Abstract
The use of interleukin 2 (IL-2) as an antineoplastic agent has been limited by the serious toxicities that accompany the doses necessary for a tumor response. Elevation of nitric oxide (NO) and tumor necrosis factor (TNF) both have been implicated in IL-2 toxicities. CNI-1493, a tetravalent guanylhydrazone, is an inhibitor of macrophage activation including the synthesis of TNF and other cytokines. Doses of CNI-1493 as low as 1 mg/kg/day conferred complete protection against fatal toxicity of IL-2 with IL-2 doses tenfold higher than the safely tolerated level in Sprague–Dawley rats. Moreover, typical pathologic changes in the lungs, kidneys, and the liver caused by IL-2 infusion were blocked by cotreatment with CNI-1493. When animals bearing established hepatomas were given IL-2 and CNI-1493 combination therapy, 10 of 10 hepatomas regressed from 1 cm3 to <1 mm3. Intracytoplasmic TNF levels were increased in normal tissues from IL-2 treated animals, and treatment with CNI-1493 maintained TNF at control levels. The degree of apoptosis measured by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling staining of tumors following IL-2 therapy was not reduced compared with IL-2 cotreated with CNI-1493. In contrast, apoptosis in the liver and lung parenchyma following IL-2 therapy was blocked completely by cotreatment with CNI-1493. Taken together, these data showed that low and infrequent doses of CNI-1493 markedly protected animals from IL-2 systemic toxicities whereas not affecting tumor response to IL-2 therapy. With the protection afforded by CNI-1493 treatment, IL-2 therapy dose levels could be increased to provide significant antitumor effects in animals with established hepatomas.
A decade ago there was excitement in the oncology community over the introduction of interleukin 2 (IL-2) as a new treatment modality for melanoma and renal cell carcinoma, two tumors that had been refractory to chemotherapeutic intervention. A number of studies using IL-2 alone or in combination with adoptively transferred cells as therapy for these cancers were published at that time (1–4). The results of the clinical trials were disappointing, to a great extent, because of the severe toxicities that accompanied the high doses of IL-2 necessary for a tumor response. Because many of the toxic manifestations of IL-2 resembled the signs of endotoxic shock, it was postulated that (tumor necrosis factor) TNF and NO might mediate IL-2 toxicity. Subsequent studies showed a direct relationship between IL-2 toxicity and high TNF and NO levels (5–8). When TNF activity was blocked by using an anti-TNF antibody, IL-2 toxicity was reduced greatly (9). Unfortunately, clinical trials of two TNF inhibitors, pentoxifylline and lisofylline, as well as clinical trials using the soluble TNF receptor have failed to demonstrate a decrease in the toxicities of IL-2 (8, 10). The mechanisms of IL-2-mediated tumor regression in vivo are not well understood. Some researchers have implicated TNF, but the TNF inhibitor pentoxifylline did not cause a decrease in tumor response when used in conjunction with IL-2 (8, 9). CNI-1493 is a recently described tetravalent guanylhydrazone compound that inhibits activation of proinflammatory cytokine production in macrophages (11). The mechanism of action is by preventing the phosphorylation of p38 MAP kinase, which is required for the translation of the mRNA for TNF and other proinflammatory mediators (22). In the present study CNI-1493 was used to prevent the toxicities of high doses of IL-2 therapy, without decreasing its antitumor effect. This approach enabled the administration of curative doses of IL-2, tenfold higher than was otherwise tolerated.
MATERIALS AND METHODS
Animals.
Sprague–Dawley rats (185–200 g) were housed in the North Shore University Hospital animal facility and fed on a diet of rat chow. The following treatment protocols were reviewed and accepted by the North Shore University Hospital Institutional Review Board and its Animal Care Committee.
Infusional Therapy.
Continuous hepatic artery infusion (CHAI) of IL-2 was accomplished via an indwelling hepatic artery catheter. The catheter was introduced into the hepatic artery via the gastro-duodenal artery and connected to an external Intelliject pump (Ivion, Bloomfield, CO). The technique for catheter placement and long term hepatic artery infusions has been described (12). After catheter placement, the animals were begun immediately on a continuous infusion of a heparinized solution 4 cc/day (25 units/cc) for the first 24 hr.
For i.v. infusions, a catheter was placed into the jugular vein of an anesthetized rat. The catheter was then tunneled s.c. to the midscapular area. This was the site of the percutaneous exit. From this point, the catheters were treated identically to the hepatic artery catheters described in the preceding paragraph.
Human recombinant interleukin 2 (IL-2) generously was supplied by Amgen (Thousand Oaks, CA) (specific activity = 3 × 106 units/mg). IL-2 was given either as a constant hepatic artery infusion or a constant i.v. infusion of 4 ml/day. All of the continuous infusions of IL-2 were administered for 14 days. At the end of this treatment period, the animals were killed. Their liver, hearts, lungs, and kidneys were placed in formalin and processed routinely for pathologic examination.
Tumor.
The tumor used was a hepatoma line initiated by A. B. Novikoff (23) using dimethylamino-azobenzene injections into rats. Tumor was maintained at the North Shore University Hospital animal facility by successive s.c. injections of single-cell tumor suspensions into the thighs of Sprague–Dawley rats. Experimental tumors were induced within the liver capsule according to established methods (13) at a tumor cell dose of 1 × 106 cells. The presence of tumor was recorded at the reoperation with tumor measurements in three dimensions by using calipers. Livers were removed from all animals for tumor measurement in three dimensions and histologic evaluation.
CNI-1493.
This tetravalent guanylhydrazone compound was diluted in distilled water to make a solution of 5 mg/cc. The compound was given daily by i.p. injections at ≈11 a.m. for a duration of 14 days. If CNI-1493 was used in combination with IL-2, then the daily i.p. injection of CNI-1493 would precede the induction of IL-2 infusions by 2 hr.
Tissue Preparation.
Animals were anesthetized deeply and perfused transcardially with 100 ml of saline followed by 250 ml of 4% paraformaldehyde in 0.1 M PBS (pH 7.3) as described by Breder et al. (14). Tissues of interest were removed and placed in sucrose solution overnight at 4°C. Sections (10 μm thick) were cut on a cryostat and air dried for 1 hr. Adjacent sections were used for immunocytochemical, histological, and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling (TUNEL) staining.
Immunocytochemistry.
Sections were incubated in a blocking solution containing 1% BSA, 5% rat serum, and 0.3% Triton X-100 in 0.01 M PBS for 1 hr at room temperature and then incubated for 24 hr at 4°C with diluted primary antibodies: 1:100 dilution of the monoclonal hamster antimurine TNF, with 0.3% Triton X-100, 0.1% BSA, and 3% normal serum in 0.01 M PBS. Washed sections were incubated for 1–2 hr at room temperature in 0.5% biotinylated antihamster IgG (Vector Laboratories) or biotinylated anti-Armenian hamster IgG (Jackson ImmunoResearch) for 1–2 hr. The reaction product was visualized with 0.01% hydrogen peroxide and 0.05% diaminobenzidine as a chromogen. Specificity of immunostaining was determined by incubation of tissue without primary antibodies or after preincubation of diluted anti-TNF with recombinant mouse TNF (Genzyme).
TUNEL Staining.
We used a TUNEL method (ApopTag kit, Oncor) on 10 μm thick dry cryostat sections for specific staining of DNA fragmentation in apoptotic cells according to the manufacturer’s instructions. As a negative control, we used sections incubated with a reaction buffer and distilled water instead of terminal deoxynucleotidyltransferase enzyme. Sections stained with the TUNEL method also were stained with hematoxylin. Adjacent sections were stained with hematoxylin as a nuclear stain and eosin. Sections were examined by light microscopy.
RESULTS
Nontumor Bearing Animals.
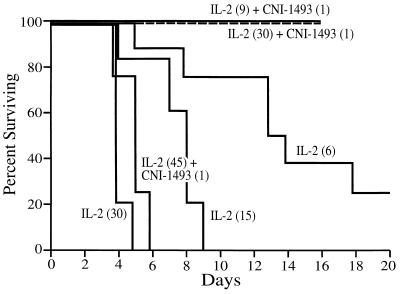
Previous work has established that when IL-2 is administered by CHAI in a rat, it is lethal at any dose ≥6 × 106 units/M2/day (15). A dose response study (Table 1) established that the length of survival was directly proportional to the dose of IL-2, and a dose of 30 × 106 units/M2/day was 100% lethal within 5 days.
Table 1.
Outcome and survival for animals treated with IL2 with or without CNI-1493
| IL-2 Dose*, units/M2/day | Number of rats | CNI-1493†, mg/kg mg/kg | Outcome | Mean survival, days ± SD |
|---|---|---|---|---|
| 6 × 106 | 8 | 0 | all died | 14.25 ± 7.8 |
| 15 × 106 | 5 | 0 | all died | 7.0 ± 2.0 |
| 30 × 106 | 5 | 0 | all died | 4.4 ± 7.8 |
| 9 × 106 | 2 | 1 | no side effects | >14 |
| 9 × 106 | 2 | 5 | no side effects | >14 |
| 9 × 106 | 2 | 10 | no side effects | >14 |
| 30 × 106 | 5 | 1 | no side effects | >14 |
| 45 × 106 | 5 | 1 | all died | 5.7 ± 2.0 |
| 45 × 106 | 5 | 5 | 2 died | |
| 45 × 106 | 5 | 10 | no side effects | >14 |
Administered CHAI.
Single i.p. administration daily.
To assess the protective effects of CNI-1493, a group of six rats were given CHAI of IL-2 at a dose of 9 × 106 units/M2/day and treated with daily CNI-1493 at doses of 1, 5, and 10 mg/kg/day (Table 1). All of the CNI-1493 treated animals survived the 14-day treatment without any obvious side effects.
In separate experiments, a group of five rats were treated with daily i.p. CNI-1493 (1 mg/kg/day) while receiving IL-2 by CHAI at 30 × 106 units/M2/day. All of the animals survived the full 14 days of IL-2 therapy at 30 × 106 units/M2/day with no obvious side effects (diarrhea and lethargy). Animals treated with 45 × 106 units/M2/day of IL-2 and 1 mg/kg/day of CNI-1493, however, died of toxicity within 9 days (Fig. 1). A log rank analysis of these survival curves showed a P value of 0.0001. In more recent experiments, two of five animals treated with 45 × 106 units/M2/day of IL-2 and 5 mg/kg/day of CNI-1493 experienced a fatal toxicity. When the dose of CNI-1493 was raised to 10 mg/kg/day, then all of the animals survived without toxicity.
Figure 1.
Survival curves for animals treated with IL-2 alone (dose of IL-2 indicated in parenthesis is ×106 units/M2/day) or IL-2 plus CNI-1493 (dose of CNI-1493 is mg/kg). Log rank analysis, P = 0.0001.
Tumor Bearing Animals.
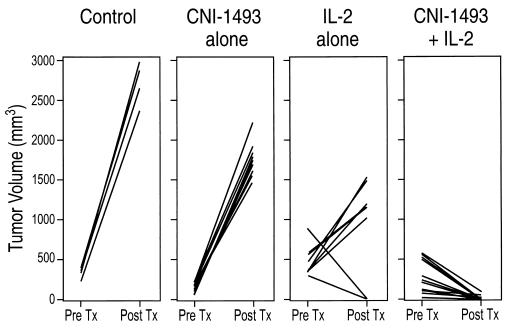
Because the above results indicated that CNI-1493 significantly protected against IL-2 toxicity, we examined whether the drug interfered with the antitumor effect of the IL-2 infusion. Animals with established hepatomas, tumor volume ≈1 cm3, were then treated with CHAI of IL-2 (30 × 106 units/M2/day) and CNI-1493 (1 mg/kg/day given i.p.). After 14 days, five had significant reduction of the tumor mass (<1 mm3) and five had no detectable tumor (Fig. 2). Histologic examination corroborated all of these objective findings.
Figure 2.
Tumor volumes (product of measurements in three directions) in animals with no treatment (control), treated with CNI-1493 (1 mg/kg/d) alone, IL-2 alone (3 × 106 units/M2/d), or CNI-1493 plus IL-2 (30 × 106 units/M2/d) in combination. Pretreatment (Pre Tx) measurements were taken 6 days after injection of tumor cells into the liver. Post-treatment (Post Tx) measurements were taken after 14 days of treatment or at death of animal.
As a control, four animals were given hepatoma cells followed by no treatment. One of the four died after 10 days with a tumor ≈10 times as large as the original measurement. The other three were sacrificed at 14 days. All tumors had grown by a factor of 10 or more. As an additional control, five hepatoma-bearing animals were treated with daily i.p. injections of CNI-1493 at 1 mg/kg/day alone. All animals had a marked increase in tumor size after 14 days (Fig. 2). Because of dose-limiting toxicity, animals in the control group scheduled to receive IL-2 infusion alone, could only receive 3 × 106 units/M2/day without fatal toxicity. At this dose, two of eight animals had marked diminution of tumor and six had a marked increase (Fig. 2).
Histologic Confirmation of CNI-1493 Protective Effect.
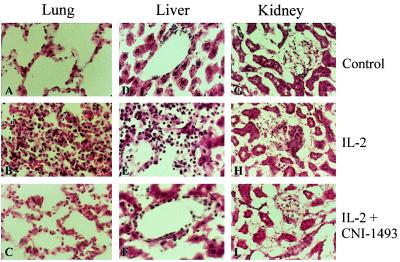
CHAI of IL-2 produced severe, multiple organ damage. Histological analysis of hemotoxylin/eosin and TUNEL-stained sections revealed significant abnormalities within the lungs, liver, and kidneys of animals treated with IL-2 (3 × 106 u/M2/d) alone (Fig. 3 B, E, and H). The lungs showed thickening of the alveolar walls with congestion, mononuclear cell infiltration, interstitial and intra-alveolar edema, fibrin deposition, and hyaline membrane appearance. The erythrocyte infiltration (see arrows in 3B) is consistent with the most common side effect of IL-2 treatment, vascular leak syndrome. The liver parenchyma of animals treated with IL-2 exhibited proliferation of small bile ducts and a pleomorphic mononuclear cell infiltration in periportal and sinusoidal spaces (Fig. 3E). The kidneys showed a minimal interstitial mononuclear cell infiltration extending into the glomerular capsule (Fig 3H). By contrast, histologic sections from the lungs, liver, and kidneys from animals treated with IL-2 and CNI-1493, resembled nontreated controls in their histologic appearance (Fig. 3 C, F, and I).
Figure 3.
Hematoxylin/eosin stained sections of lung (A–C), liver (D–F), and kidney (G–I) samples from animals with no treatment (A, D, and G), IL-2 infusions (B, E, and H), and IL-2 plus CNI-1493 treatments (C, F, and I). The lung after IL-2 treatment (B) shows thickened alveolar walls and erythrocytic infiltration (arrows), the liver (E) shows bile duct proliferation and mononuclear cell infiltration, and the kidney (H) shows minimal interstitial mononuclear cell infiltration. Histologic appearance of tissue from animals treated with IL-2 plus CNI-1493 from the lung (C), liver (F), and kidney (I) resembled untreated controls.
Tumor Cell Death.
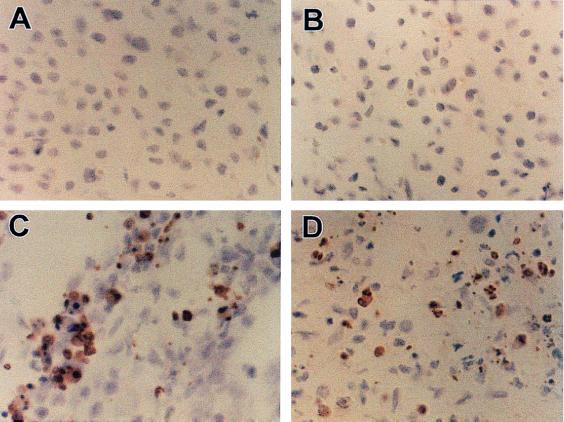
Immunocytochemical examination of the tumors in animals after CHAI of IL-2 alone revealed extremely high levels of apoptotic cell death in the tumor tissue with all stages of chromatin condensation and the appearance of multiple apoptotic bodies (Fig. 4C). No TUNEL positive cells were detected within the hepatomas from animals not treated with IL-2 (Fig. 4A) or animals treated with CNI-1493 alone (Fig. 4B). The addition of CNI1493 to the IL-2 treatment did not decrease the level of apoptosis observed in the hepatomas sections (Fig. 4D).
Figure 4.
Immunocytochemical analysis by TUNEL staining of experimental hepatoma in the animals with no treatment (A), given CNI-1493 only (B), given IL-2 only (3 × 106 units/M2/d) (C), and given IL-2 (30 × 106 units/M2/d) plus CNI-1493 (D). Notice the apoptotic cells with positive brown granules in C and D.
Apoptosis in Normal Tissues.
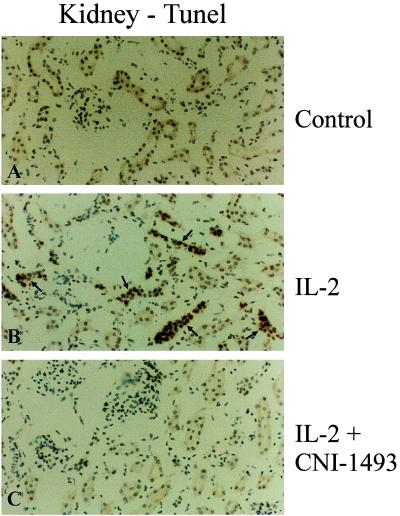
Sections of lung, liver, and kidneys from untreated animals showed no evidence of apoptosis. In animals treated with IL-2, however, there was marked evidence of apoptosis in all three organs. By contrast, in those animals treated with IL-2 and CNI-1493 the lungs, livers, and kidneys again showed no evidence of apoptosis. Fig. 5 shows this described effect in representative sections of kidney.
Figure 5.
Immunocytochemical analysis of cellular apoptosis in the kidney by TUNEL staining in untreated control animals (A), IL-2 alone treated animals (B), and IL-2 plus CNI-1493-treated animals (C). Notice the apoptotic cells (arrows) in the sample from an IL-2 alone treated animal (B). Few or no apoptotic profiles were found in comparable sections from controls (A) or the animals treated with IL-2 plus CNI-1493 (C).
TNF Expression.
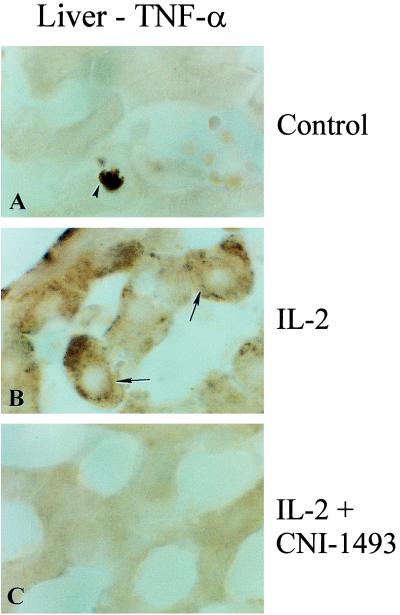
Sections of the lungs, livers, and kidneys of animals given IL-2 alone, IL-2 and CNI-1493, or control treatments also were stained for immunoreactive TNF. No TNF expression was noted in liver or lungs obtained from untreated animals (liver shown in Fig. 6A). By contrast, tissue from the livers of animals treated with CHAI of IL-2 showed a high expression of TNF immunoreactive protein (Fig. 6B). Liver and lung samples from animals treated with both CNI-1493 and CHAI of IL-2 showed minimal TNF expression similar to control animals (Fig. 6C).
Figure 6.
Immunocytochemical analysis of the liver for TNF protein. High power light microscopy fields of liver sections taken from animals with no treatment (A), with CHAI of IL-2 (B), and with both CNI-1493 plus CHAI of IL-2 (C). TNF protein is manifested by brown granules.
DISCUSSION
Although IL-2 is the most potent therapy known for metastatic renal cell cancer and metastatic melanoma, the associated dose-limiting toxicities such as hypotension and pulmonary edema attributed to the vascular leak syndrome have prevented its successful use in most patients with this disease. Previous work in our laboratory established a rat model using continuous infusion IL-2, which demonstrated all of the toxicities observed in IL-2 treated patients (15). When administered at any dose over 3 × 106 units/M2/day, continuous IL-2 infusion is uniformly fatal in this rat model. Because the features of IL-2 toxicity are so similar to those seen in endotoxic shock, it has been postulated that the major toxicities of IL-2 are mediated by TNF. Previous work showed that when anti-TNF antibody was given with IL-2 in a mouse model, the toxic effects of IL-2 were attenuated and the antitumor activity seemed to be unchanged (7). Unfortunately, recent clinical data using a soluble TNF receptor in a similar paradigm have been disappointing. Studies with pentoxifylline, which inhibits TNF by blocking its transcription, followed a similar history (8, 9). In the laboratory, pentoxifylline was able to block the toxicity of IL-2, without interfering with its antitumor activity. However, when pentoxifylline was used in a clinical Phase I trial with IL-2, a dose of pentoxifylline high enough to block plasma TNF levels in humans proved intolerably toxic itself. Another demonstrated TNF inhibitor, lisofylline, was studied in a randomized placebo-controlled trial (10), but again, no evaluation of prevention of IL-2 toxicity could be made because of the dose limitations of lisofylline toxicity.
Although TNF may be a critical mediator of IL-2 toxicity, other factors also may play important roles. IL-2 is a potent inducer not only of proinflammatory cytokines such as TNF but also of other effectors such as NO (16). Studies using a p75 TNF receptor IgG chimera in patients receiving IL-2 treatment showed no decrease in NO production (17). This treatment led to a decrease in serum TNF bioactivity but did not reduce the toxicity of IL-2. This result suggests that, in addition to interfering with TNF, other factors such as NO might have to be blocked for effective reduction of IL-2 toxicity.
CNI-1493, a tetravalent guanylhydrazone, is a potent post-transcriptional inhibitor of macrophage activation that appears to act by blocking the phosphorylation of p38 MAP kinase (11, 22). A large body of evidence in multiple animal models suggests that this compound can be useful to prevent the macrophage mediated sequelae of disease and trauma, including sepsis, cerebral ischemia, and endotoxemia through inhibition of proinflammatory cytokine responses (11, 22, 24–27). Because IL-2 toxicity is thought to be mainly mediated by the products of macrophage activation including TNF and NO, we reasoned that CNI-1493 might prevent IL-2 toxicity. We have considered the possibility that the protective effects of CNI-1493 in vivo may be in part due to inhibiting endothelial cell activation. This possibility does not seem likely because evidence previously obtained shows that CNI-1493 does not inhibit endothelial-derived NO production (24).
The present series of experiments demonstrated that CNI-1493 was well tolerated at doses that suppressed the toxic effects of continuous infusion IL-2, such that >10 times the normally fatal dose of IL-2 could be administered safely to rats when given in conjunction with CNI-1493. Microscopic evaluation of tissues from the lungs, liver, and kidneys of animals that received infusional IL-2 showed marked abnormalities consistent with the pathologic changes seen in vascular leak syndrome. When animals were treated with IL-2 and CNI-1493, these pathologic changes nearly were prevented completely. Thus, CNI-1493 protected against microscopic tissue and cellular damage as well as fatality and overt organ toxicity.
These rat hepatomas have shown poor response to treatment with continuous hepatic artery infusion (CHAI) of IL-2 given at a constant rate (18), a result that may be attributable to dose-limiting toxicity of the IL-2 treatment. When CNI-1493 was administered in conjunction with continuous CHAI of IL-2 to animals with preexisting tumors, allowing tenfold higher doses of IL-2 to be infused, all animals had a significant tumor response. One-half of all tumors were eradicated completely whereas one-half were reduced from ≈200 mm3 to slightly <1 mm3. Thus, CNI-1493 although nearly completely attenuating host toxicities to IL-2, did not interfere with the antitumor activity of the IL-2. These data also indicate that the higher doses of IL-2 that can be tolerated in the context of protective dosing with CNI-1492 may provide significantly improved antitumor activity.
On a cellular level, tumors that were treated with IL-2 alone showed evidence of apoptosis on microscopic examination. If tumor bearing animals were treated with both IL-2 and CNI-1493, the amount of apoptosis in the tumors was unchanged. In the liver parenchyma of nontumor bearing animals, apoptosis was less extensive when both CNI-1493 and IL-2 were used than after IL-2 alone. Thus, CNI-1492 protected normal cells and tissue from the apoptosis induced by IL-2 treatment but did not interfere with the desired therapeutic induction of apoptosis in tumor cells.
The mechanism of the IL-2 antitumor effect is not understood. However, because CNI-1493 cotreatment with IL-2 significantly reduced tissue TNF levels, it seems unlikely that the main antitumor effects of IL-2 are mediated through TNF. It does seem likely, by contrast, that the potentially fatal and dose-limiting toxic effects of IL-2 are mediated by TNF, possibly in combination with other proinflammatory cytokines that also are suppressed by CNI-1493.
CNI-1493 may prove useful as a research tool to elucidate the distinct mechanisms involved in IL-2 host toxicity vs. tumor cell toxicity in the setting of normal immunocompetence. The efficacy of CNI-1493 coadministration with IL-2 in this animal model argues strongly for assessment of its protective effect in humans where in the clinical setting IL-2 has proven somewhat effective in the treatment of renal cell carcinomas and melanomas and may be useful at higher doses for a wide number of malignancies.
Acknowledgments
We would like to thank Dr. Glenn Rice and Dr. Kurt Manogue for help on the manuscript.
ABBREVIATIONS
- CHAI
Continuous hepatic artery infusion
- TNF
tumor necrosis factor
- TUNEL
terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end-labeling
References
- 1.Rosenberg S A, Lotze M T, Muul L M, Leitman M T, Chang A E, Ettinghausen S E, Matory Y L, Skibber J M, Shiloni E, Vetto J T, Seipp C A, Simpson C, Reichert C M. N Engl J Med. 1985;313:1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- 2.West W H, Tauer K W, Yannelli J R, Marshall G D, Orr D W, Thurman G B, Oldham R K. N Engl J Med. 1987;316:898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]
- 3.Lotze M T, Chang A E, Seipp G A, Simpson C, Vetto J T, Rosenberg S A. JAMA. 1986;256:3117–3124. [PubMed] [Google Scholar]
- 4.Rosenberg S A, Lotze M T, Yang J C, Aebersold P M, Linehan W M, Seipp C A, White D E. Ann Surg. 1989;210(4):474–483. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gemlo B T, Palladino M A, Jaffe H S, Espevik T P, Rayner A A. Cancer Res. 1988;48:5864–5867. [PubMed] [Google Scholar]
- 6.Kasid A, Director E P, Rosenberg S A. J Immunol. 1989;143:736–739. [PubMed] [Google Scholar]
- 7.Fraker D L, Langstein H N, Norton J A. J Exper Med. 1989;170:1015–1020. doi: 10.1084/jem.170.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards M J, Abney D L, Miller F N. Surgery. 1991;110:199–204. [PubMed] [Google Scholar]
- 9.Edwards M J, Heniford T B, Klar E A, Doak K W, Miller F N. J Clin Invest. 1992;90:637–641. doi: 10.1172/JCI115904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolin K, Atkins M, Sparano J, Sosman J, Weiss G, Lotze M, Doroshow J, Mier J, O’Boyle K, Fisher R, Campbell E, Rubin J, Federighi D, Bursten S. Clin Cancer Res. 1997;3:565–572. [PubMed] [Google Scholar]
- 11.Bianchi M, Bloom O, Raabe T, Cohen P S, Chesney J, Sherry B, Schmidtmayerova H, Calandra T, Zhang X, Bukrinsky M, et al. J Exp Med. 1996;183:927–936. doi: 10.1084/jem.183.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kemeny M M, Alava G, Oliver J M, Smith F B. HPB Surg. 1992;5:185–194. doi: 10.1155/1992/28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemeny M M, Alava G, Oliver J M. HPB Surg. 1994;7:219–224. doi: 10.1155/1994/54058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breder C D, Tsujimoto M, Terano Y, Scott D W, Saper C B. J Comp Neurol. 1993;337:543–567. doi: 10.1002/cne.903370403. [DOI] [PubMed] [Google Scholar]
- 15.Kemeny M M, Alava G, Oliver J M, Smith F B. Reg Cancer Treat. 1992;4:260–264. [Google Scholar]
- 16.Hibbs J B, Westenfelder C, Taintor R, Vavrin Z, Kablitz C, Baranowski R L, Ward J H, Menlove R L, McMurry M P, Kushner J P, et al. J Clin Invest. 1992;89:867–877. doi: 10.1172/JCI115666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuBois J S, Trehu E G, Mier J W, Shapiro L, Epstein M, Klempner M, Dinarello C, Kappler K, Ronayne L, Rand W, et al. J Clin Oncol. 1997;15:1052–1062. doi: 10.1200/JCO.1997.15.3.1052. [DOI] [PubMed] [Google Scholar]
- 18.Kemeny M M, Alava G, Oliver J M. J Immunother. 1992;12:219–223. doi: 10.1097/00002371-199211000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Montel A H, Bochan M R, Hobbs J A, Lynch D H, Brahmi Z. Cell Immunol. 1995;166:236–246. doi: 10.1006/cimm.1995.9974. [DOI] [PubMed] [Google Scholar]
- 20.Strieter R M, Remick D G, Lynch J P, Spengler R N, Kunkel S L. Am Rev Respir Dis. 1988;139:335–342. doi: 10.1164/ajrccm/139.2.335. [DOI] [PubMed] [Google Scholar]
- 21.Nakagawa K, Miller F N, Sims D E, Lentsch A B, Miyazaki M, Edwards M J. Cancer Res. 1996;56:507–510. [PubMed] [Google Scholar]
- 22.Cohen P S, Schmidtmayerova H, Dennis J, Dubrovsky L, Sherry B, Wang H, Bukrinsky M, Tracey K J. Mol Med. 1997;3(5):339–346. [PMC free article] [PubMed] [Google Scholar]
- 23.Novikoff A B. Cancer Res. 1957;17:1010–1027. [PubMed] [Google Scholar]
- 24.Bianchi M, Ulrich P, Bloom O, Meistrell M E, Zimmerman G A, Schmidtmayerova H, Bukrinsky M, Donnelley T, Bucala R, Sherry B, et al. Mol Med. 1995;1(3):254–266. [PMC free article] [PubMed] [Google Scholar]
- 25.Meistrell M E, Botchkina G I, Wang H, DeSanto E, Cockroft K M, Bloom O, Vishnubhakat J M, Ghezzi P, Tracey K J. Shock. 1997;8(5):341–348. [PubMed] [Google Scholar]
- 26.Villa P, Meazza C, Sironi M, Bianchi M, Ulrich P, Botchkina G, Tracey K J, Ghezzi P. J Endotoxin Res. 1997;4(3):197–204. [Google Scholar]
- 27.Denham W, Fink G, Yang J, Ulrich P, Tracey K, Norman J. Am Surg. 1997;63(12):1045–1049. [PubMed] [Google Scholar]