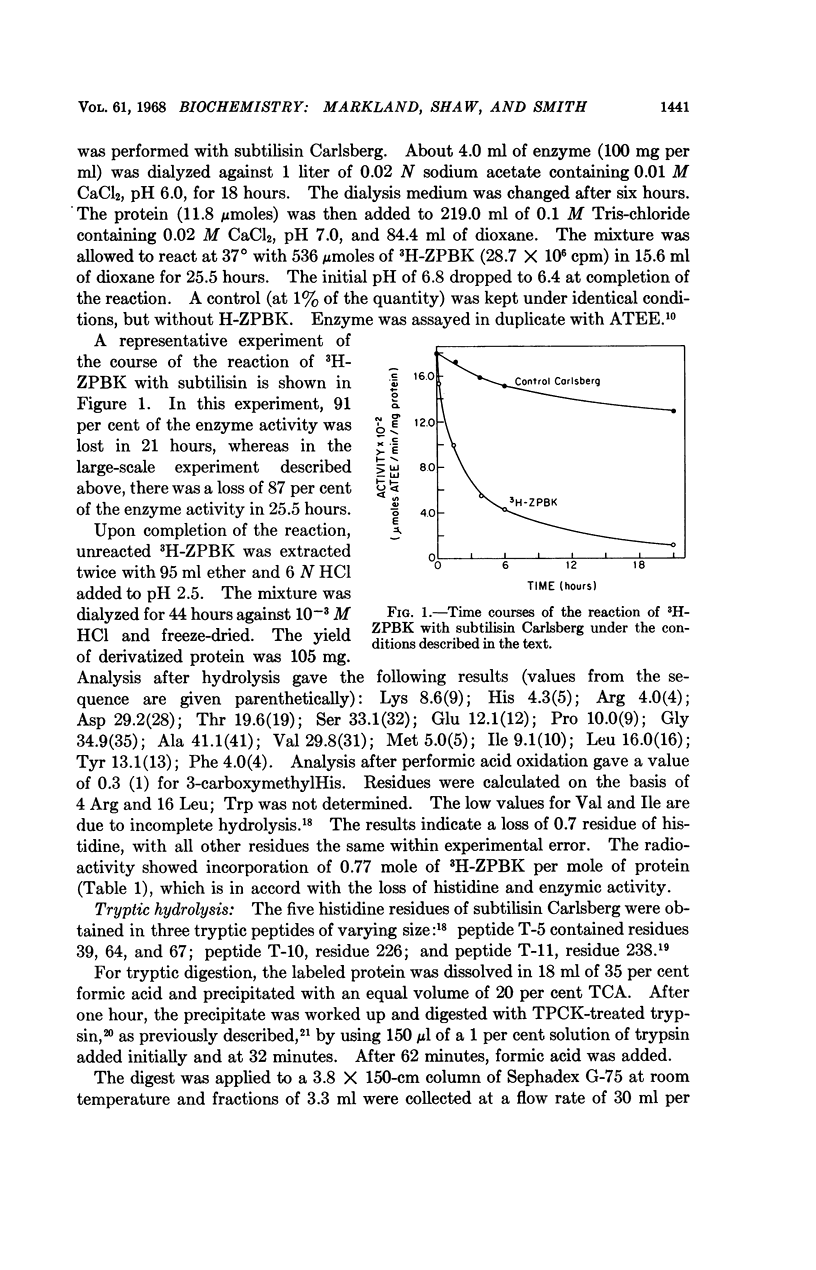
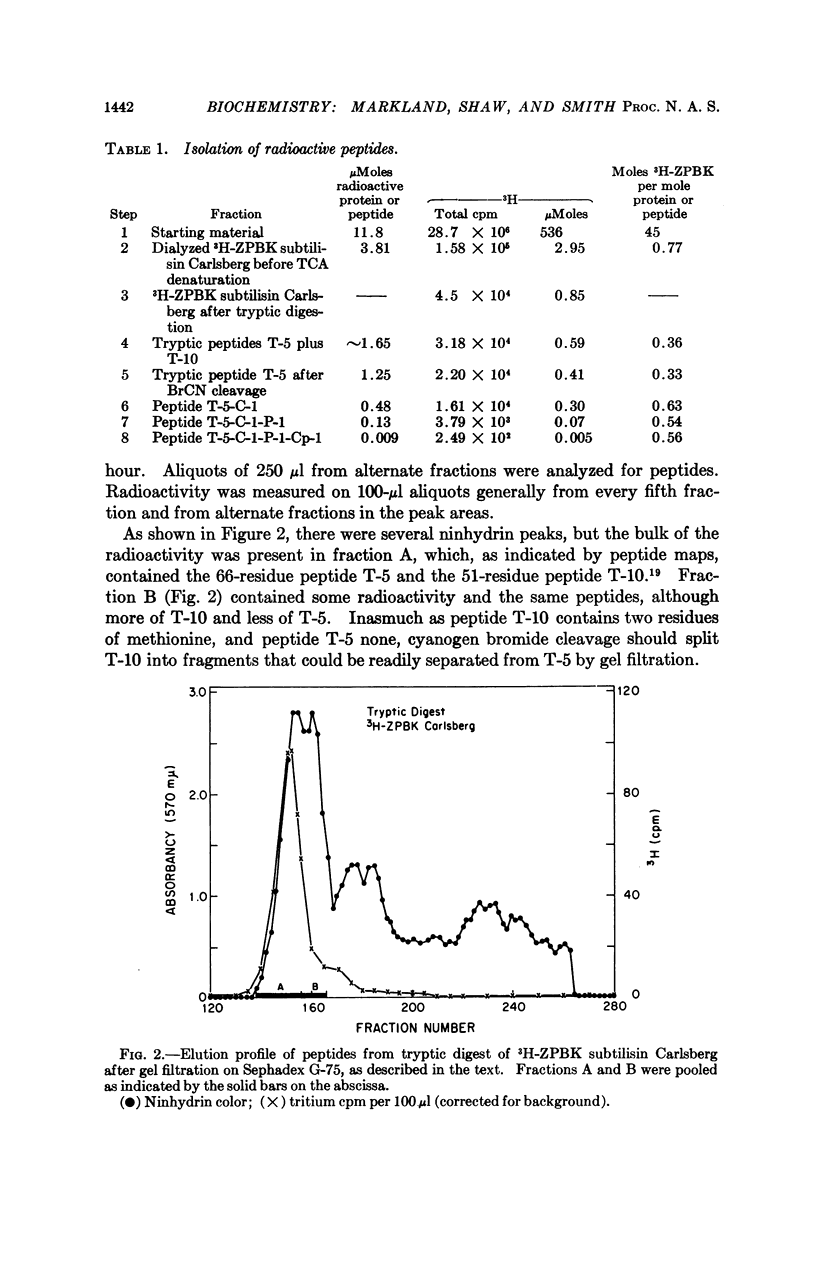
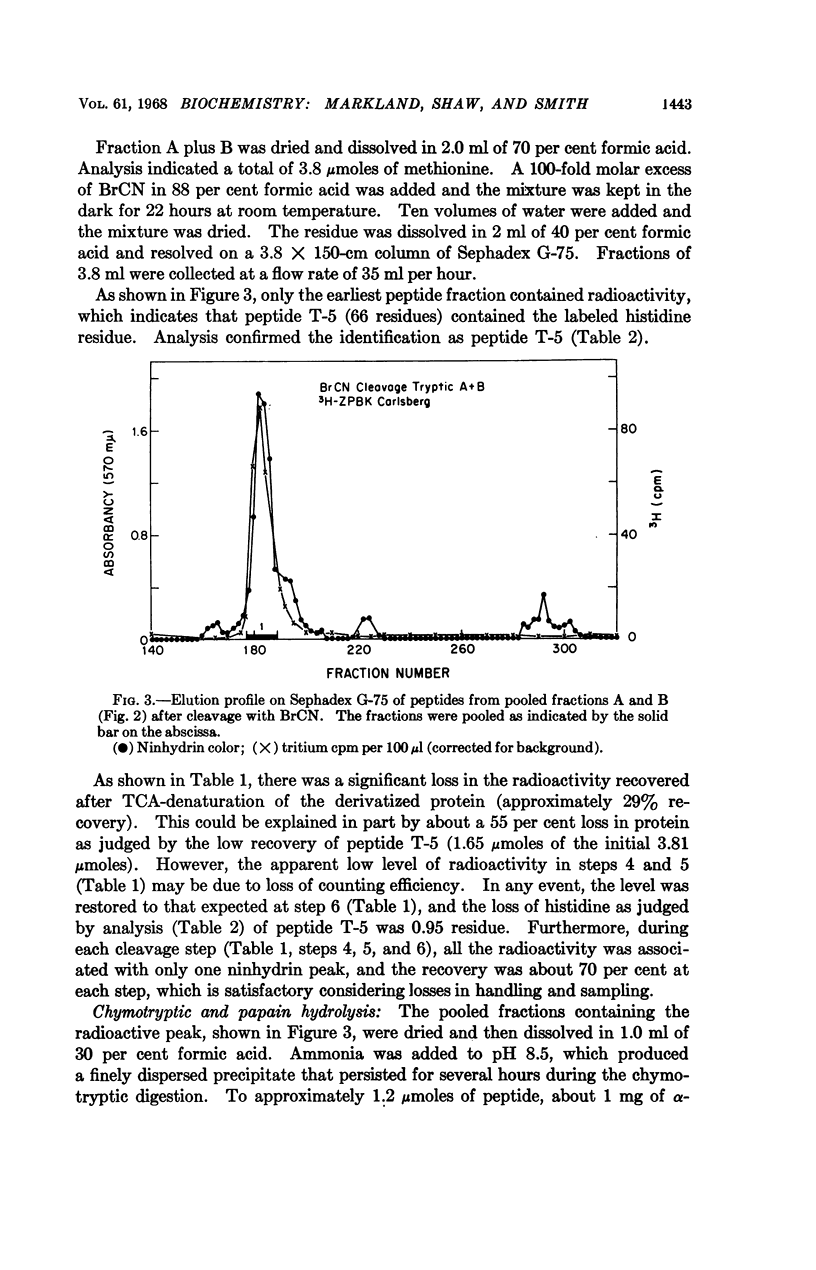
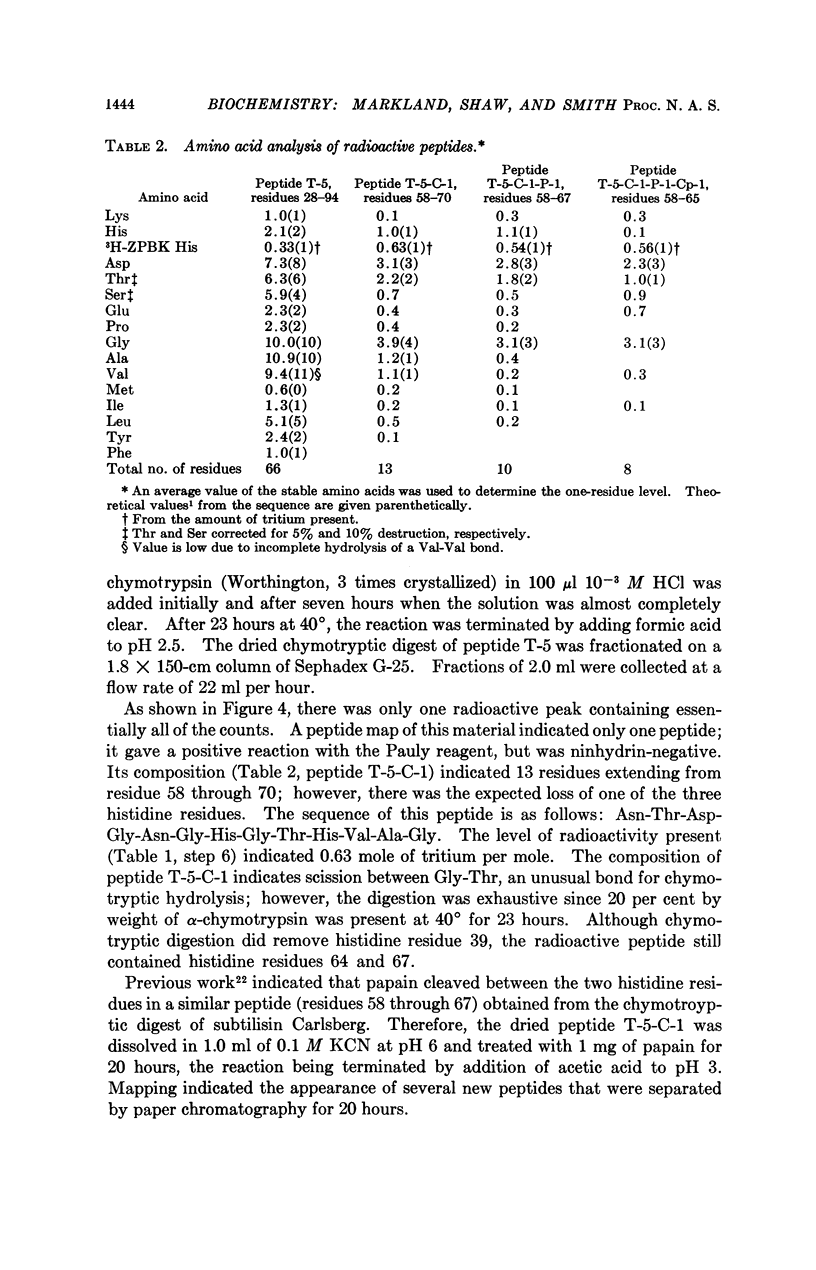
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENSON J. V., Jr, PATTERSON J. A. ACCELERATED AUTOMATIC CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS ON A SPHERICAL RESIN. Anal Chem. 1965 Aug;37:1108–1110. doi: 10.1021/ac60228a008. [DOI] [PubMed] [Google Scholar]
- Barel A. O., Glazer A. N. Comparative studies of the enzymatic properties of Novo and Carlsberg subtilisins. J Biol Chem. 1968 Apr 10;243(7):1344–1348. [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLange R. J., Smith E. L. Subtilisin Carlsberg. I. Amino acid composition; isolation and composition of peptides from the tryptic hydrolysate. J Biol Chem. 1968 May 10;243(9):2134–2142. [PubMed] [Google Scholar]
- Evans W. H., Landon M., Smith E. L. Subtilisin Carlsberg. IV. Amino acid sequences of the chymotryptic peptides. J Biol Chem. 1968 May 10;243(9):2172–2183. [PubMed] [Google Scholar]
- Glazer A. N. Esteratic reactions catalyzed by subtilisins. J Biol Chem. 1967 Feb 10;242(3):433–436. [PubMed] [Google Scholar]
- HIRS C. H., MOORE S., STEIN W. H. Peptides obtained by tryptic hydrolysis of performic acid-oxidized ribonuclease. J Biol Chem. 1956 Apr;219(2):623–642. [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hartley B. S., Brown J. R., Kauffman D. L., Smillie L. B. Evolutionary similarities between pancreatic proteolytic enzymes. Nature. 1965 Sep 11;207(5002):1157–1159. doi: 10.1038/2071157a0. [DOI] [PubMed] [Google Scholar]
- Hubbard R. W., Kremen D. M. Increased sensitivity of accelerated amino acid ion-exchange chromatography. Anal Biochem. 1965 Sep;12(3):593–602. doi: 10.1016/0003-2697(65)90227-7. [DOI] [PubMed] [Google Scholar]
- KOSTKA V., CARPENTER F. H. INHIBITION OF CHYMOTRYPSIN ACTIVITY IN CRYSTALLINE TRYPSIN PREPARATIONS. J Biol Chem. 1964 Jun;239:1799–1803. [PubMed] [Google Scholar]
- Kasper C. B., Smith E. L. Subtilisin BPN'. 3. Isolation and sequence of 10 peptides from the tryptic digest. J Biol Chem. 1966 Aug 25;241(16):3754–3770. [PubMed] [Google Scholar]
- Markland F. S., Kreil G., Ribadeau-Dumas B., Smith E. L. Subtilisin BPN'. V. Isolation and sequence of chymotryptic peptides. J Biol Chem. 1966 Oct 25;241(20):4642–4664. [PubMed] [Google Scholar]
- Markland F. S., Smith E. L. Subtilisin BPN. VII. Isolation of cyanogen bromide peptides and the complete amino acid sequence. J Biol Chem. 1967 Nov 25;242(22):5198–5211. [PubMed] [Google Scholar]
- Mikes O., Holeysovský V., Tomásek V., Sorm F. Covalent structure of bovine trypsinogen. The position of the remaining amides. Biochem Biophys Res Commun. 1966 Aug 12;24(3):346–352. doi: 10.1016/0006-291x(66)90162-8. [DOI] [PubMed] [Google Scholar]
- ONG E. B., SHAW E., SCHOELLMANN G. THE IDENTIFICATION OF THE HISTIDINE RESIDUE AT THE ACTIVE CENTER OF CHYMOTRYPSIN. J Biol Chem. 1965 Feb;240:694–698. [PubMed] [Google Scholar]
- Petra P. H., Cohen W., Shaw E. N. Isolation and characterization of the alkylated histidine from TLCK inhibited trypsin. Biochem Biophys Res Commun. 1965 Dec 21;21(6):612–618. doi: 10.1016/0006-291x(65)90530-9. [DOI] [PubMed] [Google Scholar]
- SANGER F., SHAW D. C. Amino-acid sequence about the reactive serine of a proteolytic enzyme from Bacillus subtilis. Nature. 1960 Sep 3;187:872–873. doi: 10.1038/187872a0. [DOI] [PubMed] [Google Scholar]
- Smith E. L., DeLange R. J., Evans W. H., Landon M., Markland F. S. Subtilisin Carlsberg. V. The complete sequence; comparison with subtilisin BPN'; evolutionary relationships. J Biol Chem. 1968 May 10;243(9):2184–2191. [PubMed] [Google Scholar]
- Smith E. L., Markland F. S., Kasper C. B., DeLange R. J., Landon M., Evans W. H. The complete amino acid sequence of two types of subtilisin, BPN' and Carlsberg. J Biol Chem. 1966 Dec 25;241(24):5974–5976. [PubMed] [Google Scholar]
- Stevenson K. J., Smillie L. B. The reaction of phenoxymethyl chloromethyl ketone with nitrogen 3 of histidine-57 of chymotrypsin. J Mol Biol. 1965 Jul;12(3):937–941. doi: 10.1016/s0022-2836(65)80342-4. [DOI] [PubMed] [Google Scholar]
- WALSH K. A., NEURATH H. TRYPSINOGEN AND CHYMOTRYPSINOGEN AS HOMOLOGOUS PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:884–889. doi: 10.1073/pnas.52.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]


