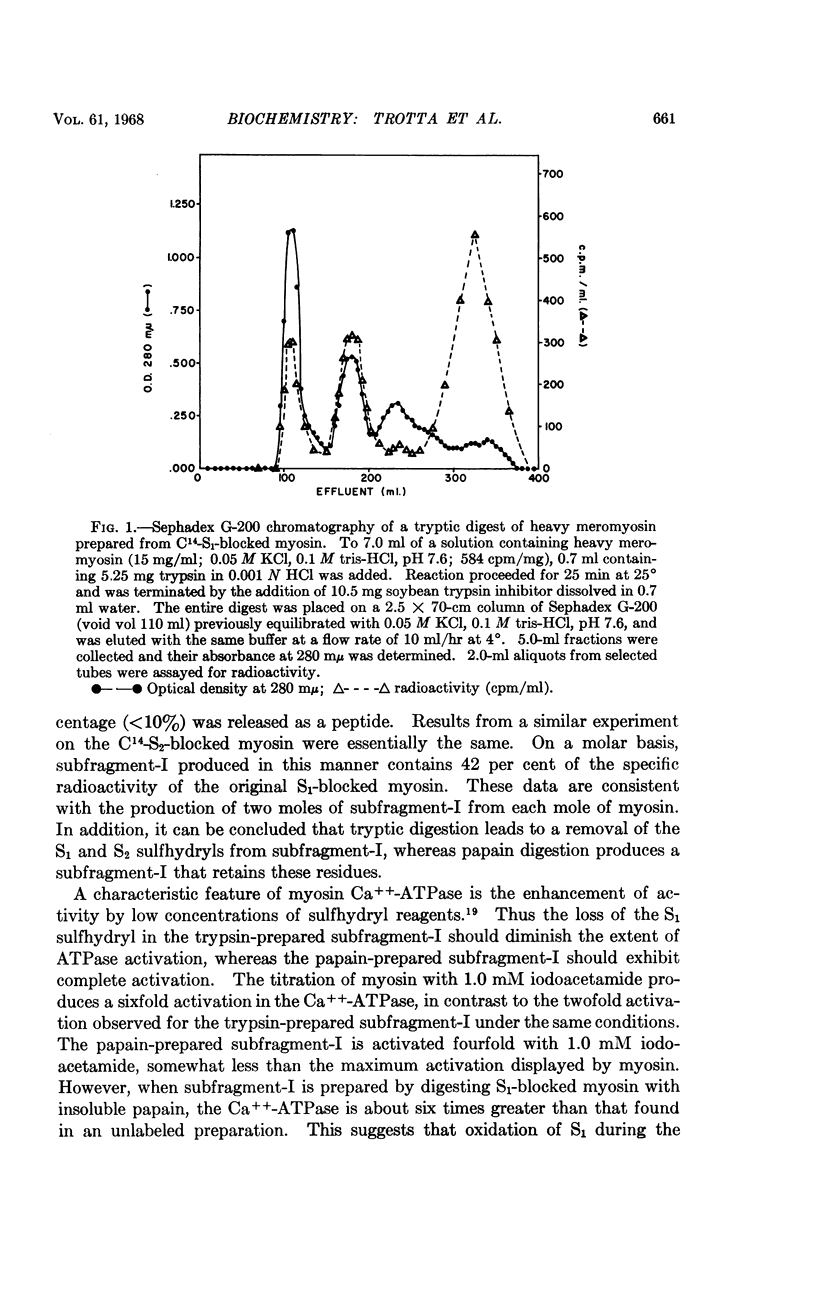
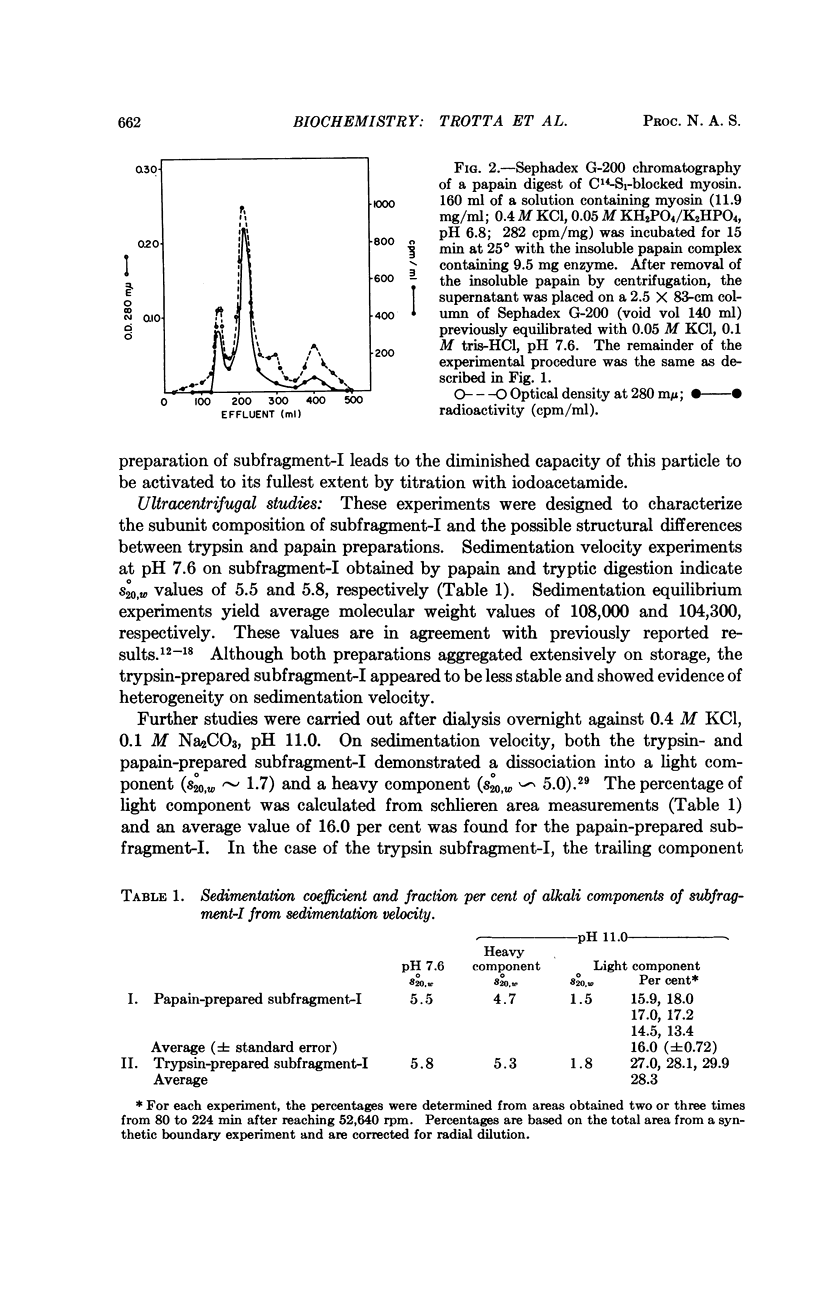
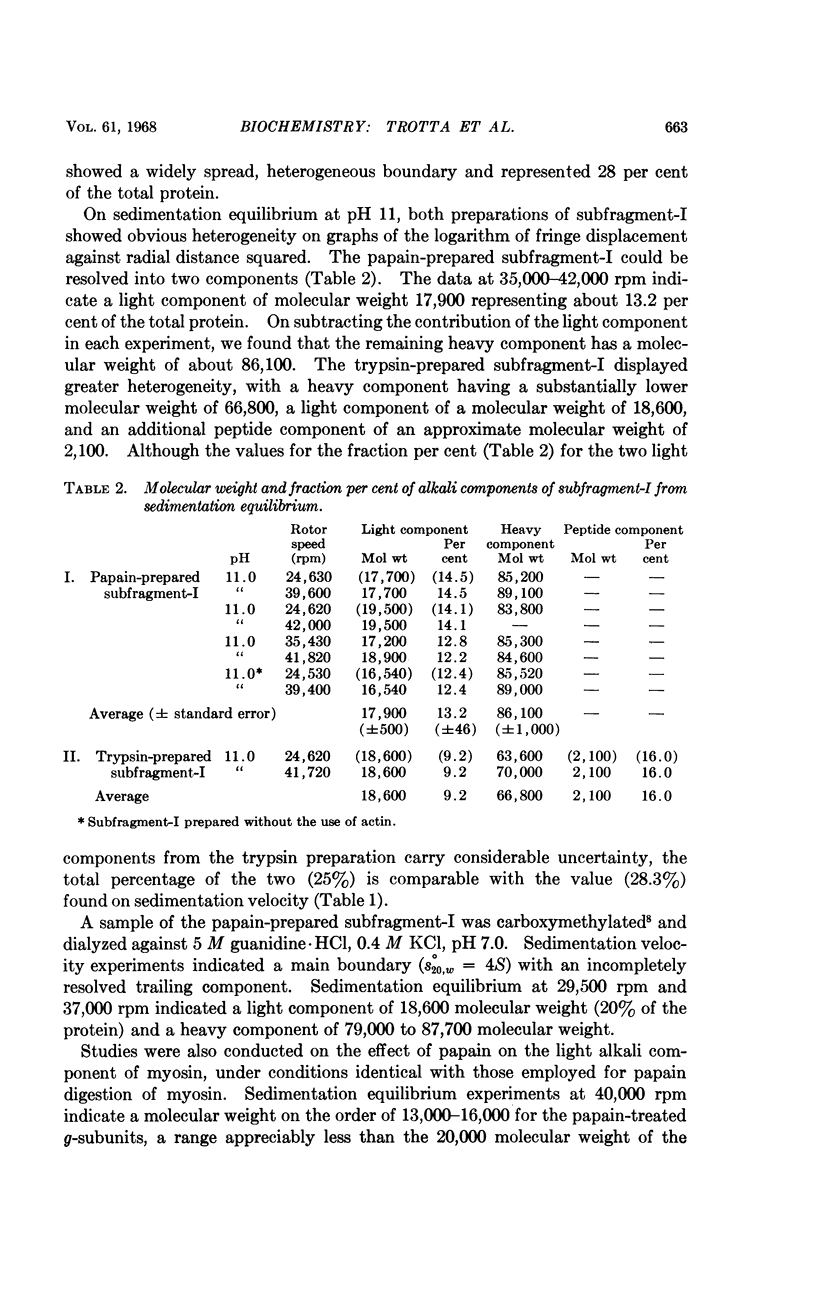
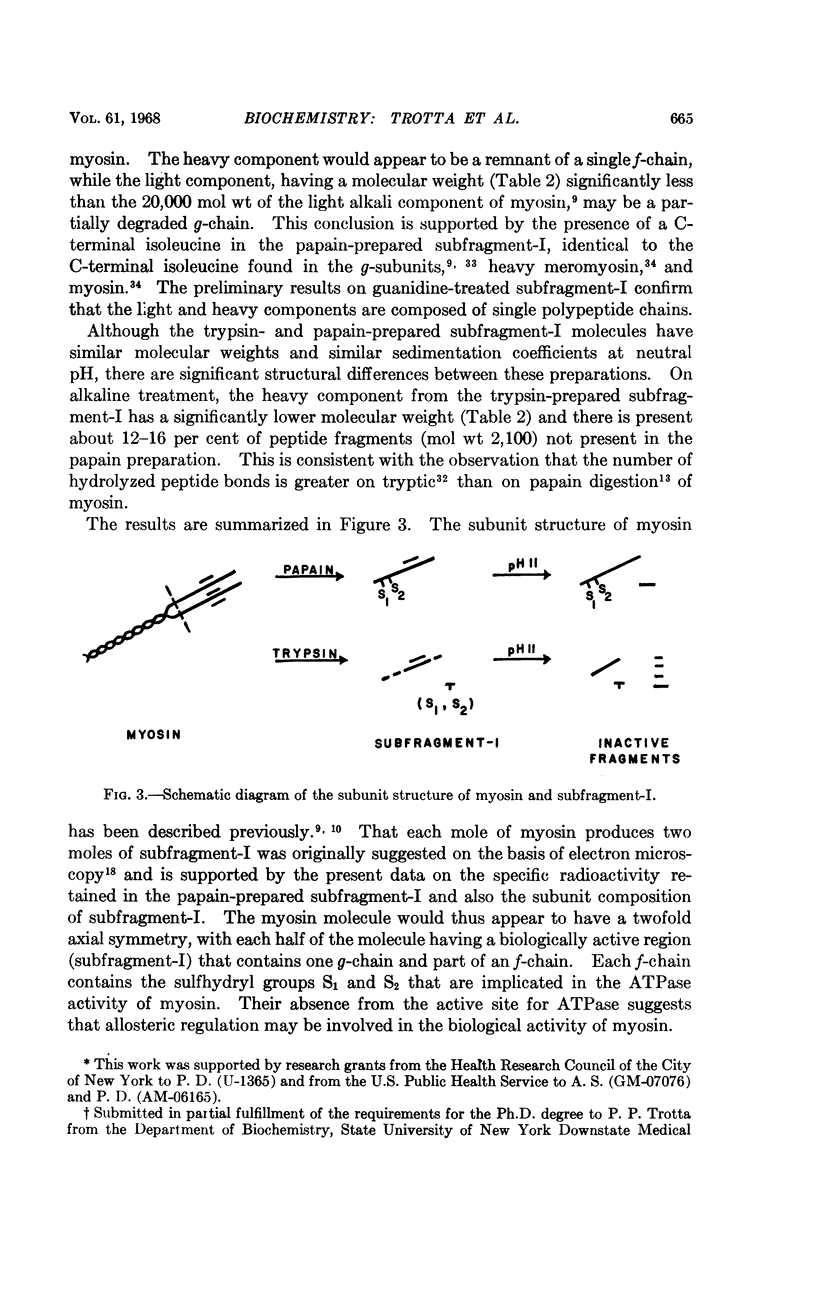
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARSTEN M. E., MOMMAERTS W. F. A study of actin by means of starch gel electrophoresis. Biochemistry. 1963 Jan-Feb;2:28–32. doi: 10.1021/bi00901a006. [DOI] [PubMed] [Google Scholar]
- Dreizen P., Hartshorne D. J., Stracher A. The subunit structure of myosin. I. Polydispersity in 5 M guanidine. J Biol Chem. 1966 Jan 25;241(2):443–448. [PubMed] [Google Scholar]
- GILMOUR D., GELLERT M. The binding of p-chloromercuribenzoate by myosin. Arch Biochem Biophys. 1961 Jun;93:605–616. doi: 10.1016/s0003-9861(61)80059-3. [DOI] [PubMed] [Google Scholar]
- Gershman L. C., Dreizen P., Stracher A. Subunit structure of myosin, II. Heavy and light alkali components. Proc Natl Acad Sci U S A. 1966 Sep;56(3):966–973. doi: 10.1073/pnas.56.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. M., Perry S. V. The biological activity of subfragment 1 prepared from heavy meromyosin. Biochem J. 1966 Jul;100(1):120–129. doi: 10.1042/bj1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELLEY W. W., BRADLEY L. B. The relationship between sulfhydryl groups and the activation of myosin adenosinetriphosphatase. J Biol Chem. 1956 Feb;218(2):653–659. [PubMed] [Google Scholar]
- LOWEY S., COHEN C. Studies on the structure of myosin. J Mol Biol. 1962 Apr;4:293–308. doi: 10.1016/s0022-2836(62)80007-2. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MUELLER H., PERRY S. V. The degradation of heavy meromyosin by trypsin. Biochem J. 1962 Dec;85:431–439. doi: 10.1042/bj0850431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller H. Characterization of the molecular region containing the active sites of myosin. J Biol Chem. 1965 Oct;240(10):3816–3828. [PubMed] [Google Scholar]
- Nihei T., Kay C. M. Isolation and properties of an enzymatically active fragment from papain-digested myosin. Biochim Biophys Acta. 1968 May 6;160(1):46–52. doi: 10.1016/0005-2795(68)90062-7. [DOI] [PubMed] [Google Scholar]
- SEKINE T., BARNETT L. M., KIELLEY W. W. The active site of myosin adenosine triphosphatase. I. Localization of one of the sulfhydryl groups. J Biol Chem. 1962 Sep;237:2769–2772. [PubMed] [Google Scholar]
- Slayter H. S., Lowey S. Substructure of the myosin molecule as visualized by electron microscopy. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1611–1618. doi: 10.1073/pnas.58.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAO T. C. Fragmentation of the myosin molecule. Biochim Biophys Acta. 1953 Jul;11(3):368–382. doi: 10.1016/0006-3002(53)90056-0. [DOI] [PubMed] [Google Scholar]
- YAMASHITA T., SOMA Y., KOBAYASHI S., SEKINE T., TITANI K., NARITA K. THE AMINO ACID SEQUENCE AT THE ACTIVE SITE OF MYOSIN A ADENOSINE TRIPHOSPHATASE ACTIVATED BY EDTA. J Biochem. 1964 May;55:576–577. [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. ON THE STRUCTURAL ASSEMBLY OF THE POLYPEPTIDE CHAINS OF HEAVY MEROMYOSIN. J Biol Chem. 1965 Jun;240:2428–2436. [PubMed] [Google Scholar]
- YOUNG D. M., HIMMELFARB S., HARRINGTON W. F. THE RELATIONSHIP OF THE MEROMYOSINS TO THE MOLECULAR STRUCTURE OF MYOSIN. J Biol Chem. 1964 Sep;239:2822–2829. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



