Abstract
Activators of σ54-RNA polymerase holoenzyme couple ATP hydrolysis to formation of an open promoter complex. DctDΔ1-142, a truncated and constitutively active form of the σ54-dependent activator DctD from Sinorhizobium meliloti, displayed an altered DNase I footprint at its binding site located upstream of the dctA promoter in the presence of ATP. The altered footprint was not observed for a mutant protein with a substitution at or near the putative arginine finger, a conserved arginine residue thought to contact the nucleotide. These data suggest that structural changes in DctDΔ1-142 during ATP hydrolysis can be detected by alterations in the DNase I footprint of the protein and may be communicated by interactions between bound nucleotide and the arginine finger. In addition, kinetic data for changes in fluorescence energy transfer upon binding of 2′(3′)-O-(N-methylanthraniloyl)-ATP (Mant-ATP) to DctDΔ1-142 and DctD suggested that these proteins undergo multiple conformational changes following ATP binding.
RNA polymerase holoenzyme containing the alternate sigma factor σ54 (σ54-holoenzyme) is an alternative form of RNA polymerase that is required for the transcription of genes involved in such diverse functions as nitrogen assimilation and fixation, C4-dicarboxylate transport, flagellar biosynthesis, hydrogen metabolism, and degradation of aromatic compounds (6, 8). σ54-holoenzyme forms a stable closed complex with the promoter that does not undergo isomerization to an open complex competent to initiate transcription in the absence of an activator and ATP (9, 14, 19). Activators of σ54-holoenzyme generally bind to sites located upstream of the promoter and contact the closed complex through DNA looping to activate transcription (16, 23) in a reaction that requires ATP hydrolysis by the activator (14, 27).
Activators of σ54-holoenzyme consist of two or more functional domains, one of which is conserved among all known σ54-dependent activators and is responsible for ATP hydrolysis and transcriptional activation (12). The activation domain belongs to the AAA+ (for “ATPases associated with various cellular activities”) superfamily, members of which are found in all kingdoms of life and participate in diverse cellular functions, including membrane fusion, proteolysis, DNA replication, and transcription (10, 11). In general, the variety of functions carried out by AAA+ proteins is manifested through chaperone-like activities of these proteins. Cryoelectron microscopy of p97, a multifunctional ATPase that participates in membrane fusion and extraction of proteins from the endoplasmic reticulum in animal cells, revealed that binding of ATP analogs to this AAA+ protein induced various conformational changes (18). Such conformational changes are thought to occur during the ATPase cycle and allow AAA+ proteins to impose forces on bound substrates and perform their various functions (11).
Activators of σ54-holoenzyme appear to similarly undergo conformational changes that are associated with protein function during the ATP hydrolysis cycle. For example, Escherichia coli phage shock protein F (PspF) forms a stable complex with σ54 in the presence of the transition state analog ADP-aluminum fluoride (ADP-AlFx, where x is 3 or 4) (4). We report here on conformational changes associated with the ATP hydrolysis cycle in the σ54-dependent activator Sinorhizobium meliloti C4-dicarboxylic acid transport protein D (DctD), at least one of which appears to be communicated to the DNA-binding domain of the protein. DctDΔ1-142, a truncated form of DctD that lacks the amino-terminal domain and is constitutively active for both ATP hydrolysis and transcriptional activation (7), was included in these studies.
ATP hydrolysis induces a structural change in DctDΔ1-142 as assessed by DNase I footprinting.
Structural changes in DNA-binding proteins can be reflected by changes in the DNA footprinting patterns of these proteins. For example, binding of either ATP or adenosine 5′-[γ-thio]triphosphate (ATPγS) to Pseudomonas putida XylR stimulates cooperative binding of this σ54-dependent activator to the upstream activation sequence (UAS) of the Pu promoter (13). To determine if ATP or intermediate states in the ATP hydrolysis cycle altered the footprinting pattern of DctDΔ1-142 at the S. meliloti dctA UAS, DNase I footprinting assays were done in the presence of ATP or ATP analogs. These ATP analogs, which included ADP, ATPγS, 5′-adenylylimidodiphosphate (AMP-PNP), ADP-beryllium fluoride (ADP-BeFx where x is 1 to 4), and ADP-AlFx, mimic various nucleotide states during the ATP hydrolysis cycle (3, 5, 20).
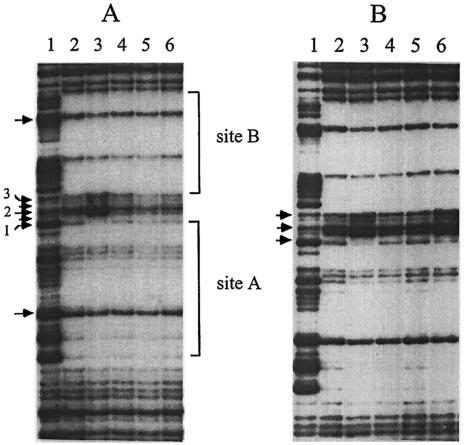
DctDΔ1-142 binds cooperatively to two tandem binding sites (sites A and B) that comprise the S. meliloti dctA UAS (21). DNase I footprinting experiments with DctDΔ1-142 were done as described previously (24) using a 195-bp BamHI-EcoRI DNA fragment that carried the UAS from S. meliloti dctA. Footprinting reactions contained 800 nM DctD monomer and 0.3 pmol (2 × 105 dpm) of DNA probe in a 20-μl reaction mixture. When ATP was included in the DNase I footprinting assay, an altered pattern of protection was observed in the region between the two DctD-binding sites of the dctA UAS (Fig. 1A, lane 3). Quantitative data were obtained by comparing the relative intensities of three of the five bands between sites A and B of the dctA UAS that were affected by the addition of ATP, as well as two bands within sites A and B that did not appear to be affected by ATP (Fig. 1). These data were normalized to bands that were outside the region of DNA protected by DctDΔ1-142. In the presence of ATP, one of the phosphodiester bonds between sites A and B (band 1) was protected ∼3-fold relative to the footprinting assay without ATP, while the other two phosphodiester bonds in this region that we examined (bands 2 and 3) displayed ∼3-fold-enhanced cleavages when ATP was included in the assay (Table 1). These changes in the footprint were specific for the region between sites A and B, as the relative intensities of the bands within the DctD-binding sites varied by 25% or less upon the addition of ATP. The altered protection pattern was not observed when ATPγS, AMP-PNP, or ADP was included in the DNase I footprinting assay (Fig. 1; Table 1). These results suggest that the changes in the footprint within the dctA UAS resulted from a conformational change in DctDΔ1-142 during the ATP hydrolysis cycle.
FIG. 1.
DNase I footprinting of DctDΔ1-142 at the dctA UAS in the presence of ATP or ATP analogs. Reaction mixtures contained 800 nM DctDΔ1-142 monomer unless indicated otherwise. (A) Lane 1, no-protein control; lane 2, no addition of nucleotide; lane 3, addition of 3 mM ATP; lane 4, addition of 3 mM ATPγS; lane 5, addition of 3 mM AMP-PNP; lane 6, addition of 3 mM ADP. The locations of DctD-binding sites A and B are indicated. The arrows within sites A and B indicate the two bands whose relative intensities were compared. The arrows between the two sites indicate the bands whose relative intensities were altered in the presence of ATP. Quantitative data were obtained with a PhosphorImager using PhosphorImager SI Scanner Control and ImageQuant 1.0 software and are reported in Table 1 for bands 1, 2, and 3. (B) Lane 1, no-protein control; lane 2, no addition of nucleotide; lane 3, addition of 3 mM ATP; lane 4, addition of 2 mM ADP; lane 5, addition of 4 mM NaF and 0.1 mM AlCl3; lane 6, addition of 2 mM ADP, 4 mM NaF, and 0.1 mM AlCl3.
TABLE 1.
Quantitation of DNase I footprinting assays with DctDΔ1-142
| Nucleotide | Relative intensitya
|
||||
|---|---|---|---|---|---|
| Band 1 | Band 2 | Band 3 | Band A | Band B | |
| None | 1 | 1 | 1 | 1 | 1 |
| ATP | 0.31 | 3.03 | 3.45 | 0.87 | 0.75 |
| ATPγS | 0.85 | 0.79 | 0.96 | 0.73 | 0.84 |
| ADP | 0.81 | 0.79 | 1.05 | 0.78 | 0.83 |
| ADP-AlFx | 0.25 | 2.63 | 2.42 | 0.78 | 0.74 |
Bands correspond to those indicated in Fig. 1. For each band, the intensity is relative to that of the corresponding band in the no-nucleotide control. Values that are greater than 1 indicate enhanced cleavage relative to the control, while values that are less than 1 indicate increased protection relative to the control.
ADP-AlFx alters the DNase I footprint of DctDΔ1-142 similarly to ATP.
We determined if the phosphate analogs BeFx and AlFx, either alone or in combination with ADP, influenced the footprint of DctDΔ1-142 observed at the dctA UAS. Aluminum fluoride and beryllium fluoride solutions were prepared freshly by mixing sodium fluoride in 20 mM EPPS (N-[2-hydroxyethyl]piperazine-N′-[3-propanesulfonic acid]), pH 8.0, with aluminum chloride or beryllium sulfate solutions. Neither BeFx by itself nor BeFx with ADP altered the protection pattern (data not shown). Although AlFx did not alter the protection pattern, the combination of ADP and AlFx resulted in an altered footprint pattern that was the same as that observed with ATP (Fig. 1B; Table 1). These data suggest that binding of an ADP-AlFx complex to DctDΔ1-142 stabilizes a conformation that is similar to that observed with ATP in the DNase I footprinting assay.
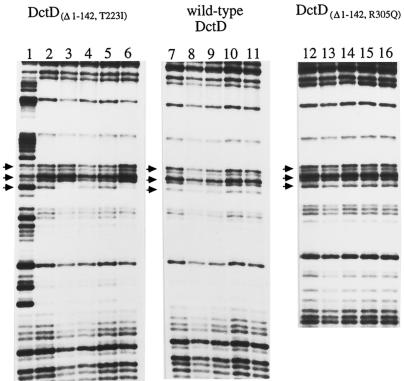
Mutant forms of DctDΔ1-142 that were unable to activate transcription were examined to determine if any failed to undergo ATP-dependent changes in their footprinting pattern at the dctA UAS. Several mutant forms of DctDΔ1-142 that failed to activate transcription but still hydrolyzed ATP had been isolated previously (25). Three of these mutant proteins, DctDΔ1-142,S212I, DctDΔ1-142,T223I, and DctDΔ1-142,A225T, were examined in the DNase I footprinting assay. All three of these mutant proteins showed the same changes in the footprint pattern in the presence of ATP or ADP-AlFx that were seen with DctDΔ1-142 (Fig. 2; data shown only for DctDΔ1-142,T223I). Quantitation of these assays showed that changes in the protection pattern for these mutant proteins in the presence of ATP were similar to those observed with DctDΔ1-142 (data not shown). These data indicate that the inability of these mutant proteins to activate transcription was not due to failure to undergo the conformational change that registered with the DNase I footprinting assay.
FIG. 2.
DNase I footprinting of wild-type DctD and mutant forms of DctDΔ1-142 in the presence of ATP or ATP analogs. DctDΔ1-142,T223I (lanes 2 to 6), wild-type DctD (lanes 7 to 11), and DctDΔ1-142,R305Q (lanes 12 to 16) were examined in a DNase I footprinting assay. Reaction mixtures contained 800 nM concentrations of monomers of the indicated DctD proteins. Lane 1, no-protein control; lanes 2, 7, and 12, no addition of nucleotide; lanes 3, 8, and 13, addition of 3 mM ATP; lanes 4, 9, and 14, addition of 3 mM ATPγS; lanes 5, 10, and 15, addition of 2 mM ADP; and lanes 6, 11, and 16, addition of 2 mM ADP, 4 mM NaF, and 0.1 mM AlCl3. The bands marked with arrows correspond to bands 1, 2, and 3 in Fig. 1.
We also examined the DNase I footprint patterns for wild-type DctD protein, which cannot hydrolyze ATP in its unphosphorylated form, and DctDΔ1-142,R305Q, a mutant form of DctDΔ1-142 that is deficient in ATPase activity (24). In the absence of nucleotides, both of these proteins protected the dctA UAS from DNase I digestion in a manner that was similar to that observed with DctDΔ1-142 (Fig. 2). The DNase I footprints with these proteins at the dctA UAS, however, were unaffected by ATP or ADP-AlFx.
Binding of ATP analogs induces structural changes in DctD and DctDΔ1-142 as assessed by fluorescence energy transfer.
The ATP analogs 2′(3′)-O-(N-methylanthraniloyl)-ADP (Mant-ADP) and Mant-ATP have been used to study nucleotide binding by proteins in fluorescence energy transfer experiments (28). Structural changes in a protein that follow the initial binding event can appear in the time course of a binding assay, since the altered structure can influence the efficiency of the energy transfer.
Mant-ATP and Mant-ADP were synthesized as described elsewhere (28). Fluorescence energy transfer was observed between the DctD proteins and Mant-ATP, as indicated by the loss of emission from DctD and the gain of emission from Mant-ATP (Fig. 3). Both DctD and DctDΔ1-142 have two tryptophan residues that could participate in fluorescence energy transfer. Equilibrium binding assays corrected for inner filtering at high nucleotide concentrations gave a binding constant of 170 μM nucleotide (data not shown).
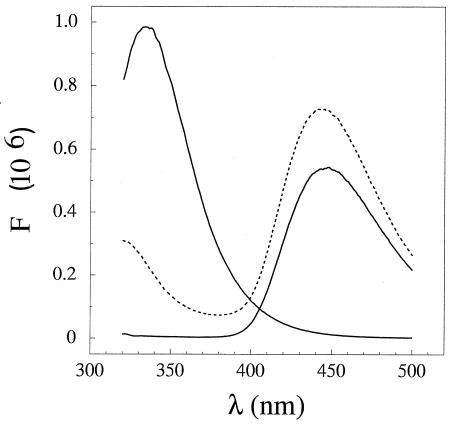
FIG. 3.
Energy transfer from DctD to Mant-ATP. Fluorescence emission was scanned from 320 to 500 nm for DctD (peak emission at 340 nm), Mant-ATP (peak emission at 444 nm), and a mixture of the two (dashed line). Energy transfer from DctD proteins to Mant nucleotide was used to indirectly assay ATP binding. For steady-state data, 290-nm light and 2-nm band pass were used to excite protein (3 μM monomer) or Mant-ATP (100 μM) solutions that were either separate or had been mixed and allowed to set for several minutes at 20°C. Data provided by the manufacturer (PTI, Inc.) were used to correct for variation in the sensitivity of the photomultiplier tube to light of different wavelengths.
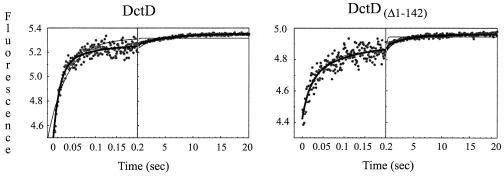
Kinetic data for binding of Mant nucleotides to DctD proteins was obtained using a stop-flow instrument (KinTek Corp.) to rapidly mix protein (4 μM monomer equivalents) and Mant nucleotides (200 μM) in a 3/2 ratio and deliver them to an observation cell that was bathed in 290-nm light (2-nm band pass). A photomultiplier tube containing a 443 to 456-nm band-pass filter (Ealing Electro-Optics, Inc.) was used to limit the detection of fluorescence to that emitted by Mant nucleotides. Changes in fluorescence amplitude that occurred upon mixing of protein and Mant nucleotide were indicative of energy transfer from the former to the latter, giving an indirect readout of nucleotide binding and subsequent environment changes. Transient kinetic data showed a complex change in fluorescence upon mixing of DctDΔ1-142 with Mant-ATP (Fig. 4). Photobleaching did not contribute to the change in fluorescence under the assay conditions (data not shown). The data fit poorly to a single exponential function, better to a sum of two exponentials, and best to a sum of three exponentials (Table 2), suggesting that at least three processes were being observed. Similar results were seen with DctD, except that the amplitude of the first phase for DctD was about twice that seen for DctDΔ1-142. Since unphosphorylated DctD does not hydrolyze ATP, these observations suggest that the apparent structural changes observed for both proteins result from ATP binding rather than hydrolysis. More extensive experiments are needed to determine if the first observed phase is the initial binding event or a rapid conformational change and to associate the additional phases with specific structural species.
FIG. 4.
Time course of changes in fluorescence upon mixing Mant-ATP with DctD and DctDΔ1-142. Fluorescence was monitored in two time domains (200 data points from 0 to 100 or 200 ms and 300 data points from then until 20 s). The observed rates of reactions were determined by nonlinear least-squares regression to exponential functions using the KinTek Stop Flow or KinTekSim software program (KinTek Corp.). Heavy lines indicate the best-fitting model. Thin lines indicate models that were less complex but displayed larger error or nonrandom residuals (Table 2).
TABLE 2.
Exponential models for Mant-ATP bindinga
| Protein | Variance of fit (residual)
|
k1 | amp1 | k2 | amp2 | k3 | amp3 | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 Exponential | 2 Exponentials | 3 Exponentials | |||||||
| DctD | 1.09 (systematic) | 0.38 (systematic) | 0.34 (random) | 67.7 (5.5) | 0.58 (0.03) | 12.7 (2.8) | 0.18 (0.03) | 0.19 (0.04) | 0.09 (0.006) |
| DctDΔ1-142 | 0.64 (systematic) | 0.42 (systematic) | 0.39 (random) | 58.0 (11.8) | 0.24 (0.03) | 10.7 (2.0) | 0.23 (0.03) | 0.24 (0.06) | 0.07 (0.008) |
Rate (k) and amplitude (amp) are given (with standard errors in parentheses) only for the simplest models that had random residuals.
Stopped-flow fluorescence energy transfer experiments were also done with Mant-ADP in the presence and absence of AlFx. Interpretation of the results of these experiments, however, was complicated by the large scatter at the early time points (data not shown). The data, however, demonstrated binding of the Mant-ADP derivatives to both DctD and DctDΔ1-142.
Conclusions.
Activators of σ54-holoenzyme act as molecular machines to transduce energy released from ATP hydrolysis to open complex formation (26). Based on recent studies of conformational changes that occur in AAA+ ATPases when different nucleotide forms are bound (1, 15, 18, 22), the catalytic cycle for σ54-dependent activators and thus interactions with RNA polymerase is likely a complicated process. The kinetic data we report for the binding of Mant-ATP to DctD and DctDΔ1-142 are consistent with multiple changes in structure following binding of nucleotides to these proteins. None of these initial conformational changes, however, appear to be directly related to open complex formation, since DctD does not activate transcription unless it is phosphorylated, which stimulates the ATPase activity of the protein. Moreover, neither ATPγS (7, 14) nor ADP-AlFx (4) replaces ATP in open complex formation. Thus, the activator conformation involved in remodeling σ54 and its holoenzyme probably occurs at some point following the transition state of ATP hydrolysis.
ADP-AlFx stabilizes a conformation of E. coli PspFΔHTH, a truncated form of the σ54-dependent activator PspF that lacks most of the carboxy-terminal DNA-binding domain, with increased affinity for σ54 (4). Formation of a ternary complex between σ54, PspFΔHTH, and ADP-AlFx appears to involve direct interactions between the GAFTGA motif of PspFΔHTH and σ54 (2, 4). The GAFTGA motif is part of a loop structure that functions in coupling ATP hydrolysis to open complex formation (25, 27, 29) and is predicted to relocate upon ATP hydrolysis (2, 30). Two of the mutants examined in this study, DctDΔ1-142,T223I and DctDΔ1-142,A225T, have substitutions within the GAFTGA motif yet displayed the altered footprint in the presence of ATP and ADP-AlFx, indicating that the GAFTGA motif is not involved in the structural change associated with the altered footprint of DctDΔ1-142.
The nucleotide-dependent changes in the pattern of protection from DNase I were not observed with DctDΔ1-142,R305Q, which is deficient in its ability to hydrolyze ATP. Arg-305 corresponds to a highly conserved residue within the AAA+ minimum consensus that has been proposed to be necessary for catalytic activity (10). Mutations in Arg-294 of the Salmonella enterica serovar Typhimurium NtrC protein, which corresponds to Arg-299 of DctD, interfere with ATP hydrolysis but not ATP binding activity (17). The X-ray crystal structure of the ATPase domain of Aquifex aeolicus NtrC1 shows that either of these arginine residues may correspond to the arginine finger that contacts the nucleotide bound to the adjacent protomer in the oligomeric, activated ATPase (S.-K. Lee, A. De La Torre, D. Yan, S. Kustu, T. Nixon, and D. E. Wemmer, submitted for publication). These data suggest that the nucleotide-dependent changes in the footprint of DctDΔ1-142 at the dctA UAS depend upon changes occurring in the AAA+ minimum consensus, perhaps through sensing of the bound nucleotide by the arginine finger. This sensing process evidently occurs even in the absence of readout through the GAFTGA region.
Acknowledgments
This work was funded by award MCB-9974558 to T.R.H. from the National Science Foundation and award 9703546 to B.T.N. from the U.S. Department of Agriculture.
We thank Ken Johnson and Greg Ferry for access to stop-flow fluorescence equipment.
REFERENCES
- 1.Bochtler, M., C. Hartmann, H. K. Song, G. P. Bourenkov, H. D. Bartunik, and R. Huber. 2000. The structures of HslU and the ATP-dependent protease HslU-HslV. Nature 403:800-805. [DOI] [PubMed] [Google Scholar]
- 2.Bordes, P., S. R. Wigneshweraraj, J. Schumacher, X. Zhang, M. Chaney, and M. Buck. 2003. The ATP hydrolyzing transcription activator phage shock protein F of Escherichia coli: identifying a surface that binds σ54. Proc. Natl. Acad. Sci. USA 100:2278-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chabre, M. 1990. Aluminofluoride and beryllofluoride complexes: new phosphate analogs in enzymology. Trends Biochem. Sci. 15:6-10. [DOI] [PubMed] [Google Scholar]
- 4.Chaney, M., R. Grande, S. R. Wigneshweraraj, W. Cannon, P. Casaz, M.-T. Gallegos, J. Schumacher, S. Jones, S. Elderkin, A. E. Dago, E. Morett, and M. Buck. 2001. Binding of transcriptional activators to σ54 in the presence of the transition state analog ADP-aluminum fluoride: insights into activator mechanochemical action. Genes Dev. 15:2282-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher, A. J. 1995. X-ray structures of the myosin motor domain of Dictyostelium discoideum complexed with MgADP-BeFx and MgADP-AlF4−. Biochemistry 34:8960-8972. [DOI] [PubMed] [Google Scholar]
- 6.Kustu, S., A. K. North, and D. S. Weiss. 1991. Prokaryotic transcriptional enhancers and enhancer-binding proteins. Trends Biochem. Sci. 16:397-402. [DOI] [PubMed] [Google Scholar]
- 7.Lee, J. H., D. Scholl, B. T. Nixon, and T. R. Hoover. 1994. Constitutive ATP hydrolysis and transcriptional activation by a stable, truncated form of Rhizobium meliloti DCTD, a σ54-dependent transcriptional activator. J. Biol. Chem. 269:20401-20409. [PubMed] [Google Scholar]
- 8.Merrick, M. J. 1993. In a class of its own—the RNA polymerase sigma factor σ54 (σN). Mol. Microbiol. 10:903-909. [DOI] [PubMed] [Google Scholar]
- 9.Morett, E., and M. Buck. 1989. In vivo studies on the interaction of RNA polymerase-σ54 with the Klebsiella pneumoniae and Rhizobium meliloti nifH promoters: the role of NIFA in the formation of an open promoter complex. J. Mol. Biol. 210:65-77. [DOI] [PubMed] [Google Scholar]
- 10.Neuwald, A. F., L. Aravind, J. L. Spouge, and E. V. Koonin. 1999. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9:27-43. [PubMed] [Google Scholar]
- 11.Ogura, T., and A. J. Wilkinson. 2001. AAA+ superfamily ATPases: common structure—diverse function. Genes Cells 6:575-597. [DOI] [PubMed] [Google Scholar]
- 12.Osuna, J., X. Soberon, and E. Morett. 1997. A proposed architecture for the central domain of the bacterial enhancer-binding proteins based on secondary structure prediction and fold recognition. Protein Sci. 6:543-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Martin, J., and V. de Lorenzo. 1996. ATP binding to the σ54-dependent activator XylR triggers a protein multimerization cycle catalyzed by UAS DNA. Cell 86:331-339. [DOI] [PubMed] [Google Scholar]
- 14.Popham, D., D. Szeto, J. Keener, and S. Kustu. 1989. Function of a bacterial activator protein that binds to transcriptional enhancers. Science 243:629-635. [DOI] [PubMed] [Google Scholar]
- 15.Putnam, C. D., S. B. Clancy, H. Tsuruta, S. Gonzalez, J. G. Wetmur, and J. A. Tainer. 2001. Structure and mechanism of the RuvB Holliday junction branch migration motor. J. Mol. Biol. 311:297-310. [DOI] [PubMed] [Google Scholar]
- 16.Rippe, K., M. Guthold, P. H. von Hippel, and C. Bustamante. 1997. Transcriptional activation via DNA-looping: visualization of intermediates in the activation pathway of E. coli RNA polymerase σ54 holoenzyme by scanning force microscopy. J. Mol. Biol. 270:125-138. [DOI] [PubMed] [Google Scholar]
- 17.Rombel, I., P. Peters-Wendisch, A. Mesecar, T. Thorgeirsson, Y.-K. Shin, and S. Kustu. 1999. MgATP binding and hydrolysis determinants of NtrC, a bacterial enhancer-binding protein. J. Bacteriol. 181:4628-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rouiller, I., B. DeLaBarre, A. P. May, W. I. Weis, A. T. Brunger, R. A. Mulligan, and E. M. Wilson-Kubalek. 2002. Conformational changes of the multifunction p97 AAA ATPase during its ATPase cycle. Nat. Struct. Biol. 9:950-957. [DOI] [PubMed] [Google Scholar]
- 19.Sasse-Dwight, S., and J. D. Gralla. 1988. Probing the Escherichia coli glnALG upstream activation mechanism in vivo. Proc. Natl. Acad. Sci. USA 85:8934-8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindelin, H., C. Kisker, L. Schlessman, J. B. Howard, and D. C. Rees. 1997. Structure of ADP-AlF4−-stabilized nitrogenase complex and its implications for signal transduction. Nature 387:370-376. [DOI] [PubMed] [Google Scholar]
- 21.Scholl, D., and B. T. Nixon. 1996. Cooperative binding of DctD to the dctA UAS of Rhizobium meliloti is enhanced in a constitutively active truncated mutant. J. Biol. Chem. 271:26435-26442. [DOI] [PubMed] [Google Scholar]
- 22.Sousa, M. C., C. B. Trame, H. Tsuruta, S. M. Wilbanks, V. S. Reddy, and D. B. McKay. 2000. Crystal and solution structures of an HslUV protease-chaperone complex. Cell 103:633-643. [DOI] [PubMed] [Google Scholar]
- 23.Su, W., S. Porter, S. Kustu, and H. Echols. 1990. DNA-looping and enhancer activity: association between DNA-bound NTRC activator and RNA polymerase at the bacterial glnA promoter. Proc. Natl. Acad. Sci. USA 87:5504-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, Y.-K., and T. R. Hoover. 1997. Alterations within the activation domain of the σ54-dependent activator DctD that prevent transcriptional activation. J. Bacteriol. 179:5812-5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang, Y.-K., J. H. Lee, J. M. Brewer, and T. R. Hoover. 1997. A conserved region in the σ54-dependent activator DctD is involved in both binding to RNA polymerase and coupling ATP hydrolysis to activation. Mol. Microbiol. 26:373-386. [DOI] [PubMed] [Google Scholar]
- 26.Wedel, A. B., and S. Kustu. 1995. The bacterial enhancer-binding protein NtrC is a molecular machine: ATP hydrolysis is coupled to transcriptional activation. Genes Dev. 9:2042-2052. [DOI] [PubMed] [Google Scholar]
- 27.Weiss, D. S., J. Batut, K. E. Klose, J. Keener, and S. Kustu. 1991. The phosphorylated form of the enhancer-binding protein NTRC has an ATPase activity that is essential for activation of transcription. Cell 67:155-167. [DOI] [PubMed] [Google Scholar]
- 28.Woodward, S. K. A., J. F. Eccleston, and M. A. Greeves. 1991. Kinetics of the interaction of 2′(3′)-O-(N-methylanthraniloyl)-ATP with myosin subfragment 1 and actomyosin subfragments 1: characterization of two acto-S1-ADP complexes. Biochemistry 30:422-430. [DOI] [PubMed] [Google Scholar]
- 29.Yan, D., and S. Kustu. 1999. “Switch I” mutant forms of the bacterial enhancer-binding protein NtrC that perturb the response to DNA. Proc. Natl. Acad. Sci. USA 96:13142-13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, X., M. Chaney, S. R. Wigneshweraraj, J. Schumacher, P. Bordes, W. Cannon, and M. Buck. 2002. Mechanochemical ATPases and transcriptional activation. Mol. Microbiol. 45:895-903. [DOI] [PubMed] [Google Scholar]