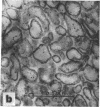
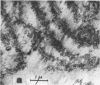
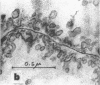
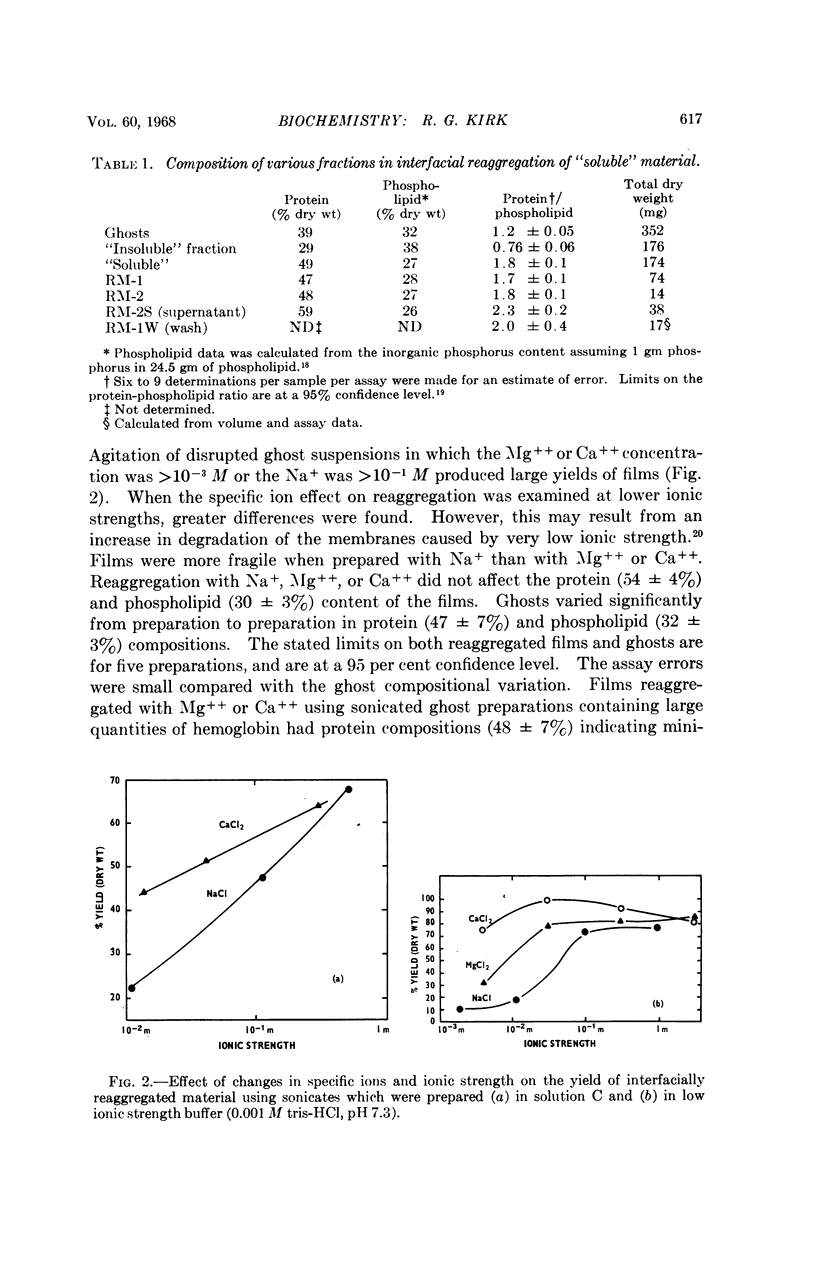
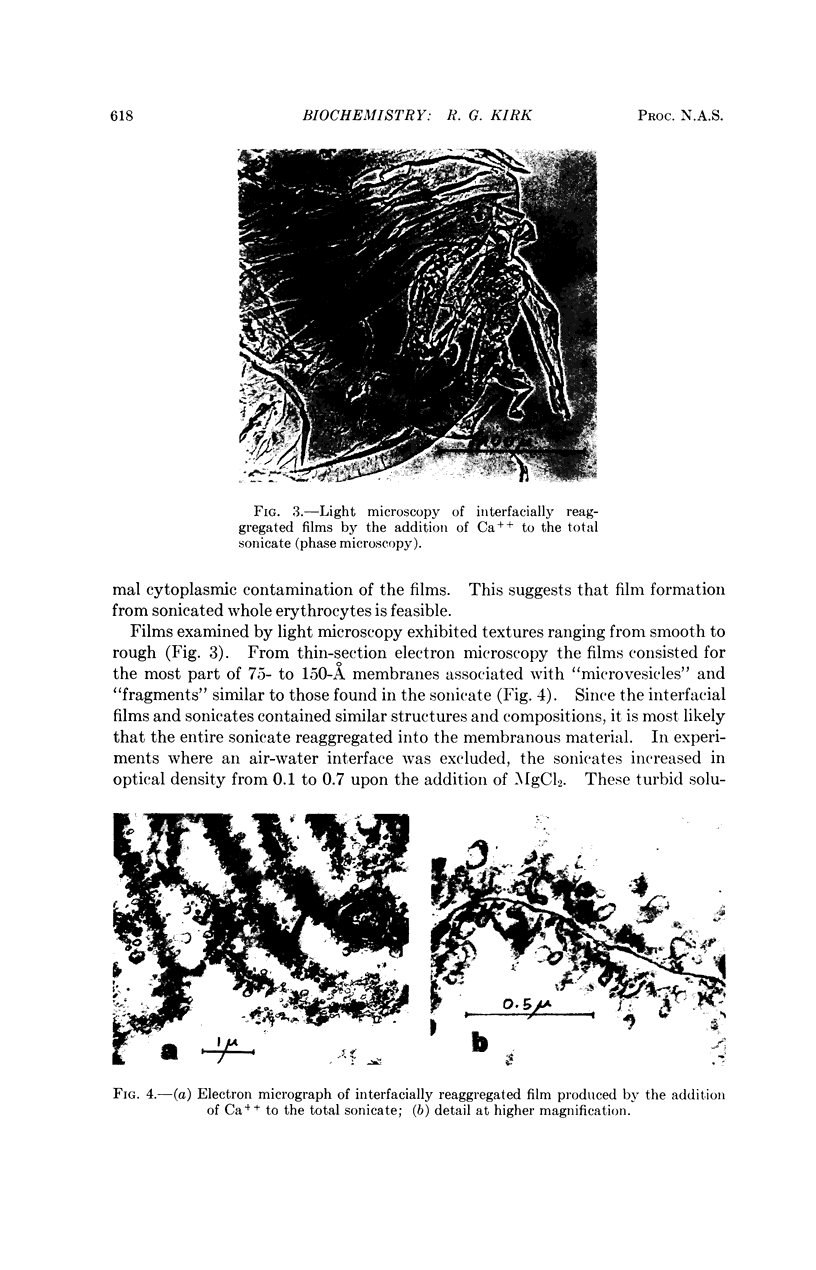
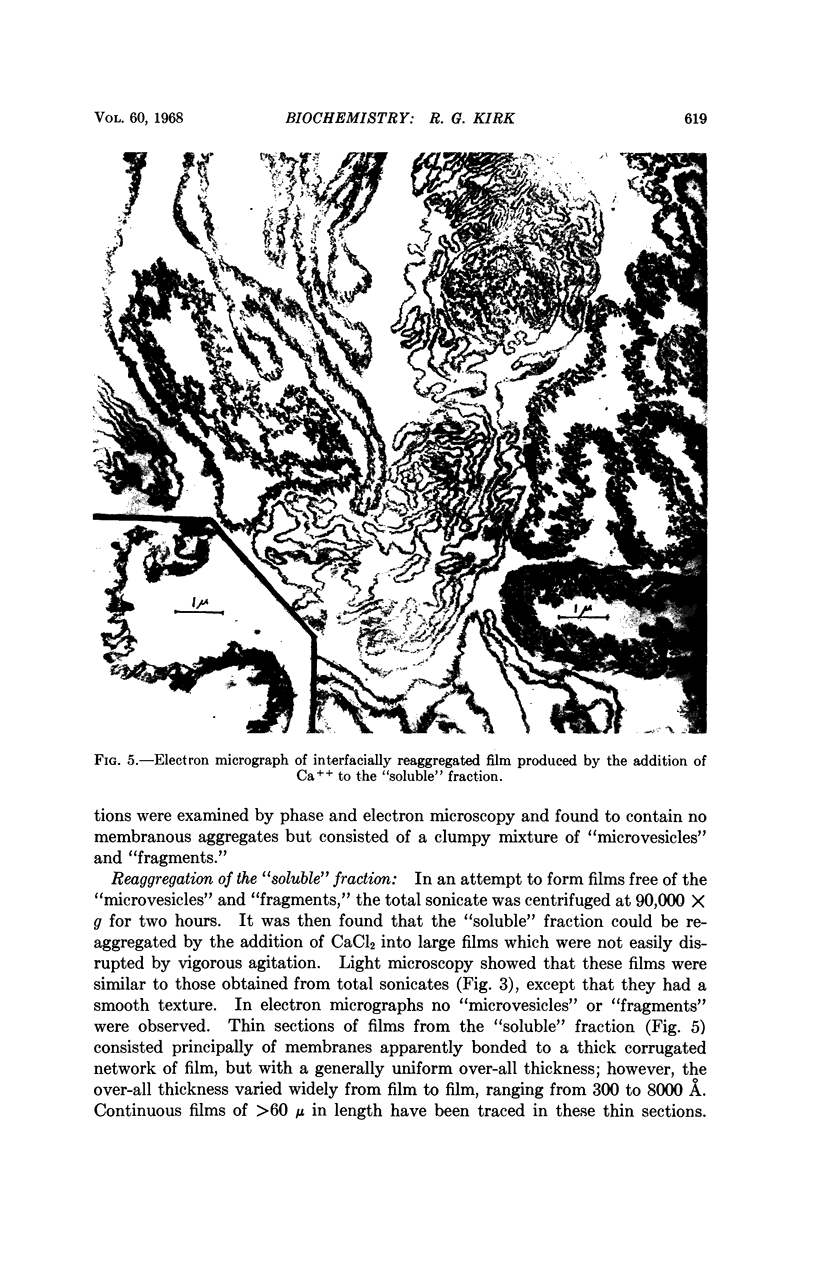
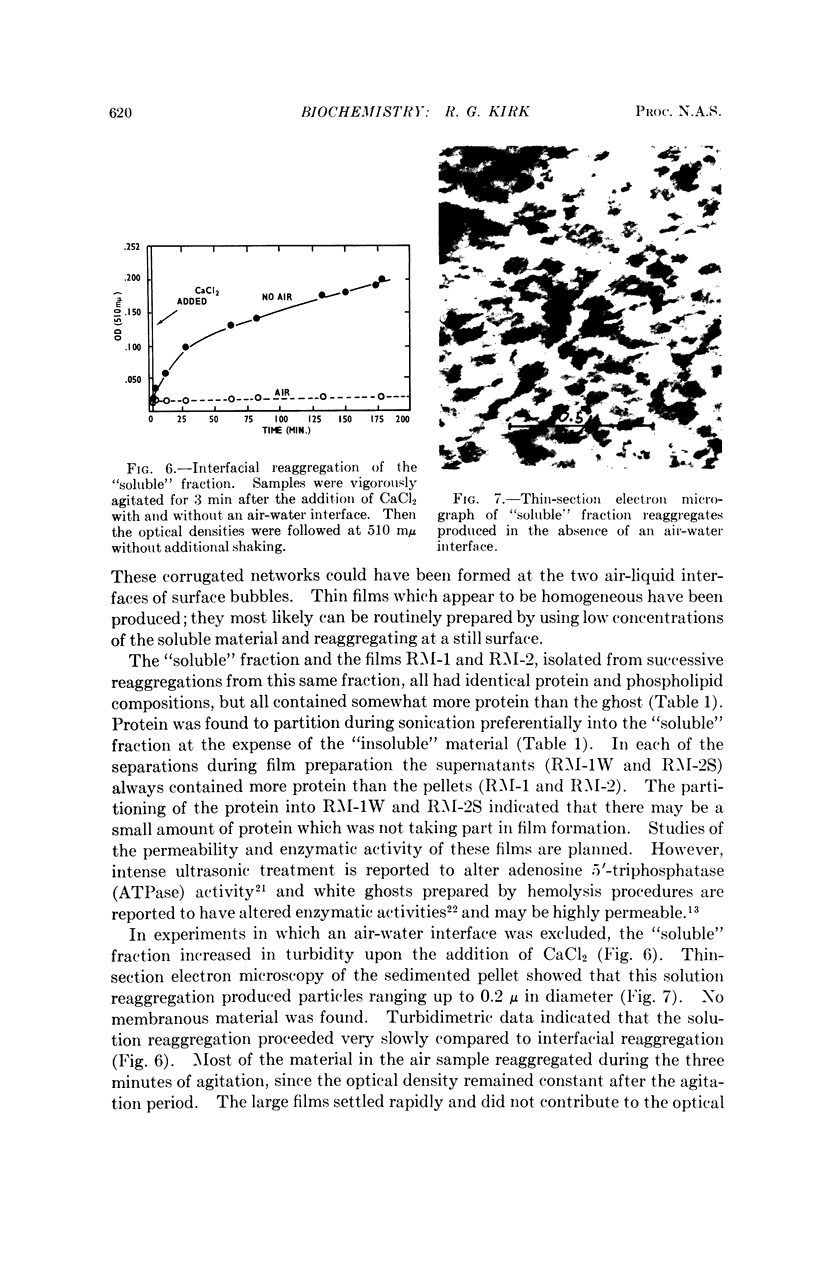
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Butler T. F., Smith G. L., Grula E. A. Bacterial cell membranes. I. Reaggregation of membrane subunits from Micrococcus lysodeikticus. Can J Microbiol. 1967 Nov;13(11):1471–1479. doi: 10.1139/m67-195. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Terry T. M., Morowitz H. J. Characterization of the plasma membrane of Mycoplasma laidlawii. I. Sodium dodecyl sulfate solubilization. Biochim Biophys Acta. 1967 Jul 3;135(3):381–390. doi: 10.1016/0005-2736(67)90028-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MANILOFF J., MOROWITZ H. J., BARRNETT R. J. STUDIES OF THE ULTRASTRUCTURE AND RIBOSOMAL ARRANGEMENTS OF THE PLEUROPNEUMONIA-LIKE ORGANISM A5969. J Cell Biol. 1965 Apr;25:139–150. doi: 10.1083/jcb.25.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Palade G. E. The localization of Mg-Na-K-activated adenosine triphosphatase on red cell ghost membranes. J Cell Biol. 1967 Nov;35(2):385–404. doi: 10.1083/jcb.35.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Action potential phenomena in experimental bimolecular lipid membranes. Nature. 1967 Feb 11;213(5076):603–604. doi: 10.1038/213603a0. [DOI] [PubMed] [Google Scholar]
- Razin S., Morowitz H. J., Terry T. M. Membrane subunits of Mycoplasma laidlawii and their assembly to membranelike structures. Proc Natl Acad Sci U S A. 1965 Jul;54(1):219–225. doi: 10.1073/pnas.54.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Netschey A. Physical chemistry of isolated bacterial membranes. Biochim Biophys Acta. 1965 Oct 18;107(3):539–545. doi: 10.1016/0304-4165(65)90198-4. [DOI] [PubMed] [Google Scholar]
- Tosteson D. C. Some properties of HK and LK sheep red cell membrane fragements and their relevance to active transport of K and NA. Trans N Y Acad Sci. 1965 Jun;27(8):970–977. doi: 10.1111/j.2164-0947.1965.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Wheeler K. P., Whittam R. Structural and enzymic aspects of the hydrolysis of adenosine triphosphate by membranes of kidney cortex and erythrocytes. Biochem J. 1964 Nov;93(2):349–363. doi: 10.1042/bj0930349. [DOI] [PMC free article] [PubMed] [Google Scholar]