Abstract
A new operon (designated the puc2BA operon) displaying a high degree of similarity to the original pucBA genes of Rhodobacter sphaeroides 2.4.1 (designated puc1) was identified and studied genetically and biochemically. The puc2B-encoded polypeptide is predicted to exhibit 94% identity with the original β-apoprotein. The puc2A-encoded polypeptide is predicted to be much larger (263 amino acids) than the 54-amino-acid puc1A-encoded polypeptide. In the first 48 amino acids of the puc2A-encoded polypeptide there is 58% amino acid sequence identity to the original puc1A-encoded polypeptide. We found that puc2BA is expressed, and DNA sequence data suggested that puc2BA is regulated by the PpsR/AppA repressor-antirepressor and FnrL. Employing genetic and biochemical approaches, we obtained evidence that the puc2B-encoded polypeptide is able to enter into LH2 complex formation, but neither the full-length puc2A-encoded polypeptide nor its N-terminal 48-amino-acid derivative is able to enter into LH2 complex formation. Thus, the sole source of α-polypeptides for the LH2 complex is puc1A. The role of the puc1C-encoded polypeptide was also determined. We found that the presence of this polypeptide is essential for normal levels of transcription and translation of the puc1 operon but not for transcription and translation of the puc2 operon. Thus, the puc1C gene product appears to have both transcriptional and posttranscriptional roles in LH2 formation. Finally, the absence of any LH2 complex when puc1B was deleted in frame was surprising since we know that in the presence of functional puc2BA, approximately 30% of the LH2 complexes normally observed contain a puc2B-encoded β-polypeptide.
In response to decreasing oxygen tensions the purple nonsulfur bacterium Rhodobacter sphaeroides is capable of developing a series of intracytoplasmic invaginations of the cell membrane, which house the photosynthetic apparatus of this organism. In addition to a lower oxygen tension, light intensity ultimately determines the cellular level of the photosynthetic apparatus. The photosynthetic apparatus contains three majorcarotenoid-bacteriochlorophyll (Bchl)-protein complexes,B800-850 (LH2) and B875 (LH1), which are light-harvesting complexes, and the reaction center complex (21). The LH2 complex is located peripherally with respect to the LH1 complex, and the ratio of B800-850 to B875 is variable, because the amounts are differentially regulated depending on the incident light intensity (9, 19). On the other hand, the reaction center complex is surrounded by the core antenna complex (LH1), and there is a fixed ratio of the latter to the former of approximately 12:1 to 15:1 irrespective of the light intensity (1, 4).
It has been reported previously that the puc operon of R. sphaeroides consists of the pucBA structural genes encoding the LH2 β- and α-polypeptides and an additional gene (pucC) which extends approximately 1.8 kb immediately downstream of pucBA. PucC appears to affect the posttranscriptional expression of the LH2 β- and α-polypeptides (21, 26). In R. sphaeroides, the puc operon gives rise to 0.5- and 2.3-kb puc-specific transcripts. These transcripts have the same 5′ end, which is localized 117 nucleotides upstream of the translational start of the pucB gene. Expression of both transcripts is regulated by oxygen and light intensity. The 0.5-kb transcript is about 10- to 25-fold more abundant than the 2.3-kb transcript (21, 26). Previously, in a study of regulation of the pucBAC operon it was observed that following deletion of the pucBA genes there was a highly homologous (as judged by hybridization intensity and stability) second transcript that was approximately 1.1 to 1.3 kb long. However, no LH2 complexes were found in the mutant despite the presence of the second transcript (26).
It has been observed that the genomes of Rhodopseudomonas acidophila and Rhodopseudomonas palustris contain additional, highly homologous copies of the puc operon encoding the LH2 β- and α-polypeptides. It was shown previously that all five copies of the puc operon in Rhodopseudomonas palustris were expressed and were regulated by light intensity, However, only two copies of the puc gene products have been detected in purified LH2 complexes from Rhodopseudomonas palustris (15, 40, 41).
Recently, work on the R. sphaeroides 2.4.1 genome in our laboratory revealed that there is a second copy of the pucBA genes (designated puc2BA in this study; gene numbers RSP1556 and RSP1557, respectively [www.rhodobacter.org]) (5, 28), which are located on chromosome I at approximately 1.36 Mbp clockwise from the original pucBAC operon (designated puc1BAC in this study). Therefore, what is the role of the puc2BA operon in R. sphaeroides? Is this operon translated, and if so, can the puc2BA-encoded polypeptides enter into formation of LH2 complexes? What is the structure of the second puc operon? What is the role of the presumed polypeptide extension of the puc2A-encoded polypeptide? Is this extension processed, or does it enter LH2 complex formation? In this genetic and biochemical study, we addressed these and other questions pertaining to the puc2BA operon, as well as the interaction between puc1BAC and puc2BA.
MATERIALS AND METHODS
Strains, plasmids, and cell growth.
All bacterial strains and plasmids used in this study are described in Table 1. R. sphaeroides 2.4.1 and its derivatives were grown as previously described (9). When required, antibiotics were added to Sistrom's minimal A medium at the following final concentrations: tetracycline, 1 μg/ml; kanamycin, 50 μg/ml; streptomycin, 50 μg/ml; spectinomycin, 50 μg/ml; and trimethoprim, 50 μg/ml.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli strains | ||
| DH5αphe | DH5αphe::Tn10dCm | 10 |
| S17-1 | C600::RP-4 2-(Tc::Mu)(Km::Tn7) thi pro hsdR hsdM+recA | 39 |
| R. sphaeroides strains | ||
| 2.4.1 | Wild type | W. R. Sistrom |
| ΔPUC1B | 2.4.1 Δpuc1B (in-frame deletion of the DNA sequence between positions 7 and 141 from the start codon of puc1B) | This study |
| ΔPUC1A | 2.4.1 Δpuc1A (in-frame deletion of the DNA sequence between positions 7 and 150 from the start codon of puc1A) | This study |
| ΔPUC1BA | 2.4.1 Δpuc1B Δpuc1A (in-frame deletion of the DNA sequence between position 7 from the start codon of puc1B and position 150 from the start codon of puc1A) | This study |
| ΔPUC2B | 2.4.1 Δpuc2B (in-frame deletion of the DNA sequence between positions 7 and 141 from the start codon of puc2B) | This study |
| ΔPUC2AS | 2.4.1 Δpuc2AS (in-frame deletion of the DNA sequence between positions 10 and 144 from the start codon of puc2A, which is a puc1A-like sequence) | This study |
| ΔPUC2A | 2.4.1 Δpuc2A (in-frame deletion of the DNA sequence between positions 10 and 780 from the start codon of puc2A) | This study |
| ΔPUC2BAS | 2.4.1 Δpuc2B Δpuc2AS (in-frame deletion of the DNA sequence between position 7 from the start codon of puc2B and position 144 from the start codon of puc2A [puc1A-like]) | This study |
| ΔPUC2BA | 2.4.1 Δpuc2BA (in-frame deletion of the DNA sequence between position 7 from the start codon of puc2B and position 780 from the start codon of puc2A) | This study |
| ΔPUC-C | 2.4.1 Δpuc1C::Tp (deletion of a 1,140-bp fragment of puc1C replaced with a 1.5-kb Tpr::Ω fragment), Tpr | This study |
| ΔPUC1BA-C | 2.4.1 Δpuc1BA Δpuc1C::Tp, Tpr | |
| ΔPUC1BA-2BA | 2.4.1 Δpuc1BA (in-frame deletion) Δpuc2BA (in-frame deletion) | This study |
| ΔPUC1BA-2BA-C | 2.4.1 Δpuc1BA (in-frame deletion) Δpuc2BA (in-frame deletion) Δpuc1C::Tp, Tpr | This study |
| Plasmids | ||
| pRK415 | Tcr | 20 |
| PBSIIKS+ | Apr, T3 and T7 promoters | Stratagene |
| pCF1010Tc | Smr/Spr TcrlacZ | M. Wood and S. Kaplan |
| pLO1 | Kmr, sacB RP4-oriT ColE1-oriV Kmr, sacB RP4-oriT ColE1-oriV | 27 |
| pUI1158 | Apr, pBSIIKS+ carrying phoA from pUI310 introduced as an XbaI and SacI fragment, phoA is in reading frame 3 with respect to the upstream polylinker sequence | M. Wood and S. Kaplan |
| pUC2P-PhoA | pRK415 containing phoA under control of the promoter of puc2BA, Tcr | This study |
| pUC35B-PhoA | pRK415 containing translational fusion of phoA to the 35 amino acid residues of the N-terminal portion of the puc2B-encoded protein, Tcr | This study |
| pUC2B-PhoA (pUC51B-PhoA) | pRK415 containing translational fusion of phoA to the C-terminal portion of the puc2B-encoded protein, Tcr | This study |
| pUC25A-PhoA | pRK415 containing translational fusion of phoA to the 25 amino acid residues of the N-terminal portion of the puc2A-encoded protein, Tcr | This study |
| pUC2AS-PhoA (pUC48A-PhoA) | pRK415 containing translational fusion of phoA to the N-terminal 48 amino acid residues of the puc2A-encoded protein (Puc1A-like), Tcr | This study |
| pUC99A-PhoA | pRK415 containing translational fusion of phoA to the 99 amino acid residues of the N-terminal portion of the puc2A-encoded protein, Tcr | This study |
| pUC147A-PhoA | pRK415 containing translational fusion of phoA to the 147 amino acid residues of the N-terminal portion of the puc2A-encoded protein, Tcr | This study |
| pUC207A-PhoA | pRK415 containing translational fusion of phoA to the 207 amino acid residues of the N-terminal portion of the puc2A-encoded protein, Tcr | This study |
| pUC232A-PhoA | pRK415 containing translational fusion of phoA to the 232 amino acid residues of the N-terminal portion of the puc2A-encoded protein, Tcr | This study |
| pUC2A-PhoA (pUC263A-phoA) | pRK415 containing translational fusion of phoA to the C-terminal region of the puc2A-encoded protein, Tcr | This study |
| pUI523A | Tcr, lacZ is in reading frame 3 with respect to the upstream polylinker sequence | M. Wood and S. Kaplan |
| pUI-2B523A | pUI523A containing translational fusion of lacZ to the N-terminal 15 amino acid residues of the puc2B-encoded protein under control of the puc2P promotor, Tcr | This study |
| pUI-2A523A | pUI523A containing translational fusion of lacZ to the N-terminal 25 amino acid residues of the puc2A-encoded protein under control of the puc2P promotor, Tcr | This study |
| pUI-1B523A | pUI523A containing translational fusion of lacZ to the N-terminal 10 amino acid residues of the puc1B-encoded protein under control of the puc1P promotor, Tcr | This study |
| pUI-1A523A | pUI523A containing translational fusion of lacZ to the N-terminal 12 amino acid residues of the puc1A-encoded protein under control of the puc1P promotor, Tcr | This study |
| pUI-1C523A | pUI523A containing translational fusion of lacZ to the N-terminal 19 amino acid residues of the puc1C-encoded protein under control of the puc1P promotor, Tcr | This study |
| pCF200Km | Smr/Spr Km+puc::lacZ | 24 |
| pUI-2BA-1010TC | pUI1010Tc containing transcriptional fusion of lacZ to the puc2BA operon (positions −536 to 70 of 5′-puc2BA), Smr/Spr Tcr | This study |
| pUC-1C | pRK415 containing a fragment which starts at position −35 upstream from the start codon of puc1C and ends at position 105 downstream from the stop codon of puc1C at the HindIII-KpnI site of pRK415, tet promotor, Tcr | This study |
| pUC1BA | pRK415 containing a fragment which starts at position −649 upstream from the start codon of puc1B and ends at position 77 downstream from the stop codon of puc1A at the HindIII-KpnI site of pRK415, Tc r | This study |
| pUC1BA-C | pRK415 containing a fragment which starts at position −649 upstream from the start codon of puc1B and ends at position 105 downstream from the stop codon of puc1C at the HindIII-Kpn1 site of pRK415, Tcr | This study |
| pUC2BAS | pRK415 containing a fragment which starts at position −536 upstream from the start codon of puc2B and ends at position 144 downstream from the start codon of puc2A (puc1A-like) at the HindIII-KpnI site of pRK415, Tcr | This study |
| pUC2BA | pRK415 containing a fragment which starts at position −536 upstream from the start codon of puc2B and ends at position 95 downstream from the stop codon of puc2A at the HindIII-KpnI site of pRK41 5, Tcr | This study |
| pUC2P-2B1A | pUC2BA derivative with puc2A replaced by puc1A, Tcr | This study |
| pUC1P-2B1A | pUC1BA derivative with puc1B replaced by puc2B, Tcr | This study |
| pUC2P-1BA | pUC1BA derivative with 649-bp upstream regulatory region replaced by a 536-bp upstream regulatory region of puc2BA, Tcr | This study |
| pUC1P-1B2AS | pUC1BA derivative with puc1A replaced by puc2AS, Tcr | This study |
| pUC1P-1B2A | pUC1BA derivative with puc1A replaced by puc2A, Tcr | This study |
Escherichia coli was grown at 37°C in Luria broth (29). When required, antibiotics were added at the following final concentrations: tetracycline, 10 μg/ml; kanamycin, 50 μg/ml; streptomycin, 50 μg/ml; spectinomycin, 50 μg/ml; trimethoprim, 50 μg/ml; and ampicillin, 50 μg/ml.
Molecular techniques.
Standard procedures were used for plasmid isolation, restriction endonuclease digestion, ligation, and other molecular biological techniques (29, 37). Sequence analyses were performed with the DNA Strider computer programs (Institut de Recherche Foundamentale, Commissariat a l'Energie Atomique, Paris, France).
Plasmid construction.
To construct vectors for expression of puc1BAC or puc2BA in pRK415, HindIII/KpnI fragments containing more than 500 bp of the upstream regulatory region and the puc2BA, puc2BAS (the first 144 bp of puc2A, which encodes a puc1A-like amino acid sequence), or puc1BA structural gene were subcloned into the HindIII/KpnI site of pBSIIKS+. These constructs were digested with HindIII/KpnI and introduced into the HindIII/KpnI site of pRK415. Reconstitution of chimeric operons between genes from puc1BA and genes from puc2BA was conducted by using methods described previously (18). The primers used for construction of all of the operon chimeras are shown in Table 2. All of the resulting constructions were confirmed by DNA sequencing.
TABLE 2.
Primers used in this study for operon fusion construction
| Plasmid | Primers | Cloned sequence(s) |
|---|---|---|
| pUC2P-2B1A | P1 (5′-CCAAGCTTGGATCGTGCTCTGCCGGCTC-3′) and P2 (5′-CATCTTTACTTCTCCCTCAG-3′) | puc2P and puc2B |
| P3 (5′-CTGAGGGAGAAGTAAAGATGACCAACGGCAAAATCT-3′) and P4 (5′-CCGGTACCGAGTAGCCTTTTGCACCTCC-3′) | puc1A | |
| pUC1P-2B1A | P5 (5′-CCAAGCTTCCCGCGTGCTGATCTCGGCC-3′) and P6 (5′-CACTGTGTCGTCTCCCAACT-3′) | puc1P |
| P4 and P7 (5′-AGTTGGGAGACGACACAGTGACCGATGATCCGAAAAA-3′) | puc2B and puc1A | |
| pUC2P-1BA | P1 and P8 (5′-TTGTACTCTCCCGAATGTGC-3′) | puc2P |
| P4 and P9 (5′-ACATTCGGGAGAGTACAAGTGACTGACGATCTGAAC-3′) | puc1BA | |
| pUC1P-1B2AS | P5 and P10 (5′-CATGTCAGTCTTCTCCTATC-3′) | puc1P and puc1B |
| P11 (5′-GATAGGAGAAGACTGACATGAACAACTCGAAGATGTG-3′) and P12, (5′-CCGGTACCGGCCACGGCTACGAGCCCTG-3′) | First 144 bp of puc2A DNA (puc1A-like) | |
| pUC1P-1B2A | P5 and P10 | puc1P and puc1B |
| P11 and P13 (5′-CCGGTACCGGTGGAACACCGGCCACAAC-3′) | puc2A |
To construct the transcriptional fusion puc2BA::lacZ, the puc2BA upstream DNA was subcloned into the multiple cloning region of pCF1010Tc. To construct translational fusions of puc2BA::lacZ and puc1BAC::lacZ in vector pUI523A, fragments containing the 536-bp upstream region and the first 45 and 244 bp of the DNA sequence of puc2BA were subcloned to produce the puc2B::lacZ and puc2A::lacZ translational fusions, respectively. Fragments containing the 649-bp upstream region and the first 30, 207, and 542 bp of the sequence of puc1BAC were subcloned to produce the puc1B::lacZ, puc1A::lacZ, and puc1C::lacZ translational fusions, respectively. KpnI and SmaI sites were introduced at the 5′ and 3′ ends of the fragments, respectively. These fragments were subcloned into the KpnI/SmaI site of pUI523A. The resulting constructions were confirmed by DNA sequencing.
Construction of in-frame mutations and plasmid derivatives.
Construction of ΔPUC1BA is described below to explain the protocol used for construction of in-frame mutations. A fragment containing the 649-bp upstream sequence of puc1BA and the start codon of puc1B was subcloned by performing PCR with primers pUC1BA55 (5′-CC GAG CTC CCC GCG TGC TGA TCT CGG-3′) (a SacI site was introduced at the 5′ end of this primer) and pUC1BA53 (5′-CC AAG CTT CAC TGT GTC GTC TCC CAA-3′) (a HindIII site was introduced at the 5′ end of this primer). A fragment containing the stop codon of puc1A and the 480-bp downstream sequence was subcloned by performing PCR with primers pUC1BA35 (5′-CC AAG CTT TAA TGC GCA AGG CGC GGG-3′) (a HindIII site was introduced at the 5′ end of this primer) and pUC1BA33 (5′-CC GTC GAC CGA GTG CGG AGA CAT-3′) (a SalI site was introduced at the 5′ end of this primer). These two fragments were confirmed by DNA sequencing and were joined in plasmid pBSIIKS+ to produce a SacI/SalI fragment in which 303 bp of puc1BA (from position 4 to position 307 bp) was removed. The SacI/SalI fragment was ligated into the SacI/SalI site of the suicide vector pLO1 and confirmed by DNA sequencing, and the resulting plasmid was transferred into E. coli S17-1. Conjugation and selection of mutant strains were carried out by using methods described previously (34). The mutant ΔPUC-C was constructed by deleting an internal 1,140-bp fragment of puc1C and replacing it with a 1.5-kb Tpr::Ω fragment. Mutants were screened by selecting for Tpr Kms colonies (8). All mutants were further confirmed by PCR amplification and DNA sequencing from the chromosome.
Construction of puc2BA::phoA fusions.
For construction of phoA fusions to puc2BA, we generated a HindIII/XbaI DNA fragment which had the same 5′ ends located approximately 500 bp upstream of the start codon of puc2B with the 3′ end of digested puc2BA located at different positions prior to the stop codon. The 3′ ends of the different fragments of puc2BA were located 0, 105, 153, 244, 313, 466, 610, 790, 865, and 958 bp downstream of the start codon of puc2B. These fragments encode 0, 35, and 51 amino acid residues of Puc2B and 25, 48, 99, 147, 207, 232, and 263 amino acid residues of Puc2A, respectively. The resulting DNA fragments were ligated into the HindIII/XbaI site of plasmid pUI1158, which contained the structural phoA gene. The HindIII/KpnI fragments derived from the resulting plasmids containing puc2B-phoA or puc2A-phoA fusions were ligated into the HindIII/KpnI site of plasmid pRK415. The constructions were confirmed by DNA sequencing.
Conjugation techniques.
Plasmid DNA was mobilized into R. sphaeroides strains by using the biparental conjugation system as previously described (8). Exconjugants were selected on SIS plates with the appropriate antibiotics. To select for mutant strains containing puc2BA::phoA fusion plasmids, cells were grown on SIS plates with 40 μg of 5-bromo-4-chloro-3-indolylphosphate (XP) per ml and 1 μg of tetracycline per ml.
Cell fractionation, spectroscopy, and β-galactosidase and alkaline phosphatase assays.
Crude cell extracts were prepared by the method of Tai et al. (42). Cell breakage and preparation of crude extracts were performed as described previously (44). Absorption spectra were recorded with a UV 1601 PC spectrophotometer (Shimadzu Corp., Columbia, Md.). The amount of B800-850 could be measured at an optical density at 849 nm (OD849) to OD900(ɛ = 96 ± 4 mM−1 cm−1) by using 3 mol of Bchl a per mol of complex, and the amount of B875 light-harvesting complexes could be measured at OD875 to OD820(ɛ = 73 ± 2.5 mM−1 cm−1) by using 2 mol of Bchl a per mol of complex, as described elsewhere (33). To determine the β-galactosidase activity, R. sphaeroides cultures were harvested at an OD600 of approximately 0.3 to 0.4, and chloramphenicol was added to a final concentration of 80 μg/ml to terminate protein synthesis. The β-galactosidase activity in cell extracts was measured in triplicate as described previously (25). The β-galactosidase activity was expressed in units, and 1 U was equal to 1 μmol of o-nitrophenyl-β-d-galactopyranoside hydrolyzed/min/mg of protein. For the alkaline phosphatase assay, cells carrying the puc2BA::phoA fusion plasmids were harvested at an OD600 of 0.3 to 0.4 by low-speed centrifugation at 4°C and were treated by using the method described previously for R. sphaeroides 2.4.1 (35). Each experiment was repeated three times with three separate cultures, and 1 U of alkaline phosphatase activity was defined as a change of 1 OD420 unit per min per 106 cells (30).
Spectral complex purification.
Cells of R. sphaeroides 2.4.1 and its puc1BA or puc2BA mutant derivatives were grown anaerobically at a light intensity of 100 W/m2 to an OD600 of 0.3 to 0.4. Cell breakage and preparation of membrane fragments were performed as described previously (44). Membrane fragments were solubilized with 20 mM Tris-HCl buffer (pH 8.0) containing 100 mM NaCl and 1% lauryl N,N-dimethylamine-N-oxide (LDAO). The OD800 of the suspension was adjusted to 30, and the resulting suspension was vortexed and incubated overnight at room temperature. The solubilized suspension was centrifuged at 12,000 × g for 30 min at 4°C to remove the particulate material, and a portion of the resulting supernatant was kept for later use. The remainder of the soluble fraction was centrifuged at 260,000 × g for 16 h at 4°C on a 20 to 40% sucrose gradient in 20 mM Tris-HCl buffer (pH 8.0) containing 0.1% LDAO (TL buffer). Pigmented layers containing LH2 complexes were removed carefully, filtered, and concentrated by centrifugation through Centricon YM-3 (regenerated cellulose; molecular weight cutoff, 3,000 Da) three times, and then they were resuspended in TL buffer. The samples were kept at −20°C for further use or for additional purification.
To purifiy the LH2 complexes, the sample described above was used for DEAE-52 cellulose chromatography by monitoring the spectral absorption at 800 and 850 nm. The fraction eluting between 0.15 and 0.20 M NaCl displayed an absorption profile typical of the B800-850 complex, and it was collected, desalted, and concentrated by centrifugation through Centricon YM-3 (regenerated cellulose; molecular weight cutoff, 3,000) three times with TL buffer. The samples were kept at −20°C until they were used.
SDS-polyacrylamide gel electrophoresis (PAGE), native electrophoresis, Western blotting, and immunoprecipitation.
Polypeptide profiles of purified LH2 complexes were determined by using a sodium dodecyl sulfate (SDS)-Tricine buffer system which resolves low-molecular-weight proteins (38).
For native electrophoresis, purified LH2 complexes were redissolved in 2% n-octyl-β-d-glucopyranoside (β-OG). To replace LDAO with β-OG in solution, the sample buffer was washed with Centricon YM-3 (regenerated cellulose; molecular weight cutoff, 3,000) three times by using 20 mM Tris-HCl (pH 8.0) containing 2% β-OG and 1 mM EDTA. Electrophoresis was performed on a 7.5% polyacrylamide gel by using the method of Laemmli (23) with 0.1% β-OG used in place of SDS.
For Western blotting, SDS-PAGE was performed by the method of Laemmli (23) on a 10% acrylamine gel. Western blotting was performed as described previously (44). Monoclonal anti-PhoA antibody was added at a dilution of 1:1,000, and the anti-rabbit alkaline phosphatase secondary antibody was used at a dilution of 1:3,000.
Immunoprecipitation was performed as described previously, with a slight modification to avoid the possibility that the strong detergent, SDS, might destroy the spectral complexes (37). The membrane fractions described above dissolved in 20 mM Tris-HCl buffer with 1% LDAO containing 100 mM NaCl were resuspended in TL buffer by using Centricon YM-3 (regenerated cellulose; molecular weight cutoff, 3,000). The sample was centrifuged at 12,000 × g for 30 min to remove undissolved materials and mixed 1:10 (vol/vol) with NET-gelatin buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% Nonidet P-40, 1 mM EDTA, 0.25% gelatin, 0.02% sodium azide). The mixture was incubated at 4°C for 1 h with slow shaking (50 to 100 rpm) in the presence of anti-PhoA antibody (1,000:5, vol/vol) to bind the Puc2BA-PhoA fusion proteins. The immune complexes were precipitated by addition of 50 μl of protein A-agarose and were incubated for another 1 h at 4°C. The mixture was centrifuged at 13,800 × g for 20 s, and the supernatant was removed. The resulting pellet was washed with NET-gelatin buffer three times and boiled in sample buffer for 3 min to dissolve the antibody-PhoA fusion protein complex, which was then used for Tricine-SDS gel electrophoresis analysis. To measure the spectral absorption of the immunoprecipitated complexes, the resulting pellet was resuspended in antibody-NET-gelatin buffer (1:10, vol/vol) and incubated at 4°C overnight to release the complex from the protein A-agarose. The mixture was centrifuged at 13,800 × g for 1 min at 4°C, and the supernatant was used for spectral absorption. A sample extract of mutant strain ΔPUC2BA containing plasmid pRK415 was used as a nonspecific control throughout.
Materials.
Antibiotics, o-nitrophenyl-β-d-galactopyranoside, XP, β-OG, and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) were obtained from Sigma (St. Louis, Mo.). Restriction endonucleases and nucleic acid-modifying enzyme were purchased from New English Biolabs, Inc. (Beverly, Mass.). LDAO was obtained from Stepan Company (Northfield, Ill.).
RESULTS
Identification and features of the puc2BA locus of R. sphaeroides 2.4.1.
The puc2BA locus and the derived proteins were found to exhibit high levels of similarity to the proteins encoded by the puc1BAC operon, in agreement with previous observations (5, 28) (Fig. 1). However, the puc2BA operon was not present in the photosynthesis gene cluster; it was flanked by genes coding for a putative hypothetical transthyretin-like protein and a putative 2-dehydropantoate 2-reductase. Interestingly, the length of the mRNA from the start codon of puc2B to the termination codon of puc2A was estimated to be approximately 963 bp, which is substantially longer than the corresponding length of the puc1BA mRNA (337 bp). The size of the puc2BA transcript is consistent with the purported 1.1- to 1.3-kb transcript detected previously, as determined by using puc1BA as a probe (26). Figure 1 shows that the levels of identity and similarity between the puc1B-encoded β-apoprotein and the puc2B-encoded polypeptide are 94 and 95%, respectively. Three amino acid residues in the puc1B-encoded β-apoprotein, Leu5, Asn6, and Val15, are replaced by Pro5, Lys6, and Ile15 in the puc2B-encoded polypeptide. A comparison of the puc1A-encoded α-apoprotein and the first 48 amino acid residues of the puc2A-encoded polypeptide (designated Puc2AS) revealed 58% identity and 72% similarity. Further examination of Puc2A revealed the presence of a long carboxy-terminal extension starting at amino acid 49 of Puc2A and extending to the end of the polypeptide. This extension or tail of Puc2A contained a repeating amino acid sequence (PVAAAPAEEAAA or PV[A]EAAAPV[A]AEAAA). Transmembrane hidden Markov model 2.0 computer predictions suggested that the puc2A polypeptide contains only one transmembrane helix domain between Asn13 and Thr37. The long extension from Glu49 to the C terminus contained no apparent membrane-spanning region (Fig. 1).
FIG. 1.
Comparison of the LH2 sequences of R. sphaeroides 2.4.1 (RS 2.4.1) and Rhodopseudomonas acidophila 10050 (RA 10050). (A) β-Apoprotein. (B) α-Apoprotein. The transmembrane helices are indicated by boldface type.
By searching the 500-bp upstream DNA sequence to the start codon of puc2B, we found two PpsR binding motifs (TGT-N12-ACA) centered at positions −144 and −169 relative to the start codon. These locations are similar to the locations of the same DNA sequences found in puc1BA. An FnrL binding sequence (TTG-N8-CAA) was found to be centered at position −80 from the start codon of puc2B, while an FnrL binding sequence was located at position −229 for puc1BA. No integration host factor (IHF) binding sequence was found upstream of the start codon of puc2BA. It appeared that expression of puc2BA, like expression of puc1BAC, may be regulated by the repressor-antirepressor PpsR/AppA, as well as FnrL (16, 44); this remains to be determined. We have not mapped the 5′ end of the puc2BA transcript, which is necessary to firmly fix these purported regulatory binding sequences relative to expression of the puc1BA locus; such work is now being performed.
Since the three-dimensional crystal structure of the LH2 complex from Rhodopseudomonas acidophila 10050 has been determined with a high degree of resolution (6, 31), we compared the derived amino acid sequences of the puc1BA-encoded α- and β-apoproteins and the puc2BA-encoded polypeptides of R. sphaeroides 2.4.1 with the amino acid sequences of the α- and β-apoproteins of Rhodopseudomonas acidophila 10050 (Fig. 1). Two His residues (β-His40 and α-His31), which are presumed to provide the fifth ligand for the central Mg2+ of Bchl a in the LH2 complex (6, 22, 31), were found in the puc2B- and puc2A-encoded polypeptides, respectively. Two conserved Tyr residues (Tyr44 and Tyr45 in the α-apoprotein) were present in the puc2A-encoded polypeptide. These residues have been shown to be involved in binding the 2-acetyl carbonyl groups of the B850 pigments of R. sphaeroides (12, 17). There was one Arg residue at position 30 in the puc2B-encoded polypeptide, which has been found to form part of the binding site for the B800 Bchl a in the LH2 complex of Rhodopseudomonas acidophila (11, 14).
Are the puc2BA polypeptides produced?
In order to rapidly assess whether the α- and β-polypeptides encoded by the puc2BA operon are actually formed, even in the absence of any LH2 complex, such as in a puc1BA deletion (26), we tagged these polypeptides by construction of PhoA fusion proteins.
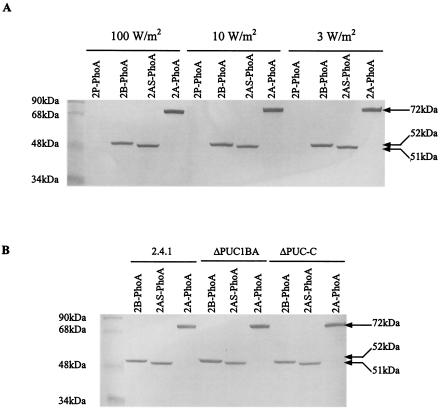
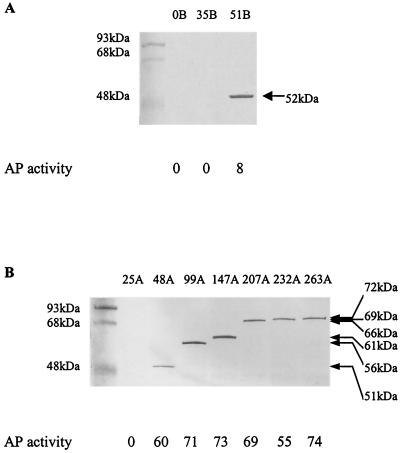
Figure 2A shows a Western blot used to analyze a series of phoA constructions with puc2B, puc2AS, and puc2A, as well as the control puc2P expressed in mutant strain ΔPUC2BA. Plasmids pUI2B-PhoA and pUI2A-PhoA encoded the entire Puc2B and Puc2A amino acid sequences, respectively. Plasmid pUI2AS-PhoA was a puc2A construction lacking the extension of the coding sequence which went beyond the identifiable puc1A-encoded orthologue (namely, the portion of puc2A encoding the first NH2-terminal 48 amino acids). As shown in Fig. 2, the observed molecular masses of the hybrid proteins comprising Puc2B-PhoA and Puc2AS-PhoA were 52 and 51 kDa, respectively. The observed molecular mass of Puc2A-PhoA was 75 kDa, compared to a calculated value of 72 kDa. What was immediately apparent was that the puc2A-encoded polypeptide was translated and was apparently not processed to any significant extent. In addition, the PhoA fusion proteins corresponding to Puc2B, Puc2AS, and Puc2A were present even when the chromosomal puc2BA genes were deleted. More importantly, if we carried this analysis one step further (i.e., expressing the same fusion constructions in puc1BA and puc1C deletion strains in which no LH2 complex was produced), these fusion proteins were still formed (Fig. 2B).
FIG. 2.
Western blot of Puc2BA-PhoA fusion proteins from cells of R. sphaeroides 2.4.1 and mutants of this strain. (A) Western blot of cells of mutant ΔPUC2BA carrying plasmids pUC2P-PhoA (2P-PhoA), pUC2B-PhoA/pRK415 (2B-PhoA), pUC2AS-PhoA/pRK415 (2AS-PhoA), and pUC2A-PhoA/pRK415 (2A-PhoA) grown anaerobically at different light intensities. (B) Western blot of cells of R. sphaeroides 2.4.1, mutant ΔPUC1BA, and mutant ΔPUC-C carrying plasmids pUC2B-PhoA/pRK415, pUC2AS-PhoA/pRK415, and pUC2A-PhoA/pRK415 grown anaerobically at 100 W/m2. Fifty micrograms of protein from each cell extract was applied to an SDS-PAGE gel, and anti-PhoA antibody was used to detect the PhoA fusion protein. The sizes of the hybrid proteins are indicated on the right. Cells were grown to an OD600 of 0.3 to 0.4.
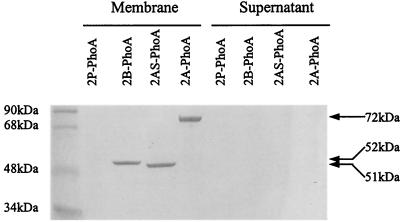
Therefore, expression of puc2BA and its encoded polypeptides is independent of the presence of puc1BA and puc1C. Since we were dealing with PhoA fusion proteins, our assumption was that these chimeric proteins pass through the membrane in the normal manner. The structure of LH2 (6, 31) predicts that the fusion junction should lie in the periplasm. To address this question, we fractionated the particulate and soluble fractions shown in Fig. 2. Figure 3 shows that all of the fusion proteins, including Puc2AS-PhoA, were membrane localized. None of these chimeric proteins was found to be associated with the supernatant fraction of the cell extract.
FIG. 3.
Localization of Puc2BA-PhoA fusion proteins in R. sphaeroides mutant ΔPUC2BA containing plasmids pUC2P-PhoA/pRK415 (2P-PhoA), pUC2B-PhoA/pRK415 (2B-PhoA), pUC2AS-PhoA/pRK415 (2AS-PhoA), and pUC2A-PhoA/pRK415 (2A-PhoA). Cells were grown anaerobically at 100 W/m2 to an OD600 of 0.3 to 0.4. Fifty micrograms of protein was applied to each lane of an SDS-PAGE gel. Membranes were isolated as described in Materials and Methods. The sizes of the hybrid proteins are indicated on the right.
Immunoprecipitation of Puc2BA-PhoA fusion proteins.
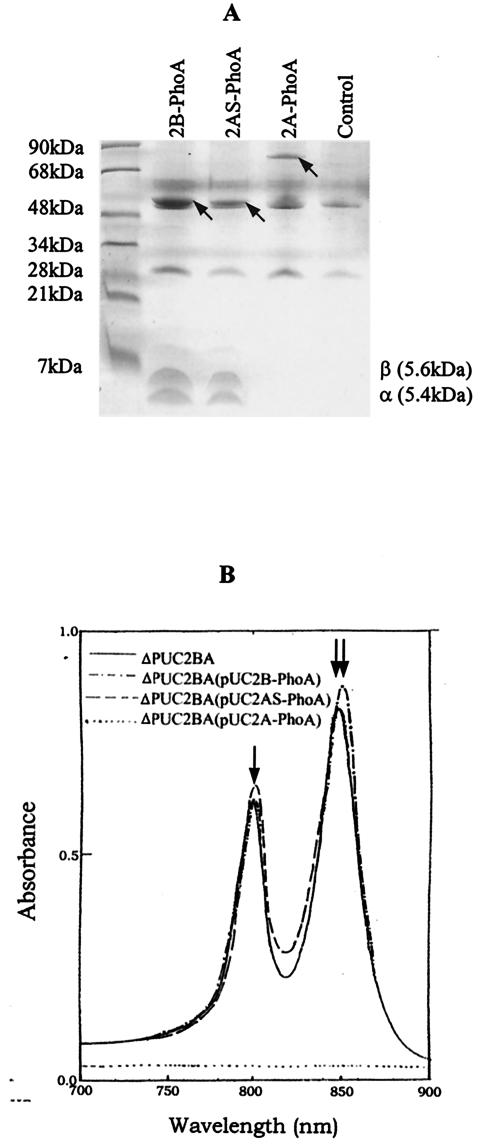
To directly demonstrate the existence of an interaction between the puc2BA-encoded proteins and the β and α subunits encoded by the puc1BAC operon, we immunoprecipitated LH2 spectral complexes which contained the Puc2B, Puc2A, or Puc2AS-PhoA chimeric proteins by using anti-PhoA antibodies and protein A-agarose beads. The polypeptides were released from the immunoprecipitated LH2 complexes as described above. Figure 4A shows the SDS-PAGE profiles of the released LH2-anti-PhoA complexes. The free β- and α-polypeptides derived from the puc1BAC-encoded operon migrated to the front of the gel. However, these polypeptides were not present in the lane containing Puc2A-PhoA, as described below.
FIG. 4.
Immunoprecipitation of Puc2BA-PhoA of R. sphaeroides mutant strain ΔPUC2BA containing plasmids pUC2B-PhoA (2B-PhoA), pUC2AS-PhoA (2AS-PhoA), and pUC2A-PhoA/pRK415 (2A-PhoA). Mutant strain ΔPUC2BA containing plasmid pRK415 was used as a control. (A) Tricine-SDS-PAGE of immunoprecipitated complexes. The stained bands corresponding to the chimeric proteins Puc2A-PhoA, Puc2AS-PhoA, and Puc2A-PhoA are indicated by arrows. (B) Absorption spectra of the immunoprecipitated complexes and purified LH2 complex from mutant strain ΔPUC2BA (solid line). The arrows indicate the absorption peaks for B800 and B850 of LH2, respectively.
By scanning the immunoprecipitated complexes, we obtained the absorption spectra of the complexes, as shown in Fig. 4B. To more accurately show the features of the immunoprecipitated complexes, the absorption profile of purified LH2 from mutant strain ΔPUC2BA is also shown in Fig. 4B. Notably, the complexes containing either Puc2B-PhoA or Puc2AS-PhoA showed the typical absorption spectrum of the LH2 complex. Complexes containing Puc2AS-PhoA had absorption maxima at 800.1 and 849.5 nm. When complexes containing a Puc2B-PhoA fusion protein were analyzed, absorption maxima at 800.5 and 852.0 nm were observed. However, the immunoprecipitated complexes containing the Puc2A-PhoA fusion protein exhibited no spectral absorption and did not form a discernible LH2 complex. When we compared the ratios of B800 to B850 derived from the immunoprecipitated complexes, the complex containing Puc2AS-PhoA had a ratio of 0.73, whereas the complex containing the Puc2B-PhoA fusion protein had a ratio of 0.62. The ratio for LH2 of the control ΔPUC2BA was 0.67. These data show that not only do the fusion proteins enter the membrane, but they can do so in the form of discernible LH2 complexes. When the polypeptide extension of Puc2A was added to Puc1A, no LH2 spectral complex of any kind was observed (unpublished results).
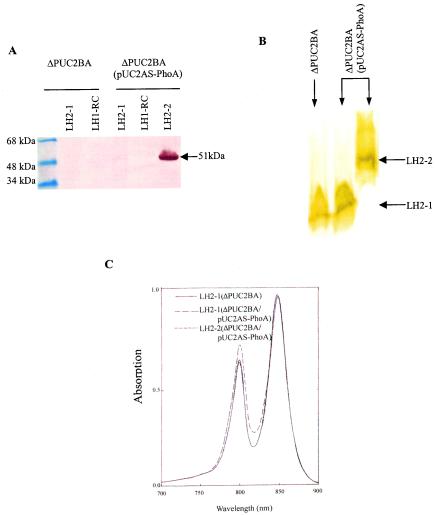
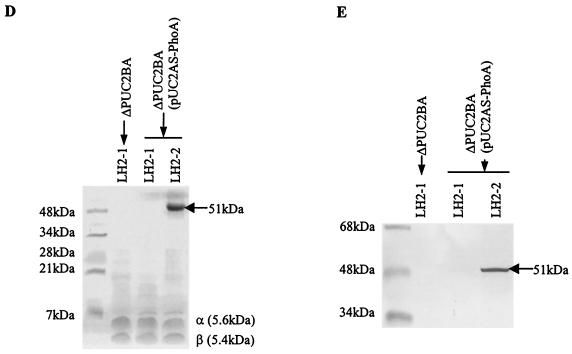
Purification of the Puc2AS-PhoA fusion protein.
To further investigate the biochemical features of the LH2 complexes containing either the puc2B- or puc2AS-encoded polypeptides, the LH2 complex containing Puc2AS-PhoA was partially purified from mutant ΔPUC2BA(pUC2AS-PhoA) by using the detergent LDAO combined with sucrose gradient centrifugation as described in Materials and Methods. Gradient centrifugation separated the photosynthetic membranes into three pigment-protein fractions (data not shown). The three fractions were examined by SDS-PAGE and Western blotting (Fig. 5A). Protein Puc2AS-PhoA was detected by using anti-PhoA antibody and was found only in the fraction corresponding to the heavier form of LH2, LH2-2. Thus, within the membrane there are two fractions of LH2, one designated LH2-1 containing none of the fusion protein and the other, LH2-2, containing the fusion protein. This suggests that the fusion protein normally behaves as a native β- or α-polypeptide. It was difficult to accurately determine the amount of LH2-2 relative to the amount of LH2-1 in the sucrose gradient, but we estimated that this fraction accounted for between 10 and 20% of the total LH2 (data not shown).
FIG. 5.
Purification and biochemical analysis of LH2 from mutants ΔPUC2BA and ΔPUC2BA(pUC2AS-PhoA). Cells were grown anaerobically in the light at 100 W/m2 to an OD600 of 0.3 to 0.4. Membrane fractions were obtained and dissolved in 20 mM Tris-HCl (pH 8.0) containing 1% LDAO and 0.1 M NaCl as described in Materials and Methods. The sample was centrifuged on a 20 to 40% sucrose gradient at 260,000 × g for 16 h at 4°C. Three pigmented bands were obtained, and a Western blot for each band is shown in panel A. Anti-PhoA antibody was used to detect the PhoA fusion protein. RC, reaction center. (B) Native electrophoresis of the LH2 complexes purified by sucrose gradient centrifugation and DEAE-52 chromatography, as described in Materials and Methods. (C) Absorption spectra of the purified LH2 complexes from panel B. The same concentration of protein (100 μg/ml) was applied for each sample. (D) Tricine-SDS-PAGE of the purified LH2 complex from panel B. (E) Western blot of the fusion protein from panel B with anti-PhoA antibodies. Fifty micrograms of protein from each of the separated pigment bands was applied to the SDS-PAGE gel. Anti-PhoA antibody was used to detect the PhoA fusion protein. The sizes of the hybrid proteins are indicated on the right (arrows).
The membrane fractions from the sucrose gradient were purified further, as described above, in order to isolate the various LH2 fractions. The resulting isolated complexes are shown in Fig. 5B, in which the LH2-2 complex is apparent only in the strain containing the puc2BAS::phoA fusion. The spectral absorption data for the complexes were determined and are shown in Fig. 5C. Analysis of the absorption spectra shown in Fig. 5C revealed that the two near-infrared absorption bands due to Qy absorption of B800/Bchl a and B850/Bchl a of the LH2 complex containing the Puc2AS-PhoA fusion protein were located at 799.5 and 848.7 nm, respectively. These bands exhibited little blue shift when they were compared with the bands found in the normal LH2 complexes made up of the native β- and α-apoproteins. However, the ratio of B800 to B850 of the LH2-2 complex in mutant ΔPUC2BA(pUC2AS-PhoA) was greater than that of the LH2-1 complex in mutant ΔPUC2BA (0.67 and 0.75, respectively). The results suggest that the Bchl a moieties have a slightly altered binding environment in the puc2B- and puc2AS-encoded polypeptides compared to that of the normal α and β subunits. However, the relative amount of B800 or B850 in the LH2-2 complex had a slightly different profile when it was compared with the amount observed for the LH2-1complex made up of α- and β-apoproteins (Fig. 5C). Nonetheless, it appears that the α-polypeptide derived from pUC2AS-PhoA can enter into a membrane complex of some sort.
The Tricine-SDS-PAGE profiles derived from the column fractions shown in Fig. 5B are shown in Fig. 5D. The results show that the PhoA fusion protein was found only in the fraction designated LH2-2 and that its size was identical to the size of the band stained with anti-PhoA antibody shown in Fig. 5E.
Native PAGE of the LH2 complexes shown in Fig. 5B suggested that LH2-2 containing the Puc2AS-PhoA hybrid protein had a different molecular weight and a slightly increased B800 absorption band compared to the normal LH2-1 complex. The Tricine-SDS-PAGE profiles shown in Fig. 5D further confirmed that the LH2-1 complex from ΔPUC2BA(pUC2AS-PhoA) was identical to that from ΔPUC2BA, which consisted of only the normal α- and β-apoproteins encoded by puc1BA. Note the appearance of the normal α- and β-polypeptides that migrated to the 5- to 6-kDa region of the gel for all of the fractions from Fig. 5B. This complex was also found in the complemented mutant, as expected.
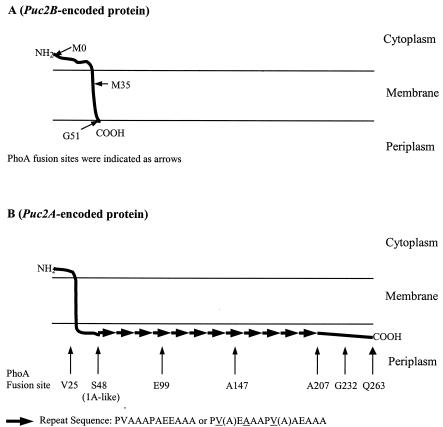
Topological analysis of puc2BA-encoded proteins.
The crystal structure of the B800-850 light-harvesting complex of Rhodopseudomonas acidophila has revealed that the N termini of both the α- and β-apoproteins are located in the cytoplasmic side of the membrane and that the C terminus of each protein is located in the periplasmic region (6, 31). To investigate the topology of the puc2BA-encoded polypeptides, a hydropathy profile-based computer analysis (transmembrane hidden Markov model 2.0) was performed, and the predicted topology of the puc2BA-encoded polypeptides is shown in Fig. 6. The predicted topology of the puc2B-encoded or puc2AS-encoded polypeptide included only one transmembrane helix domain, as expected. It is important to point out that even though the puc2A-encoded polypeptide is much larger than the puc1A-encoded α-apoprotein, there is still only one transmembrane helix that is predicted, which is located in the N-terminal 48 amino acid residues. The extended region of Puc2A has no computer-predicted transmembrane helix. The predicted topology of puc2B-, puc2AS-, or puc2A-encoded polypeptides is therefore similar to the known structure of the corresponding subunit (α or β subuit) of LH2; i.e., the C terminus of the puc2B- or puc2A-encoded protein is localized in the periplasmic region, and the N terminus is located on the cytoplasmic side of the membrane.
FIG. 6.
Summary of hydropathy analyses by transmembrane hidden Markov model 2.0. The arrows indicate the positions where fusions of puc2B::phoA (A) and puc2A::phoA (B) were made.
To confirm the predicted topology of the puc2BA-encoded polypeptides, PhoA fusion proteins were constructed. Alkaline phosphatase activities were determined in cells of R. sphaeroides mutant strain ΔPUC2BA in which different lengths of puc2B or puc2A-encoded polypeptides were fused with the PhoA protein, as shown in Fig. 7.
FIG. 7.
Alkaline phosphatase (AP) activities and Western blot analysis of the fusion proteins from the different fusion positions of puc2BA and phoA. The positions of the junction sites are identified by the most C-terminal amino acid residues of Puc2B and Puc2A prior to the PhoA sequence (indicated by arrows in Fig. 6). The cells were grown anaerobically at 100 W/m2 to an OD600 of 0.3 to 0.4. Fifty micrograms of protein from each cell extract was applied to a lane of an SDS-PAGE gel, and anti-PhoA antibody was used to detect the PhoA fusion protein. Assays were performed in triplicate, and the standard deviations were within ± 10%. The values are the averages for the three independent experiments rounded to the nearest integer; a value multiplied by 10−7 is equal to the number of units per cell. The sizes of the hybrid proteins are indicated on the right.
Cells were observed as white colonies on plates containing 40 μg of XP per ml when the Puc2B-PhoA fusions mapped to position 0 or 35 and also for the Puc2A-PhoA fusion, which was fused at position 25 (Fig. 7). These results revealed both the physical absence of the fusion proteins and the resulting lack of alkaline phosphatase activities. When the Puc2B-PhoA fusion, which mapped to the C terminus of Puc2B (position 51) (Fig. 6A), and the Puc2A-PhoA fusions, which mapped to positions 48, 99, 147, 207, 232, and 263 (Fig. 6B), were constructed, the cells were observed as blue colonies. The results shown in Fig. 7 are in agreement with the topological diagrams shown in Fig. 6, suggesting that the N-terminal regions of puc2B- and puc2A-encoded polypeptides are located on the cytoplasmic side of the membrane and that the C terminus is located on the periplasmic side. Finally, it appeared that the entire extension of the Puc2A polypeptide is located on the periplasmic side of the cell membrane. In all fusion constructions showing PhoA activity, the proteins produced appeared to be stable and functional.
It is important to point out that all of the presumed chimeric proteins with the fusion junction assumed to be in the cytoplasm or in the membrane, which displayed no alkaline phosphatase activity and appeared as white colonies, were assumed to be unstable and could not be detected by Western blotting.
Mutation of the puc2BA operon.
To further assess the role of puc2BA and its relationship to puc1BA, we constructed a series of mutant strains in which puc2B, puc2A, or puc2BA was deleted in frame. The results are shown in Table 3. These results revealed that the absence of Puc2B resulted in a decrease ranging from 25% (3 W/m2) to 30% (10 W/m2) in the level of the LH2 complex. On the other hand, the absence of Puc2A led to no obvious decline in LH2 abundance, since the values observed were all within statistical variation. When both Puc2B and Puc2A were absent, the level of LH2 was reduced to a level similar to that observed in the absence of Puc2B alone. There was no effect upon the relative levels of the B875 complex in any of these experiments (data not shown). Previous studies showed that a mutation in puc1BA (usually polar) resulted in a complete absence of the LH2 complex, despite the presence of an intact puc2BA operon, as we now know. It was also found that the puc1C gene is essential for LH2 formation, and it was concluded that the puc1C gene product is involved in LH2 assembly (24-26). On the basis of these previous findings and our findings, it appears that the β-polypeptide encoded by puc2BA is likely to be involved in the formation of LH2 complexes and that LH2 complexes involving Puc2B may go through the same assembly pathway as the β- and α-polypeptides encoded by puc1BA.
TABLE 3.
Light-harvesting complex LH2 in R. sphaeroides 2.4.1 and mutants of this straina
| Strain | Amt of LH2 complex (nmol/mg of protein)
|
||
|---|---|---|---|
| 100 W/m2 | 10 W/m2 | 3 W/m2 | |
| 2.4.1 | 13.0 | 26.0 | 36.3 |
| ΔPUC2B | 9.5 | 18.3 | 29.0 |
| ΔPUC2AS | 12.0 | 23.1 | 33.9 |
| ΔPUC2A | 11.9 | 24.0 | 34.1 |
| ΔPUC2BAS | 8.8 | 15.9 | 27.0 |
| ΔPUC2BA | 8.8 | 16.6 | 26.2 |
| ΔPUC1B | 0.8 | 1.1 | 0.9 |
| ΔPUC1A | NDb | ND | 0.6 |
| ΔPUC1BA | ND | 0.1 | 0.3 |
| ΔPUC-C | ND | ND | 0.3 |
| ΔPUC1BA-C | ND | 0.4 | 0.5 |
| ΔPUC1BA-2BA | 0.2 | 0.2 | ND |
| ΔPUC1BA-2BA-C | ND | ND | 0.1 |
Cells were grown anaerobically in the light to an OD600 of 0.3 to 0.4. Three independent experiments were performed, and the standard deviations were within ±10%. The values are averages for the three independent experiments.
ND, not detectable.
Table 3 also shows that in the absence of either Puc1B or Puc1A, no LH2 complex was formed. We predicted at least a low level of LH2 in the presence of functional Puc1A, since the data revealed that Puc2B can enter into LH2 formation (see below). Finally, the results shown in Table 3 confirmed the inverse dependence between light intensity and LH2 abundance, when data were considered in the present context.
Use of transcriptional and translational fusions of puc2BA and puc1BAC.
The results shown in Table 3 raise the question of why no LH2 was observed in the absence of puc1B. The PhoA fusion data showed that at least some Puc2B and Puc2A were produced. Was this effect upon puc2BA expression transcriptional or posttranscriptional? To address this question, we employed a transcriptional fusion plasmid, pCF1010Tc, involving a puc2BA::lacZ reporter. This construction was introduced into R. sphaeroides 2.4.1 and puc1BA, puc1C, and puc2BA derivatives of this strain. Using wild-type R. sphaeroides 2.4.1 as a control, we obtained β-galactosidase activities of 667, 761, and 918 U/min/mg of protein when cells were grown photosynthetically at 100, 10, and 3 W/m2, respectively. When the same construction was introduced into any of the mutant strains described above, identical levels of β-galactosidase activity were observed for the different light intensities.
The data revealed that expression of the puc2BA operon fusions was regulated inversely with respect to light intensity, but not to the same extent that the abundance of LH2 was regulated under similar conditions. More importantly, mutation of puc1BAC or any of the individual genes comprising this operon did not affect transcription of the puc2BA operon in trans. The results show that the puc2BA operon was functional at the transcriptional level when either or both chromosomal copies of either puc operon were mutated. These results and those shown in Table 3, as well as the PhoA fusion data, appear to present a paradox: puc2BA was transcribed at apparently normal levels under all conditions, but LH2 was not observed in the puc1BAC-containing mutant strains.
In order to determine if there is a translational effect upon the puc2BA mRNA in the genetic backgrounds affecting puc1BAC, we constructed lacZ translational fusions to the puc2B and puc2A genes of puc2BA. When the translational fusions to the puc2B gene were introduced into wild-type strain 2.4.1 growing photosynthetically, we obtained β-galactosidase values of 610, 690, and 791 U/min/mg of protein at 100, 10, and 3 W/m2, respectively. The β-galactosidase values obtained for the puc2A translational fusion were 448, 512, and 575 U/min/mg of protein, respectively. It is evident that, as observed for expression of the transcriptional fusions, expression of the translational fusions was independent of the mutant background, since virtually identical levels of β-galactosidase activity were obtained for all of the combinations of puc1BAC and puc2BA mutational backgrounds. Thus, if there is a posttranscriptional effect upon puc2BA expression in the puc1BAC-containing mutant strains, it is likely to be beyond transcriptional or translational processes.
As a result of the findings described above, it is apparent that expression at the level of spectral complex formation encoded by the puc2BA operon is obligately dependent upon a functioning puc1BAC operon. However, it is equally apparent that neither transcription and translation of puc2BA nor membrane association of the derived polypeptides is affected by the absence of puc1BAC. Therefore, the question immediately arises, does the absence of puc2BA affect the transcription and/or translation of puc1BAC? Table 4 summarizes the results obtained when we used a puc1BA::lacZ transcriptional fusion in various puc-containing mutant strains. We observed a very strong but inverse effect of light intensity upon puc1BA expression. Such an effect has been noted previously (24-26), but when the effect was compared to expression of puc2BA, it was severalfold greater and was consistent with the levels of the LH2 complex present. However, it was also immediately obvious that when the puc1C gene was absent, the level of expression of the puc1BA::lacZ transcriptional fusion was reduced by ∼67%. This was not observed with the puc2BA::lacZ fusion in the same genetic background and has not been reported previously. It was also evident that the puc1BAC promoter (Table 4) was more active than the puc2BA promoter. When puc1C was present in trans, LacZ activity was increased to nearly normal levels. Given the fact that puc1C expression in the complemented strains was likely to be far from optimal (the tet promoter drives puc1C expression), the results shown in Table 4 demonstrate for the first time that puc1C has a major effect upon the transcription of puc1BAC but not on puc2BA. Furthermore, the puc1C gene product also had a major effect upon the assembly of both forms of the LH2 spectral complex, as noted previously (26).
TABLE 4.
β-Galactosidase activities of cell extracts of R. sphaeroides 2.4.1 and mutant derivatives of this strain containing the pCF200km (puc1BAC::lacZ) transcriptional fusion plasmida
| Strain | β-Galactosidase activity (U/min/mg of protein)
|
||
|---|---|---|---|
| 100 W/m2 | 10 W/m2 | 3 W/m2 | |
| 2.4.1 | 1,038 | 2,043 | 3,121 |
| ΔPUC2BA | 1,121 | 2,197 | 3,213 |
| ΔPUC1BA | 1,029 | 2,098 | 3,191 |
| ΔPUC-C | 381 | 593 | 1,127 |
| ΔPUC1BA-C | 410 | 506 | 1,092 |
| ΔPUC1BA-2BA-C | 379 | 510 | 1,009 |
| ΔPUC-C (pUC-1C) | 827 | 1,903 | 2,527 |
| ΔPUC1BA-C(pUC-1C) | 902 | 1,706 | 2,592 |
| ΔPUC1BA-2BA-C(pUC-1C) | 879 | 1,810 | 2,409 |
Cells were grown anaerobically in the light to an OD600 of 0.3 to 0.4. Three independent experiments were performed, and the standard deviations were within ±10%. The values are averages for the three independent experiments rounded off to the nearest integer.
Using translational fusions to puc1B, puc1A, and puc1C in the genetic backgrounds as shown in Table 4 further revealed the complex interactions between the two puc operons. Table 5 shows that all genes of the puc1BAC operon regardless of the genetic background are light regulated; this includes puc1C. Also, it appeared that approximately 30% more β-polypeptide than α-polypeptide was translated. The amount of Puc1C translated is generally 10% of the amount of the β-polypeptide translated and is consistent with the general abundance of the 2.3-kb puc1BAC transcript relative to the abundance of the 0.5-kb puc1BA transcript (24-26). As observed for the puc1BA transcriptional fusion, the absence of puc1C had a profound effect upon the different puc1BAC translational fusions. Such an effect was not observed for the puc2BA translational fusions. Finally, the presence of puc2BA had no effect upon expression of the translational fusions involving puc1BAC.
TABLE 5.
β-Galactosidase activities of cell extracts of R. sphaeroides 2.4.1 and mutant derivatives of this strain containing the puc1B::lacZ (pUI-1B523A), puc1A::lacZ (pUI1A523A), or puc1C::lacZ (pUI1C523A) translational fusion plasmida
| Strain | Plasmid | β-Galactosidase activity (U/min/mg of protein)
|
||
|---|---|---|---|---|
| 100 W/m2 | 10 W/m2 | 3 W/m2 | ||
| 2.4.1 | pUI1B523A | 640 | 978 | 1,234 |
| pUI1A523A | 546 | 712 | 866 | |
| pUI1C523A | 48 | 91 | 221 | |
| ΔPUC2BA | pUI1B523A | 601 | 918 | 1,381 |
| pUI1A523A | 519 | 691 | 871 | |
| pUI1C523A | 51 | 93 | 226 | |
| ΔPUC1BA | pUI1B523A | 550 | 992 | 1,377 |
| pUI1A523A | 532 | 627 | 829 | |
| pUI1C523A | 51 | 85 | 233 | |
| ΔPUC-C | pUI1B523A | 84 | 222 | 351 |
| pUI1A523A | 104 | 173 | 247 | |
| pUI1C523A | 45 | 73 | 174 | |
Cells were grown anaerobically in the light to an OD600 of 0.3 to 0.4. Three independent experiments were performed, and the standard deviations were within ±10%. The values are averages for the three independent experiments rounded off to the nearest integer.
Interactions between the puc1BAC and puc2BA operons.
Based on the results described above, it seems clear that mutation of puc2BA does not lead to changes in expression of the puc1BAC operon at either the transcriptional or translational level. On the other hand, the ∼30% decrease in the level of LH2 complexes in the absence of a functional puc2BA operon revealed that this operon contributes to LH2 complex abundance. It is also clear that the contribution of the puc2BA operon to LH2 formation is dependent not only upon expression of puc1C, as is the case for puc1BA, but also upon expression of puc1BA. To gain additional insight into this complex relationship between puc1BAC and puc2BA, a series of experiments that included mixing and matching of promoters and structural genes between the two operons was performed. The results are shown in Table 6.
TABLE 6.
Amounts of LH2 in R. sphaeroides 2.4.1. and puc2BA- or puc1BAC-containing mutant strains complemented in transa
| Plasmid | Amt of LH2 (nmol/mg of protein)
|
||||
|---|---|---|---|---|---|
| 2.4.1 | ΔPUC1BA | ΔPUC2BA | ΔPUC1BA-2BA | ΔPUC-C | |
| pRK415 | 36.8 | 0.3 | 25.1 | 0.1 | NDb |
| pUC1BA | 38.1 | 35.4 | 27.8 | 0.3 | 0.2 |
| pUC-1C | 38.4 | ND | 0.8 | ND | 20.9 |
| pUC1BA-C | 37.1 | 37.9 | 26.2 | 26.6 | 36.4 |
| pUC2BAS | 37.0 | ND | 34.9 | 0.2 | ND |
| pUC2BA | 38.4 | ND | 37.4 | ND | 0.2 |
| pUC2P-1BA | 37.8 | 15.4 | 26.9 | 11.1 | 1.1 |
| pUC2P-2B1A | 38.3 | 14.1 | 35.0 | 10.2 | 0.9 |
| pUC1P-2B1A | 38.6 | 38.1 | 38.8 | 36.9 | 0.7 |
| pUC1P-1B2AS | 37.6 | ND | 26.7 | ND | 0.4 |
| pUC1P-1B2A | 36.9 | ND | 27.5 | 0.6 | ND |
Cells were grown anaerobically in the light at 3 W/m2 to an OD600 of 0.3 to 0.4. Three independent experiments were performed, and the standard deviations were within ±10%.
ND, not detected.
First, the absence of puc2BA led to an approximately 30% decline in the total levels of LH2, as previously noted, and normal levels of LH2 could be restored only by addition of puc2BA in trans. Not even puc1BA in trans could restore normal levels of LH2 when puc2BA was absent. This is a remarkable finding and is critically important, since it demonstrates that the levels of puc1BAC-encoded complexes are under critical control and are not affected by extra copies of puc1BAC. Therefore, puc2BA expression is required for normal LH2 abundance. The formation of all LH2 is dependent upon a functional puc1C even though both puc operons are expressed in its absence. Therefore, Puc1C has a posttranslational effect upon LH2 assembly.
In addition, puc2P is weaker than puc1P. The presence of the extended portion of the puc2A-encoded polypeptide appears to be irrelevant to the formation of the puc2BA-encoded fraction of LH2. Finally, and most importantly, the data suggest that it is the puc2B-encoded polypeptide which is essential for the formation of the puc2BA-encoded portion of LH2. Conversely, the data indicate that the puc2A-encoded gene product, either native or shortened, does not take part in LH2 formation, although it does enter the membrane. This finding raises an obvious question: what is the role of the extended puc2A-encoded polypeptide? As mentioned above, when this polypeptide is added to the Puc1A protein, no LH2 spectral complexes are produced.
DISCUSSION
In this study we identified a new genetic locus, puc2BA, showing a high degree of similarity to the puc1BAC locus described previously for R. sphaeroides 2.4.1 (21, 24-26). Our results suggest that under standard conditions, the puc2BA locus is not as highly expressed as the puc1BAC operon, although expression is both oxygen and light dependent. However, the extent of the light control of puc2BA is substantially less than the extent of the light control of puc1BAC. This could be a reflection of the upstream regulatory region. puc2BA possesses both repressor PpsR and FnrL binding sequences. The former sequences are in the same relative location with respect to the start of puc2B as the puc1B sequences, but the presumed FnrL sequence is not in the same relative location. Another major difference between the puc1BAC and puc2BA loci is in the primary sequences of the encoded polypeptides. puc2BA has no puc1C gene homologue, nor were we able to find such a homologue elsewhere in the genome, The puc2B-encoded polypeptide is nearly identical to the puc1B-encoded polypeptide. However, the puc2A-encoded polypeptide is significantly different from the puc1A-encoded gene product. The N-terminal 48 amino acid residues of Puc2A exhibit only 58% identity and 72% similarity to the puc1A-encoded product. Beyond the N-terminal 48 amino acids, the remainder of the derived polypeptide (215 amino acids) bears no resemblance to any other sequence encoded in the genome or present in the databases. This extended protein possesses a unique repeating sequence throughout much of its length. It has been reported that the C termini of the two encoded α-polypeptides of Rhodopseudomonas palustris are shortened by approximately 13 amino acid residues. It was also noted that the carboxy ends of these α-polypeptides are rich in alanine and proline residues; although these findings are superficially similar to findings obtained with the puc2A-encoded protein, the differences are significant. Only one copy of the α-polypeptides was reportedly found in the LH2 complexes of Rhodopseudomonas palustris (40, 41).
We reported previously that the puc1C gene product most likely is involved in assembly of the α- and β-polypeptides, Bchl and Crt, into a functional LH2 complex. This conclusion was based on the finding that mutation of puc1C resulted in a complete absence of the LH2 complex despite the presence of puc1BA mRNA and the encoded polypeptides (24-26). The data presented here extend that role to the puc2BA-encoded polypeptides. Not only does the Puc1C protein affect assembly of the LH2 complex, but the absence of this protein also appears to have a significant effect upon transcription of the puc1BA genes. The transcription activity of puc1BAC was reduced by 67% in a Puc1C mutant background. However, no such effect was observed for expression of the puc2BA genes. In addition, the absence of puc1C has an even more pronounced effect upon translation of the puc1BAC gene products. This observation introduces the possibility that in the absence of Puc1C, the transient accumulation of intermediates during assembly of the LH2 complex has a feedback-like effect upon transcription and translation of the puc1BAC genes and gene products, respectively. This finding clearly opens a new dimension in the regulation of photosynthesis gene expression, and the process is superficially similar to regulatory processes described for the pilin or flagellum systems (2).
Mix and match constructions shown in the Table 6 suggest that neither Puc2A nor engineered Puc2AS participates in discernible LH2 complex formation, although the PhoA fusion data reveal that these molecules enter the membrane. Conversely, the data suggest that the Puc2B polypeptide does form productive LH2 complexes, which must be derived from the interaction of Puc2B with Puc1A. Thus, the observed LH2 is a mixture of ∼70 to 75% Puc1 α- and β-polypeptides and 30 to 25% Puc1 α- and Puc2 β-polypeptides. This explains why the presence of LH2 is entirely dependent upon expression of the puc1BA genes. Despite the validity of this finding, it is curious, since the translational fusion data suggest that an excess of the Puc1 β-polypeptide is produced relative to the amount of the Puc1 α-polypeptide. Given the fact that puc2P is weaker than puc1P, it may be that the Puc2B β-polypeptide is a more effective partner for the Puc1A α-polypeptide than the Puc1B β-polypeptide is. However, this conclusion appears to be in opposition to the finding that in the absence of the puc1B-encoded polypeptide, no LH2 complex is produced, despite the presence of a functional puc1A gene and a functional puc2B gene.
Although we had to resort to the use of PhoA fusion proteins to dramatically increase the size of the complexes containing the Puc2A α-polypeptide, we do not believe that this altered the primary finding, namely, that the Puc2A α-polypeptide may reside in some form of membrane-associated complex which fractionates with the LH2 complexes. Furthermore, productive expression of the puc2BA operon is entirely dependent upon expression of both the puc1BA structural genes and the puc1C gene. It is also apparent that the use of PhoA fusions alters neither the stability nor the function of the apoproteins to which they are fused when they reside in the periplasm. The Puc2B-PhoA fusion protein appears to form normal and stable spectral complexes.
Finally, we are left with the proposed role of the Puc2A polypeptide. As shown in Fig. 3, the Puc2A polypeptide is inserted into the membranes and appears to be part of the membrane-associated complexes, but its presence does not lead to the characteristic absorption properties of the LH2 complex. We cannot say whether this polypeptide binds Bchl or Crt or whether it forms a complex with the β-polypeptides from any source. It could form a complex of Puc2A polypeptides. Puc2A has the basic topology of the Puc1A polypeptide, with the extended portion lying entirely within the periplasm. We suggest several possible roles for the puc2A gene product. One possibility is that the extended region facilitates, but is not essential for, the interaction of the LH2 with the LH1 complex, in the same way that the PufX polypeptide appears to facilitate the interaction between LH1 and the reaction center (13). Another possibility is that the extended region of the Puc2A protein is required to provide the structural information which ultimately gives rise to the intracytoplasmic invaginations characteristic of wild-type R. sphaeroides (21).
Finally, even the Puc2AS polypeptide does not appear to enter into formation of discernible LH2 complexes. It has been proposed that the transmembrane helix domains of the α-polypeptides show a low level of sequence conservation when different proteobacteria are compared; the one exception is His31. The major determinant for the specific Bchl-B850 geometry may be found predominantly in the residues outside the transmembrane region (3, 7, 32, 36, 43). It has been found that insertion of four residues (TTWA) towards the carboxy terminus of the transmembrane helix of the α-apoprotein leads to the absence of LH2 complexes (3). Thus, it is reasonable to point out that when the PWIAN residues are inserted towards the carboxy terminus of the transmembrane helix of Puc2AS, they might play a different role than the role of the α-apoprotein from standard LH2 complexes. However, there is another possibility, the possibility that the weak interaction of the N or C terminus of Puc2A or Puc2AS with other apoproteins or with themselves results in the failure of Puc2A or Puc2AS to enter into the assembly and formation of LH2 spectral complexes. This appears to be the case when the extended region of Puc2A is added to Puc1A. For example, it appears that the Puc1B β-polypeptide is overproduced compared to the Puc1A α-polypeptide, and it is therefore possible that the Puc2A α-polypeptide forms a nonproductive complex with the excess β-polypeptide. This still leaves open the question of the physiological role(s) of such complexes or associations.
It is also possible that the puc2A gene is a pseudogene and is actually in the process of assuming a new function.
In conclusion, our studies demonstrated that there is an interaction(s) between puc1BAC and puc2BA; the former operon is dominant, and the latter is dependent upon the former. The data also revealed major differences in the regulation of these two operons; puc1BAC is subject to greater regulatory control than puc2BA. Finally, the presence of LH2 is entirely dependent upon puc1BAC, but the ultimate cellular level of LH2 is dependent upon puc2BA.
Acknowledgments
This work was supported by National Institutes of Health grant GM15590 and by DOE OBER DE-FG02-01ER63232.
REFERENCES
- 1.Aagaard, J., and W. R. Sistrom. 1972. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem. Photobiol. 15:209-225. [DOI] [PubMed] [Google Scholar]
- 2.Aldridge, P., and K. T. Hughes. 2002. Regulation of flagellar assembly. Curr. Opin. Microbiol. 5:160-165. [DOI] [PubMed] [Google Scholar]
- 3.Braun, P., J. D. Olsen, B. Strohmann, C. N. Hunter, and H. Scheer. 2002. Assembly of light-harvesting bacteriochlorophyll in a model transmembrane helix in its natural environment. J. Mol. Biol. 318:1085-1095. [DOI] [PubMed] [Google Scholar]
- 4.Chory, J., T. J. Donohue, A. R. Varga, L. A. Staehelin, and S. Kaplan. 1984. Induction of the photosynthetic membranes of Rhodopseudomonas sphaeroides: biochemical and morphological studies. J. Bacteriol. 159:540-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choudhary, M., and S. Kaplan. 2000. DNA sequence analysis of the photosynthesis region of Rhodobacter sphaeroides 2.4.1. Nucleic Acids Res. 28:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cogdell, R. J., N. W. Isaacs, A. A. Freer, J. Arrelano, T. D. Howard, M. Z. Papiz, A. M. Hawthornthwaite-Lawless, and S. Prince. 1997. The structure and function of the LH2 (B800-850) complex from the purple photosynthetic bacterium Rhodopseudomonas acidophila strain 10050. Prog. Biophys. Mol. Biol. 68:1-27. [DOI] [PubMed] [Google Scholar]
- 7.Davis, C. M., P. L. Bustamante, J. B. Todd, P. S. Parkes-Loach, P. McGlynn, J. D. Olsen, L. McMaster, C. N. Hunter, and P. A. Loach. 1997. Evaluation of structure-function relationships in the core light-harvesting complex of photosynthetic bacteria by reconstitution with mutant polypeptides. Biochemistry 36:3671-3679. [DOI] [PubMed] [Google Scholar]
- 8.Davis, J., T. J. Donohue, and S. Kaplan. 1988. Construction, characterization, and complementation of a Puf− mutant of Rhodobacter sphaeroides. J. Bacteriol. 170:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donohue, T. J., J. H. Hoger, and S. Kaplan. 1986. Cloning and expression of the Rhodobacter sphaeroides reaction center H gene. J. Bacteriol. 168:953-961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eraso, J. M., and S. Kaplan. 1994. prrA, a putative response regulator involved in oxygen regulation of photosynthesis gene expression in Rhodobacter sphaeroides. J. Bacteriol. 176:32-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler, G. J., S. Hess, T. Pullerits, V. Sundstrom, and C. N. Hunter. 1997. The role of beta Arg-10 in the B800 bacteriochlorophyll and carotenoid pigment environment within the light-harvesting LH2 complex of Rhodobacter sphaeroides. Biochemistry 36:11282-11291. [DOI] [PubMed] [Google Scholar]
- 12.Fowler, G. J., R. W. Visschers, G. G. Grief, R. van Grondelle, and C. N. Hunter. 1992. Genetically modified photosynthetic antenna complexes with blue-shifted absorbance bands. Nature 355:848-850. [DOI] [PubMed] [Google Scholar]
- 13.Frese, R. N., J. D. Olsen, R. Branvall, W. H. Westerhuis, C. N. Hunter, and R. van Grondelle. 2000. The long-range supraorganization of the bacterial photosynthetic unit: a key role for PufX. Proc. Natl. Acad. Sci. USA 97:5197-5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gall, A., G. J. Fowler, C. N. Hunter, and B. Robert. 1997. Influence of the protein binding site on the absorption properties of the monomeric bacteriochlorophyll in Rhodobacter sphaeroides LH2 complex. Biochemistry 36:16282-16287. [DOI] [PubMed] [Google Scholar]
- 15.Gardiner, A. T., R. C. Mackenzie, S. J. Barrett, K. Kaiser, and R. J. Cogdell. 1996. The purple photosynthetic bacterium Rhodopseudomonas acidophila contains multiple puc peripheral antenna complex (LH2) genes: cloning and initial characterisation of four β/α pairs. Photosynth. Res. 49:223-235. [DOI] [PubMed] [Google Scholar]
- 16.Gomelsky, M., and S. Kaplan. 1995. appA, a novel gene encoding a trans-acting factor involved in the regulation of photosynthesis gene expression in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 177:4609-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hess, S., K. J. Visscher, T. Pullerits, V. Sundstrom, G. J. Fowler, and C. N. Hunter. 1994. Enhanced rates of subpicosecond energy transfer in blue-shifted light harvesting LH2 mutants of Rhodobacter sphaeroides. Biochemistry 33:8300-8305. [DOI] [PubMed] [Google Scholar]
- 18.Joseph-Liauzun, E., P. Delmas, D. Shire, and P. Ferrara. 1998. Topological analysis of the peripheral benzodiazepine receptor in yeast mitochondrial membranes supports a five-transmembrane structure. J. Biol. Chem. 273:2146-2152. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan, S., B. D. Cain, T. J. Donohue, W. D. Shepherd, and G. S. Yen. 1983. Biosynthesis of the photosynthetic membranes of Rhodopseudomonas sphaeroides. J. Cell. Biochem. 22:15-29. [DOI] [PubMed] [Google Scholar]
- 20.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 21.Kiley, P. J., A. Varga, and S. Kaplan. 1988. Physiological and structural analysis of light-harvesting mutants of Rhodobacter sphaeroides. J. Bacteriol. 170:1103-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koepke, J., X. Hu, C. Muenke, K. Schulten, and H. Michel. 1996. The crystal structure of the light-harvesting complex II (B800-850) from Rhodospirillum molischianum. Structure 4:581-597. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J. K., and S. Kaplan. 1992. cis-Acting regulatory elements involved in oxygen and light control of puc operon transcription in Rhodobacter sphaeroides. J. Bacteriol. 174:1146-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, J. K., and S. Kaplan. 1992. Isolation and characterization of trans-acting mutations involved in oxygen regulation of puc operon transcription in Rhodobacter sphaeroides. J. Bacteriol. 174:1158-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, J. K., P. J. Kiley, and S. Kaplan. 1989. Posttranscriptional control of puc operon expression of B800-850 light-harvesting complex formation in Rhodobacter sphaeroides. J. Bacteriol. 171:3391-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz, O., E. Schwartz, J. Dernedde, M. Eitinger, and B. Friedrich. 1994. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J. Bacteriol. 176:4385-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mackenzie, C., M. Choudhary, F. W. Larimer, P. F. Predki, S. Stilwagen, J. P. Armotage, R. D. Barber, T. J. Donohue, J. P. Hosler, J. F. Newman, J. P. Shapleigh, R. E. Sockett, J. Zeilstra-Ryalls, and S. Kaplan. 2001. The home stretch, a first analysis of the nearly completed genome of Rhodobacter sphaeroides 2.4.1. Photosynth. Res. 70:19-41. [DOI] [PubMed] [Google Scholar]
- 29.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Manoil, C. 1991. Analysis of membrane protein topology using alkaline phosphatase and beta-galactosidase gene fusions. Methods Cell Biol. 34:61-75. [DOI] [PubMed] [Google Scholar]
- 31.McDermott, G., S. M. Prines, A. A. Freer, A. M. Hawthornthwaite-Lawless, M. Z. Paplz, R. J. Cogdell, and N. W. Isaacs. 1995. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374:517-521. [Google Scholar]
- 32.Meadows, K. A., P. S. Parkes-Loach, J. W. Kehoe, and P. A. Loach. 1998. Reconstitution of core light-harvesting complexes of photosynthetic bacteria using chemically synthesized polypeptides. 1. Minimal requirements for subunit formation. Biochemistry 37:3411-3417. [DOI] [PubMed] [Google Scholar]
- 33.Meinhardt, S. W., P. J. Kiley, S. Kaplan, A. R. Crofts, and S. Harayama. 1985. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of energy transfer from light-harvesting complexes to the reaction center. Arch. Biochem. Biophys. 236:130-139. [DOI] [PubMed] [Google Scholar]
- 34.Oh, J. I., and S. Kaplan. 2002. Oxygen adaptation. The role of the CcoQ subunit of the cbb3 cytochrome c oxidase of Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 277:16220-16228. [DOI] [PubMed] [Google Scholar]
- 35.Ouchane, S., and S. Kaplan. 1999. Topological analysis of the membrane-localized redox-responsive sensor kinase PrrB from Rhodobacter sphaeroides 2.4.1. J. Biol. Chem. 274:17290-17296. [DOI] [PubMed] [Google Scholar]
- 36.Rau, H. K., H. Snigula, A. Struck, B. Robert, H. Scheer, and W. Haehnel. 2001. Design, synthesis and properties of synthetic chlorophyll proteins. Eur. J. Biochem. 268:3284-3295. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 38.Schagger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 39.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Bio/Technology 1:37-45. [Google Scholar]
- 40.Tadros, M. H., and K. Waterkamp. 1989. Multiple copies of the coding regions for the light-harvesting B800-850 α and β-polypeptides are present in the Rhodopseudomonas palustris genome. EMBO J. 8:1303-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tadros, M. H., E. Katsiou, M. A. Hoon, N. Yurkova, and D. P. Ramji. 1993. Cloning of a new antenna gene cluster and expression and expression analysis of the antenna gene family of Rhodopseudomonas palustris. Eur. J. Biochem. 217:867-875. [DOI] [PubMed] [Google Scholar]
- 42.Tai, T. N., W. A. Havelka, and S. Kaplan. 1988. A broad-host-range vector system for cloning and translational lacZ fusion analysis. Plasmid 19:175-188. [DOI] [PubMed] [Google Scholar]
- 43.Todd, J. B., P. S. Parkes-Loach, J. F. Leykam, and P. A. Loach. 1998. In vitro reconstitution of the core and peripheral light-harvesting complexes of Rhodospirillum molischianum from separately isolated components. Biochemistry 37:17458-17468. [DOI] [PubMed] [Google Scholar]
- 44.Zeng, X., and S. Kaplan. 2001. TspO as a modulator of the repressor/antirepressor (PpsR/AppA) regulatory system in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 183:6355-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]