Abstract
Human umbilical cord blood T lymphocytes (CBTL) respond to primary allostimulation but they do not proliferate upon rechallenge with alloantigen. Using PKH-26-labeled cells created a proliferative block that was observed only in CBTL that have divided during primary stimulation (PKH-26dim) but not in unstimulated (PKH-26bright) CBTL. CBTL’s secondary unresponsiveness resembles anergy and can be overcome by treatment with phorbol myristate acetate (PMA) and ionomycin or by high doses (50–100 units/ml) of interleukin 2. Addition of interleukin 2 to the primary cultures does not prevent the induction of secondary unresponsiveness. Defective Ras activation is detected in PKH-26dim CBTL during secondary response to alloantigen or after antibody-mediated T cell receptor stimulation whereas Ras is activated and proliferation is induced in CBTL during primary alloantigenic stimulation. Upon stimulation with PMA plus ionomycin, PMA plus alloantigen, but not alloantigen plus ionomycin, Ras is activated in PKH-26dim CBTL, and the block in proliferation is overcome. Correction of PKH-26dim CBTL’s proliferative defect correlates with PMA-induced Ras activation, suggesting a defect in the signaling pathway leading to Ras. Ras-independent signals, necessary but not sufficient to induce PKH-26dim CBTL proliferation, are provided by alloantigen exposure, as evident by the ability of PMA plus alloantigen but not PMA alone to overcome the proliferative block. Functional signal transduction through CD28 in PKH-26dim CBTL is supported by detectable CD28-mediated PI-3 kinase activation after PKH-26dim CBTL’s exposure to alloantigen or CD28 cross-linking. These results suggest that defective activation of Ras plays a key role in PKH-26dim CBTL’s secondary unresponsiveness and point to a defect along the T cell receptor rather than the CD28 signaling pathway.
The successful outcome of allogeneic bone marrow transplantation is significantly limited by the risk of graft versus host disease, which correlates with the number of mature T cells present in the graft and with the degree of genetic disparity between the donor and the host (1). Preliminary clinical data on cord blood stem cell (CBSC) transplantation suggest a reduced graft versus host disease-inducing potential, despite the presence of large numbers of T cells in the grafts and the use of partially HLA mismatched donors (2, 3). These intriguing observations have elicited many questions on the immunological reactivity of umbilical CBSC grafts and the alloreactive potential of cord blood T lymphocytes (CBTL). We have shown that CBTL respond normally to primary allostimulation (4) and that mitogen- or alloantigen-activated CBTL express equivalent amounts of CD3, CD28, and CD25 as compared with similarly activated peripheral blood T lymphocytes (PBTL) (5). In addition, CBTL switch from a mostly CD45RA+ phenotype to a mostly CD45RO+ phenotype after stimulation and culture (5). However, unlike PBTL, alloantigen-primed CBTL are not induced to proliferate when restimulated with the same or a third-party alloantigen, a phenomenon we refer to as secondary unresponsiveness (4). The mechanism of secondary unresponsiveness is currently unknown, and although suppressor cells and activation-induced cell death have not been conclusively excluded, alloantigen-induced anergy seems to be a more likely mechanism. Currently accepted models of anergy induction are consistent with an abnormal T cell receptor (TCR)-mediated activation of cytoplasmic protein tyrosine kinases and of the Ras/mitogen-activated protein kinase pathway after T cell stimulation in the presence of insufficient costimulatory signals (6, 7). The secondary unresponsiveness induced by alloantigen exposure in freshly isolated CBTL, however, is peculiar because it occurs in spite of both TCR and costimulatory molecule activation. In this report we present evidence indicating that defective Ras activation is directly associated with secondary unresponsiveness.
MATERIALS AND METHODS
Antibodies.
The following antibodies were used: OKT3 (mouse anti-human CD3), obtained from the America Type Culture Collection; mouse IgG1 anti-human CD28 and mouse IgG1 anti-human CD2 (BioSource International, Camarillo, CA); Y13–259 rat anti-Ras antibody (Oncogene Research, Cambridge, MA); and goat anti-mouse IgG serum (Sigma).
Cells.
Human cord blood was obtained via heparinized syringes from normal deliveries at Wishard Memorial Hospital in Indianapolis, as approved by the Institutional Review Board of the Indiana University School of Medicine. Heparinized adult peripheral blood was obtained after informed consent from healthy volunteer donors. Mononuclear cells were obtained from all samples by centrifugation over Ficoll/Hypaque (Pharmacia LKB) gradients. Purified T cells were obtained by incubating 15–25 × 106 mononuclear cells with Lymphokwik-T (One Lambda, Los Angeles) as previously described (4). Cell purity was >90% CD3+. For PKH-26 staining, cells were prepared and stained according to the manufacturer’s instructions (Sigma).
Proliferation Assays.
Primary mixed leukocyte cultures (MLC) were established in 75-cm3 tissue culture flasks with 10–20 × 106 responding PKH-26-labeled T cells and irradiated (10,000 rad) stimulating allogeneic cells at a final density of 2 × 106 cells/ml in complete medium consisting of RPMI 1640, 2 mM glutamine, 1 mM sodium pyruvate, and 50 μg/ml of penicillin/streptomycin (GIBCO/BRL). All cultures were supplemented with 10% (vol/vol) human AB serum (Sigma). Flasks were incubated at 37°C in 5% CO2/95% air for 7–10 days. The lymphoblastoid cell line Sweig (kindly provided by Janice Blum, Indiana University School of Medicine, Indianapolis) served as alloantigen. The responder to stimulator ratio was 1:1. At day 7–10 of primary culture, cells were washed once in PBS and sorted into PKH-26dim and PKH-26bright populations by using a FACStar Plus cell sorter (Becton Dickinson) (8). Viability was determined by trypan blue exclusion. Secondary MLC were then generated with purified PKH-26dim and PKH-26bright cells. For stimulation experiments performed with mAbs, cells were cultured at a final density of 1 × 106 cells/ml and stimulated with 10 μg/ml of antibody. Goat anti-mouse IgG was added to the cultures to cross-link anti-CD3 antibodies.
Detection of Guanine Nucleotides Bound to Ras.
Detection of GTP-bound Ras was performed following the protocol described by Gibbs et al. (9). Briefly, CBTL and PBTL were incubated with serum-free RPMI 1640 medium for 18 hr, washed, and incubated for 4 hr with serum-free, phosphate-minus RPMI 1640 medium (GIBCO/BRL) containing 0.5 μCi of carrier-free 32P-labeled orthophosphate (Amersham). Cells were then stimulated for 15 min at 37°C and lysed with lysis buffer containing 100 μg/ml of anti-Ras Y13–259 mAb. Immunoprecipitations were performed in 1.5-ml microcentrifuge tubes with 150 μl of a 40% slurry of Sepharose CL-4B-protein A (Pharmacia LKB) previously coupled with rabbit anti-rat Ig (Sigma). Control immunoprecipitations were done with Sepharose CL-4B. After 2 hr at 4°C, the immunocomplexes were washed with lysis buffer (without Y13–259 antibody) and resuspended in 200 μl of 1M KH2PO4, pH 3.4, and heated for 5 min at 90°C. The solution was clarified by centrifugation, and 15 μl was spotted onto a sheet of polyethylene imine-cellulose (J.T. Baker). Chromatograms were then developed with 1 M KH2PO4, pH 3.4, and exposed to Kodak XAR-2 film for 5–10 days at −70°C.
In Vitro Phosphatidylinositol-3′-Kinase (PI-3K) Assay.
Measurement of CD28-associated PI-3K activity was performed following the method described by Truitt et al. (10) with minor modifications. Briefly, 5 × 106 cells were stimulated with antibodies (10 μg/ml) for 15 min or with allogeneic cells for 30 min at 37°C and lysed with 1 ml of ice-cold lysis buffer. Immunoprecipitation was performed with 5 μg of anti-CD28 antibody, anti-CD3 antibody, or with goat anti-mouse antibody as control, followed by overnight incubation with Protein G-coupled Sepharose CL-4B (Sigma) at 4°C. The immunoprecipitate was then resuspended in 50 μl of kinase reaction buffer (10) containing 10 μCi of [γ-32P]ATP and 10 μg of phosphatidylinositol-biphosphate (PIP2) (Sigma) and incubated for 10 min at 37°C. The organic phase was then extracted with 1:1 chloroform/methanol (vol/vol) and dried under vacuum, and the lipids were resolved by thin layer chromatography on Si250-PA plates (J. T. Baker). The plates were then exposed to Kodak-XAR film for 5–10 days at −70°C.
RESULTS
Secondary Unresponsiveness Is Due to an Intrinsic Proliferative Defect Detectable Only in Successfully Primed CBTL.
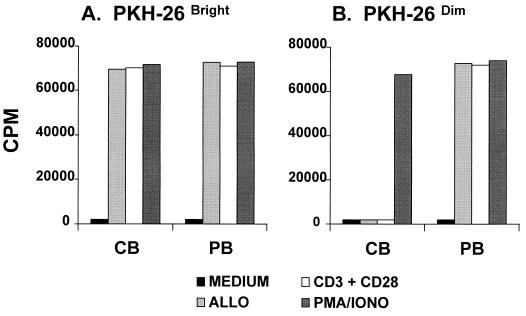
Most of the data describing the proliferative response of CBTL after allostimulation thus far have been obtained by using bulk cultures. Even if CBTL are repurified at the end of primary culture and replated in equal numbers, remarkable sample-to-sample variability in the secondary stimulation experiments may still result because of varying percentages of CBTL being successfully primed during primary allostimulation. To overcome this problem, freshly isolated CBTL were labeled with PKH-26 and then cultured in the presence of irradiated human allogeneic cells. At the end of a typical 7- to 10-day primary allostimulation experiment, the majority of the cells are PKH-26dim, indicating that they have completed one or more cycles of cell division, whereas a minority of cells, not successfully stimulated, remain PKH-26bright (Fig. 1). PKH-26dim and PKH-26bright CBTL (and similarly labeled PBTL as controls) were subsequently sorted by FACS and plated separately in secondary stimulation experiments. Fig. 2 shows the secondary proliferative responses of PKH-26-sorted CBTL and PBTL. PKH-26dim CBTL, unlike PKH-26bright CBTL and PKH-26dim and PKH-26bright PBTL, are unable to proliferate when rechallenged with TCR-dependent stimuli, such as alloantigen or CD3 plus CD28 cross-linking. Of note, cell surface expression of CD3, CD28, and CD25 does not significantly differ between PKH-26dim CBTL and PKH-26dim PBTL (data not shown). That PKH-26bright CBTL can be induced to proliferate in secondary cultures whereas PKH-26dim CBTL cannot suggests that the proliferative block is not due to nonspecific soluble factors produced by cells with suppressor activity in MLC but, rather, to an intrinsic resistance to reactivation that develops only in CBTL after a productive primary response to antigen. To determine whether PKH-26dim CBTL’s unresponsiveness is due to a proximal or distal transmembrane signaling defect, cells were treated with a combination of PMA and ionomycin, which are known to activate mitogenic pathways by stimulation of protein kinase C (PKC) and calcium-dependent messenger systems (11). As shown in Fig. 2, PKH-26dim CBTL can be induced to proliferate by PMA plus ionomycin, indicating that signaling pathways downstream of PKC (including Ras) and the calcium messenger system are intact and functional.
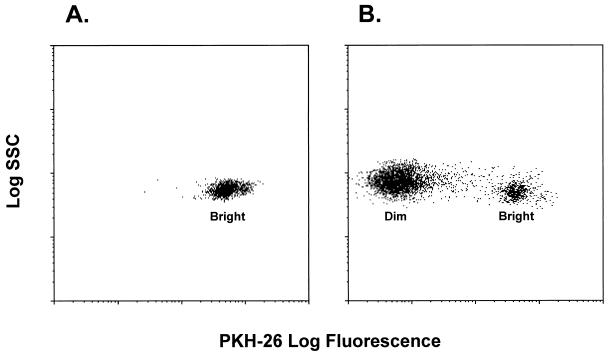
Figure 1.
Flow cytometric analysis of CBTL after PKH-26 staining. CBTL were purified and stained with PKH-26 on day 0 of primary MLC. They are identified as a homogeneously PKH-26bright population (A). After allostimulation and culture for 7–10 days, a major population of PKH-26dim CBTL is detectable, together with a residual minor population of PKH-26bright cells (B). PKH-26 staining was performed in all MLC experiments.
Figure 2.
(B) Alloantigen-primed PKH-26dim CBTL (CB) do not proliferate after secondary stimulation with alloantigen or CD3 plus CD28 activation, whereas PKH-26dim PBTL (PB) do. PKH-26bright CBTL and PKH-26bright PBTL, which were not successfully activated during primary alloresponse, exhibit a vigorous proliferative response when rechallenged with alloantigen or CD3 plus CD28 activation (A). Treatment of PKH-26dim CBTL with PMA and ionomycin overcomes the proliferative block (B). Results represent the mean of three different experiments. Each experiment was performed in triplicate.
Analysis of the Stimulatory Signals Able to Overcome Secondary Unresponsiveness.
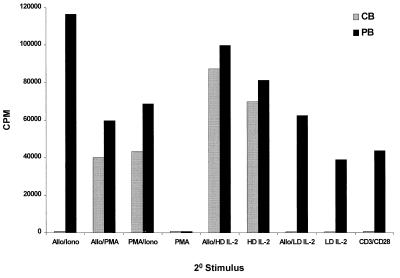
T cell allostimulation, as studied in MLC, requires complex molecular interactions with antigen-presenting cells (12). Different families of membrane-bound receptors and ligands, chief among which are TCR and CD28, are engaged in the induction of T cell proliferation, which is a hallmark of a productive alloresponse (13). Because this complexity makes the delineation of the biochemical mechanisms responsible for secondary unresponsiveness a difficult task, we investigated the outcome of secondary stimulation of alloantigen-primed CBTL by selective activation of distinct signaling pathways. Alloantigen-primed PKH-26dim CBTL and PKH-26dim PBTL were initially plated in secondary cultures in the presence of various combinations of stimulatory signals, including alloantigen, anti-CD3, and anti-CD28 antibodies, PMA, ionomycin, and interleukin 2 (IL-2) (at low or high doses) (Fig. 3). As already shown in Fig. 2, anti-CD3 plus anti-CD28 antibodies had no effect on PKH-26dim CBTL, whereas PMA + ionomycin induced vigorous proliferation. The combination of alloantigen and PMA induced a proliferative response comparable to PMA plus ionomycin, whereas alloantigen plus ionomycin had no effect. Addition of low doses (5 units/ml) of IL-2, either alone or in combination with anti-CD3 and anti-CD28, had no effect. On the other hand, high-dose IL-2 (50–100 units/ml), in the presence or the absence of alloantigen, stimulated PKH-26dim CBTL to proliferate, indicating that IL-2-dependent mitogenic pathways can still be activated in these cells if they are exposed to high concentrations of IL-2 and that TCR signaling does not provide a dominant negative signal. To determine whether exposure to IL-2R common γ chain-activating cytokines could block the induction of secondary unresponsiveness, similar to what has been described for anergy induction in human T cell clones (14), we performed primary alloantigenic stimulation experiments in the presence of exogenous IL-2 (10–100 units/ml) and examined secondary alloresponses as described above. PKH-26dim CBTL exposed to low- or high-dose IL-2 during primary stimulation were still unresponsive to subsequent alloantigenic challenge or to CD3 plus CD28 cross-linking (data not shown).
Figure 3.
Differences in the proliferative response to secondary stimulation between alloantigen-primed PKH-26dim CBTL and PKH-26dim PBTL. Alloantigen-primed PKH-26dim CBTL do not proliferate upon secondary stimulation with CD3 plus CD28 (CD3/CD28), even in the presence of low-dose (5 units/ml) IL-2 (CD3/CD28/LD IL2). Secondary stimulation with PMA plus ionomycin (PMA/Iono) or PMA plus alloantigen (PMA/Allo) induces proliferation but not stimulation with ionomycin plus alloantigen (Iono/Allo). High-dose (50–100 units/ml) IL-2, either alone (HD IL2) or combined with alloantigen (HD IL2/Allo), overcomes the secondary proliferative block of PKH-26dim CBTL. Experiments with low-dose IL-2 alone (LD-IL2) and PMA alone (PMA) are also presented, showing that neither can induce cell proliferation in PKH-26dim CBTL. Results represent the mean of three different experiments. Each experiment was performed in triplicate.
Secondary Unresponsiveness in PKH-26dim CBTL Is Associated with Defective Activation of Ras.
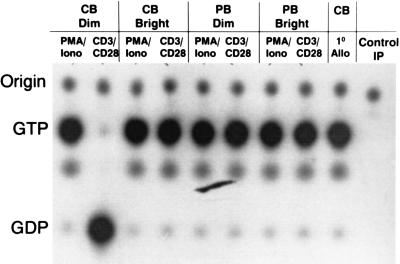
Based on the fact that (i) treatment of T cells with PMA + ionomycin is known to induce activation of Ras, (ii) Ras is crucial for TCR-mediated T cell proliferation, and (iii) RAS activation has been shown to be defective in murine models of T cell anergy (15–17), we examined Ras activation in PKH-26dim CBTL as compared with PLH-26bright CBTL and similarly labeled PBTL. In the experiment shown in Fig. 4, CB and PB T cells were stimulated with alloantigen during the primary stimulation and with anti-CD3 and anti-CD28 antibodies during the secondary stimulation. This was done to avoid the use of large amounts of antibodies during bulk primary cultures as well as to give specific stimulation during the secondary culture. Similar results were obtained when either alloantigen or antibodies were used for both primary and secondary stimulation (data not shown). As shown in Fig. 4, freshly isolated CBTL demonstrated activation of Ras after primary allostimulation (CB 1° Allo), which paralleled the robust proliferative response induced under the same conditions (4). Ras activation was also seen when PKH-26bright CBTL (CB Bright) and PKH-26bright PBTL (PB Bright) were stimulated in secondary cultures with anti-CD3 plus anti-CD28 antibodies or alloantigen (data not shown), again in agreement with the proliferation assays described in Fig. 2. The same was true for PKH-26dim PBTL (PB Dim). PKH-26dim CBTL (CB Dim), on the other hand, fail to activate Ras when stimulated with anti-CD3 plus anti-CD28 antibodies, although they can be induced to activate Ras (Fig. 4) as well as proliferate (Fig. 2) by treatment with PMA and ionomycin. This close correlation between Ras activation and the ability to proliferate upon TCR-dependent stimulation suggests that Ras is crucial for the proliferative alloresponse of CBTL and that conditions leading to defective Ras activation during secondary stimulation may result in secondary unresponsiveness.
Figure 4.
Alloantigen-primed PKH-26dim CBTL have defective Ras activation after restimulation with CD3 plus CD28. Ras can be activated in PKH-26dim CBTL by treatment with PMA plus ionomycin. The ability of freshly isolated CBTL to activate Ras during primary allostimulation is shown (CB 1o Allo), indicating that defective Ras activation develops in PKH-26dim CBTL only after a primary proliferative response. PKH-26dim PBTL, PKH-26bright CBTL, and PKH-26bright PBTL show normal Ras activation. Similar results were obtained when either alloantigen or antibodies were used for both primary and secondary experiments (data not shown). Control IP, immunoprecipitation with rat anti-mouse Ig.
Role of TCR and CD28 Signaling in the Defective Activation of Ras in PKH-26dim CBTL.
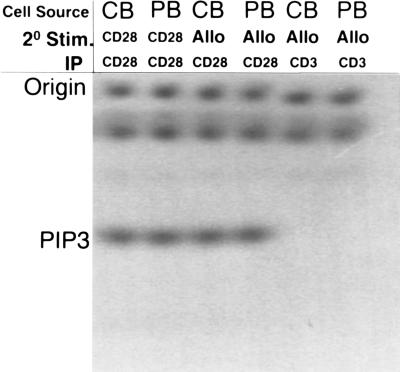
Ras activation in T cells is believed to be mediated mainly through a TCR-dependent pathway, although the coupling mechanism between the two currently is not clear (18). It has also been shown that cross-talk between the TCR and CD28 pathways exists and that CD28 signaling may cause synergistic activation of Ras and some of its downstream effectors, possibly through interactions with adapter molecules such as Grb-2 (19). To determine whether CD28-mediated signal transduction was intact in PKH-26dim CBTL, CD28 signaling was examined by measuring the amount of PI-3K enzymatic activity that could be coimmunoprecipitated with anti-CD28 antibodies from alloantigen-primed PKH-26dim CBTL after CD28 cross-linking. Although it is unclear whether CD28-mediated costimulation depends on PI3K binding and activation (20, 21), it has been shown that signaling through CD28 results in PI3K binding and activation. Therefore, PI3K activation can be used as a surrogate marker for CD28-mediated signaling even if the dependence of various CD28-mediated intracellular events on PI3K activation is still under scrutiny and far from being conclusively sorted out (22). Fig. 5 shows that CD28 immunoprecipitation after stimulation of PKH-26dim CBTL and PKH-26dim PBTL with anti-CD28 antibodies (CB/CD28/CD28 and PB/CD28/CD28), followed by in vitro PI-3 kinase assay, results in the detection of similar amounts of PIP3. As a control, the same immunoprecipitation and in vitro kinase experiments were performed after stimulating cells with irrelevant (goat anti-mouse) antibodies (not shown). To examine whether these findings were reproducible in experimental conditions simulating a more physiological antigen encounter, alloantigen-primed PKH-26dim CBTL and PKH-26dim were exposed to irradiated allogeneic cells and rapidly solubilized. CD28 immunoprecipitation was then performed on the cell lysate (CB/Allo/CD28 and PB/Allo/CD28) and was followed by in vitro kinase assays. Again, PIP3 was detectable in similar amounts. Control immunoprecipitations with anti-CD3 antibodies yielded no detectable amount of PIP3 (CB/Allo/CD3 and PB/Allo/CD3). Together with the data on the proliferative effect of the combination of alloantigen and PMA, these results strongly suggest that CD28-mediated signaling can be efficiently activated in PKH-26dim CBTL.
Figure 5.
In vitro PI-3K assay after secondary stimulation of alloantigen-primed PKH-26dim CBTL and PKH-26dim PBTL. Cells were stimulated by CD28 cross-linking or with alloantigen and lysed, and immunoprecipitation with anti-CD28 antibodies was performed. The PI-3K activity of the immunoprecipitate was then measured as the amount of radioactive PIP3 generated. Control immunoprecipitation was performed with anti-CD3 antibodies.
DISCUSSION
A large body of experimental evidence in laboratory animals clearly indicates that specific immune tolerance can be induced in neonatal T lymphocytes (23). Less direct evidence of such predisposition is available in human T lymphocytes from experiments showing a generic immaturity in cytotoxic activity, lymphokine production, and expression of activation markers (24, 25). More recently, defective IL-2 receptor γ chain and CD40 ligand (CD40L) expression and/or function have been reported in neonatal T cells by some (26, 27) but not all investigators (28), and this was proposed as an explanation for neonatal T cells’ reduced antigenic response and helper function for B cells (27). Few of these studies, however, have clearly established the molecular basis of well characterized functional defects and investigated their relevance to the phenomenon of neonatal tolerance induction, mainly because of the lack of a convenient and reproducible model and the difficulty of performing signaling assays with heterogeneous populations and limited numbers of cells. To this regard, CBTL represent a unique model of antigen-induced unresponsiveness that may be relevant for the study of anergy and that may provide an insight into the cellular and molecular interactions occurring in vivo after CBSC transplantation. In this paper, we establish a biochemical link between the altered response to alloantigen observed in CBTL in vitro and the signaling machinery leading to their activation and proliferation.
PKH-26 cell labeling and sorting allow the purification of successfully alloantigen-primed CBTL from primary MLC and the performance of cell culture and biochemical assays on functionally homogeneous cell populations (as defined by proliferation assays). Our data clearly indicate that the blunted response originally observed after secondary allostimulation of CBTL in bulk cultures (4) is not due to apoptosis or to cellular or humoral factors with suppressive activity. This is evident from the fact that PKH-26bright CBTL isolated from the same primary culture flasks as PKH-26dim CBTL respond normally to secondary stimulation and from the ability of PKH-26dim CBTL to respond in secondary cultures if provided with adequate stimuli (PMA + lonomycin or high-dose IL-2). In addition, viability in both PKH-26dim and PKH-26bright cell populations at the end of primary cultures is consistently >90%. Conversely, the complete lack of response to secondary stimulation observed by using homogeneous PKH-26dim CBTL, as opposed to the blunted response in bulk cultures, is most likely a result of the elimination of the small residual population of PKH-26bright CBTL. Based on these findings, we postulated that alloantigen-primed PKH-26dim CBTL acquire a defect in their signaling response to antigen, which results in a block of cell proliferation upon further TCR-dependent stimulation. We now provide experimental confirmation of our hypothesis showing that the block is at the level of Ras activation.
The role of Ras in T cell proliferation clearly has been established by the work of others (15), and the importance of the Ras/mitogen-activated protein kinase pathway in TCR signaling is underscored by studies of this pathway in anergic murine T cells (16, 17). To date, little information has been available on the role of the Ras signaling pathway in anergic human cells. Recently, preliminary studies have suggested that TCR-dependent stimulation of human T cell clones in the absence of costimulation, which induces anergy, results in the preferential formation of a cbl-crkL-C3G complex and the consequent activation of Rap-1, which functions as a down-regulator of Ras, as opposed to a Shc-Grb2-SOS complex detected in T cell clones that received both TCR stimulation and full costimulation (29). However, direct demonstration of an inhibitory effect of Rap-1 on Ras activation in the anergic cells was not provided. The activation status of Ras in T cells depends on a delicate balance between positive and negative regulators, both of which potentially can be modulated by TCR-mediated signals and synergistically affected by costimulatory signals mediated by CD28 (18). Although we do not provide definitive evidence indicating which of the above pathways is involved in the altered Ras activation observed in PKH-26dim CBTL, the ability of PMA to complement alloantigen as a proliferative stimulus for PKH-26dim CBTL and the evidence of detectable signal transduction through CD28 in these cells, as shown by PI-3 kinase activation, suggest that the TCR signaling pathway leading to Ras activation, rather than the CD28 pathway, is defective.
Although CBTL’s secondary unresponsiveness shows a close resemblance to current anergy models, there are a number of important differences. In Schwartz’s classic in vitro anergy model (27), anergy induction requires occupancy of TCR (signal 1) in the absence of costimulation (signal 2). However, CBTL’s secondary unresponsiveness is clearly inducible in the presence of both signals (delivered either by allogeneic cells or by CD3 + CD28 cross-linking). Provision of additional costimulation in the form of common γ chain IL-2R activation (with IL-2), which has been shown to block anergy induction in human T cell clones (14), does not prevent CBTL from entering an unresponsive state. Altered signal transduction through the TCR or CD28 during the primary response, leading to an altered pattern of nonreceptor protein tyrosine kinase activation, as described by others (7), cannot be excluded based on our current data. However, it is unclear which role similar differences would have in the induction of secondary unresponsiveness because Ras and PI-3K activation occurs normally in CBTL during primary allostimulation and their primary proliferative response is indistinguishable from that of PBTL in terms of kinetics, CD25 up-regulation, and cell viability at the end of culture. It is likely that differences in signal transduction during primary alloantigen stimulation exist between CBTL and PBTL that are responsible for the opposite functional outcome following secondary antigen encounter. Their nature and functional relevance, though, is likely to be more easily determined when the specific signaling mechanism leading to defective Ras activation upon secondary stimulation is identified.
It is unclear at this time whether these findings bear any relevance to the graft versus host disease-inducing potential of CBSC grafts and whether secondary unresponsiveness develops in vivo after CBSC transplantation. Additional in vitro experiments are necessary to determine whether the unresponsive status is reversible or not and how prolonged it is. Finally, further delineation of the functional status of CBTL in vivo remains an important goal, which unfortunately is hampered by the necessity for prolonged immunosuppression and by the lack of a sensitive assay to follow the destiny of these cells after transplant.
Acknowledgments
These studies were supported by U.S. Public Health Service Grants R01 HL54037, R01 HL46416, and a project in P01 HL53586 from the National Institutes of Health to H.E.B. P.P. and J.G. were supported by Training Program T32DK07519 from the National Institutes of Health to H.E.B.
ABBREVIATIONS
- CBTL and PBTL
umbilical cord and peripheral blood T lymphocytes, respectively
- PMA
phorbol myristate acetate
- IL-2
interleukin 2
- TCR
T cell receptor
- CBSC
cord blood stem cell
- MLC
mixed leukocyte cultures
- PI-3K
phosphatidylinositol-3′-kinase
References
- 1.Deeg H J. Semin Hematol. 1993;30:110–117. [PubMed] [Google Scholar]
- 2.Kurtzberg J, Laughlin M, Graham M L, Smith C, Olson J F, Halperin E C, Ciocci G, Carrier C, Stevens C E, Rubinstein P. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 3.Wagner J E, Rosenthal J, Sweetman R, Shu X O, Davies S M, Ramsay N K, McGlave P B, Sender L, Cairo M S. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 4.Risdon G, Gaddy J, Horie M, Broxmeyer H E. Proc Natl Acad Sci USA. 1995;92:2413–2417. doi: 10.1073/pnas.92.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risdon G, Gaddy J, Stehman J B, Broxmeyer H E. Cell Immunol. 1994;154:14–24. doi: 10.1006/cimm.1994.1053. [DOI] [PubMed] [Google Scholar]
- 6.Gajewski T F, Qian D, Fields P, Fitch F W. Proc Natl Acad Sci USA. 1994;91:38–42. doi: 10.1073/pnas.91.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boussiotis V A, Barber D L, Lee B J, Gribben J G, Freeman G J, Nadler L M. J Exp Med. 1996;184:365–376. doi: 10.1084/jem.184.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verfaillie C M, Miller J S. Blood. 1995;86:2137–2145. [PubMed] [Google Scholar]
- 9.Gibbs J B, Marshall M S, Scolnick E M, Dixon R A F, Vogel U S. J Biol Chem. 1990;265:20437–20442. [PubMed] [Google Scholar]
- 10.Truitt K E, Hicks C M, Imboden J B. J Exp Med. 1994;179:1071–1076. doi: 10.1084/jem.179.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truneh A, Albert F, Golstein P, Schmitt-Verhulst A M. J Immunol. 1985;135:2262–2267. [PubMed] [Google Scholar]
- 12.Boussiotis V A, Freeman G J, Gribben J G, Nadler L M. Immunol Rev. 1996;153:5–26. doi: 10.1111/j.1600-065x.1996.tb00918.x. [DOI] [PubMed] [Google Scholar]
- 13.Qian D, Weiss A. Curr Opin Cell Biol. 1997;9:205–212. doi: 10.1016/s0955-0674(97)80064-6. [DOI] [PubMed] [Google Scholar]
- 14.Boussiotis V A, Barber D L, Nakarai T, Freeman G J, Gribben J G, Bernstein G M, D’Andrea A D, Ritz J, Nadler L M. Science. 1994;266:1039–1042. doi: 10.1126/science.7973657. [DOI] [PubMed] [Google Scholar]
- 15.Downward J, Graves J D, Warne P H, Rayter S, Cantrell D A. Nature (London) 1990;346:719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- 16.Fields P E, Gajewski T F, Fitch F W. Science. 1996;271:1276–1278. doi: 10.1126/science.271.5253.1276. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Whaley C D, Mondino A, Mueller D L. Science. 1996;271:1272–1276. doi: 10.1126/science.271.5253.1272. [DOI] [PubMed] [Google Scholar]
- 18.Cantrell D. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 19.Raab M, Cai Y C, Bunnell S C, Heyeck S D, Berg L J, Rudd C E. Proc Natl Acad Sci USA. 1995;92:8891–8895. doi: 10.1073/pnas.92.19.8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Phillips C A, Trevellyan J M. Eur J Immunol. 1995;25:533–537. doi: 10.1002/eji.1830250234. [DOI] [PubMed] [Google Scholar]
- 21.Truitt K E, Shi J, Gibson S, Segal L G, Mills G B, Imboden J B. J Immunol. 1995;155:4702–4710. [PubMed] [Google Scholar]
- 22.Ward S G. Biochem J. 1996;318:361–377. doi: 10.1042/bj3180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz H R. In: Fundamental Immunology. Paul W E, editor. New York: Raven; 1993. pp. 677–731. [Google Scholar]
- 24.Muller K, Zak M, Nielsen S, Redersen F K, de Nully P, Bendtzen K. Pediatr All Immunol. 1996;7:117–124. doi: 10.1111/j.1399-3038.1996.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 25.Amlot P L, Tahami F, Chinn D, Rawlings E. Clin Exp Immunol. 1996;105:176–182. doi: 10.1046/j.1365-2249.1996.d01-722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nonayama S, Penix L A, Edwards C P, Lewis D B, Ito S, Aruffo A, Wilson C B, Ochs H D. J Clin Invest. 1995;95:66–75. doi: 10.1172/JCI117677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito S, Morii T, Umekage H, Makita K, Nishikawa K, Narita N, Ichijo M, Morikawa H, Ishii N, Nakamura M, Sugamura K. Blood. 1996;87:3344–3350. [PubMed] [Google Scholar]
- 28.Splawski J B, Nishioka J, Nishioka Y, Lipsky P E. J Immunol. 1996;156:119–127. [PubMed] [Google Scholar]
- 29.Boussiotis V A, Barber D A, Berezovskaya A, Freeman G J, Gibben J G, Nadler L M. Blood. 1996;88:465a. (abstr.). [Google Scholar]
- 30.Schwartz R H. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]