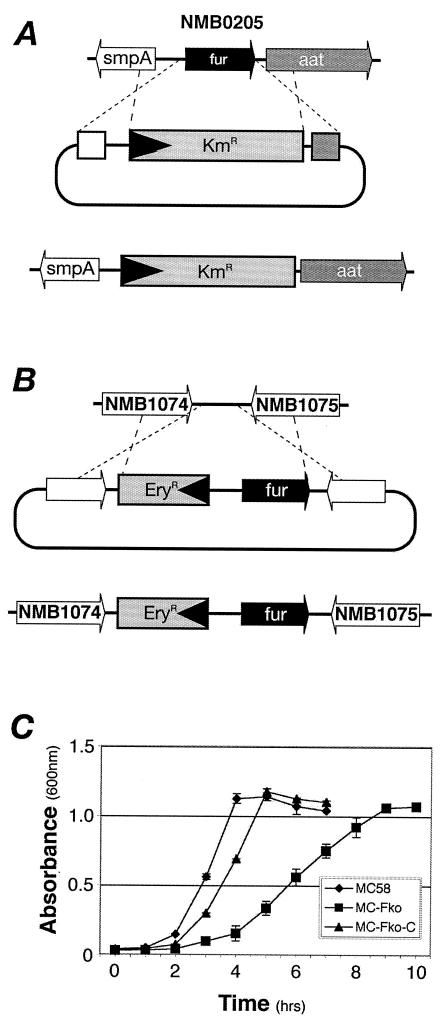
Previous attempts to isolate a fur null mutant of N. meningitidis by insertional replacement through allelic exchange were unsuccessful (33), and as a result, it has been hypothesized that fur is an essential gene of N. meningitidis. Seventy-two base pairs downstream of the fur gene maps a homolog (ORF NMB0206) of the leucyl, phenylalanyl-tRNA-protein transferase gene aat from E. coli (27), which is oriented in the same direction. Nucleotide sequence analysis of this region showed no obvious promoter elements, suggesting that the fur and aat genes may represent an operon. We attempted to insertionally inactivate the fur gene by allelic exchange, as shown in Fig. 1A, by replacing it with a kanamycin cassette oriented similarly to the fur gene; this replacement may drive expression of the downstream hypothetical aat gene, thereby minimizing the polar effect. On transformation of the MC58 strain with the allelic-replacement construct, pFkoB:Km, only 1 out of 15 Kmr transformants had the correct PCR profile and this transformant, named MC-Fko, was selected for further analysis.
FIG. 1.
Mutation and complementation of the fur gene of N. meningitidis. (A) Schematic representation of the strategy used to construct a fur mutant; (B) schematic representation of the strategy used for complementation of Fur; (C) growth curves of the wild-type MC58 strain, the fur MC-Fko mutant, and the MC-Fko-C complemented mutant in supplemented GC medium.
The MC-Fko fur mutant when grown on solid medium has an obvious small-colony phenotype; i.e., freshly grown overnight cultures result in pinpoint colonies on GC plates. In order to verify that this phenotype was indeed due to the lack of the Fur protein, we complemented the MC-Fko mutant by inserting the fur gene with its own promoter region and an upstream erythromycin cassette as a selection marker between ORFs NMB1074 and NMB1075 as described in Materials and Methods and shown in Fig. 1B. The size of the colonies of the complemented mutant, named MC-Fko-C, is intermediate between that of the pinpoint colonies of the mutant and that of the normal round colonies of the wild type. We determined the growth curves of the three strains to further analyze the apparent growth defect of the mutant, and the results are shown in Fig. 1C. The doubling time of the mutant at 63 (±3) min is considerably longer than that of the wild type at 40 (±2) min. Nevertheless, the fast-growth phenotype is restored almost to wild-type levels in the complemented strain whose doubling time is 51 (±3) min. In growth experiments with supplemented GC medium with low [1 to 10 μM Fe(NO3)3] or trace [no Fe(NO3)3 added] concentrations of iron, the slow-growth phenotype did not match that of the wild type and, therefore, the slow-growth phenotype appears not to be a result of possible cellular iron overload due to the derepression of iron uptake systems in the mutant (data not shown).
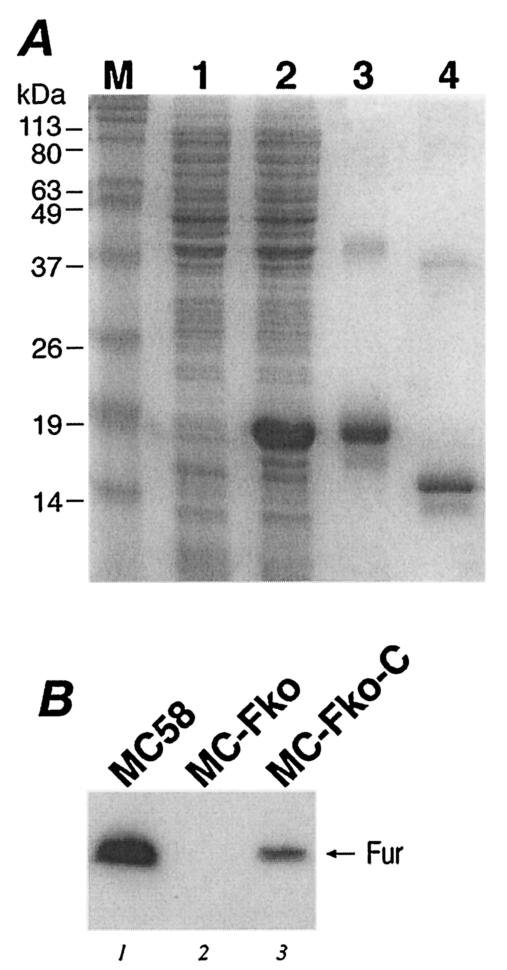
In order to investigate the expression of the Fur protein in these strains, we purified the Fur protein and raised antibodies against Fur in mice. The fur gene was cloned into an expression vector in E. coli, and the protein was expressed and purified by Ni2+ affinity chromatography by virtue of an N-terminally located His tag, which was then cleaved and removed after purification. Samples from the expression and purification steps of the recombinant Fur protein are shown in Fig. 2A. After SDS-PAGE of the purified tagged and untagged Fur proteins, we clearly observed the major protein bands migrating close to the expected positions and minor slowly migrating bands in each case (lanes 3 and 4). The nature of these slowly migrating bands has not been investigated and may probably correspond to dimeric forms of the proteins.
FIG. 2.
(A) Expression and purification of the Fur protein. Lane 1, protein extracts from noninduced E. coli cells harboring plasmid pET15furB; lane 2, protein extracts from cells induced for 3 h with IPTG; lane 3, purified His-tagged Fur protein; lane 4, untagged Fur protein. (B) Western blot analysis showing Fur expression in the wild-type MC58 (lane 1), the MC-Fko fur mutant (lane 2), and the MC-Fko-C complemented mutant (lane 3) strains. Lane M, molecular size standards.
Figure 2B shows the immunoblot of total protein extracts from N. meningitidis wild-type MC58, the MC-Fko mutant, and the MC-Fko-C mutant strains. The antibodies recognize a protein band migrating to a position corresponding to approximately 15 kDa in the wild-type MC58 and in the complemented MC-Fko-C mutant but not in the MC-Fko deletion mutant (Fig. 2B). This result verifies that, indeed, the Fur protein is not expressed in the MC-Fko mutant and confirms the complementation of the fur mutant. The signal from the Fur protein is weaker in the MC-Fko-C complemented mutant, however, than in the wild-type strains, indicating that the level of expression of fur from the heterologous location on the chromosome is not at the level of expression of the wild type. This observation may account for the fact that the complemented mutant has a lower growth rate than the wild-type strain. In conclusion, we successfully constructed a fur null mutant, which strongly suggests that the fur gene is not essential in N. meningitidis as it is currently considered to be. The growth rate of the fur null mutant of N. meningitidis is affected.
Iron- and Fur-mediated regulation of protein expression.
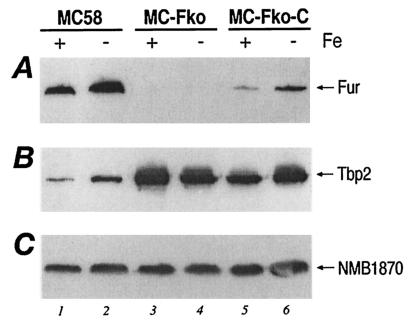
The Tbp2 protein is a subunit of the transferrin receptor of N. meningitidis and is known to be iron regulated (2). To establish whether this iron regulation is Fur mediated and to gain insight into the regulation of the fur gene in response to iron, we decided to monitor the fates of the Tbp2 and Fur proteins in total protein extracts of N. meningitidis strains cultured under iron-replete and iron-limiting conditions. We grew parallel cultures of the MC58, MC-Fko, and MC-Fko-C strains under iron-replete [GC medium with 12 μM Fe(NO3)3] and iron-limiting (GC medium with 25 μM desferal) conditions to an OD600 of 0.4 to 0.6 and analyzed the expression of the Fur and Tbp2 proteins by Western blot analysis. Equal quantities of total protein from each strain under iron-replete and iron-limiting conditions were separated by SDS-PAGE, blotted onto nitrocellulose filters, and tested with antiserum raised against the Fur protein, the Tbp2 protein, and a constitutive protein as a negative control (NMB1870). Figure 3 shows the results of the analysis. As shown in Fig. 2B, there is no signal recognized by the Fur antiserum in the fur mutant, as this gene has been deleted (Fig. 3A, lanes 3 and 4) and the relative quantities of Fur in the complemented mutant are less than in the wild type (Fig. 3A, lanes 5 and 6 versus lanes 1 and 2). In the MC58 and MC-Fko-C strains, however, there is an approximately threefold difference in the signal intensities of the Fur protein between the corresponding cultures grown under iron-replete and iron-limiting conditions (Fig. 3A, lane 1 versus lane 2 and lane 5 versus lane 6), indicating that the expression of Fur is iron regulated in both these backgrounds. Moreover, the expression of the Fur protein is down-regulated by iron. In the MC58 strain, the band corresponding to Tbp2 represents approximately threefold less protein in iron-replete culture than in the iron-limited culture, thus confirming that the Tbp2 protein is similarly regulated by iron (Fig. 3B, lane 1 versus lane 2). In the fur mutant the expression of Tbp2 is derepressed under both iron conditions (lanes 3 and 4), and in the complemented mutant, although the expression is not repressed to wild-type levels, iron regulation can once more be detected, as the amount of protein expressed is less under iron-replete conditions (lanes 5 and 6). Figure 3C shows the results of the Western blot analysis with anti-NMB1870 antiserum, a protein whose amount is not affected by the iron content of the culture or by the Fur background; thus, its expression is neither regulated by iron nor by Fur and the protein serves as a negative control.
FIG. 3.
Iron and Fur regulation of gene expression. Western blot analysis showing iron-regulated protein expression. Wild-type MC58, the MC-Fko fur mutant, and the MC-Fko-C complemented mutant were grown under iron-replete (+) (supplemented GC medium) and iron-limiting (−) (supplemented GC medium with 25 μM desferal) conditions and were harvested at an OD600 of 0.4 to 0.6. Equal amounts of total protein from each culture were fractionated by SDS-PAGE, blotted onto nitrocellulose filters, and stained with antiserum raised against the Fur protein (A), the Tbp2 protein (B), and constitutive protein NMB1870 as the negative control (C).
In summary, through Western blot analysis we can detect iron regulation of both the Fur and the Tbp2 protein. Furthermore, iron regulation of the Tbp2 protein is Fur mediated.
Binding of Fur to the fur promoter region.
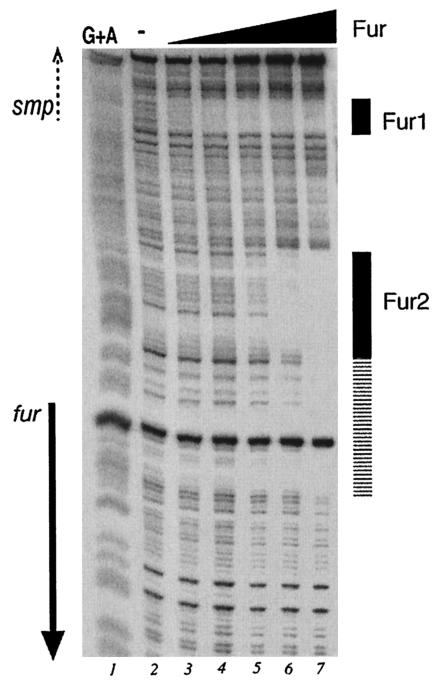
In order to investigate whether the meningococcal Fur protein interacts directly with its promoter, we carried out in vitro binding assays with the purified Fur protein (Fig. 2A). We prepared a probe of the fur upstream region and carried out DNase I footprinting analysis, with the results shown in Fig. 4. Two binding sites were identified in the fur upstream region: with the addition of 40 nM Fur (lane 3), protection occurs at the Fur1 binding site, and with the addition of a 1.1 μM concentration of the protein (lane 6), protection occurs at the Fur2 binding site. Interestingly, with increased amounts of the Fur protein in the reaction mixture, the Fur2 binding site extends towards the translational initiation site of the fur gene. The binding ability of the Fur protein was found to be dependent on the presence of a divalent metal ion when footprinting was performed with the addition of 100 μM MnCl2, and no protection was observed when EDTA or 2,2′-dipyridyl was added to the binding reaction mixtures (data not shown). On analysis of the nucleotide sequences corresponding to the protected regions of the Fur1 and Fur2 binding sites, we can identify a Fur box-like element in both binding sites (Fig. 5C) corresponding to seven and eight mismatches, respectively, from the Fur box consensus from E. coli.
FIG. 4.
Footprinting analysis of purified Fur on the NMB204-fur intergenic probe. The probe was labeled at one extremity and prepared as described in Materials and Methods. Lanes 2 to 7 contain reaction mixtures to which purified Fur protein at 13.4 nM, 40 nM, 122 nM, 366 nM, 1.1 μM, and 3.2 μM, respectively, was added. Lane 1 represents the G+A sequence reaction (19) obtained with the same probe and used as a molecular weight marker. The solid arrow shows the position and the orientation of the fur coding region. The dashed arrow indicates the orientation of the smpA gene, which is not contained in the probe.
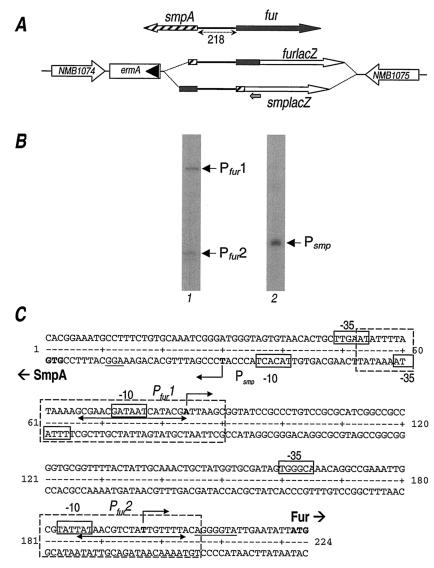
FIG. 5.
Mapping the promoters. (A) Schematic representation (not to scale) of the fur locus (top) and chromosomal promoter fusions to the lacZ gene (bottom) inserted between the NMB1074 and NMB1075 loci. Hatched and filled arrows and boxes indicate all or part of the smpA and fur genes, respectively. The 218-bp intergenic region is highlighted. Open arrows indicate the lacZ gene. The position of the lacZ-PE primer is indicated with a small grey arrow. (B) Primer extension reactions with total RNA extracted from strains MC-furlacZ (lane 1) and MC-smpAlacZ (lane 2). Arrows mark the elongated primer products, Pfur1, Pfur2, and Psmp. (C) Nucleotide sequence of the smpA-fur intergenic region. Deduced initiations of RNA transcription are in boldface and marked with bent arrows. Putative promoter DNA elements are boxed and marked −10 and −35 at Pfur1, Pfur2, and Psmp. Nucleotides protected by Fur in DNase I protection assays are boxed with broken lines. Double-ended arrows indicate the core Fur box sequences. The translation start sites of the smpA and fur genes are in boldface and labeled accordingly.
We conclude that Fur binds to two distinct sites of the fur upstream region with different affinities.
Mapping of the promoters.
Within the 218 bp of DNA upstream of the fur gene, there is a possibility of at least two promoter elements controlling the transcription of the divergent genes fur and smpA, which codes for a hypothetical lipoprotein (Fig. 1A). To identify the positions of these promoters with respect to the two binding sites identified, we decided to map the 5′ end of the RNA in both directions on both strands. We incurred difficulties in finding primers, within the two divergent genes, which in primer extension experiments gave specific reactions and clear results. Therefore, we generated chromosomally located genes transcriptionally fused to the lacZ gene by cloning the promoter region of each gene upstream of a promoterless lacZ gene of E. coli and introducing this into the chromosome between the NMB1074 and NMB1075 ORFs as described in Materials and Methods and schematically represented in Fig. 5A. The resulting strains, namely, MC-furlacZ and MC-smpAlacZ, carry the chromosomally located transcriptional lacZ fusion of the fur and smpA genes, respectively. RNA was prepared from logarithmic-phase cultures of each of these strains, and primer extension was performed on the RNA preparations with a lacZ-specific primer (Fig. 5B). Two elongation products from the primer extension reaction of RNA prepared from the MC-furlacZ strain mapping were observed at 138 and 25 bp upstream from the ATG start codon of the fur gene, defining the position of the Pfur1 and Pfur2 promoters, respectively. One elongation product was observed from reactions of the RNA that was prepared from the MC-smpAlacZ strain and that mapped at 27 bp upstream from the ATG start of translation of the smpA gene, defining the position of the Psmp promoter. The nucleotide sequences in each case upstream of the elongated primers showed the presence of elements similar to the −10 and the −35 hexamers of sigma 70-dependent promoters from E. coli, and these are shown in Fig. 5C. The Psmp promoter maps divergently upstream from Pfur1 so that their respective −35 hexamers overlap by 16 bp. The Fur binding sites identified by footprinting, Fur1 and Fur2, overlap the Pfur1 and Pfur2 promoter elements, respectively. The Psmp promoter maps in a position proximal to the Fur1 binding site (Fig. 5C).
Iron regulation of fur gene transcription.
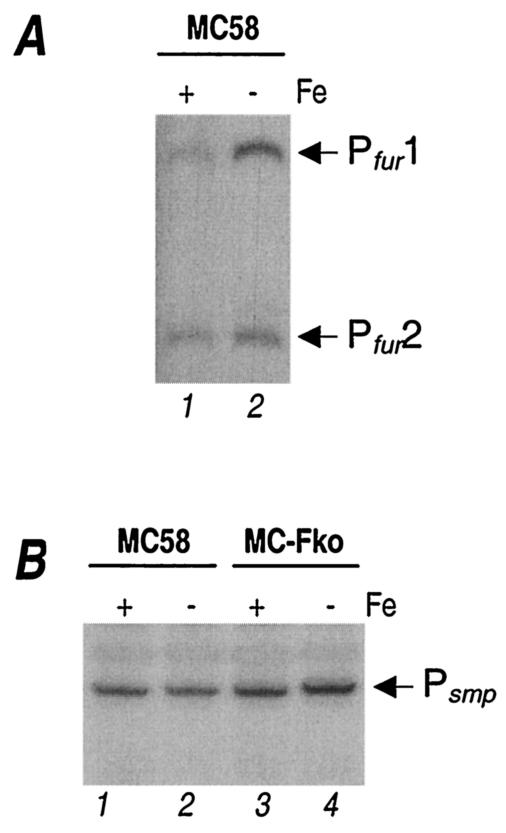
To study the iron-mediated regulation of transcription at the Pfur1, Pfur2, and Psmp promoters, we monitored the accumulation of specific transcripts in total RNA extracted from cells exposed to iron-replete or iron-limiting conditions by carrying out S1 nuclease protection experiments. The MC58 and MC-Fko strains were grown under iron-replete conditions (supplemented GC medium) to logarithmic phase and treated for 15 min with 100 μM 2,2′-dipyridyl (iron limitation), and total RNA was extracted from the cells before and after treatment. Radioactive probes were prepared as described in Materials and Methods for S1 nuclease protection assays of the fur and smpA transcripts. The labeled probes were hybridized to equal amounts of total RNA and digested with S1 nuclease, and the RNA-protected digestion products were separated on a denaturing gel, the results of which are shown in Fig. 6.
FIG. 6.
Analysis of gene transcription by S1 nuclease mappings. (A) Regulation of fur transcripts. Total RNA extracted from wild-type MC58 cells exposed to iron-replete (+) or iron-limiting (−) conditions was hybridized to a 533-bp NsiI-EcoRI probe labeled at the EcoRI site and digested with S1 nuclease. Bands corresponding to S1 nuclease-resistant products were fractionated on denaturing gel and are indicated by arrows and labeled Pfur1 and Pfur2. (B) Regulation of smpA transcripts. A 325-bp NsiI-EcoRI smpA probe labeled at the EcoRI site was used to hybridize total RNA extracted from wild-type MC58 and MC-Fko strains exposed to iron-replete (+) or iron-limiting (−) conditions and processed as described for panel A. An arrow marks the S1 nuclease-resistant product, Psmp.
A 533-bp NsiI-EcoRI fur-specific probe was used to study RNA transcription in RNA extracted from the wild-type strain under different iron conditions; however, we did not perform S1 nuclease experiments with the fur probe and the mutant, as the fur gene and, hence, the specific transcript are not synthesized. The results (Fig. 6A) show an increase (almost threefold) in the fur transcript initiating at Pfur1 but no significant increase in the transcript initiating at Pfur2 after treatment of the MC58 strain with the iron chelator. It therefore appears that the two fur promoters are differentially regulated: Pfur1 responds to iron in that it is repressed with high concentrations of iron and induced under iron limitation; however, there was no evidence of the iron regulation of Pfur2. An S1 protection experiment carried out with RNA extracted from wild-type and mutant strains grown under high- and low-iron conditions and a 325-bp NsiI-EcoRI smpA probe is shown in Fig. 6B. Results show that, independent of the growth conditions and of the fur background, the amount of transcripts at the Psmp promoter is not changed, suggesting that under the experimental conditions used, neither iron nor Fur affects transcription from this promoter.
DISCUSSION
In the present study, we initiate the characterization of the regulatory role of the Fur protein of N. meningitidis. The most surprising result is the ability to construct a fur null mutant. It has generally been accepted, due to unsuccessful attempts to make fur null mutants of Neisseria spp. (33, 34), that the fur gene was essential in this species. The reason for this remains unclear. The strategy that we attempted was to replace the fur gene with a similarly oriented kanamycin cassette, possibly to reduce the polar effects on the downstream aat gene (NMB0206), which codes for a putative leucyl, phenylalanyl-tRNA-protein transferase (27, 32). E. coli strains containing mutant versions of this gene are viable (11), and we did not investigate whether this gene was essential in meningococcus. Many unsuccessful attempts have been made to replace the fur gene of neisserial strains with an allele containing a point mutation that strongly suggests that severe selection against the fur mutation takes place (26). It may be that the MC58 strain is sufficiently different to allow the survival of the mutant under the in vitro culture conditions used. The fur mutant was, however, affected in its growth rate, having a doubling time significantly longer than that of the wild type. We found that this effect was not solely a result of cellular iron overload due to derepression of iron uptake systems as had been reported for the slow-growth phenotype that was also observed for the gonococcal point mutant (34). We complemented the MC-Fko mutant by inserting the entire fur gene along with its two promoters into a heterologous chromosomal locus, but this strain does not express Fur to wild-type levels. The incomplete complementation does not seem to be dependent on chromosomal location as insertion of the fur locus at another location in the mutant downstream of PorA (NMB1429) resulted in similarly reduced expression of Fur (data not shown), and the reason for this phenomenon was not further investigated. We speculate that it may be due to changes in the stability of the non-wild-type fur mRNA.
Although we did not get full complementation of Fur in the MC-Fko-C strain, the slow-growth phenotype is strongly suppressed in the complemented mutant (Fig. 1C). This result, furthermore, supports the hypothesis that secondary mutations have not been selected. Nevertheless, the generation of a fur null mutant allowed us to initiate transcriptional studies of strongly suspected iron-regulated and fur-mediated genes, the tbp2 and fur genes. Moreover, we present a valuable tool for regulatory studies and the definition of the Fur regulon in meningococcus.
The Tbp2 protein is known to be iron regulated, and here we demonstrate that this regulation is Fur mediated, as its expression is derepressed in the fur mutant. We also demonstrate that the fur gene itself is classically iron regulated, and we provide strong evidence that this is through a mechanism of autoregulation. Through primer extension and S1 nuclease experiments, we discovered that transcription of the fur gene is controlled by two independent promoters, the Pfur1 distal and the Pfur2 proximal promoters, which are differentially regulated in response to iron, and sequence analysis implies that recognition occurs by the vegetative sigma of RNA polymerase. Fur binds with high affinity to a site directly overlapping the Pfur1 promoter, and this binding may be responsible for the observed regulation by iron at this promoter. Although this binding site is proximal to the Psmp promoter, we were unable to detect iron regulation of this promoter. The core Fur box of the Fur1 binding site lies well within the total protected region observed in in vitro footprinting and is upstream of the Psmp promoter elements (Fig. 5C). Therefore, binding of Fur to this site in vivo may not be sufficient to occlude RNA polymerase entry at the Psmp promoter, which has been demonstrated as the mechanism of repression of this protein (9).
A low-affinity binding site was identified over the Pfur2 promoter, and although no iron regulation was observed under the conditions of the experiments, Fur binds to Fur2 in the footprinting experiment at a concentration that is possibly physiologically significant. Fur is known to be a highly expressed and cellularly abundant regulator protein, and its concentration within the bacterial cell has been estimated as approximately 4 μM (37, 39). This fact suggests that this promoter may also respond to Fur under different environmental conditions, perhaps when concentrations of Fur itself reach a high threshold level. The presence of two differentially regulated fur promoters possibly allows meningococcus to fine-tune expression of this important regulator in response to cellular iron as well as to other signals, such as oxidative stress and acid stress, as has been reported for other bacteria (3, 6, 39).
Acknowledgments
We thank S. Savino for the donation of antiserum raised against the Tbp2 and NMB1870 proteins. We thank C. Mallia for manuscript editing and G. Corsi for artwork.
This work has been supported by Chiron and partially by a grant from MIUR and a grant from the University of Bologna to V.S.
REFERENCES
- 1.Ala'Aldeen, D. A., H. A. Davies, and S. P. Borriello. 1994. Vaccine potential of meningococcal FrpB: studies on surface exposure and functional attributes of common epitopes. Vaccine 12:535-541. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee-Bhatnagar, N., and C. E. Frasch. 1990. Expression of Neisseria meningitidis iron-regulated outer membrane proteins, including a 70-kilodalton transferrin receptor, and their potential for use as vaccines. Infect. Immun. 58:2875-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bijlsma, J. J. E., B. Waidner, A. H. M. van Vliet, N. J. Hughes, S. Häg, S. Bereswill, D. J. Kelly, C. M. J. E. Vandenbroucke-Grauls, M. Kist, and J. G. Kusters. 2002. The Helicobacter pylori homologue of the ferric uptake regulator is involved in acid resistance. Infect. Immun. 70:606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calver, G. A., C. P. Kenny, and G. Lavergne. 1976. Iron as a replacement for mucin in the establishment of meningococcal infection in mice. Can. J. Microbiol. 22:832-838. [DOI] [PubMed] [Google Scholar]
- 5.Carson, S. D. B. G., P. E. Klebba, S. M. C. Newton, and P. F. Sparling. 1999. Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J. Bacteriol. 181:2895-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delany, I., G. Spohn, A.-B. F. Pacheco, R. Ieva, C. Alaimo, R. Rappuoli, and V. Scarlato. 2002. Autoregulation of Helicobacter pylori Fur revealed by functional analysis of the iron-binding site. Mol. Microbiol. 46:1107-1122. [DOI] [PubMed] [Google Scholar]
- 7.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2001. The Fur repressor controls transcription of iron-activated and iron-repressed genes in Helicobacter pylori. Mol. Microbiol. 42:1297-1309. [DOI] [PubMed] [Google Scholar]
- 8.Escolar, L., J. Pérez-Martín, and V. de Lorenzo. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Escolar, L., V. de Lorenzo, and J. Perez-Martin. 1997. Metaloregulation in vitro of the aerobactin promoter of Escherichia coli by Fur (ferric uptake regulation) protein. Mol. Microbiol. 26:799-808. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 11.Ichetovkin, I. E., G. Abramochkin, and T. E. Shrader. 1997. Substrate recognition by the leucyl/phenylalanyl-tRNA-protein transferase: conservation within the enzyme family and localization to the trypsin-resistant domain. J. Biol. Chem. 272:33009-33014. [DOI] [PubMed] [Google Scholar]
- 12.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klee, S. R., X. Nassif, B. Kusecek, P. Merker, J.-L. Beretti, M. Achtman, and C. R. Tinsley. 2000. Molecular and biological analysis of eight genetic islands that distinguish Neisseria meningitidis from the closely related pathogen Neisseria gonorrhoeae. Infect. Immun. 68:2082-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Labigne-Roussel, A., P. Courcoux, and L. Tompkins. 1988. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J. Bacteriol. 170:1704-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larson, J. A., D. L. Higashi, I. Stojiljkovic, and M. So. 2002. Replication of Neisseria meningitidis within epithelial cells requires TonB-dependent acquisition of host cell iron. Infect. Immun. 70:1461-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis, L. A., E. Gray, Y. P. Wang, B. A. Roe, and D. W. Dyer. 1997. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol. Microbiol. 23:737-749. [DOI] [PubMed] [Google Scholar]
- 17.Lissolo, L., G. Maitre-Wilmotte, P. Dumas, M. Mignon, B. Danve, and M. J. Quentin-Millet. 1995. Evaluation of transferrin-binding protein 2 within the transferrin-binding protein complex as a potential antigen for future meningococcal vaccines. Infect. Immun. 63:884-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackinnon, F. G., A. R. Gorringe, S. G. Funnell, and A. Robinson. 1992. Intranasal infection of infant mice with Neisseria meningitidis. Microb. Pathog. 12:415-420. [DOI] [PubMed] [Google Scholar]
- 19.Maxam, A. M., and W. Gilbert. 1977. A new method for sequencing DNA. Proc. Natl. Acad. Sci. USA 74:560-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ochsner, U. A., and M. L. Vasil. 1996. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc. Natl. Acad. Sci. USA 93:4409-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettersson, A., A. Maas, D. van Wassenaar, P. van der Ley, and J. Tommassen. 1995. Molecular characterization of FrpB, the 70-kilodalton iron-regulated outer membrane protein of Neisseria meningitidis. Infect. Immun. 63:4181-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettersson, A., T. Prinz, A. Umar, J. van der Biezen, and J. Tommassen. 1998. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol. Microbiol. 27:599-610. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Schryvers, A. B., and I. Stojiljkovic. 1999. Iron acquisition systems in the pathogenic Neisseria. Mol. Microbiol. 32:1117-1123. [DOI] [PubMed] [Google Scholar]
- 25.Sebastian, S., S. Agarwal, J. R. Murphy, and C. A. Genco. 2002. The gonococcal Fur regulon: identification of additional genes involved in major catabolic, recombination, and secretory pathways. J. Bacteriol. 184:3965-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serkin, C. D., and H. S. Seifert. 2000. Iron availability regulates DNA recombination in Neisseria gonorrhoeae. Mol. Microbiol. 37:1075-1086. [DOI] [PubMed] [Google Scholar]
- 27.Shrader, T. E., J. W. Tobias, and A. Varshavsky. 1993. The N-end rule in Escherichia coli: cloning and analysis of the leucyl, phenylalanyl-tRNA-protein transferase gene aat. J. Bacteriol. 175:4364-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spohn, G., D. Beier, R. Rappuoli, and V. Scarlato. 1997. Transcriptional analysis of the divergent cagAB genes encoded by the pathogenicity island of Helicobacter pylori. Mol. Microbiol. 26:361-372. [DOI] [PubMed] [Google Scholar]
- 29.Stojiljkovic, I., V. Hwa, L. de Saint Martin, P. O'Gaora, X. Nassif, F. Heffron, and M. So. 1995. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol. Microbiol. 15:531-541. [DOI] [PubMed] [Google Scholar]
- 30.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 31.Sun, Y. H., S. Bakshi, R. Chalmers, and C. M. Tang. 2000. Functional genomics of Neisseria meningitidis pathogenesis. Nat. Med. 6:1269-1273. [DOI] [PubMed] [Google Scholar]
- 32.Tettelin, H., N. J. Saunders, J. Heidelberg, A. C. Jeffries, K. E. Nelson, J. A. Eisen, K. A. Ketchum, D. W. Hood, J. F. Peden, R. J. Dodson, W. C. Nelson, M. L. Gwinn, R. DeBoy, J. D. Peterson, E. K. Hickey, D. H. Haft, S. L. Salzberg, O. White, R. D. Fleischmann, B. A. Dougherty, T. Mason, A. Ciecko, D. S. Parksey, E. Blair, H. Cittone, E. B. Clark, M. D. Cotton, T. R. Utterback, H. Khouri, H. Qin, J. Vamathevan, J. Gill, V. Scarlato, V. Masignani, M. Pizza, G. Grandi, L. Sun, H. O. Smith, C. M. Fraser, E. R. Moxon, R. Rappuoli, and J. C. Venter. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809-1815. [DOI] [PubMed] [Google Scholar]
- 33.Thomas, C. E., and P. F. Sparling. 1994. Identification and cloning of a fur homologue from Neisseria meningitidis. Mol. Microbiol. 11:725-737. [DOI] [PubMed] [Google Scholar]
- 34.Thomas, C. E., and P. F. Sparling. 1996. Isolation and analysis of a fur mutant of Neisseria gonorrhoeae. J. Bacteriol. 178:4224-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson, S. A., L. L. Wang, A. West, and P. F. Sparling. 1993. Neisseria meningitidis produces iron-regulated proteins related to the RTX family of exoproteins. J. Bacteriol. 175:811-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trieu-Cuot, P., C. Poyart-Salmeron, C. Carlier, and P. Courvalin. 1990. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon TN1545. Nucleic Acids Res. 18:3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watnick, P. I., T. Eto, H. Takahashi, and S. B. Calderwood. 1997. Purification of Vibrio cholerae Fur and estimation of its intracellular abundance by antibody sandwich enzyme-linked immunosorbent assay. J. Bacteriol. 179:243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheleznova, E. E., J. H. Crosa, and R. G. Brennan. 2000. Characterization of the DNA- and metal-binding properties of Vibrio anguillarum Fur reveals conservation of a structural Zn2+ ion. J. Bacteriol. 182:6264-6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng, M., B. Doan, T. D. Schneider, and G. Storz. 1999. OxyR and SoxRS regulation of fur. J. Bacteriol. 181:4639-4643. [DOI] [PMC free article] [PubMed] [Google Scholar]