Abstract
Swordtail fish (Poeciliidae: genus Xiphophorus) are a paradigmatic case of sexual selection by sensory exploitation. Female preference for males with a conspicuous “sword” ornament is ancestral, suggesting that male morphology has evolved in response to a preexisting bias. The perceptual mechanisms underlying female mate choice have not been identified, complicating efforts to understand the selection pressures acting on ornament design. We consider two alternative models of receiver behavior, each consistent with previous results. Females could respond either to specific characteristics of the sword or to more general cues, such as the apparent size of potential mates. We showed female swordtails a series of computer-altered video sequences depicting a courting male. Footage of an intact male was preferred strongly to otherwise identical sequences in which portions of the sword had been deleted selectively, but a disembodied courting sword was less attractive than an intact male. There was no difference between responses to an isolated sword and to a swordless male of comparable length, or between an isolated sword and a homogenous background. Female preference for a sworded male was abolished by enlarging the image of a swordless male to compensate for the reduction in length caused by removing the ornament. This pattern of results is consistent with mate choice being mediated by a general preference for large males rather than by specific characters. Similar processes may account for the evolution of exaggerated traits in other systems.
Keywords: sexual selection, visual signals, preexisting bias, video playback, Poeciliidae
The evolution of the conspicuous signals used in mate choice is constrained by the perceptual properties of receivers. This constraint is most evident in cases of “sensory exploitation,” in which male signals have evolved to match preexisting female biases (1–5). The resulting ornaments are relatively stereotyped, exhibiting the same distinguishing features within a species. The specific nature of ornaments that have evolved in response to ancestral biases suggests two possible accounts of their origin. Females may exercise a specific, narrowly defined, preference for a particular stimulus. Sexual selection would then substantially constrain the design of the male ornament. Alternatively, females may have a predilection for broad classes of stimuli. If this scenario was true, a wide variety of phenotypes might increase male mating success. The specific form of an ornament would then be constrained by factors other than mate choice, such as intrasexual competition, natural selection, and evolutionary history.
Male swordtails (Xiphophorus) were cited by Darwin (6) as an example of extreme sexual ornamentation. The lower rays of the caudal fin are elongated into a conspicuous, usually pigmented, “sword.” Xiphophorus includes the swordtails, in which most species have males with swords, and the platies, in which all of the species lack swords. Males of the sister group Priapella are also swordless. Females from two platy species, X. maculatus (2) and X. variatus (7), and female P. olmecae (1) all prefer conspecific males with artificial swords attached, indicating that a female preference for the ornament predates the appearance of the structure in males.
There are at least two distinct ways in which female perceptual processes may have shaped male morphology in this system. It is possible that female swordtails have a narrow preference for swords or sword-like structures. In this case, swords effectively would be acting as a releaser of female mating behavior (8). Alternatively, a more general preference may have interacted with specific factors limiting male expression of conspicuous traits. For example, fin elongations elsewhere on the body may bear a higher hydrodynamic cost or be subject to greater developmental constraint. There also might be simply a lack of genetic variation for all but one or a few traits. A permissive bias could manifest itself in a preference for males of larger total size, which is a widespread trait in poeciliids (1, 9–11) and many other taxa (12).
Assessing the specificity of female mate choice criteria requires highly controlled manipulations of male morphology. It has been historically difficult to conduct experimental analyses of the responses evoked by visual signals, but recent studies have used video playback techniques to explore successfully a diverse array of systems (13–18). A major advantage over static models is that video stimuli retain sexual display characteristics. Female green swordtails prefer analog video sequences of a courting male to sequences of the same male engaged in noncourting movements or remaining inactive (17), suggesting that courtship-specific motor patterns are an important part of the visual signal.
We used computer-generated animations to analyze the perceptual basis of female response to visual signals in green swordtails. Two sets of experiments were conducted. In the first, we assessed female preferences for different components of the sword by presenting an animation of a normal male engaged in courtship display and two sequences, derived from this, in which portions of the sword had been removed digitally. In the second experiment, we tested between alternative hypotheses concerning the specificity of the female preference by presenting components of the sword in isolation and by manipulating stimulus size.
MATERIALS AND METHODS
Subjects.
Female X. helleri were obtained from the Xiphophorus Genetic Stock Center at Southwest Texas State University (San Marcos, TX). All of the subjects were descendants of individuals collected at various sites in southern Mexico between 1963 and 1980. We maintained the fish in the laboratory on a 14:10 h light:dark cycle, with both natural and fluorescent light available during the day. TetraMin flake food (TetraWerke, Melle, Germany), supplemented by live brine shrimp nauplii and frozen bloodworms, was provided daily. Females were housed in groups of 3–5 in 20-, 40-, and 60-l aquaria at a temperature of 26–27°C with various aquatic plants provided for shelter. All of the subjects were sexually mature and had prior experience with adult males. Females were isolated from males for at least one month prior to testing to standardize sexual responsiveness (19).
Preparation of Stimuli.
Stimuli were based on the videotaped sequence of a displaying male that had elicited the strongest response from females in a previous study (17). The original footage had been videotaped so that the area occupied by the image of an unmanipulated male was life-sized on the 9-inch monitors used for stimulus presentations. We selected a 10.8-s sequence that could be “looped” without discontinuity in movement. Every other video frame (15 frames/s) in this sequence was digitized using a FrameGrabber real time video image digitizer (Progressive Peripherals, Denver, CO). Maximum sampling rate at which the resultant animation could reproduce the fish’s movements at natural speed. Digitized frames were then loaded into an image manipulation program (deluxepaint IV, Electronic Arts, San Mateo, CA) on an Amiga 2000 computer, which was used to isolate the image of the male against a homogeneous background. 168 such frames were created to produce a 10.8-s sequence. Differences between successive animation frames were small, giving the illusion, to a human observer, of continuous motion.
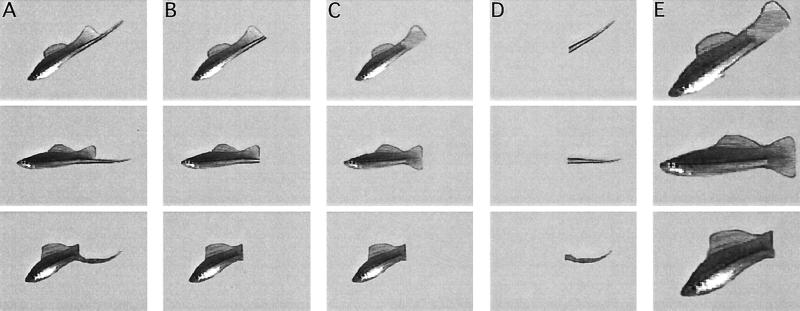
The resulting stimulus (intact; Fig. 1A) had a standard length (snout to caudal peduncle, SL) of 40 mm and a total body length (TL) of 70 mm. Males collected from wild populations range from 24 to >60 mm SL (20). The mean area of the intact stimulus on the screen, based on a sample of every tenth frame, was 601 mm2. This original sequence subsequently was altered, frame-by-frame, to generate the other stimuli. We created the partial sword stimulus(Fig. 1B; SL 40 mm, TL 49 mm, mean area 546 mm2) by erasing the sword extension protruding behind the distal margin of the dorsal half of the caudal fin. This stimulus retained the upper and lower stripes on the caudal fin but not the protruding extension. The no sword stimulus (Fig. 1C; SL 40 mm, TL 49 mm, mean area 548 mm2) was created by pasting a rotated copy of the dorsal half of the caudal fin onto the ventral half, thus simulating a complete tail lacking all components of the sword. For the sword only stimulus (Fig. 1D; length 33 mm, mean area 100 mm2), we erased the body and the dorsal part of the caudal fin. The enlarged stimulus (Fig. 1E; SL 69 mm, TL 83 mm, mean area 1095 mm2) consisted of the no sword stimulus doubled in size both vertically and horizontally to maintain natural proportions. It was necessary to use an integer multiple for this scaling operation to avoid the introduction of artifacts. The blank screen stimulus was the monochromatic background only. We used elan performer (Elan Design, San Francisco, CA), a program which plays computer-generated animations at specified intervals, to create five 100-s stimulus sequences, each consisting of 9.26 looped iterations of one of the animations. Completed stimuli were transferred to an S-VHS master videotape by using a Panasonic AG-7750 videocassette recorder and a Digital Creations (Rancho Cordova, CA) SuperGen genlock (a device that converts the signal produced by a computer into a composite video signal). Stimulus sequences subsequently were copied to VHS videotape for use in presentations.
Figure 1.
Stimuli used in playback experiments: (A) intact; (B) partial sword; (C) no sword; (D) sword only; and (E) enlarged. Successive frames show a portion of the sequence at 1-s (15-frame) intervals.
Videorecorded sequences are made up of a series of discrete fields. The NTSC (National Television Standards Committee) television standard displays 60 such images/s. The maximum critical flicker fusion frequency for X. helleri is 43.1 Hz (21), appreciably lower than this refresh rate and below most published values for humans. The subjects in this study and in a previous one (17) showed a pattern of responses consistent with recognition of the video sequences as conspecifics, suggesting that any perceived flicker was not producing critical deficiencies in image sequences. Systematic quantitative comparisons of female X. helleri responses to digitized video sequences and to live male conspecifics reveal no differences in the attractiveness of these two stimulus types when presented against null stimuli (B. Trainor and A. Basolo, unpublished data). Female guppies (Poecilia reticulata) similarly spend the same amount of time associating with video images of conspecific males as they do associating with live males behind a one-way glass barrier (22).
Playback Presentations.
Females were tested in a 76.8 × 31.8 cm aquarium filled to a height of 25.1 cm. The aquarium was divided into three 25.6-cm sections (left, right, and center) with a small clump of Java moss provided for cover in the center section. A Panasonic TR-930B 9-inch monochrome monitor abutted either end of the aquarium. Monochrome images were used because they are likely to reproduce intensity and contrast information more accurately than color monitors, which have been designed specifically for human spectral sensitivity. Monitor output was matched prior to each test by placing the monitors adjacent to each other, playing identical sequences, and then adjusting the controls until the images were indistinguishable. Presentation sequences were played on Panasonic AG-1970 S-VHS videocassette players. The experimenter was concealed behind an opaque barrier.
Subjects were acclimated in the test tank with the monitors on for 20 min prior to the start of each test. For the experiment I trials, females were presented with 10 min of monochromatic screen on both sides followed by simultaneous presentation of the 100-s test stimuli. We recorded the amount of time the female spent in each section for 100 s immediately before stimulus onset and for the duration of the stimulus. Females that spent >190 s in any one section were excluded from analyses. Side biases were controlled across subjects by alternating stimulus location from one trial to the next.
In experiment I, we presented a series of stimuli that lacked one or more components of the male ornament. We compared female responses to a fully sworded male, possessing both the prominent stripe on the caudal fin and the pigmented extension of the lower caudal rays (intact; Fig. 1A) vs. the same male with the protruding part of the sword (i.e., the sword extension, ref. 7) removed (partial sword; Fig. 1B), intact vs. the same male with the entire sword removed (no sword; Fig. 1C), and partial sword vs. no sword. Each female was shown all of the three stimulus pairs in a random order.
In experiment II, negative results were potentially relevant to the hypotheses we were testing, and we therefore wanted to rule out low sexual motivation as an explanation for a lack of female preference. Procedures for the simultaneous choice tests in experiment II were identical to those for experiment I, but we introduced a pretest screening procedure. We used an assay based on results from a previous study (17). After acclimation, females were shown a 10-min background sequence followed by simultaneous presentation of two 3-min analog video sequences of a male that was different from the one used to generate the stimuli. One sequence depicted courtship displays whereas the other depicted the same male inactive. After a 3-min blank screen interval, the sequences were reversed. Only females that spent at least 60 s with the courting male in both intervals, and that spent a majority of time with the courting male in each interval, were retained for testing. To control for any effects of the screening process, we varied the position of the test stimuli so that each stimulus would appear on the same side as each of the screening stimuli with equal frequency. In addition, the initial side of both the screening and test stimuli were alternated to control for side biases. Females that failed the screening procedure were reevaluated after a minimum of 24 h. Approximately one in four of the screening trials were successful, suggesting that this procedure ensured a high level of sexual responsiveness.
Females that met the screening criteria were immediately shown a 3-min background sequence on each monitor, followed by the 100-s stimulus pair. We allowed a minimum interval of 24 h between tests. The presentation order of stimulus pairs was randomized to control for possible order effects. We examined female response to four pairs of stimuli: intact (Fig. 1A) vs. a disembodied courting sword (sword only; Fig. 1D), no sword (Fig. 1C) vs. sword only, sword only vs. a homogeneous background screen (blank screen), and intact vs. an enlarged version of the swordless male (enlarged; Fig. 1E).
The logic behind the choice of stimulus pairs was as follows. If females attend specifically to the ornament, then the sword should be both necessary and sufficient to elicit female response. A releaser model clearly predicts that females should prefer stimuli with swords over swordless stimuli and that females should respond to swords presented in isolation. In contrast, if females simply prefer stimuli of greater total length, then they should do so whether or not the ornament is present. A male with a sword should thus be preferred over a disembodied sword, but there should be no difference in response to stimuli of comparable size.
Data Analysis.
The measure of female choice employed was association time, which is a standard assay of sexual response in Xiphophorus (1–2, 11, 23–25). Female association time with live males confined behind glass partitions is an excellent predictor of poeciliid male reproductive success in nature and of mating patterns in “open field” trials (e.g. 10, 25). Differences in mean association time for each stimulus pair were analyzed with Wilcoxon-signed rank-matched pairs tests. We also conducted a post hoc analysis of the strength of preference, measured as the net amount of time spent with the favored male, for female responses to the stimulus pairs in experiment I.
Previous studies using surgically manipulated live male swordtails (26) and males with artificial ornaments (1, 2, 7, 23) have demonstrated reliably a female preference for males with swords over those without and for males with longer swords over those with shorter ones. Female X. variatus (whose males lack swords) similarly prefer static wooden models with swords or with long tails (24). These results suggest that females should prefer males with a full sword (intact) to males with shorter swords (partial sword and no sword). If a preference for males with a caudal stripe (7) is ancestral to the genus, either as a specific preference for this trait or as part of a more general preference, then female X. helleri also should prefer males with caudal stripes (partial sword) to those lacking this character (no sword). Previous work thus provided clear a priori predictions for each of the comparisons in experiment I, and for this reason we used one-tailed tests of significance. For experiment II, predictions were available for the intact vs. sword only comparison, in which the body size hypothesis predicted a preference for intact, and for the sword only vs. blank screen comparison, in which the sign stimulus hypothesis suggested that there should be a preference for sword only. We used one-tailed tests of significance for these comparisons; all other tests in experiment II were two-tailed.
RESULTS
Experiment I.
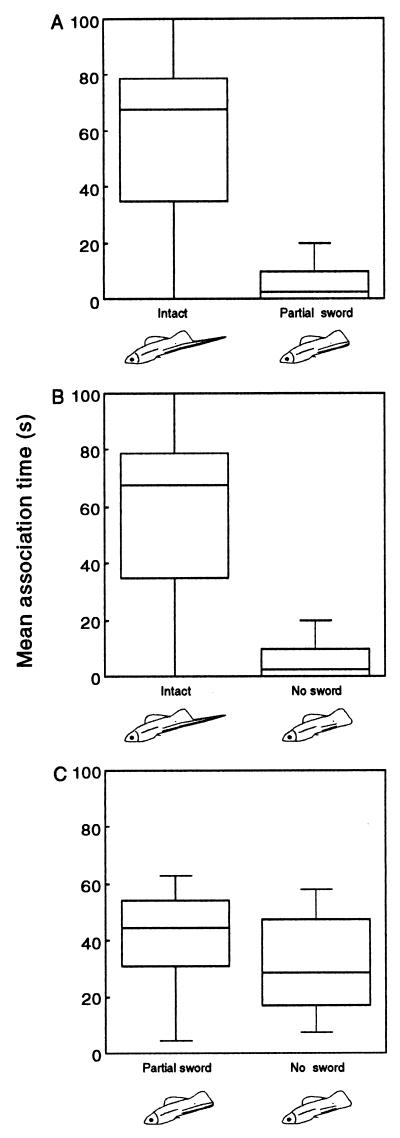
Females showed a strong preference for an intact over a partial sword sequence (Fig. 2A; Z = −2.12, P < .02), and spent an order of magnitude more time with intact than with no sword (Fig. 2B; Z = −2.97, P < 0.002) . Females tended to spend more time associating with the partial sword sequence than with the no sword sequence (Fig. 2C), although this failed to achieve statistical significance (Z = −1.49, P = 0.064). The strength of preference for intact over no sword was significantly greater than that for partial sword over no sword (Mann–Whitney U test; P < 0.01), but not significantly different from that for intact over partial sword (Mann–Whitney U test; P = 0.17) sequences.
Figure 2.
Association time (s) of female X. helleri in experiment I with simultaneously presented stimuli. (A) Intact vs. partial sword (n = 17); (B) intact vs. no sword (n = 14); and (C) partial sword vs. no sword (n = 17). The line in the box denotes the median, the lower and upper edges of the box the 25% and 75% values, and the two whiskers the 10% and 90% values.
Experiment II.
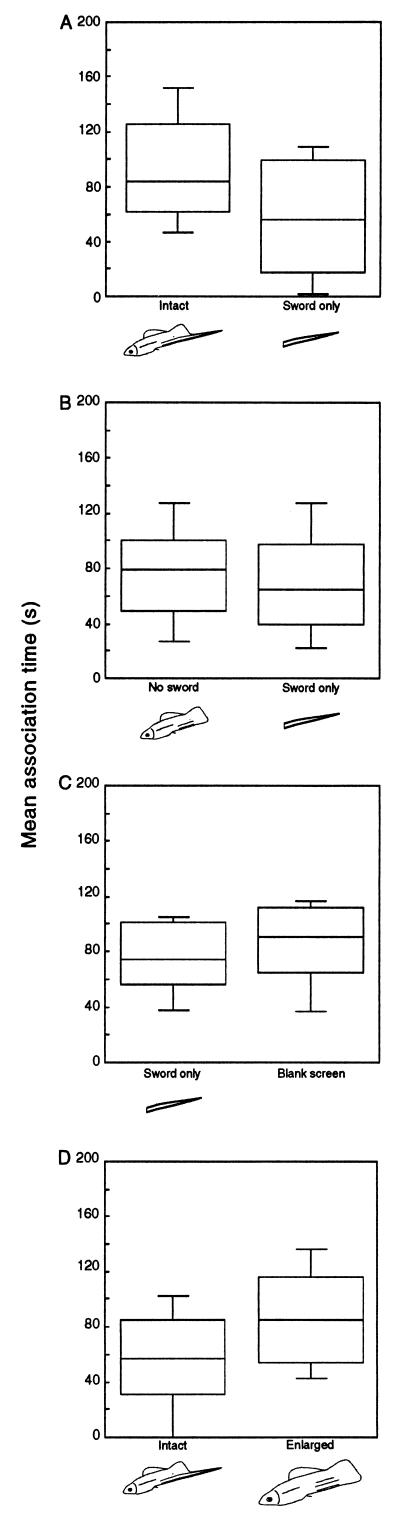
Females significantly preferred the intact stimulus over the sword only stimulus (Fig. 3A; Z = −1.66, P < 0.05). There was no significant difference in association times between the no sword and sword only stimuli (Fig. 3B; Z = −0.414; p = 0.49). Sword only also was not preferred over a blank background screen (Fig. 3C; Z = 0.691; p = 0.34).
Figure 3.
Association time (s) of female X. helleri in experiment II with simultaneously presented stimuli. (A) Intact vs. sword only (n = 17); (B) no sword vs. sword only (n = 18); (C) sword only vs. blank screen (n = 14); and (D) intact vs. enlarged (n = 14).
The robust preference for the intact over the no sword stimulus that had been apparent in experiment I (Fig. 2B) was abolished by enlarging the swordless stimulus, and there was a nonsignificant trend in favor of the enlarged stimulus (Fig. 3D; Z = −1.503; p = 0.13).
DISCUSSION
Our findings are consistent with the hypothesis that responsiveness to the sword is a consequence of the increase in size that it confers. Removal of the sword caused an order of magnitude reduction in female response, but enlargement of the body was sufficient to compensate fully for the loss of the ornament. By using digital video sequences, we were able to retain courtship motor patterns on a disembodied sword. This result allowed us to rule out the possibility that females might have a specific preference for swords contingent on these structures moving in a natural way.
Females had a strong preference for image sequences depicting a male with an intact sword over sequences in which the ornament had been removed either partially or fully (Figs. 2 A and B). This finding replicates with video stimuli results obtained in previous studies using manipulations of the appearance of live males (23, 26). Our results also extend earlier work: by using digital video sequences depicting identical courtship motor patterns, we have been able to exclude the possibility that female preference might by influenced by changes in the behavior of manipulated males. Enlarging the swordless male stimulus made it just as attractive as the image of a normal sworded male (Fig. 3D). The qualitative difference in association time between intact and no sword that had been apparent in experiment I (Fig. 2B) was eliminated simply by scaling the no sword image, even though caudal morphology was unchanged. The sword is thus not a necessary trait for eliciting female preference.
Females preferred the intact stimulus to the sword only stimulus (Fig. 3A), demonstrating that an isolated courting sword is not sufficient to elicit a full sexual response. Sword only also was not preferred over a swordless male (Fig. 3B) or a blank background screen (Fig. 3C), suggesting that a disembodied sword is not especially salient. It is therefore unlikely that swords are perceived as prey items (2). It is important to note that the association times in the sword only vs. no sword and sword only vs. blank screen comparisons were nearly identical for the two stimuli.
Basolo (7) found that female platies, X. maculatus, preferred males with a stripe on the lower margin of the caudal fin both to males that naturally lacked these markings and to males from which they had been surgically removed. Our failure to find a similar effect in X. helleri may have been caused by low statistical power or to deficiencies in the video stimuli such that they were less discriminable than live males with equivalent morphology. Nevertheless, preference for the intact over the no sword sequence was significantly stronger than that for partial sword over no sword, but not significantly different from that for intact over partial sword. This comparison suggests that the magnitude of any contribution made by the caudal stripe to overall male attractiveness in X. helleri is smaller than that of the protruding sword, which confers an increase in total body size.
The experimental results demonstrate that the sword is neither necessary nor sufficient for eliciting sexual responses in X. helleri. We conclude that the male ornament does not function as a sign stimulus. However, the sword definitely enhances the attractiveness of males. We suggest that female preference may be constructed hierarchically. In some species, such as the túngara frog Physalaemus pustulosus, sexually selected signal components only influence female choice when they are accompanied by a species recognition component. If the species-typical “whine” is present in a call, a wide variety of additional components, from a normal “chuck” to white noise, can enhance signal attractiveness (27). In poeciliid fishes, specific visual cues from the male’s body may be necessary for initial recognition of potential mates but a range of possible ornaments might then have equivalent effects.
This account may appear difficult to reconcile with the fact that females failed to prefer no sword—which had many of the behavioral and morphological cues provided by a conspecific male—over a disembodied sword (Fig. 3B), particularly because the latter stimulus was not preferred over a blank screen. Several factors may account for this apparent lack of preference. Females may have simply failed to attend to the small difference in length (6 mm) between the two stimuli. Alternatively, females may actively avoid males below a certain size threshold. Male X. helleri occasionally perform “sneak/chase” behavior, in which copulation is attempted without a courtship display (26). In at least two swordtails, X. multilineatus and X. nigrensis, sneak/chase behavior is correlated negatively with body size, and females avoid mating with small males (25). In addition, platyfishes—swordless, generally smaller congeners—are broadly sympatric with X. helleri (28). Females may thus avoid small males because they are perceived as heterospecifics.
Receiver permissiveness is a widespread trait. Females in several Xiphophorus (1, 7, 12) and two Poecilia species (9–10) show a preference for males of greater apparent size, suggesting that the preference is likely to be plesiomorphic in the genus Xiphophorus. A general bias toward larger males may result incidentally from greater stimulation of the visual system (29) or may have an adaptive basis (30); these accounts are not mutually exclusive (12). Ornaments that exploit preexisting biases should be more likely to evolve if the biases are permissive, because this increases the likelihood that a rare variant will have a fitness advantage with respect to sexual selection. The specific form and placement of the sword may thus be the result of idiosyncratic patterns of genetic variation in an ancestral population. The sword in Xiphophorus, the elongate gonopodium in Priapella, and the extended dorsal fin in Poecilia may represent the arbitrary evolutionary responses of different lineages to a general perceptual preference in females.
The preference–trait interaction observed in X. helleri may be an instance of a common phenomenon in the evolution of signals and receivers. Sexually dimorphic structures that increase apparent size are ubiquitous, as are directional preferences for large size (12). Male X. helleri on restricted diets tend to increase in sword length but not in body length, whereas males on ad libitum diets continue to invest in both traits (31). Swords may thus offer a metabolically inexpensive means of appearing large. Analogous traits in other animals, such as an elongated tail or crest feathers, may function in a similar way, exploiting a permissive perceptual system while minimizing metabolic costs.
Acknowledgments
We are indebted to M. J. Ryan for thorough comments on many drafts and for access to facilities. We thank D. Morizot and the Xiphophorus Genetic Stock Center for generously providing experimental animals; J. Crutchfield, L. Gilbert, and the Brackenridge Field Laboratory, University of Texas at Austin, for housing study animals; A. Basolo, K. C. Y. Cheng, E. Deinert, L. Dries, L. Evans, L. Higgins, D. Johnson, M. Kirkpatrick, M. Servedio, G. Sword, B. Trainor, K. Warkentin, P. Warren, K. Witte, and four anonymous reviewers for comments; K. Bok, S. Hoover, S. Livingston, and W. L. Miller for assistance in creating the stimuli; and K. Hall, T. Prude, T. D. Green, C. McCain, and A. McCormick for help performing the experiments. Supported by grants from the National Science Foundation and Dr. Lorraine Stengl (to G.G.R.) and by a grant from the Australian Research Council (to C.S.E.).
Footnotes
Abbreviations, SL, standard length; TL, total length.
References
- 1.Basolo A L. Proc R Soc London Ser B. 1995;259:307–311. doi: 10.1098/rspb.1995.0045. [DOI] [PubMed] [Google Scholar]
- 2.Basolo A L. Science. 1990;250:808–810. doi: 10.1126/science.250.4982.808. [DOI] [PubMed] [Google Scholar]
- 3.Ryan M J. Oxf Surv Evol Biol. 1990;7:157–195. [Google Scholar]
- 4.Endler J. Philos Trans R Soc London B. 1993;340:215–225. doi: 10.1098/rstb.1993.0060. [DOI] [PubMed] [Google Scholar]
- 5.Guilford T, Dawkins M S. Trends Neurosci. 1993;16:430–436. doi: 10.1016/0166-2236(93)90068-w. [DOI] [PubMed] [Google Scholar]
- 6.Darwin C. The Descent of Man and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 7.Basolo A L. Anim Behav. 1995;50:365–375. [Google Scholar]
- 8.Tinbergen N. The Study of Instinct. New York: Oxford Univ. Press; 1951. [Google Scholar]
- 9.Bischoff J A, Gould J L, Rubenstein D I. Behav Ecol Sociobiol. 1985;17:253–255. [Google Scholar]
- 10.Schlupp I, Marler C A, Ryan M J. Science. 1994;263:373–374. doi: 10.1126/science.8278809. [DOI] [PubMed] [Google Scholar]
- 11.Ryan M J, Wagner W E., Jr Science. 1987;236:595–597. doi: 10.1126/science.236.4801.595. [DOI] [PubMed] [Google Scholar]
- 12.Ryan M J, Keddy-Hector A. Am Nat. 1992;139:S4–S35. [Google Scholar]
- 13.Clark D L, Uetz G W. Proc Natl Acad Sci USA. 1996;90:11954–11957. doi: 10.1073/pnas.90.24.11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans C S, Macedonia J M, Marler P. Anim Behav. 1993;46:1–11. [Google Scholar]
- 15.Macedonia J M, Stamps J A. Ethology. 1994;98:246–264. [Google Scholar]
- 16.McKinnon J S, McPhail J D. Can J Zool. 1996;74:1727–1733. [Google Scholar]
- 17.Rosenthal G G, Evans C S, Miller W L. Anim Behav. 1996;51:811–820. [Google Scholar]
- 18.Rowland W J, Bolyard K J, Jenkins J J, Fowler J. Anim Behav. 1995;49:1559–1567. [Google Scholar]
- 19.Morris M R, Wagner W E, Ryan M J. Anim Behav. 1996;52:1193–1203. [Google Scholar]
- 20.Kallman K D. In: Genetic Control of Size at Maturity in Xiphophorus. In Ecology and Evolution of Livebearing Fishes (Poeciliidae) Meffe G K, Snelson F F, editors. Englewood Cliffs, NJ: Prentice–Hall; 1989. pp. 163–184. [Google Scholar]
- 21.Crozier W J, Wolf E. J Gen Physiol. 1939;22:463–485. doi: 10.1085/jgp.22.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kodric-Brown A, Nicoletto P F. Anim Behav. 1997;54:369–376. doi: 10.1006/anbe.1996.0420. [DOI] [PubMed] [Google Scholar]
- 23.Basolo A L. Anim Behav. 1990;40:332–338. [Google Scholar]
- 24.Haines S E, Gould J L. Nature (London) 1994;370:512. [Google Scholar]
- 25.Ryan M J, Hews D K, Wagner W E., Jr Behav Ecol Sociobiol. 1990;24:341–348. [Google Scholar]
- 26.Franck D. Zool Jb Physiol Bd. 1964;71:117–170. [Google Scholar]
- 27.Wilczynski W, Rand A S, Ryan M J. Anim Behav. 1995;49:911–929. doi: 10.1006/anbe.1999.1208. [DOI] [PubMed] [Google Scholar]
- 28.Rosen D E. Bull Am Mus Nat Hist. 1979;162:268–375. [Google Scholar]
- 29.Rowland W J. Anim Behav. 1989;38:112–120. [Google Scholar]
- 30.Dawkins M S, Guilford T. Am Nat. 1996;148:937–942. [Google Scholar]
- 31.Basolo, A. (1998) Anim. Behav., in press. [DOI] [PubMed]