Abstract
Antigen presentation by major histocompatibility complex (MHC) class II molecules requires the participation of different proteases in the endocytic route to degrade endocytosed antigens as well as the MHC class II-associated invariant chain (Ii). Thus far, only the cysteine protease cathepsin (Cat) S appears essential for complete destruction of Ii. The enzymes involved in degradation of the antigens themselves remain to be identified. Degradation of antigens in vitro and experiments using protease inhibitors have suggested that Cat B and Cat D, two major aspartyl and cysteine proteases, respectively, are involved in antigen degradation. We have analyzed the antigen-presenting properties of cells derived from mice deficient in either Cat B or Cat D. Although the absence of these proteases provoked a modest shift in the efficiency of presentation of some antigenic determinants, the overall capacity of Cat B−/− or Cat D−/− antigen-presenting cells was unaffected. Degradation of Ii proceeded normally in Cat B−/− splenocytes, as it did in Cat D−/− cells. We conclude that neither Cat B nor Cat D are essential for MHC class II-mediated antigen presentation.
Major histocompatibility complex (MHC) class II molecules display to CD4+ cells a variety of antigenic peptides generated by degradation of endocytosed proteins (1). MHC class II αβ dimers possess a peptide-binding site designed to accommodate a large variety of peptides differing in sequence and length (2–4). When empty, the peptide-binding site is unstable, rendering αβ dimers prone to aggregation at acidic pH (5) and to dissociation (6). This prevents expression of empty class II molecules at the cell surface, which could interact with polypeptides nonspecifically. Because most antigenic determinants are presented by newly synthesized class II molecules (7, 8), antigen-presenting cells (APC) have devised mechanisms to protect the antigen-binding site of the αβ dimers from both interacting nonspecifically with endoplasmic reticulum-resident proteins (9) and from destabilization of the dimer (10). This is accomplished by the association of newly synthesized class II αβ dimers with a third subunit, invariant chain Ii (Ii) (11). A specialized region of Ii, CLIP [from CLass II-associated Invariant chain Peptide (3)], fills the binding site of αβ dimers as do antigenic peptides (4, 12, 13). The cytoplasmic region of Ii also carries the information necessary to direct the αβIi complexes to the endocytic compartments where antigenic peptides reside (14, 15). The activation of the peptide-binding properties of class II αβ dimers occurs by degradation of Ii in a stepwise fashion, in which several proteases of the endosomal/lysosomal system are involved (16–21). This yields αβ molecules associated with CLIP-containing Ii fragments, which are then substituted by antigenic peptides in a reaction facilitated by the MHC class II-like molecule H-2M (in humans, HLA-DM) (22–24).
The generation of the antigenic determinants to be presented by MHC class II also requires the action of proteases (8). Endocytosed antigens are degraded in an as yet incompletely characterized series of proteolytic events. It is not clear whether αβ dimers associate with previously formed determinants, or rather bind larger antigenic fragments first, which are then trimmed in a process that leaves the actual epitope protected (25–28).
The identification of the proteases involved in degradation of Ii and endocytosed antigens has been a matter of intense investigation. The focus of most of the research aimed at characterizing these enzymes has been directed at cathepsins (Cat) B and D because (i) Cat B and Cat D are the most abundant cysteine and aspartyl proteases, respectively (29); (ii) APC treated with inhibitors of cysteine or of aspartyl proteases showed impairment of Ii degradation and/or altered profiles of antigen presentation to T cells (18, 30–35); (iii) both Cat B and Cat D can degrade antigens (36, 37); (iv) Cat D is present in endocytic compartments specialized in the generation of αβ-peptide complexes (38, 39). However, it is difficult to ascertain whether the effect of a protease inhibitor occurs at the level of degradation of antigen or of Ii. In addition, some inhibitors affect multiple proteases (40), and the preparations of purified enzymes used in in vitro assays may have contained other enzymes as well (H. A. Chapman, personal communication). Finally, the role of a protease in degrading Ii or in generating antigenic peptides in vitro may not be directly extrapolated to in vivo situations, given the widely differing concentrations of enzyme(s) and substrate(s) likely to be present in living cells as compared with those used in vitro. Thus, in opposition to results obtained in vitro (41), we have previously excluded the involvement of Cat D in degradation of Ii based on analysis of Cat D−/− APC (21).
Previous work has demonstrated the essential role of the minor cysteine protease Cat S in the degradation of Ii in both human and mouse APC. Inhibition of Cat S without affecting other cysteine proteases, such as Cat B, blocked complete destruction of Ii (20, 21) and impaired T-cell-mediated immune responses in vivo (51). These experiments showed that the inhibition of Cat S could not be fully compensated by Cat B. We have performed pulse-chase experiments followed by immunoprecipitation of class II molecules to confirm that degradation of Ii in Cat B−/− cells occurs normally, eliminating a requirement for Cat B in the destruction of Ii.
Which proteases degrade antigens? The requirement of Cat S to completely degrade Ii supports the notion that some proteolytic events localized in endocytic compartments may require a specific enzyme. Given the ample literature suggesting an involvement of Cat B and/or Cat D in the generation of antigenic epitopes, it was important to assess the consequences of a lack of Cat B or Cat D on antigen presentation. We have analyzed the capacity of APC from Cat B−/− and Cat D−/− mice to present antigenic determinants derived from four different protein antigens to a panel of 15 T-cell hybridomas. Our results indicate that neither Cat B nor Cat D are required for presentation of these antigens.
MATERIALS AND METHODS
Reagents.
Leupeptin came from Boehringer Mannheim. Morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl (LHVS) was provided by D. Brömme (Arris Pharmaceuticals, South San Francisco, CA). DMEM, RPMI, and methionine/cysteine-free media were obtained from GIBCO/BRL. Recombinant mouse interleukin (IL)-2 was from Boehringer Mannheim. [3H]Thymidine was from DuPont/NEN. Ovalbumin (OVA), hen egg lysozyme (HEL), pigeon cytochrome C, and myelin basic protein were obtained from Sigma.
Mice.
A detailed outline of the method followed for generation of the Cat B−/− mice will be described elsewhere (J.D. and C. Peters, unpublished work). Briefly, a 14.6-kb genomic phage clone, including exons 2–8 of the Cat B gene, was isolated from a 129/sv λ FixII teratocarcinoma phage library (Stratagene). A targeting construct carrying a neo expression cassette inserted into exon 4 of the Cat B gene was subcloned into pBluescript SK+ (Stratagene). This construct was introduced into the embryonic stem cell line E-14–1 by electroporation, and G418-resistant colonies were screened for homologous recombination of the Cat B gene. Mutated embryonic stem cells were microinjected into blastocysts of C57BL/6 mice. Resulting male chimeras were mated with C57BL/6 and 129/sv females. Intercrossing heterozygous offspring resulted in mice homozygous for the mutation. The phenotype of Cat B-deficient mice appears normal compared with wild-type littermates.
Cat D-deficient mice have been described (42). Cat D−/− mice die at around 24 days of age, and experiments involving these mice were performed with littermates 18–20 days old. To obtain Cat D−/− mice in the H-2d, H-2k, and H-2s background, adult Cat D+/− mice were crossed with BALB/c, C3H, or SJL animals, respectively (all from The Jackson Laboratory). Cat D+/− couples coexpressing mixed MHC backgrounds were selected by PCR as described (21). Cat D+/− couples with homozygous MHC backgrounds were then selected again by PCR and by fluorescence-activated cell sorter analysis in a FACScan (Becton Dickinson) by using appropriate mAb coupled to immunofluorescent moieties (PharMingen) and mated to obtain the Cat D-deficient animals used in the experiments of antigen presentation.
Pulse-Chase Analysis and Immunoprecipitations.
Metabolic labeling and chase in the presence or absence of protease inhibitors, immunoprecipitation with mAb N22 (a gift of R. M. Steinman, The Rockefeller University, New York) and SDS/PAGE analysis of immunoprecipitates was performed exactly as described in ref. 21.
T Cell Hybridomas.
DO11, 3DO18.3 (43), and 6KC27.2 were the gift of P. Marrack (National Jewish Center, Denver). 6KC27.2 was made in B10.BR mice, and it responds to I-Ek bound to peptide 88–103 of moth cytochrome C (P. Marrack, personal communication), or of pigeon cytochrome C (this work). BO1710 and BO4H9 (44) were the gift of D. Mathis (Institut de Chimie Biologique, Strasbourg, France). 1C5.1 (33) was the gift of F. Momburg (German Cancer Research Center, Heidelberg, Germany). SHEL9 and 3A1 (45) were the gift of H. Bodmer (The Edward Jenner Institute for Vaccine Research, Berkshire, United Kingdom). 11C4.2.8, P2, P22, P29, P30, P35, and P47 (32) were the gift of K. Rock (Dana–Farber Cancer Institute, Boston). All hybridomas were maintained in RPMI 1640 + Hepes, 10% fetal calf serum (FCS), 2 mM l-glutamine, penicillin, and streptomycin.
Antigen Presentation.
Single cell suspensions were prepared from spleens of Cat D+/− or Cat D−/− littermates or from Cat B+/− or Cat B−/− adult mice. After centrifugation, cells were resuspended in AcK lysis buffer (0.15 M NH4Cl/1 mM KHCO3/0.1 mM EDTA) to remove red blood cells, spun down, washed, and resuspended in RPMI 1640 + Hepes supplemented with 10% FCS, 2 mM l-glutamine, penicillin, and streptomycin. The splenocytes were then plated in 96 flat-bottom well plates (Corning–Costar, Cambridge, MA) (0.5 × 106 cells per well) and γ-irradiated (5K rads). T-hybridoma cells (105) were then added to each well, followed by the addition of antigen, and dissolved in culture media at the indicated final concentration. Plates were incubated for 18 hr at 37°C, and one-half of the supernatant (100 μl) transferred to a 96-well round-bottom plate, frozen, and thawed. The concentration of IL-2 in each well was then determined by incorporation of [3H]thymidine by the IL-2-dependent cell line CTLL2 (American Type Culture Collection) following standard methods.
RESULTS AND DISCUSSION
Degradation of Mouse Ii Proceeds Normally in Cat B-Deficient Cells.
We have described the distinct role of the cysteine protease Cat S in degrading Ii in both human and mouse APC (20, 21). Cat S cleaves Ii N terminally of the CLIP region, a critical step in the maturation of MHC class II molecules that precedes the substitution of CLIP by antigenic peptides (22–24). In humans, Cat S is highly expressed in APC, and its expression is stimulated by γ-interferon (46). Pharmacological inactivation of mouse Cat S in vivo results in the inhibition of humoral and allergic responses, underlining the importance of this enzyme in the onset of immunity (51). However, when only Cat S is blocked with the specific inhibitor LHVS, its effect on the maturation of MHC class II in human B cells and in mouse splenocytes is not as profound as when other additional cysteine proteases, including Cat B, are also inactivated with leupeptin (refs. 20 and 21 and Fig. 1). Furthermore, long-term administration of LHVS in mice results in complete inactivation of Cat S without significantly reducing the steady-state levels of mature class II molecules expressed at the cell surface (51). These observations suggested that, in addition to Cat S, another cysteine protease(s) such as Cat B, not blocked by administration of LHVS, may also cleave Ii N terminally of CLIP, albeit less efficiently.
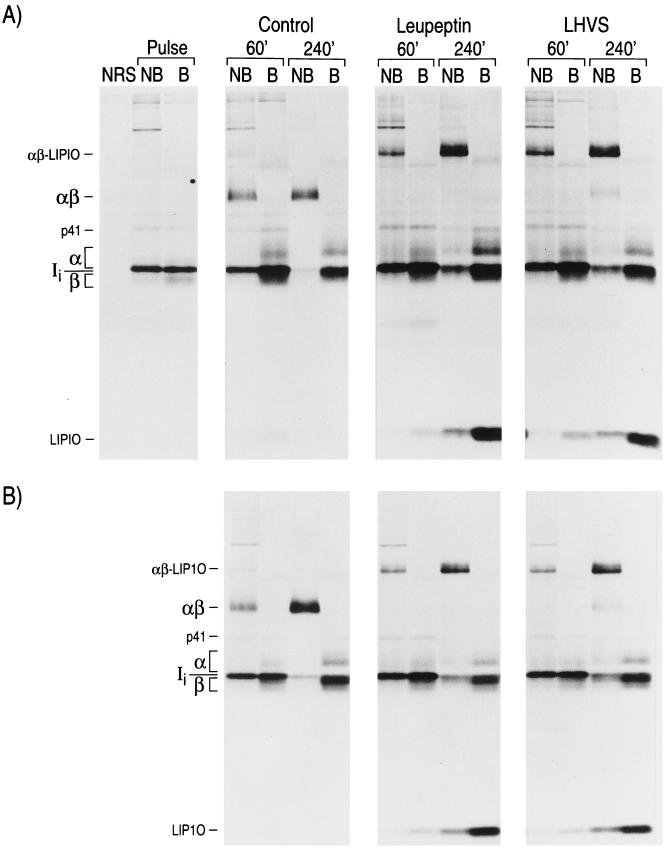
Figure 1.
Normal maturation of MHC class II in Cat B knock-out mice. Cat B+ (A) or Cat B− (B) splenocytes were metabolically labeled for 30 min and chased for the times indicated in the absence or presence of leupeptin (1 mM) or LHVS (3 nM). MHC class II molecules were immunoprecipitated with mAb N22 and run in 12.5% reducing SDS/PAGE without (NB) or after (B) boiling. The position of the class II α and β chains, Ii, the p41 form of Ii, SDS-stable (peptide-bound) αβ complexes, the leupeptin-induced polypeptide LIP10, and the I-Ab-LIP10 SDS-stable complex (21, 47, 48), are indicated
The least ambiguous approach to addressing the involvement of Cat B in Ii breakdown in vivo is to compare the degradation pattern of Ii in mice deficient in this enzyme with that of normal animals. A pulse-chase performed on Cat B+/− or Cat B−/− splenocytes, followed by immunoprecipitation of MHC class II molecules with the mAb N22 and analysis by SDS/PAGE, reveals that the pattern of degradation of Ii and of maturation of MHC class II proceeds normally in the absence of Cat B (Fig. 1, Control). The effect of leupeptin and of LHVS was also similar in both sets of splenocytes: destruction of Ii was interrupted at a stage that precedes formation of CLIP (20, 21), resulting in accumulation of class II αβ dimers associated with the LIP10 (from Leupeptin-Induced Polypeptide) intermediate (Fig. 1, Leupeptin and LHVS) (16, 17, 49, 50). The effect of LHVS on the formation of αβ-peptide SDS-stable dimers, compared with that of leupeptin, was less pronounced in both Cat B+/− and Cat B−/− cells, indicating that when Cat S is selectively inhibited with LHVS, Cat B does not substitute for it. These results rule out a requirement for Cat B in the degradation of Ii, either upstream or downstream of the formation of the LIP10 intermediate, in mouse APCs. This is consistent with our observation that maturation of T cells, as well as steady-state levels of MHC class II in splenocytes, are not affected in Cat B-deficient animals (not shown).
Generation of Antigenic Epitopes in Cat B- or Cat D-Deficient Animals.
In addressing the role of endosomal proteases in class II-mediated antigen presentation it is important to determine not only which proteases intervene, but also how critical is their function. Can different proteases substitute for each other? In the extreme case, every antigen might require cleavage at least once by one particular enzyme to generate antigenic peptides, so that APC lacking that protease would be incapable of generating any determinant at all. At the next level, some antigens, but not others, might require proteolysis by at least one particular enzyme to yield presentable epitopes. The absence of such an enzyme would lead to impaired presentation of antigenic determinants derived from some proteins but not from others. Next, the defect in one endoprotease could prevent the formation of some determinants without jeopardizing the availability of other epitopes derived from the same protein. Finally, it is conceivable that any antigenic determinant can be generated by more than one protease. In this case, the absence of any single protease would not significantly affect the processing of antigens, because another enzyme(s) would substitute for the missing protease.
Our biochemical analysis of MHC class II in Cat B−/− (Fig. 1) and in Cat D−/− (21) APC shows that formation of the bulk of SDS-stable αβ dimers occurs normally in the absence of either enzyme. However, most peptides bound by MHC class II are derived from proteins synthesized by the APC itself (2, 3), and therefore the biochemical evidence does not exclude a role of Cat B or Cat D on presentation of endocytosed antigens. We have assessed the presentation capacity of APC derived from Cat B−/− or Cat D−/− mice to a panel of T-cell hybridomas that recognize antigenic determinants derived from different proteins and from different regions of the same protein (Table 1). Because the set of hybridomas analyzed used different restriction elements, we backcrossed the Cat D-deficient mice (generated on an H-2b genetic background) with animals of the appropriate haplotype: BALB/c (H-2d), C3H (H-2k), and SJL (H-2s). Only I-Ab-restricted hybridomas were tested in experiments assessing Cat B-deficient APC.
Table 1.
T cell hybridomas used in this study
| T cell | H-2* | Antigen† | Ref. |
|---|---|---|---|
| BO1710 | I-Ab | OVA323-332 | 44 |
| DO11 | I-Ab,d | OVA323-339 | 43 |
| 3DO18.3 | I-Ad | OVA273-288 | 43 |
| 11C4.2.8 | I-As | OVA | 32 |
| P2 | I-As | OVA9-24 | 32 |
| P22 | I-As | OVA169-185 | 32 |
| P29 | I-As | OVA225-240 | 32 |
| P30 | I-As | OVA230-248 | 32 |
| P35 | I-As | OVA273-288 | 32 |
| P47 | I-As | OVA370-384 | 32 |
| BO4H9 | I-Ab | HEL74-88 | 44 |
| 1C5.1 | I-Ak | HEL46-61 | 33 |
| SHEL9 | I-As | HEL118-129 | 45 |
| 6KC27.2 | I-Ek | PCC88-103 | This work |
| 3A1 | I-As | MBP89-100 | 45 |
Indicates the MHC class II specificity of the T-cell hybridoma.
Numbers indicate the region containing the peptide recognized by the T cell in: ovalbumin (OVA), hen egg lysozyme (HEL), pigeon cytochrome C (PCC), and myelin basic protein (MBP). The peptide recognized by 11C4.2.8 is unknown.
Antigen Presentation by Cat D−/− APC.
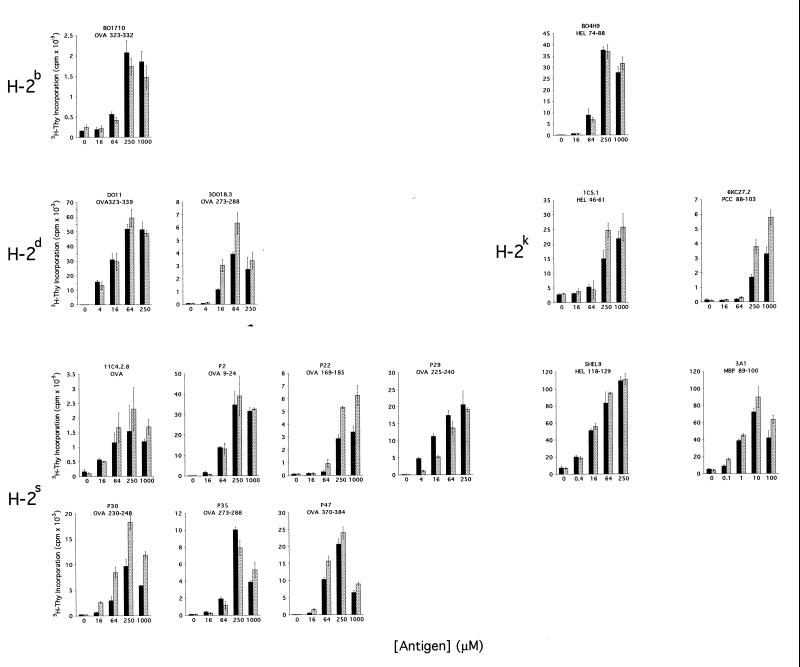
Splenocytes from Cat D+/− and Cat D−/− littermates were used as APC in assays of antigen presentation (Fig. 2). Our results show that the lack of Cat D did not abrogate the capacity of APC to process four different antigens: ovalbumin, hen egg lysozyme, pigeon cytochrome C, and myelin basic protein. These results exclude a strict requirement for Cat D in presentation of endocytosed antigens. However, recognition by some T-cell hybridomas was slightly decreased (P29) or increased (3DO18.3, 6KC27.2, 11C4.2.8, P22, P30) when the APC lacked Cat D. This heterogeneity was not caused by variability in the APC used to test different T cells because we used the same Cat D+/− or Cat D−/− splenocytes to assess all the T-cell hybridomas restricted by the same H-2 haplotype. Thus, the Cat D deficiency does not preclude uptake, processing, or presentation of the antigen to T cells. Rather, the deficit of Cat D probably results in changes in the amount of each particular peptide made available to MHC class II molecules. Even different segments of the same protein can have distinct fates as a consequence of the protease deficit, as in the case of the OVA-derived peptides.
Figure 2.
Antigen presentation by Cat D−/− APC. Splenocytes from Cat D+/− (solid bars) or Cat D−/− mice (shaded bars) were used as APC in assays of antigen recognition by the panel of hybridomas shown in Table 1 in the presence of the indicated concentration of antigen. The epitope recognized by each T cell line is shown under its name. The results shown are representative of at least two experiments in which all the hybridomas specific for the same MHC background were tested with the same combination of Cat D+/− and Cat D−/− splenocytes of the appropriate MHC specificity. All values are means of triplicate determinations, and the SD of each value is represented.
Antigen Presentation by Cat B−/− APC.
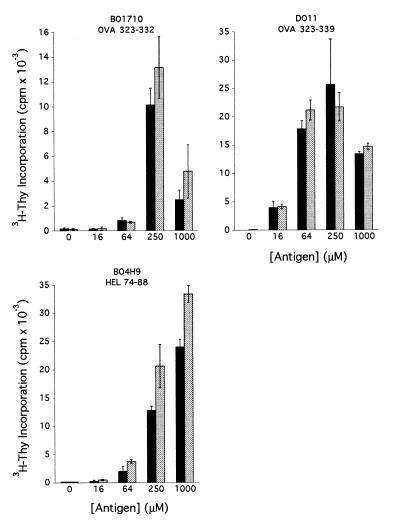
As for Cat D−/− cells, Cat B-deficient APC could present OVA- and HEL-derived peptides to T cells (Fig. 3). Also in this case, the efficiency of presentation of one determinant, HEL74–88, was slightly increased when the APC lacked Cat B. This determinant was presented with similar efficiency by Cat D+/− and Cat D−/− cells (Fig. 2), supporting the notion that formation and survival of different epitopes depend on distinct sets of proteases.
Figure 3.
Antigen presentation by Cat B−/− APC. Splenocytes from Cat B+/+ (solid bars) or Cat B−/− mice (shaded bars) were used as APC in assays of antigen recognition by the hybridomas shown in the presence of the indicated concentration of antigen. The results shown are representative of two independent experiments in which each value is the mean of triplicate determinations.
Both Cat B and Cat D Are Dispensable for MHC Class II Antigen Presentation.
Our results eliminate the two major cysteine and aspartyl proteases, Cat B and Cat D, respectively, as indispensable components of the machinery involved in antigen presentation by MHC class II molecules. Neither Cat B nor Cat D are necessary for normal degradation of Ii (ref. 21 and Fig. 1). So far, only Cat S has been shown to be essential for degradation of Ii, at least in its final stages (20, 21). We have also shown that both Cat B- and Cat D-deficient APC can present, to a variety of T-cell hybridomas, antigenic peptides derived from ovalbumin and hen egg lysozyme, and Cat D-deficient cells can also present pigeon cytochrome C- and mylein basic protein-derived peptides.
Increased Survival of Antigenic Determinants Due to Protease Deficits.
The deficit in Cat D or Cat B results in increased presentation of some peptides. Similar effects have been observed for other antigenic determinants in experiments in which APC were treated with cysteine protease inhibitors (32, 35). In both cases, the most likely explanation is that the enzyme concerned participates in the degradation of the epitope. Inhibition or elimination of the enzyme would thus result in prolonged survival of the determinant. Strikingly, one of the epitopes analyzed, OVA273–288, was presented with similar efficiency by Cat D+/− and Cat D−/− cells of the H-2s haplotype (to hybridoma P35), whereas the same defect resulted in increased presentation of the same determinant by H-2d APC (to 3DO18.3). This suggests an influence of MHC polymorphism on the susceptibility of antigenic peptides to proteolytic degradation (see below).
Multiple Proteases Can Probably Generate the Same Antigenic Determinants.
One of the objectives of studying the involvement of proteases of the endocytic route on generation of antigenic determinants is the possibility of devising specific protease inhibitors that could block the presentation of unique antigens, specifically those involved in autoimmune diseases. Cat D has been the focus of special attention because it resides in compartments where formation of MHC class II peptide complexes takes place (38, 39), and because Cat D can generate OVA- and HEL-derived peptides in vitro, which can be recognized by T cells on the surface of fixed APC (36, 37). It is likely that Cat D is also capable in vivo of generating antigenic peptides in the compartments where it resides. However, all antigenic determinants analyzed were presented by Cat D-deficient APC. This suggests that the immune system has developed a machinery for MHC class II-mediated antigen presentation in which several proteases can generate the same or closely similar antigenic determinants. If the association of class II molecules with antigenic peptides occurs according to the “antigen capture” model, in which the binding cavity formed by the class II αβ dimers binds to, and protects from degradation, distinct stretches of the antigen (25–28), the absence of one protease would perhaps delay, but not preclude, the presentation of a determinant. The combined action of other endo- and exo-proteases would suffice for complete removal of the unprotected regions. Our results support this model, but also suggest that certain regions of antigens are susceptible to degradation before the class II molecules can bind and protect them, which would explain why the absence of Cat B or D, or the use of cysteine protease inhibitors (32, 35), results in increased presentation of some determinants. The observation that the absence of Cat D results in increased survival of OVA273–288 in the H-2d but not in the H-2s background could therefore be explained by differences in the affinity of the interaction of each corresponding I-A allelic product with OVA273–288.
In conclusion, we have not found any determinant whose presentation is abolished by the absence of either Cat B or Cat D. Although we cannot exclude that some determinants not analyzed here may require these enzymes, our results suggest that the machinery involved in generation of peptides presented by MHC class II is indeed degenerate. It is unlikely that a therapy based on inhibition of proteases involved in degradation of antigens would succeed in selectively preventing autoimmune responses. The action of Cat S on Ii, in contrast, remains an attractive target for pharmacological intervention.
Acknowledgments
We thank Drs. H. Bodmer, P. Marrack, D. Mathis, F. Momburg, and K. Rock for their T-cell hybridomas, and Drs. R. J. Riese and H. A. Chapman for providing unpublished data. This work was supported by National Institutes of Health Grants 5-RO-AI34893 and 2-P30-CA14051 (H.L.P.), and by the Deutsche Forschungsgemeinschaft (C.P.). J.A.V. was supported by a fellowship from the Lady Tata Memorial Foundation (United Kingdom). J.D. was supported by a fellowship from the Fonds der Chemischen Industrie (Germany).
ABBREVIATIONS
- MHC
major histocompatibility complex
- Cat
cathepsin
- APC
antigen-presenting cell
- LHVS
morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl
- OVA
ovalbumin
- HEL
hen egg lysozyme
References
- 1.Wolf P R, Ploegh H L. Annu Rev Cell Dev Biol. 1995;11:267–306. doi: 10.1146/annurev.cb.11.110195.001411. [DOI] [PubMed] [Google Scholar]
- 2.Rudensky A Y, Preston-Hurlburt P, Hong S C, Barlow A, Janeway C A. Nature (London) 1991;353:622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- 3.Chicz R M, Urban R G, Gorga J C, Vignali D A A, Lane W S, Strominger J L. J Exp Med. 1993;178:27–47. doi: 10.1084/jem.178.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gosh P, Amaya M, Mellins E, Wiley D C. Nature (London) 1995;378:457–462. doi: 10.1038/378457a0. [DOI] [PubMed] [Google Scholar]
- 5.Germain R N, Rinkler A G. Nature (London) 1993;363:725–728. doi: 10.1038/363725a0. [DOI] [PubMed] [Google Scholar]
- 6.Sadegh-Nasseri S, Stern L J, Wiley D C, Germain R N. Nature (London) 1994;370:647–650. doi: 10.1038/370647a0. [DOI] [PubMed] [Google Scholar]
- 7.Pinet V, Vergelli M, Martin R, Bakke O, Long E O. Nature (London) 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 8.Watts C. Annu Rev Immunol. 1997;15:821–850. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 9.Busch R, Cloutier I, Sekaly R P, Hammerling G J. EMBO J. 1996;15:418–428. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong G, Castellino F, Romagnoli P, Germain R N. J Exp Med. 1996;184:2061–2066. doi: 10.1084/jem.184.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cresswell P. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 12.Bijlmakers M J, Benaroch P, Ploegh H. J Exp Med. 1994;180:623–629. doi: 10.1084/jem.180.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romagnoli P, Germain R N. J Exp Med. 1994;180:1107–1113. doi: 10.1084/jem.180.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakke O, Dobberstein B. Cell. 1990;63:707–716. doi: 10.1016/0092-8674(90)90137-4. [DOI] [PubMed] [Google Scholar]
- 15.Lotteau V, Teyton L, Peleraux A, Nilsson T, Karlsson L, Schmid S L, Quaranta V, Peterson P A. Nature (London) 1990;348:600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 16.Blum J S, Cresswell P. Proc Natl Acad Sci USA. 1988;85:3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neefjes J J, Ploegh H L. EMBO J. 1992;11:411–416. doi: 10.1002/j.1460-2075.1992.tb05069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maric M A, Taylor M D, Blum J S. Proc Natl Acad Sci USA. 1994;91:2171–2175. doi: 10.1073/pnas.91.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morton P A, Zacheis M L, Giacoletto K S, Manning J A, Schwartz B D. J Immunol. 1995;154:137–150. [PubMed] [Google Scholar]
- 20.Riese R J, Wolf P R, Brömme D, Natkin L R, Villadangos J A, Ploegh H L, Chapman H A. Immunity. 1996;4:357–366. doi: 10.1016/s1074-7613(00)80249-6. [DOI] [PubMed] [Google Scholar]
- 21.Villadangos J A, Riese R J, Peters C, Chapman H A, Ploegh H L. J Exp Med. 1997;186:549–560. doi: 10.1084/jem.186.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sloan V S, Cameron P, Porter G, Gammon M, Amaya M, Mellins E, Zaller D M. Nature (London) 1995;375:802–806. doi: 10.1038/375802a0. [DOI] [PubMed] [Google Scholar]
- 23.Denzin L K, Cresswell P. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 24.Sanderson F C, Thomas C, Neefjes J J, Trowsdale J. Immunity. 1996;4:87–96. doi: 10.1016/s1074-7613(00)80301-5. [DOI] [PubMed] [Google Scholar]
- 25.Werdelin O. Scand J Immunol. 1986;24:625–636. doi: 10.1111/j.1365-3083.1986.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 26.Donermeyer D L, Allen P M. J Immunol. 1989;142:1063–1068. [PubMed] [Google Scholar]
- 27.Mouritsen S, Meldal M, Werdelin O, Hansen A S, Buus S. J Immunol. 1992;149:1987–1993. [PubMed] [Google Scholar]
- 28.Nelson C A, Vidavsky I, Viner N J, Gross M L, Unanue E R. Proc Natl Acad Sci USA. 1997;94:628–633. doi: 10.1073/pnas.94.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bohley P, Seglen P O. Experientia. 1992;48:151–157. doi: 10.1007/BF01923508. [DOI] [PubMed] [Google Scholar]
- 30.Diment S, Stahl P. J Biol Chem. 1985;260:15311–15317. [PubMed] [Google Scholar]
- 31.Puri J, Factorovich Y. J Immunol. 1988;141:3313–3317. [PubMed] [Google Scholar]
- 32.Vidard L, Rock K, Benacerraf B. J Immunol. 1991;147:1786–1791. [PubMed] [Google Scholar]
- 33.Adorini L, Guery J C, Fuchs S, Ortiz-Navarrete V, Hammerling G J, Momburg F. J Immunol. 1993;151:3576–3586. [PubMed] [Google Scholar]
- 34.Mizuochi T, Yee S, Kakiuchi T, Muno D, Kominami E. Immunol Lett. 1994;43:189–193. doi: 10.1016/0165-2478(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 35.Manoury-Schwartz B, Chiocchia G, Lotteau V, Fournier C. Int Immunol. 1997;9:581–589. doi: 10.1093/intimm/9.4.581. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez G M, Diment S. J Immunol. 1992;149:2894–2898. [PubMed] [Google Scholar]
- 37.van Noort J M, Jacobs M J M. Eur J Immunol. 1994;24:2175–2180. doi: 10.1002/eji.1830240936. [DOI] [PubMed] [Google Scholar]
- 38.Peters P J, Neefjes J J, Oorschot V, Ploegh H L, Geuze H J. Nature (London) 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Borja M, Verwoerd D, Sanderson F, Aerts H, Trowsdale J, Tulp A, Neefjes J. Int Immunol. 1996;8:625–640. doi: 10.1093/intimm/8.5.625. [DOI] [PubMed] [Google Scholar]
- 40.Seglen P O. Methods Enzymol. 1983;96:737–765. doi: 10.1016/s0076-6879(83)96063-9. [DOI] [PubMed] [Google Scholar]
- 41.Avva R R, Cresswell P. Immunity. 1994;9:763–774. doi: 10.1016/s1074-7613(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 42.Saftig P, Hetman M, Schmahl W, Weber K, Heine L, Mossman H, Köster A, Hess B, Evers M, von Figura K, Peters C. EMBO J. 1995;14:3599–3608. doi: 10.1002/j.1460-2075.1995.tb00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shimonkevitz R, Kappler J, Marrack P, Grey H. J Exp Med. 1983;158:303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyazaki T, Wolf P, Tourne S, Waltzinger C, Dierich A, Barois N, Ploegh H L, Benoist C, Mathis D. Cell. 1996;84:531–541. doi: 10.1016/s0092-8674(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 45.Bodmer H, Viville S, Benoist C, Mathis D. Science. 1994;263:1284–1286. doi: 10.1126/science.7510069. [DOI] [PubMed] [Google Scholar]
- 46.Shi G P, Webb A C, Foster K E, Knoll J H M, Lemere C A, Munger J S, Chapman H A. J Biol Chem. 1994;269:11530–11536. [PubMed] [Google Scholar]
- 47.Brachet V, Raposo G, Amigorena S, Mellman I. J Cell Biol. 1997;137:51–65. doi: 10.1083/jcb.137.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kasai M, Hirokawa K, Kajino K, Ogasawara K, Tatsumi M, Hermel E, Monaco J J, Mizuochi T. Eur J Immunol. 1996;26:2101–2107. doi: 10.1002/eji.1830260921. [DOI] [PubMed] [Google Scholar]
- 49.Amigorena S, Webster P, Drake J, Newcomb J, Cresswell P, Mellman I. J Exp Med. 1995;181:1729–1741. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fineschi B, Arneson L S, Naujokas M F, Miller J. Proc Natl Acad Sci USA. 1995;92:10257–10261. doi: 10.1073/pnas.92.22.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riese, R. J., Mitchell, R. N., Villadangos, J. A., Karp, E. R., De Sanctis, G. T., Ploegh, H. L. & Chapman, H. A. (1998) J. Clin. Invest., in press. [DOI] [PMC free article] [PubMed]