Abstract
Perhaps the most striking fact about early Cenozoic avian history some 70 million years ago was the rapid radiation of large, flightless, ground-living birds. It has been suggested that, for a time, there was active competition between these large terrestrial birds and the early mammals. Probably reflecting the above noted early start of Ratitae of the infraclass Eoaves, the presumptive sex chromosomes of their present day survivors, such as the emu and the ostrich, largely remained homomorphic. The signs of genetic differentiation between their still-homomorphic Z and W chromosomes were tested by using two marker genes (Z-linked ZOV3 and the gene for the iron-responsive element-binding protein) and one marker sequence of a part of a presumptive pseudogene (W-linked EE0.6 of the chicken). Their homologues, maintaining 71–92% identities to the chicken counterparts, were found in both the emu (Dromaius novaehollandiae) and the ostrich (Struthio camelus). Their locations were visualized on chromosome preparations by fluorescence in situ hybridization. In the case of the emu, these three marker sequences were localized on both members of the fifth pair of a female, thus revealing no sign yet of genetic differentiation between the Z and the W. The finding was the same with regard to both members of the fourth pair of male ostriches. In the female ostrich, however, the sequence of the gene for the iron-responsive element-binding protein was missing from one of the pairs, thus revealing the differentiation by a small deletion of the W from the Z.
It has been suggested that the sex chromosomes of higher vertebrates evolved in three stages: (i) They started as a homomorphic pair of largely homologous chromosomes; (ii) the pericentric inversion and other internal rearrangements affecting either the Y of the male heterogamety or the W of the female heterogamety started the genetic isolation between the X and the Y or the Z and the W; and (iii) after this genetic isolation, the Y or the W became progressively smaller as a result of gradual genetic degeneration while the X or the Z remained as they were (1). Indeed, among snakes with conserved karyotypes, the fourth pair of chromosomes remained homomorphic even in females of boas and pythons belonging to the ancient family Boidae while in snakes of the intermediate family Colubridae, the female-specific W chromosome became distinct from the Z, most often by a pericentric inversion. In various poisonous snakes of the advanced families Crotalidae, Elapidae, and Viperidae, the W became shrunken and heterochromatic whereas the Z remained as it had always been (2).
To fly through the air, the sternum of modern birds was markedly enlarged to anchor powerful pectoral muscles. This type of bird is classified as Carinatae of the infraclass Neoaves because such enlargement of the sternum was not at all evident in the earliest birds, such as Archaeopteryx of the Jurassic Age, some 160 million years ago. It appears that the birds originally used their stretched wings either for gliding down from trees and cliffs or as an aid to their terrestrial locomotion. At the beginning of the Cenozoic Era 70 million years ago, there was rapid radiation of large, flightless, ground-living birds with no enlarged sternum belonging to Ratitae of the infraclass Eoaves. The ostrich, emu, cassowaries, and rheas are their surviving descendants (3). Not surprisingly, as far as their sex chromosomes were concerned, the position of these emu, ostrich, rheas, and cassowaries among the birds appeared comparable to that of boas and pythons among the snakes. In carinate birds, the female-specific W chromosome is largely heterochromatic and is distinctly smaller than the Z, as is the case with poisonous snakes. On the other hand, the Z and the W of ratite birds are morphologically ambiguous in a manner similar to the Z and the W of boas and pythons. Although karyotyping and banding studies indicated that the fifth chromosome pair in the female karyotype of the emu (Dromaius novaehollandiae), the Darwin’s rhea (Pterocnemia pennata), and the American rhea (Rhea americana) was somewhat heteromorphic (4, 5), such a heteromorphic pair has not been identified in female karyotypes of the ostrich (Struthio camelus), the cassowary (Casuarius casuarius), or the kiwi (Apteryx australis) (6, 7).
We have cloned two protein-coding genes of the chicken and by fluorescence in situ hybridization (FISH) technique have located their sites on the Z chromosome. ZOV3 encoding a member of the Ig superfamily was in the middle of the short arm, whereas the gene encoding an iron-responsive element-binding protein (IREBP) was located on the long arm close to the terminal heterochromatin block (8). IREBP was once known as the cytoplasmic aconitase gene, and its Z-linkage was shown by electrophoretic study (9). Subsequently, the Z-linkage of ZOV3 as well as IREBP was confirmed on six species of carinate birds representing five divergent orders (8). EE0.6 (a 0.6-kb EcoRI fragment) was a nonrepetitive W chromosome-derived sequence located between EcoRI and XhoI family-containing domains of the heterochromatic arm, and the W linkage of EE0.6 was confirmed on 18 carinate species belonging to eight different orders (10). Although EE0.6-related sequences also were located on the Z chromosome, they usually were distinct from the real W-linked EE0.6. Admittedly, a difference became diminished in certain carinate species, such as the Oriental white stork (Ciconia boyciana) (10). With regard to this particular species, the Z-linked EE0.6-related sequence and the W-linked EE0.6 sequence shared 92% identity (11). In this paper, whether the conservation of a Z linkage group extends to ratite birds was tested with regard to ZOV3 and IREBP. The above two genes and EE0.6 also were used to detect a sign of genetic differentiation, if any, between the essentially homomorphic Z and W of ratite birds.
MATERIALS AND METHODS
Construction and Screening of Genomic Libraries.
Genomic libraries of a female emu and a female ostrich were prepared by using λGEM12 vector (Promega) as described (8) from DNAs prepared from nuclei of blood cells (10). A portion of each library (1 × 105 plaque-forming units) was screened with 32P-labeled chicken EE0.6 (10), a 2.5-kb EcoRI fragment of a chicken cDNA clone, pCIREBP (8), or a PCR-amplified 1-kb fragment (nucleotide positions 377-1402) from a chicken cDNA clone, pZOV3 (8). The phage clones obtained were digested with NotI, and the inserts were recloned by using pBluescript KS (+) vector (Stratagene); a pEG series was obtained from the emu, and a pOG series was obtained from the ostrich.
Preparation of Metaphase Chromosome Spreads.
Small pieces of skin biopsies from a female emu (Chiba Zoological Park, Chiba, Japan), a female ostrich (Kobe Municipal Oji Zoo, Kobe, Japan), a male ostrich (Ostrich Industry, Okinawa, Japan; the gender was confirmed by histological examination of gonads), and two ostriches of unknown gender (Hirakawa Zoological Park, Kagoshima, Japan) were minced with two surgeon’s knives into pieces smaller than 1 mm3. These pieces of tissue were partially air-dried in a plastic dish to be fixed to the surface of the dish and were cultured in Eagle’s minimum essential medium (Sigma) supplemented with 10% fetal calf serum, 2 mM l-glutamine, 50 μg/ml streptomycin, 100 units/ml penicillin G, and 1.25 μg/ml fungizone (GIBCO/BRL) in 5% CO2/95% air at 37°C. Metaphase chromosome spreads were prepared from fibroblasts grown from the tissue pieces as described (12).
FISH.
Plasmid clones were labeled either with biotin-14-dATP (GIBCO/BRL) or digoxigenin (DIG)-11-dUTP (Boehringer Mannheim) by using a BioNick Labeling System (GIBCO/BRL), except that 0.2 mM each of dATP, dCTP, dGTP, and 0.13 mM dTTP and 0.07 mM DIG-11-dUTP were included to prepare DIG-labeled probes. A hybridization reaction with the two mixed probes was performed in 2× standard saline citrate containing 10% dextran sulfate and 50% formamide (Boehringer Mannheim) at 37°C for 16 h. Hybridization of the biotinylated probe was visualized with fluorescein isothiocyanate fluorescence, and hybridization of the DIG-labeled probe was visualized with tetramethylrhodamine isothiocyanate fluorescence as described (10). Chromosomes were counterstained with 4′,6-diamidino-2-phenylindole.
Southern Blot Hybridization.
Genomic DNA samples prepared as above from a female and a male ostrich and from two ostriches (nos. 7 and 8) of unknown gender were digested with BglII, electrophoresed on 0.7% agarose gel, and subjected to blotting and hybridization with a 32P-labeled ostrich genomic fragment, as indicated in the figure legend, under the conditions as described (10), except that hybridization and posthybridization washes were performed at 65°C. Signals of hybridization were converted to fluorescence images and quantitated by using a BAS2000 bio-image analyzer (Fuji). The blot then was subjected to autoradiography.
RESULTS
Conservation of EE0.6, ZOV3, and IREBP Genomic Sequences in Carinate and Ratite Birds.
Genomic libraries of a female emu and a female ostrich were constructed and screened with the chicken EE0.6 genomic probe, IREBP cDNA probe, or ZOV3 cDNA probe, and the following genomic clones were obtained: pEGEE0.6 (16-kb insert), pEGIREBP (16-kb insert), and pEGZOV3 (16-kb insert) from the emu and pOGEE0.6 (14-kb insert), pOGIREBP (15-kb insert), and pOGZOV3 (13-kb insert) from the ostrich. Counterpart sequences of chicken EE0.6 in pEGEE0.6 and pOGEE0.6 and parts of sequences in genomic clones of emu and ostrich homologues of IREBP and ZOV3 were determined and deposited in the nucleotide sequence databases. Determined genomic sequences of EE0.6 and sequences of exons in IREBP and ZOV3 were compared among chicken, emu, and ostrich. Levels of identities between chicken and emu or ostrich for the above sequences were remarkably high: ≈70% for EE0.6, ≈90% for the IREBP exon(s), and ≈80% for the ZOV3 exons (Table 1).
Table 1.
Levels of identity (%) of nucleotide and deduced amino acid sequences for EE0.6, IREBP, and ZOV3 among the chicken, the emu, and the ostrich
| Species compared | EE0.6, % [bp] | IREBP, % [bp] | ZOV3, % [bp] |
|---|---|---|---|
| Chicken and emu | 74 [510] | 92 [220] | 81 [327] |
| (94) | (72) | ||
| Chicken and ostrich | 71 [635] | 90 [123] | 80 [325] |
| (100) | (73) | ||
| Ostrich and emu | 88 [488] | NC | 94 [317] |
| (95) |
Lengths of aligned nucleotide sequences compared are shown in brackets. Levels of identity of deduced amino acid sequences are shown in parentheses. For IREBP, sequences of two exons in 2,686-bp genomic sequence for the emu and that of one exon in a 1,075-bp genomic sequence for the ostrich are used for comparison. For ZOV3, sequences of two exons in 2,206 bp for the emu and those of two exons in 3,431 bp for the ostrich are used for comparison.
NC, not compared because sequences of exons in cloned genomic fragments are unrelated.
Colocalization of EE0.6, IREBP, and ZOV3 Genomic Sequences on a Pair of Chromosomes in a Female Emu and a Male Ostrich.
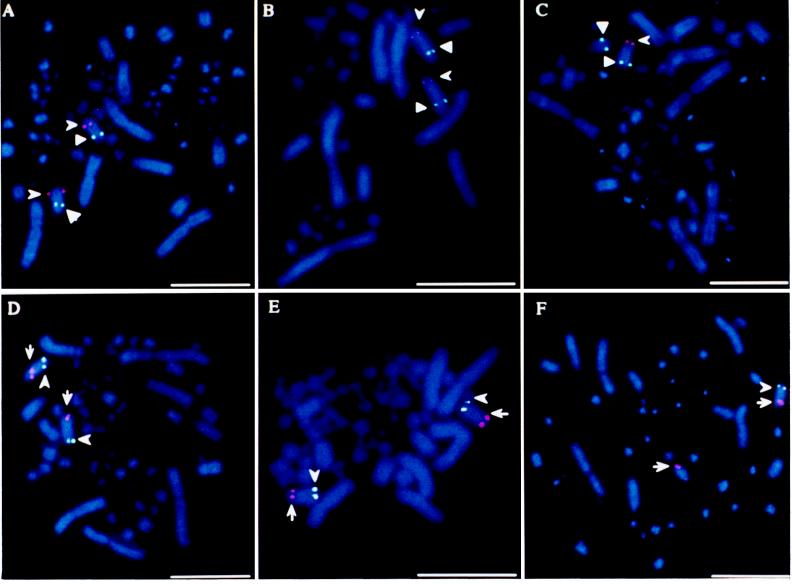
Above genomic clones for the emu and ostrich homologues of EE0.6, IREBP, and ZOV3 were used as probes to locate their chromosomal loci by FISH. When two mixed probes [DIG-labeled pEGIREBP plus biotinylated pEGZOV3 (Fig. 1A) or biotinylated pEGIREBP plus DIG-labeled pEGEE0.6 (Fig. 1D)] were hybridized to metaphase chromosome sets from the female emu, these three different probes all hybridized to the same pair of chromosomes, which was identified as the fifth pair in the female emu karyotype as shown in Fig. 2A. The loci of ZOV3 and EE0.6 are located on the arm opposite to that carrying the IREBP locus (Fig. 1 A and D). In Southern blot hybridization of genomic DNAs digested with BglII, EcoRI, EcoRV, or HindIII from the male and the female emu with 32P-labeled cDNA probes of chicken ZOV3, IREBP, or 32P-labeled chicken ET15 sequence, which is a putative exon sequence in EE0.6 (10), the same band patterns with similar intensities were produced between the male and the female (data not shown). Although a tissue sample from a male emu for preparing metaphase chromosome sets was unavailable, these results suggest that the male should have the same pair of chromosomes as the female to which those three loci are localized.
Figure 1.
Localization of IREBP, ZOV3, and EE0.6 sequences on a pair of chromosomes in metaphase sets of the emu and the ostrich. Signals of FISH shown by fluorescein isothiocyanate (white dots) or tetramethylrhodamine isothiocyanate (red dots) fluorescence indicate hybridization of the following probes: (A) DIG-pEGIREBP (arrowhead) and biotinylated pEGZOV3 (triangle) to the female emu set; (B) biotinylated pOGZOV3 (triangle) and DIG-pOGIREBP (arrowhead) to the male ostrich set; (C) as in B, but to the female ostrich set; (D) biotinylated pEGIREBP (arrowhead) and DIG-pEGEE0.6 (arrow) to the female emu set; (E) biotinylated pOGIREBP (arrowhead) and DIG-pOGEE0.6 (arrow) to the male ostrich set; and (F) as in E but to the female ostrich set. (Bars = 10 μm.)
Figure 2.
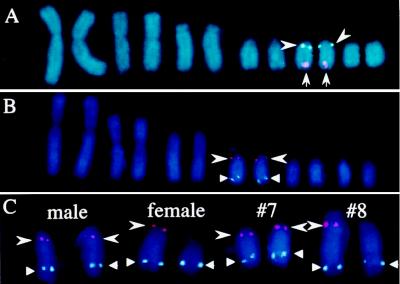
Identification of the pair of homologous chromosomes on which ZOV3, IREBP, and EE0.6 sequences are colocalized and partial morphological differentiation of the W chromosome in the ostrich. Chromosome pairs 1–6 from in situ-hybridized metaphase sets of the female emu (A) and the male ostrich (B) are shown. Signals of FISH are colocalized on chromosome 5 in the emu and chromosome 4 in the ostrich. Fluorescence signals were produced with the following probes: biotinylated pEGIREBP (arrowhead) and DIG-pEGEE0.6 (arrow) in A and DIG-pOGIREBP (arrowhead) and biotinylated pOGZOV3 (triangle) in B. (C) Pairs of chromosome 4 of the ostrich were taken from in situ-hybridized metaphase sets of the male, the female, and the individuals (nos. 7 and 8) of unknown gender. Fluorescence signals shown were produced with biotinylated pOGZOV3 (triangle) and DIG-labeled pOGIREBP (arrowhead). Partial differentiation of the W chromosome in the female and the no. 8 ostriches is noted by shortening of the arm from which the IREBP gene is missing.
Next, metaphase chromosome sets from the male and the female ostriches were subjected to FISH with the ostrich probes for IREBP, ZOV3, and EE0.6. All of these probes hybridized to a pair of chromosomes from the male ostrich (Fig. 1 B and E) with similar relative positions as in the female emu (Fig. 1 A and D). The pair of chromosomes was identified as the fourth pair in the ostrich karyotype (Fig. 2B).
Morphological Differentiation of a W Chromosome in the Ostrich.
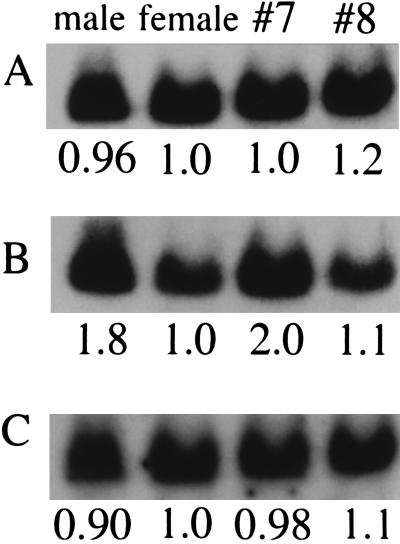
In metaphase chromosome sets of the female ostrich, the IREBP probe hybridized to only one of the chromosomes of the pair (Fig. 1 C and F). Southern blot hybridization of BglII-digested genomic DNAs [of the male and the female ostriches and of the ostriches (nos. 7 and 8) of unknown gender] with 32P-labeled ostrich genomic probes for EE0.6, IREBP, and ZOV3 produced a single band of hybridization for each probe. Intensities of hybridization signals showed an ≈2:1 male-to-female ratio with the IREBP probe (Fig. 3B), whereas ≈1:1 male-to-female intensity ratios were observed for signals with the EE0.6 and ZOV3 probes (Fig. 3 A and C). Intensities of signals with the IREBP probe for ostriches nos. 7 and 8 corresponded to those for the male and the female, respectively (Fig. 3B).
Figure 3.
Southern blot hybridization suggesting the absence of the IREBP gene on one of the homologous chromosomes in the female ostrich. The BglII-digested genomic DNA samples (5 μg/lane) from the male and female ostriches and from the two ostriches (nos. 7 and 8) of unknown gender as indicated were subjected to hybridization with the following 32P-labeled probes: (A) a 2.4-kb XbaI fragment of pOGEE0.6; (B) a 2.7-kb EcoRI–BglII fragment of pOGIREBP; and (C) a 3.5-kb BamHI fragment of pOGZOV3. The same blot was hybridized and rehybridized in this order. Relative intensities of hybridization signals are indicated.
Identifications of ostrich no. 7 as a male and ostrich no. 8 as a female were confirmed by FISH with the ostrich probes for IREBP and ZOV3 (Fig. 2C); both ZOV3 and IREBP signals were located on a pair of chromosomes of ostrich no. 7 as in the male control ostrich, whereas the IREBP signal was missing in one of the chromosomes of the pair for ostrich no. 8 as in the female control ostrich. The 4′,6-diamidino-2-phenylindole-stained chromosomes in Fig. 2C show recognizable shortening of the arm from which the IREBP gene locus is missing for the female control and for ostrich no. 8.
DISCUSSION
In the present study, we used three genomic or cDNA clones that are located on sex chromosomes of the chicken (EE0.6 on the W chromosome and IREBP and ZOV3 on the Z chromosome) to search for sex chromosome pairs in ratite birds: the emu and the ostrich. Although comparison was limited to one or two exons, sequences of both IREBP and ZOV3 homologues in the emu and the ostrich are 80–90% identical to the corresponding sequences in the chicken. The IREBP gene encodes a protein of dual functions: cytosolic aconitase and iron-responsive element-binding (13). The latter function has been shown to regulate levels of ferritin and transferrin receptor by changing the capability of IREBP to bind to those mRNAs in response to iron concentrations. The ZOV3 gene encodes a membrane-bound glycoprotein belonging to the Ig superfamily. A unique feature of the chicken ZOV3 is that its protein product is present preferentially in embryonic gonads of both genders and in sex steroid hormone-producing cells in ovarian follicles in hens. It has been speculated that ZOV3 is involved in differentiation or maintenance of the differentiated state of the sex steroid-producing cells, particularly in ovarian follicles (14). Because of those presumably essential functions, the high level conservation of genomic sequences for both IREBP and ZOV3 in carinate and ratite birds seems to be reasonable. On the other hand, the relatively high level conservation of the EE0.6 sequence is somewhat surprising because it does not seem to retain a gene function in the present-day avian species (10, 11).
The most significant findings in this study with genomic probes for the emu and the ostrich homologues of EE0.6, IREBP, and ZOV3 are that the three loci were present on a particular pair of chromosomes in both the emu and the ostrich and that a definite sign of the morphological differentiation of W chromosome was noted in the ostrich with loss of a part of the chromosome arm containing the IREBP locus. These results demonstrate at a molecular cytological level that sex chromosomes of carinate and ratite birds have evolved from a common pair of ancestral homologous chromosomes as predicted earlier by Ohno (1). During the evolution of ratite birds from a monophyletic origin (3, 15–17), morphological differentiation of sex chromosomes seems to have been frozen at early stages or has been taking place exceptionally slowly because in the present-day carinate species, not only the IREBP gene but also the ZOV3 gene on the opposite arm of the Z chromosome are absent on the W chromosome (8), and the EE0.6 sequence on the W and its related sequence on the Z are substantially different; only the sequence on the W chromosome can be detected by Southern blot hybridization or PCR in many species (10).
The present results suggest that it would be possible to compare the extent of morphological differentiation of the W chromosomes in other ratite species by using the three molecular probes used in this study and that it would be of interest to study the evolutionary relationship of avian and reptilian Z and W sex chromosomes by using the same approach adopted in this study.
Acknowledgments
We thank R. J. Etches, I. Munechika, A. Ishido, and T. Kouchi for the blood and/or skin samples from the emu or the ostrich and T. Yanai and A. Noda for histological examination of gonadal sections of an ostrich. The authors are especially grateful to Dr. Susumu Ohno for his encouragement and help during the preparation of this paper. This work was supported by a Grant-in-Aid for General Scientific Research (07456042) from the Ministry of Education, Science, Sports, and Culture of Japan.
ABBREVIATIONS
- IREBP
iron-responsive element-binding protein
- FISH
fluorescence in situ hybridization
- DIG
digoxigenin
Footnotes
References
- 1.Ohno S. Sex Chromosomes and Sex-Linked Genes. Heidelberg: Springer; 1967. [Google Scholar]
- 2.Becak W, Becak M L, Nazareth H R S, Ohno S. Chromosoma. 1964;15:606–617. doi: 10.1007/BF00319994. [DOI] [PubMed] [Google Scholar]
- 3.Sibley C G, Ahlquist J E. Phylogeny and Classification of Birds. New Haven, CT: Yale Univ. Press; 1990. pp. 810–870. [Google Scholar]
- 4.Benirschke R J, Sekulovich R E, Risser A C. Chromosome Information Service. 1976;21:13–14. [Google Scholar]
- 5.Ansari H A, Takagi N, Sasaki M. Cytogenet Cell Genet. 1988;47:185–188. doi: 10.1159/000132290. [DOI] [PubMed] [Google Scholar]
- 6.Takagi N, Itoh M, Sasaki M. Chromosoma. 1972;36:281–291. doi: 10.1007/BF00283247. [DOI] [PubMed] [Google Scholar]
- 7.de Boer L E M. Nature (London) 1980;287:84–85. doi: 10.1038/287084a0. [DOI] [PubMed] [Google Scholar]
- 8.Saitoh Y, Ogawa A, Hori T, Kunita R, Mizuno S. Chromosome Res. 1993;1:239–251. doi: 10.1007/BF00710129. [DOI] [PubMed] [Google Scholar]
- 9.Baverstock P R, Adams M, Pokinghorne R W, Gelder M. Nature (London) 1982;296:763–766. doi: 10.1038/296763a0. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa A, Solovei I, Hutchison N, Saitoh Y, Ikeda J, Macgregor H, Mizuno S. Chromosome Res. 1997;5:93–101. doi: 10.1023/a:1018461906913. [DOI] [PubMed] [Google Scholar]
- 11.Itoh Y, Ogawa A, Murata K, Hosoda T, Mizuno S. Genes Genet Syst. 1997;72:51–56. doi: 10.1266/ggs.72.51. [DOI] [PubMed] [Google Scholar]
- 12.Saitoh Y, Mizuno S. Chromosoma. 1992;101:474–477. doi: 10.1007/BF00352469. [DOI] [PubMed] [Google Scholar]
- 13.Klausner R D, Rouault T A. Mol Cell Biol. 1993;4:1–5. doi: 10.1091/mbc.4.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunita R, Nakabayashi O, Kikuchi T, Mizuno S. Differentiation. 1997;62:63–70. doi: 10.1046/j.1432-0436.1997.6220063.x. [DOI] [PubMed] [Google Scholar]
- 15.Stapel S O, Leunissen J A M, Versteeg M, Wattel J, de Jong W W. Nature (London) 1984;311:257–259. doi: 10.1038/311257a0. [DOI] [PubMed] [Google Scholar]
- 16.Lee K, Feinstein J, Cracraft J. In: Avian Molecular Evolution and Systematics. Mindell D P, editor. San Diego: Academic; 1997. pp. 173–195. [Google Scholar]
- 17.Cooper A, Penny D. Science. 1997;275:1109–1113. doi: 10.1126/science.275.5303.1109. [DOI] [PubMed] [Google Scholar]