Abstract
Heat shock protein 90α (Hsp90α) was immobilized on aminopropyl silica via the N-terminus to create the Hsp90α(NT)-column or C-terminus to create the Hsp90α(CT)-column. Binding to the exposed C-terminus on the Hsp90α(NT)-column was characterized using frontal chromatography and C-terminus ligands coumermycin A1(CA1) and novobiocin (NOVO). The calculated Kd values were 220 ± 110 nM (CA1) and 100 ± 20 nM (NOVO). Non-linear chromatography was used to determine the association and dissociation rate constants associated with the NOVO-Hsp90α complex, 22.2 (±8.8) μM−1 sec−1 and 2.7 (±0.6) sec−1, respectively. Binding to the exposed N-terminus on the Hsp90α(CT)-column was characterized using frontal chromatography. The Kd values of N-terminus ligands geldanamycin (GM) (90 ± 50 nM) 17-(Allylamino)-17-demethoxygeldanamycin (17-AAG) (210 ± 50 nM) and radicicol (RAD) (20 ± 9 nM) were consistent with previously reported values. The effect of the immobilization on ATPase activity was investigated through the determination of IC50 values for inhibition of ATPase activity on the Hsp90α(CT)-column. The IC50 for GM was 2.80 ± 0.18 μM and the relative IC50 values were 17-AAG > GM > RAD, in agreement with previously reported values and indicating that immobilization had not affected ATPase activity or sensitivity to inhibition.
Keywords: Hsp90, online screening, affinity chromatography, inhibitors
Introduction
Heat shock protein 90 (Hsp90) is a family of cellular proteins (Hsp90α, Hsp90β, Grp94, Trap 1) that act as molecular chaperones, which guide the normal folding, intracellular disposition and proteolytic turnover of many key regulators of cell growth and survival [1]. These proteins are required for the stability and function of over 100 proteins (clients) including receptors, protein kinases and transcription factors [1, 2]. Hsp90 operates through the formation of dynamic multiprotein-client complexes and inhibitors of these complexes and/or the activity of Hsp90 within these complexes have been identified. Since increased expression and activity of Hsp90 have been observed in human cancers, Hsp90 inhibitors represent a new and perhaps selective class of anti-cancer agents [1-6]. One Hsp90 inhibitor, 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) is currently in clinical trials [2,4].
The structure and function of Hsp90 has been the subject of recent reviews [1,2]. The protein contains highly conserved N-, middle- and C-terminal domains, Fig. 1, and displays an intrinsic ATPase activity, which is necessary for its function [1]. The ATPase activity has been associated with an adenine nucleotide binding pocket in the N-terminus, although the middle- and C-terminal domains also appear to play a role in this function [1]. Drug development programs have been directed at compounds that bind to either the N- or C-terminus of Hsp90 and that disrupt client-Hsp90 complexes and inhibit ATPase activity [1-6].
Figure 1.
Structure of Heat Shock 90 protein (Hsp90α) adapted from reference 1.
Since binding to Hsp90 is a critical step, the determination of binding affinities, Kd values, of potential Hsp90 inhibitors is an important aspect of drug discovery programs. These affinities have been determined using isothermal titration calorimetry (ITC) [3] or competitive binding studies [5,7]. In the latter experiments, biotinylated geldanamycin (GM) was used as the marker ligand. An additional approach to the identification and description of Hsp90 inhibitors and for determination of Kd values is the use of an online screen utilizing a liquid chromatography column containing immobilized Hsp90. Previous studies with columns containing immobilized human serum albumin or other transport proteins have been used to determine ligand binding affinities, ligand binding sites, and ligand-ligand binding interactions including allosteric interactions [8,9].
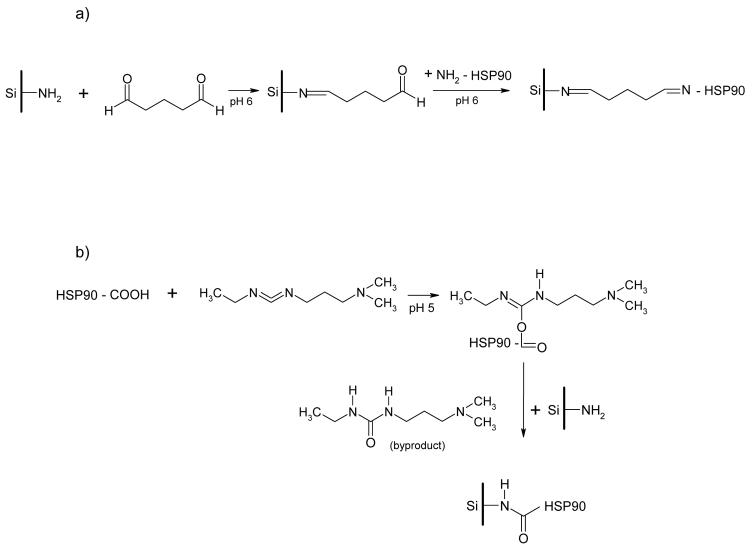
In the present study, Hsp90α has been covalently immobilized onto the surface of an aminopropyl silica liquid chromatography stationary phase. Since both the N-terminus and C-terminus of the protein are targets for drug development, the protein was immobilized via the N-terminus to create the Hsp90α(NT)-column or by the C-terminus to create the Hsp90α(CT)-column, Fig. 2. The immobilizations were accomplished using standard coupling techniques involving glutaraldehyde (N-terminus) or 1-ethyl-3-(3-methylaminopropyl)carbodiimide (C-terminus). The columns were used to study binding interactions with the immobilized Hsp90α including determination of Kd values and association and dissociation rate constants. The results from these studies are reported below.
Figure 2.
The synthetic approaches used in the covalent immobilization of Hsp90α on an aminopropyl silica liquid chromatography stationary phase via the a) amino terminus and b) carboxyl terminus.
The intrinsic ATPase activity of Hsp90 is also a key target in drug discovery. Functional studies to determine IC50 values of putative Hsp90 ATPase inhibitors have involved a pyruvate/lactate dehydrogenase-coupled enzyme assay [3] and the direct measurement of free inorganic phosphate using fluorescence [7] or colorimetric [10] assays; the latter approach having been used in a high-throughput screen. Functional assays based upon growth inhibition [4] and HER-2 degradation [5] have also been used to study Hsp90 inhibition. Because ATPase activity is a key component of the protein's function as well as a primary pharmacological target, the effect of immobilization on Hsp90 ATPase activity was also investigated in order to determine if immobilization via the C-terminus affected binding at the adenine nucleotide binding pocket in the N-terminus.
Materials and methods
Chemicals
Coumermycin A1 (CA1) and radicicol (RAD) were purchased from Biomol Int. (Plymouth Meeting, PA), geldanamycin (GM) was purchased from InvivoGen (San Diego, CA, U.S.A.), 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) and novobiocin (NOVO) were purchased from Calbiochem (Darmstadt, Germany). Recombinant human HSP90α (∼90% pure) was purchased from Stressgen Bioreagents (Ann Arbor, MI, U.S.A.). Bovine serum albumin (BSA), ammonium acetate, ATP, 1-ethyl-3-(3-methylaminopropyl)carbodiimide (EDC), glutaraldehyde, glutaric acid, glycine, pyridine (99.8%), sodium azide, and Tris buffer were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, U.S.A.). The water used in the study was prepared using a Milli-QWater Purification System (Millipore Corporation, Bedford, MA, U.S.A.). The aminopropyl silica (APS) stationary phase (Nucleosil 300-7 NH2) was purchased from Macherey-Nagel (Düren, Germany).
Immobilization of HSP90α via N-terminus (Fig 2A)
A 50 mg portion of APS was added to 10 ml of pyridine [10 mM, pH adjusted to 6.0 with 100 mM HCl] in a 15 ml conical plastic tube and the mixture was vortex-mixed for 15 min, centrifuged at 1500 × g for 10 min, and the supernatant was discarded. The APS was suspended in 10 ml of 5% glutaraldehyde, rotated at 200 rpm in an orbital shaker for 3 h and then centrifuged at 1500 × g for 10 min. The supernatant was discarded and the activated APS was washed three times with 10 ml portions of pyridine [10 mM, pH 6.0] as described above. A suspension of 200 μg human HSP90α protein in 300 μl of pyridine [10 mM, pH 6.0] was added to the activated APS, and then left for 24 h at 4°C. After the mixture had warmed to room temperature, 5 ml of glutaric acid [1M, pH 8.0] was added and the resulting mixture was rotated at 200 rpm in an orbital shaker for 30 min, centrifuged at 1500 × g for 10 min and the supernatant discarded. The HSP90α(NT)-silica was rinsed three times with 5 ml portions of Tris-HCl buffer [10 mM, pH 7.4] containing 150 mM NaCl, 0.1 % (w/v) BSA, 1mM EDTA, 0.1% sodium azide. The suspension containing the Hsp90α(NT)-silica was placed into a Tricorn 5/20 glass column (50 × 5 mm I.D., GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and allowed to settle. The fitting on the column were tightened to create a 6 × 5 mm I.D chromatographic bed. The column was washed with Tris-HCl buffer [10 mM, pH 7.4] for 2 h using a standard chromatographic pump with flow rate 0.2 ml/min at 25 °C. The Hsp90α(NT)-column was ready for immediate use or could be stored at 4°C until use.
Immobilization of HSP90α via C-terminus (Fig 2B)
A 100 mg portion of APS was placed in a 15 ml plastic tube and rinsed with 10 ml of potassium phosphate [10 mM, pH 5.5] containing 150 mM NaCl. Hsp90α protein (200μg or 400μg) was added to 400 μl of potassium phosphate [10 mM, pH 5.5] containing 150 mM NaCl and the suspension was added to the APS. The mixture was vortex-mixed for 5 min followed by the addition of 200 μl of a 10 mg/ml solution of EDC. The pH of the reaction mixture was adjusted to 5.0 using 0.1 M HCl and the mixture was then rotated at 200 rpm in an orbital shaker for 24 h at 4°C. The mixture was allowed to warm to room temperature, centrifuged at 1500 × g for 10 min and the supernatant was discarded. The Hsp90α(CT)-silica was rinsed three times with 5 ml portions of Tris-HCl buffer [10 mM, pH 7.4] containing 150 mM NaCl, 0.1 % (w/v) BSA, 1mM EDTA, 0.1% sodium azide. The suspension containing the Hsp90α(CT)-silica was placed into a Tricorn 5/20 glass column and allowed to settle. The fitting on the column were tightened to create a 12 × 5 mm I.D chromatographic bed. The column was washed with Tris-HCl buffer [10 mM, pH 7.4] for 2 h using a chromatographic pump with flow rate 0.2 ml/min at 25 °C. The Hsp90α(CT)-column was ready for immediate use or could be stored at 4°C until use.
Chromatographic system, frontal and non-linear chromatographic studies
The chromatographic system was composed of a LC-10ADvp pump, SIL-10ADvp autosampler, CTO-10ASvp column oven, SPD-10AV US-VIS detector, SCL-10ADvp controller (Shimadzu, Columbia, MD, U.S.A.). In the frontal chromatography studies a 10 ml superloop (GE Healthcare Bio-Sciences AB) was used to deliver the sample while in the non-linear chromatography studies a standard 10 μl injection loop was used. The data was acquired by CLASSvp software, v. 5.03 (Shimadzu). The mobile phase consisted of Tris-HCl [10 mM, pH 7.4] delivered at 0.2 ml/min at 25 °C. Before each injection the column was flushed for at least 6 h. The ligands were detected using UV absorption at λ = 254 nm (NOVO), λ = 280 nm (CA1), λ = 308 nm (GM), λ = 334 nm (17-AAG), or λ = 310 nm (RAD). The Hsp90α(NT) and Hsp90α(CT) columns prepared using 200 μg of the protein were used in these studies.
Frontal chromatography studies
Serial concentrations of CA1 [50, 250, 400, 500, 600 nM], RAD [10, 25, 40, 50, 60 nM], GM [10, 50, 125, 250, 500 nM], 17-AAG [100, 250, 400, 500, 1000 nM] and NOVO [50, 100, 250, 300, 400 nM] were prepared in Tris-HCl [10 mM, pH 7.4]. A 10 ml aliquot of each solution was placed in the super loop and applied as a continuous stream to the Hsp90α columns.
The observed retention volumes were used to calculate binding affinities (Kd values) of the studied HSP90α inhibitors (IHSP90α) using a previously described approach, Eqn 1 [11]:
| (1) |
where: V is the retention volume of IHSP90α measured at the midpoint of the breakthrough curve, Vmin is the retention volume of IHSP90α in the highest concentration applied of the displacer ligand and Bmax is number of the active binding sites of the immobilized target. The Kd values were obtained by plotting [IHSP90α](V-Vmin) versus [IHSP90α] and the data were analyzed by nonlinear regression with the sigmoidal response curve using Prism 4 software (Graph Pad Software Inc., San Diego, CA, U.S.A.).
Non-linear chromatography studies
Serial concentrations of NOVO [2.5, 5.0, 10.0, 15.0, 25.0, 30.0 and 50.0 μM] were prepared in Tris-HCl [10 mM, pH 7.4] and 20 μl aliquots were injected onto a HSP90α(NT) column. The column was washed with mobile phase for 1.5 h, at the end of each injection.
The observed peak asymmetries were analyzed using Impulse Input Solution, Eqn. 2, as previously described [11-13]. PeakFit v4.11 for Windows software (SPSS Inc., Chicago, IL) was used to perform the calculations.
| (2) |
where y is intensity of signal, x is reduced retention time, , I0() and I1() are modified Bessel functions, a0 is area parameter and a1 is center parameter, which determine the true thermodynamic capacity factor (k'), a2is width parameter and a3 is distortion parameter. Kinetic parameters can be calculated as follows: kd = 1/a2/t0; Ka = a3/C0; ka = Ka/kd, where: t0 is the dead time of the column; C0 is the concentration of NOVO injected multiplied by the width of the injection pulse [12].
Chromatographic system, determination of IC50 values
The HSP90α(CT)-column prepared using 400 μg of Hsp90α protein was placed in a 1100 LC/MSD liquid chromatography-mass spectrometry system (Agilent Technologies, Palo Alto, CA, U.S.A.) composed of a vacuum degasser, a quarternary pump, a thermostated autosampler, and a thermostated column compartment. The mass selective detector (MSD Quad SL) was used with an electrospray ionization interface (ESI) and on-line nitrogen generation system (Parker, Haverhill, MA, USA). The data was acquired by ChemStation software, Rev. A.10.02 (Agilent Technologies). The analyses were performed using a mobile phase composed of ammonium acetate [10 mM, pH 7.4] delivered at a flow rate of 0.2 ml/min at 25 °C.
The optimized conditions for ATP/ADP/AMP measurements were as follows: fragmentor voltage 70 V, gain 2, drying gas flow 6 L min, nebulizer pressure 60 psig, drying gas temperature 350°C, vaporizer temperature 210°C, and capillary voltage −4000. Target compounds were quantified in the single ion monitoring mode (SIM) at m/z 506 (ATP), m/z 426 (ADP) and m/z 346 (AMP). The areas under the curve associated with the analytes were determined by integration of the ion counts contained within the peaks produced by the mass spectral analysis of ATP (ATPAUC), ADP (ADPAUC) and AMP (AMPAUC) and the TotalAUC was determined as the sum of the AUCs (ATPAUC + ADPAUC + AMPAUC). The parameter X was defined as ATPAUC/TotalAUC and the parameter Y as ADPAUC/TotalAUC.
ATPase inhibition studies
GM was added to the mobile phase in sequential concentrations of 0.0, 0.5, 1.0, 1.5, 2.5, 3.0, 5.0, 10.0 μM and the resulting mobile phase was passed through the column for 10 min. ATP, 20 μl of a 50 μM solution, was injected onto the column and the AUCs of the eluted ATP, ADP and AMP were determined. The column was washed with ammonium acetate [10 mM, pH 7.4] for 30 min in between injections of ATP. Each experiment was repeated 3 times.
The IC50 value associated with the effect of GM on the hydrolysis of ATP was calculated as the relationship between the ratio Y/X and the concentration of GM in the mobile phase. The data was analyzed using a sigmoidal dose-response fitting program contained within Prism 4 software (Graph Pad Software, Inc.) running on a personal computer.
Results
Frontal chromatography studies
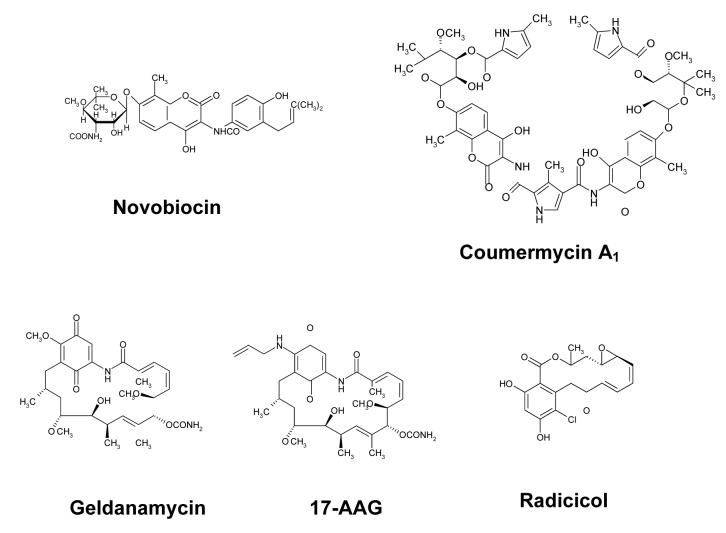
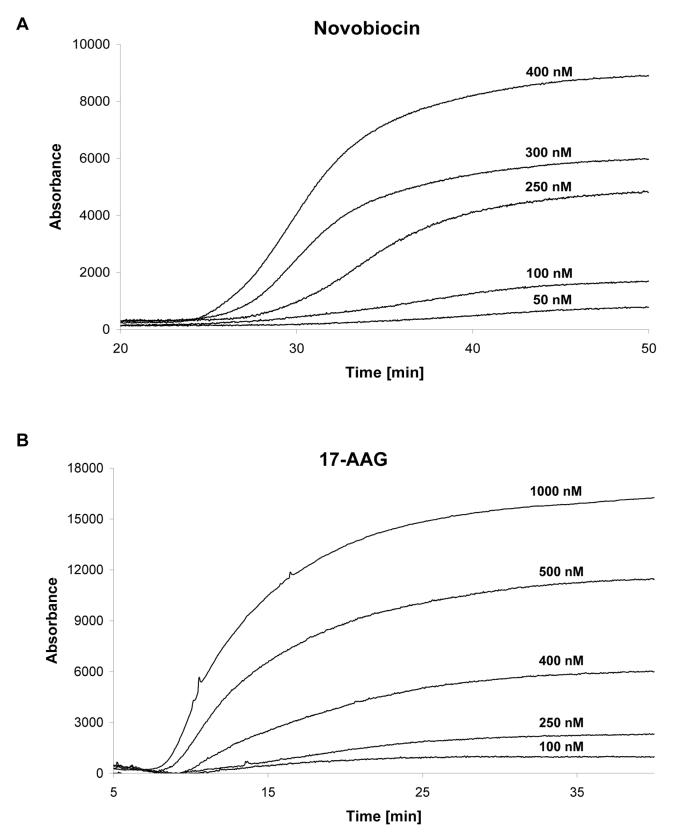
The Hsp90α columns were characterized using frontal chromatography techniques in which serial concentrations of known inhibitors, Fig. 3, were added to the mobile phase and passed through the column. In this approach, the sigmoidal-like chromatographic trace produced by the inhibitor contains a relatively flat initial portion, which represents nonspecific and specific binding of the marker to the stationary phase and target, and a vertical rise in the chromatographic trace (breakthrough), which ends, or plateaus, when the target is saturated. Representative chromatographic traces produced by frontal chromatography studies utilizing NOVO and 17-AAG are presented in Fig. 4A and 4B, respectively. The relationship between the concentration of the inhibitor and the volume required to produce the breakthrough was analyzed using Eqn. 1 in order to calculate the Kd of the inhibitor for the immobilized Hsp90α. This technique has been previously applied to the study of numerous ligand-protein interactions including binding to human serum albumin [9], cell surface receptors [11] and drug transporters [14].
Figure 3.
The Hsp90 inhibitors used in this study.
Figure 4.
Chromatographic results obtained using the immobilized Hsp90α columns in which: A. The frontal chromatography traces obtained by adding NOVO (50 - 400 nM) to the mobile phase running on the Hsp90α(NT)-column; B. The frontal chromatography traces obtained by adding 17-AAG (100 - 1000 nM) to the mobile phase running on the Hsp90α(CT)-column.
Binding to the exposed C-terminus on the Hsp90α(NT)-column was characterized using the known C-terminus ligands CA1 and NOVO, and frontal chromatography peaks with concentration-dependent breakthroughs were observed. The chromatographic traces obtained by adding NOVO (50 - 400 nM) to the mobile phase running on the Hsp90α(NT)-column are presented in Fig. 4A. Using this approach, the calculated Kd values were 220 ± 110 nM (CA1) and 100 ± 20 nM (NOVO) with an average number of binding sites (Bmax value) of 140 pmoles {range 250-30, n = 2} and correlation coefficients (r2) of 0.9488 (CA1) and 0.9795 (NOVO). The addition of GM, an N-terminus ligand, did not produce a frontal chromatogram and the addition GM to the mobile phase did not affect the breakthrough volumes of NOVO. These results indicate that GM did not specifically bind to the Hsp90α(NT)-column nor did it competitively or allosterically displace the C-terminus ligands.
Since the binding affinities of the C-terminus ligands have not been previously reported, the chromatographically determined Kd could not be directly compared to data obtained using other experimental techniques. However, the use of frontal chromatography in the online determination of binding affinities has been extensively validated. Therefore, the results demonstrate that the Hsp90α(NT)-column could be used to determine Kd values for compounds that bind at the C-terminus. In addition, the data indicate that the immobilization of Hsp90α via the N-terminus hindered binding to this domain and produced a Hsp90α(NT)-column that differentially binds C-terminus ligands.
Binding to the exposed N-terminus on the Hsp90α(CT)-column was characterized using the known N-terminus ligands GM, 17-AAG and RAD. Frontal chromatography peaks with concentration-dependent breakthroughs were observed and the frontal chromatography traces obtained by adding 17-AAG (100 - 1000 nM) to the mobile phase running on the Hsp90α(CT)-column are presented in Fig. 4B. The calculated Kd values were 90 ± 50 nM (GM), 210 ± 50 nM (17-AAG) and 20 ± 9 nM (RAD) with an average Bmax value of 130 (± 77) pmoles, n = 3, and the calculated r2 values were 0.9588 (GM), 0.9569 (17-AAG) and 0.9723 (RAD). These affinities are consistent with the previously reported Kd values for GM (1,200 nM) and RAD (19 nM) determined using ITC and yeast Hsp90 [3]. In addition, the relative affinities determined using the Hsp90α(CT)-column, RAD > GM > 17-AAG are consistent with the previously reported relative IC50 values for the inhibition of Hsp90 ATPase activity, GM (4.8 μM), 17-AAG (8.7 μM) and RAD (0.9 μM) [10]. Since IC50 values can be related to Kd using the approach described by Cheng and Prusoff [15], the results indicate that the Hsp90α(CT)-column can be used to determine the binding affinities of compounds at the N-terminus of Hsp90α.
NOVO, a C-terminus ligand, did not produce a frontal curve when it was chromatographed on the Hsp90α(CT)-column, indicating that immobilization via the C-terminus hindered specific binding to that terminus. In addition, addition of NOVO to the mobile phase did not affect the breakthrough volumes of GM demonstrating that it did not affect GM binding either competitively or allosterically. The data indicates that the Hsp90α(CT)-column selectively binds N-terminus ligands.
Non-linear chromatography studies
The shape of a chromatographic peak is the function of the specific and non-specific interactions between the solute and the stationary phase. When the stationary phase contains an immobilized protein, the mass transfer process defined by the dissociation and association of a ligand-protein complex is usually slow producing broad, non-Gaussian chromatographic peaks with significant tailing. The degree of deviation from a Gaussian distribution is a function of applied ligand concentration and the concentration-dependent asymmetry can be used with non-linear chromatography (NLC) techniques to characterize the separation processes occurring on the column, including the kinetics involved in the formation and dissolution of the solute-stationary phase complex, the association (ka) and dissociation (kd) rate constants, as well as the equilibrium constant (Ka, calculated as ka/kd) [11,16].
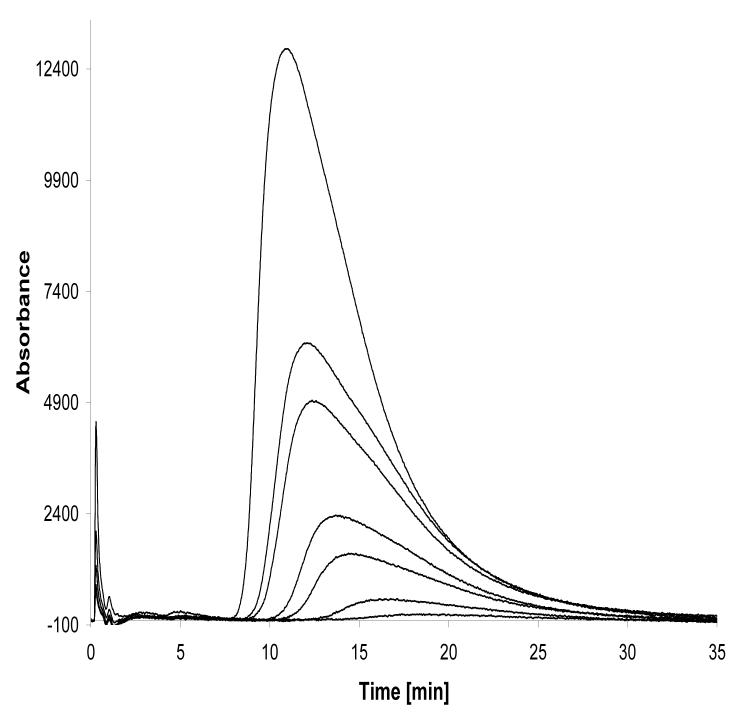
The injection of increasing concentrations of NOVO on the Hsp90α(NT)-column produced asymmetric peaks indicative of the NLC process, Fig. 5. The data was processed as previously described [16]. Analysis of the data showed that NLC parameters can be obtained from peak profiles produced by injections of 10 μl of NOVO solutions in concentrations ≤ 5 μM. Using this approach, the calculated ka and kd values associated with the interaction of NOVO and the immobilized Hsp90α were 22.2 (±8.8) μM−1sec−1 and 2.7 (±0.6) sec−1, respectively. The Ka was 8.1 (±1.5) μM and when expressed as Kd (1/Ka) the value was 122 nM, which is consistent with the Kd value determined using frontal chromatography techniques, 100 ± 20 nM.
Figure 5.
The nonlinear chromatography traces obtained by zonal injections of NOVO (2.5, 5, 10, 15, 25, 30, 50 μM) on the Hsp90α(NT)-column.
Previous NLC studies of noncompetitive inhibitors of the α3β4 nicotinic acetylcholine receptor with a cellular membrane affinity chromatography column containing this receptor have demonstrated that chromatographically derived kd values reflect the time required to recover from the functional inhibition of the receptor [17,18]. In addition, once the NLC parameters have been determined, the kd values can be determined after a single injection of a test compound [18]. Thus, the results of this study indicate that Hsp90α-columns can be used as part of drug screens and structure-activity studies to assess the relative length of binding of test compounds to Hsp90α.
Determination of ATPase inhibition
Since the ATPase activity of Hsp90 has been associated with an adenine nucleotide binding pocket in the N-terminus [1,2], the Hsp90α(CT)-column was used to determine if the ATPase activity was altered by the immobilization and if this activity could be inhibited by GM. Initial studies indicated that during the chromatographic process ATP was degraded to AMP by non-enzymatic interactions with the stationary phase. This was confirmed by the synthesis and testing of a control column prepared by treating APS with EDC without the subsequent addition of Hsp90α. The non-enzymatic production of AMP was included in the analysis of the experimental data.
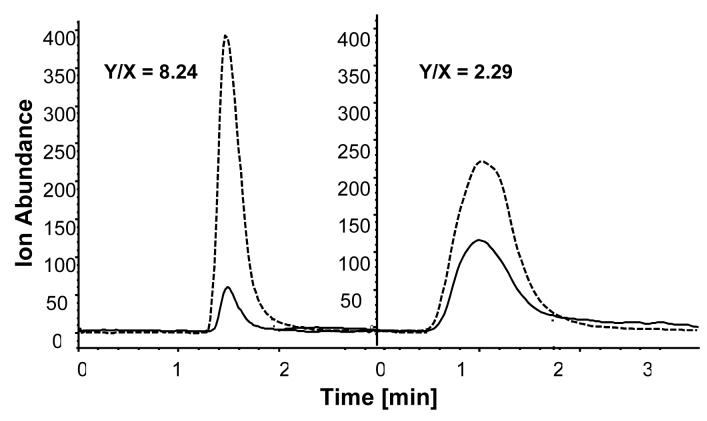
The initial studies also indicated that the enzymatic conversion of ATP to ADP occurred on the Hsp90α(CT)>-column, Fig. 6, and that this hydrolysis could be inhibited by GM. The ability of GM to inhibit the ATPase activity of the Hsp90α(CT)-column was examined by adding increasing concentrations of GM to the mobile phase and then determining the resulting effects on the ADP/ATP areas-under-the-curve ratios, expressed as Y/X as defined in Table 1. The Y/X ratios were related to GM concentrations using a sigmoidal dose-response curve, and the calculated IC50 value for GM was 2.80 ± 0.18 μM (r2 = 0.894) which is in agreement with the previously reported value of 4.80 ± 0.08 μM [10].
Figure 6.
Chromatographic traces produced by the mass spectrometric detection of ATP (solid line) and ADP (broken line) after the injection of ATP on a Hsp90α(CT)-column in the absence of GM (Y/X 8.24) and after the addition of 2.5 μM GM (Y/X 2.29) to the mobile phase.
Table 1.
The effect on the ATPase activity of an Hsp90α(CT)-column of the addition of geldanamycin (GM) to the mobile phase where: ATPAUC and ADPAUC represent the area under the curve (expressed as counts × 103) of the peak produced by the mass spectral analysis of ATP and ADP and TotalAUC represents the area-under-the-curve (expressed as ion abundance × 103) of the sum of the peaks produced by the mass spectral analysis of ATP, ADP and AMP. The data is presented as mean ± SD where n = 3.
| Added GM [μM] |
ATPAUC [counts × 103] |
ADPAUC [counts × 103] |
TotalAUC [counts × 103] |
X ATPAUC/ TotalAUC |
Y ADPAUC/TotalAUC |
Y/X |
|---|---|---|---|---|---|---|
| 0.0 | 3.5 ± 0.6 | 28.6 ± 4.7 | 167.8± 15.2 | 0.021 ± 0.003 | 0.171 ± 0.030 | 8.24 ±0.27 |
| 0.5 | 15.6 ± 4.2 | 64.9 ± 12.9 | 391.6 ± 142.4 | 0.042 ± 0.014 | 0.177 ± 0.056 | 4.21 ±0.31 |
| 1.0 | 5.5 ± 0.2 | 26.3 ± 4.3 | 284.9 ± 27.3 | 0.019 ± 0.006 | 0.091 ± 0.029 | 4.73 ±0.36 |
| 1.5 | 14.5 ± 0.8 | 53.1 ± 2.3 | 316.8 ± 10.5 | 0.046 ± 0.004 | 0.168 ± 0.009 | 3.67 ±0.14 |
| 2.5 | 3.2 ± 0.1 | 7.4 ± 0.5 | 261.9 ± 35.5 | 0.032 ± 0.001 | 0.078 ± 0.003 | 2.29 ±0.22 |
| 3.0 | 11.7 ± 3.3 | 26.3 ± 6.5 | 530.6 ± 34.0 | 0.022 ± 0.007 | 0.050 ± 0.015 | 2.26 ±0.12 |
| 5.0 | 2.3 ± 0.5 | 3.4 ± 0.2 | 419.9 ± 52.0 | 0.006 ± 0.001 | 0.008 ± 0.002 | 1.49 ±0.31 |
| 10.0 | 3.4 ± 0.3 | 3.2 ± 0.1 | 442.8 ± 54.7 | 0.018 ± 0.002 | 0.017 ± 0.002 | 0.94 ±0.09 |
In order to determine if the Hsp90α(CT)-column could differentiate between the functional inhibition produced by GM, 17-AAG and RAD, 2.5 μM concentrations of each of these compounds were added to the mobile phase and the corresponding Y/X ratios determined. A second Hsp90α(CT)-column was used in these studies and on this column the Y/X ratios were 3.24 (no inhibitor), 3.07 (17-AAG), 2.23 (GM) and 1.72 (RAD). The results are consistent with the previously reported relative IC50 values for these compounds, 17-AAG > GM > RAD, with RAD being the strongest inhibitor [10]. In addition, the addition of NOVO to the mobile phase had no affect on the observed ATPase activity. The results indicate that the Hsp90α(CT)-column is sensitive to ATPase inhibitors and that relative IC50 values can be estimated using a single injection of the test compounds.
Discussion
This study reports the first immobilization of Hsp90 within a chromatographic system and the use of the resulting columns to characterize interactions between the immobilized protein and inhibitors of its function. The results of this study demonstrate that the Hsp90α can be immobilized on the surface of APS using either the carboxylic moiety on the C-terminus or the amino moiety on the N-terminus to produce Hsp90α(CT)- and Hsp90α(NT)-columns, respectively.
The ability of the exposed N-terminus on the Hsp90α(CT)-column to bind compounds identified as N-terminus ligands was not affected by the immobilization and frontal chromatography techniques could be used to calculate Kd values associated with these ligands. However, the immobilization via the C-terminal did hinder the ability of C-terminus ligands to bind to the immobilized Hsp90α. It is unclear whether this was a function of the length of the spacer between the silica and protein or if conversion of the carboxyl moiety into an amide derivative altered the C-terminus binding pocket. Since the same effect was observed with binding to the Hsp90α(NT)-column, i.e. compounds identified as N-terminus ligands did not bind to the column while C-terminus ligands did, the effect is most likely due to steric hindrance arising from the chromatographic backbone. Future studies will explore this issue by examining the effect of increasing spacer length on binding to the immobilized protein.
While the lack of binding of a N-terminus ligand to the Hsp90α(NT)-column and of a C-terminus compound to the Hsp90α(CT)-column appears to be a problem, it also can be used to rapidly differentiate between the two binding sites. A single parallel displacement chromatography experiment using the two Hsp90α columns and the appropriate marker ligand will be able to determine if a test compound binds to HSP90α and to identify the site at which it binds. This approach has been previously used to identify and rank the interactions of test compounds with immobilized nicotinic acetylcholine receptor isoforms [11].
This paper reports the initial immobilization of Hsp90α within a chromatographic system and the characterization of the resulting columns. As with the question of the effect of the length of the spacer, the immobilization process has not been optimized. This will also be addressed in future studies. One approach will be based upon previous work with a cellular membrane affinity column containing membranes from a cell line expressing the drug transporter P-glycoprotein [14]. These studies have demonstrated that the chromatographic system can be converted from one based upon silica particles to an open tubular column format. The change to an open tubular column format eliminated non-specific interactions with the chromatographic backbone and reduced experimental time from hours to minutes without affecting the ability of the chromatographic system to accurately reflect functional transport of P-glycoprotein substrates.
In addition to the screening of recombinant Hsp90 proteins, this approach can also be adapted to the comparison of Hsp90 proteins obtained from different cell lines and different states of the same cell. The advantage of this approach is not only the direct comparison of binding affinities between the Hsp90 proteins, but also the characterization of the interaction process. Previous studies have shown that immobilized protein-based columns and cellular membrane affinity columns can be used to determine the thermodynamic parameters associated with the ligand-protein process [11,17] as well as determine allosteric interactions between binding sites on the immobilized protein [9,11]. The Hsp90α columns developed in this study can be used in the same manner. The results from the initial thermodynamic studies of the binding of compounds to the N-terminus and C-terminus of immobilized Hsp90α and the effect of middle-terminus interaction on the binding to the N-terminus and C-terminus will be reported elsewhere.
The ability of the Hsp90α(CT) column to detect ATPase inhibition and to estimate relative IC50 suggest that this column may be of use in the online screening for ATPase inhibitors. High throughput chromatographic screens for enzyme inhibitors using immobilized enzymes have been developed [19,20] and the immobilized Hsp90α should be readily adaptable to this technology.
Acknowledgements
This work was supported by funds from the Intramural Research Program of the National Institutes of Health. KJ was supported in part by FOCUS (4/2006) provided by the Foundation for Polish Science.
Abbreviations
- Hsp90
Heat shock protein 90
- CA1
coumermycin A1
- RAD
radicicol
- GM
geldanamycin
- 17-AAG
17-(allylamino)-17-demethoxygeldanamycin
- NOVO
novobiocin
- EDC
1-ethyl-3-(3-methylaminopropyl)carbodiimide
- APS
aminopropyl silica
- NLC
non-linear chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer. 2005;5:671–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 2.Calderwood SK, Khaleque MD, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem. Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Roe SM, Prodromou Ch., O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J. Med. Chem. 1999;42:260–266. doi: 10.1021/jm980403y. [DOI] [PubMed] [Google Scholar]
- 4.Chiosis G, Huezo H, Rosen N, Mimnaugh E, Whitesell L, Neckers L. 17AAG: Low target binding affinity and potent cell activity-finding an explanation. Molec. Cancer Ther. 2003;2:123–129. [PubMed] [Google Scholar]
- 5.Kasibhatla SR, Hong K, Biamonte MA, Busch DJ, Karjian PL, Sensintaffar JL, Kamal A, Lough RE, Brekken J, Lundgren K, Grecko R, Timony GA, Mansfield RR, Fritz LC, Ulm E, Burrows FJ, Boehm MF. Rationally designed high-affinity 2-amino-6-halopurine heat shock protein 90 inhibitors that exhibit potent antitumor activity. J. Med. Chem. 2007;50:2767–2778. doi: 10.1021/jm050752+. [DOI] [PubMed] [Google Scholar]
- 6.Workman P. Altered states: selectively drugging the Hsp90 cancer chaperone. Trends Molec. Med. 2004;10:47–51. doi: 10.1016/j.molmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ. A high-affinity conformation of Hsp90 confers tumor selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Hage DS. Quantitative analysis of allosteric drug-protein binding by biointeraction chromatography. Nature Biotech. 2004;22:1445–1449. doi: 10.1038/nbt1022. [DOI] [PubMed] [Google Scholar]
- 9.Bertucci C, Bartolini M, Gotti R, Andrisano V. Drug affinity to immobilized target bio-polymers by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. B. 2003;797:111–129. doi: 10.1016/j.jchromb.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Rowlands MR, Newbatt YM, Prodromou Ch., Pearl LH, Workman P, Aherne W. High-throughput screening assay for inhibitors of heat-shock protein 90 ATPase activity. Anal. Biochem. 2004;327:176–183. doi: 10.1016/j.ab.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 11.Moaddel R, Jozwiak K, Wainer IW. Allosteric modifiers of neuronal nicotinic acetylcholine receptors: New methods, new opportunities. Med. Res. Rev. doi: 10.1002/med.20091. DOI 10.1002/med.20091. [DOI] [PubMed] [Google Scholar]
- 12.Wade J, Bergold AF, Carr PW. Theoretical description of nonlinear chromatography, with applications to physicochemical measurements in affinity chromatography and implications for preparative-scale separations. Anal. Chem. 1987;59:1286–1295. [Google Scholar]
- 13.Jaulmes A, Vidal-Madjar C. Theoretical aspects of quantitative affinity chromatography: an overview. In: Giddings J, Grushka E, Brown PR, editors. Advances in Chromatography. Vol. 28. Marcel Dekker; New York, USA: 1989. pp. 1–64. [Google Scholar]
- 14.Moaddel R, Hamid R, Patel S, Bullock P, Wainer IW. Identification of P-glycoprotein substrates using open tubular chromatography on an immobilized P-glycoprotein column: comparison of chromatographic results with Caco-2 permeability. Anal. Chem. Acta. 2006;578:25–30. doi: 10.1016/j.aca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Prusoff W. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 16.Jozwiak K, Haginaka J, Moaddel R, Wainer IW. Displacement and non-linear chromatographic techniques in the investigation of the interaction of noncompetitive inhibitors with an immobilized α3β4 nicotinic acetylcholine receptor liquid chromatographic stationary phase. Anal. Chem. 2002;74:4618–4624. doi: 10.1021/ac0202029. [DOI] [PubMed] [Google Scholar]
- 17.Jozwiak K, Hernandez SC, Kellar KJ, Wainer IW. The enantioselective interactions of dextromethorphan and levomethorphan with the α3β4-nicotinic acetylcholine receptor: Comparison of chromatographic and functional data. J. Chromatogr. B. 2003;797:373–379. doi: 10.1016/s1570-0232(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 18.Moaddel R, Jozwiak K, Yamaguchi R, Wainer IW. Direct chromatographic determination of dissociation rate constants of ligand-receptor complexes: Assessment of the interaction of noncompetitive inhibitors with an immobilized nicotinic acetylcholine receptor-based liquid chromatography stationary phase. Anal. Chem. 2005;77:5421–5426. doi: 10.1021/ac0504464. [DOI] [PubMed] [Google Scholar]
- 19.Ng, Yang ESMF, Kameyama A, Palcic MM, Hindsgaul O, Schriemer DC. High-throughput screening for enzyme inhibitors using frontal affinity chromatography and liquid chromatography and mass spectrometry. Anal. Chem. 2005;77:6125–6133. doi: 10.1021/ac051131r. [DOI] [PubMed] [Google Scholar]
- 20.Hodgson RJ, Besanger TR, Brook MA, Brennan JD. Inhibitor screening using immobilized enzyme reactor chromatography/mass spectrometry. Anal. Chem. 2005;77:7512–7519. doi: 10.1021/ac050761q. [DOI] [PubMed] [Google Scholar]