Abstract
Multiple human skeletal and craniosynostosis disorders, including Crouzon, Pfeiffer, Jackson–Weiss, and Apert syndromes, result from numerous point mutations in the extracellular region of fibroblast growth factor receptor 2 (FGFR2). Many of these mutations create a free cysteine residue that potentially leads to abnormal disulfide bond formation and receptor activation; however, for noncysteine mutations, the mechanism of receptor activation remains unclear. We examined the effect of two of these mutations, W290G and T341P, on receptor dimerization and activation. These mutations resulted in cellular transformation when expressed as FGFR2/Neu chimeric receptors. Additionally, in full-length FGFR2, the mutations induced receptor dimerization and elevated levels of tyrosine kinase activity. Interestingly, transformation by the chimeric receptors, dimerization, and enhanced kinase activity were all abolished if either the W290G or the T341P mutation was expressed in conjunction with mutations that eliminate the disulfide bond in the third immunoglobulin-like domain (Ig-3). These results demonstrate a requirement for the Ig-3 cysteine residues in the activation of FGFR2 by noncysteine mutations. Molecular modeling also reveals that noncysteine mutations may activate FGFR2 by altering the conformation of the Ig-3 domain near the disulfide bond, preventing the formation of an intramolecular bond. This allows the unbonded cysteine residues to participate in intermolecular disulfide bonding, resulting in constitutive activation of the receptor.
Keywords: Crouzon syndrome, receptor activation, Neu chimera
Fibroblast growth factor receptors (FGFRs) form a family of at least four receptor tyrosine kinases that share several structural features, including three extracellular immunoglobulin-like domains (Ig), a single transmembrane domain, and an intracellular split tyrosine kinase domain (1, 2). Binding of fibroblast growth factors in the presence of heparan sulfate proteoglycans leads to dimerization of receptor molecules, followed by tyrosine autophosphorylation (3–5). Mutations have been identified in FGFR1, FGFR2, and FGFR3 that give rise to human developmental syndromes such as skeletal dwarfism and craniosynostosis syndromes (for review, see refs. 6 and 7). Constitutive receptor activation appears to represent the underlying defect for these developmental syndromes, consistent with their autosomal dominant transmission (8–12).
Mutations in the extracellular domain of FGFR2 have been associated with several craniosynostosis syndromes, including Crouzon, Pfeiffer, Jackson–Weiss, and Apert syndromes (Table 1) (13–27), all of which exhibit premature closure of the cranial sutures. Crouzon syndrome is characterized by maxillary hypoplasia, shallow eye orbits, and ocular proptosis. Jackson–Weiss syndrome is further distinguished by broadened toes, Pfeiffer syndrome is further distinguished by broadened thumbs and toes, and Apert syndrome is further distinguished by severe fusion of the bones of the hands and feet (28). Some of these mutations (Table 1A), such as C278F or C342Y, destroy one of the cysteine residues forming the disulfide bond in the Ig-3 domain, leaving the other cysteine potentially unpaired to participate in intermolecular disulfide bonding. Similarly, other mutations create a new cysteine residue that could participate in aberrant disulfide bonding (Table 1B). However, many other craniosynostosis mutations, shown in Table 1 C and D, do not result in the creation of an unpaired cysteine, offering no obvious explanation for the observed phenotype in these cases.
Table 1.
Craniosynostosis mutations in FGFR2 extracellular domain
| Mutation | Syndrome(s) | Ref(s). |
|---|---|---|
| A–Mutations destroying a Cys residue | ||
| C278F | Crouzon; Pfeiffer | 13–15 |
| C342R | Crouzon; Pfeiffer; Jackson–Weiss | 14, 16–19 |
| C342S | Crouzon; Pfeiffer | 14, 18, 20 |
| C342Y | Crouzon; Pfeiffer | 14, 15, 18–21 |
| C342W | Crouzon | 16, 21 |
| C342F | Crouzon | 13–15 |
| B–Mutations creating a new Cys residue | ||
| Y105C | Crouzon | 22 |
| Y328C | Crouzon | 23 |
| S347C | Crouzon | 15, 23 |
| S351C | Crouzon | 22 |
| S354C | Crouzon | 15, 16, 18, 20 |
| S372C | Beare–Stevenson cutis gyrata | 24 |
| Y375C | Beare–Stevenson cutis gyrata | 24 |
| C–Noncysteine mutations in Ig-3 | ||
| ΔH287–Q289 | Crouzon | 15 |
| Q289P | Crouzon; Jackson–Weiss | 13–15, 20 |
| W290G | Crouzon | 16 |
| W290R | Crouzon | 15 |
| D321A | Pfeiffer | 25 |
| G338R | Crouzon | 20 |
| G338E | Crouzon | 22 |
| Y340H | Crouzon | 18, 21, 23 |
| T342P | Pfeiffer | 19 |
| A344P | Pfeiffer | 14 |
| A344G | Crouzon; Jackson–Weiss | 20, 23 |
| ΔG345–P361 | Crouzon; Pfeiffer | 14, 16, 18, 23, 26 |
| D–Other noncysteine mutations | ||
| S252W | Apert | 14, 27 |
| P253R | Apert | 14, 27 |
| S267P | Crouzon | 13, 15 |
| Insertion G269 | Crouzon | 14 |
| V359F | Pfeiffer | 14 |
In this work, we have systematically examined the importance of each of the cysteine residues that participate in the three disulfide bonds in the FGFR2 extracellular domain. We also examined FGFR2 activation in response to two of the noncysteine mutations in the Ig-3 domain, W20G and T341P. We show herein that these mutations result in a constitutively activated receptor, characterized by dimer formation and kinase activation. Surprisingly, this activation is dependent on the presence of the cysteine residues that normally form the disulfide bond in the Ig-3 domain. We propose a model whereby the noncysteine craniosynostosis mutations disrupt formation of the native Ig-3 disulfide bond, allowing these cysteines to participate in aberrant intermolecular dimerization and resulting in constitutive receptor activation.
MATERIALS AND METHODS
Construction of FGFR2 Constructs.
Constructs used in this study are presented in Fig. 1 and were constructed largely as described (11). The correct sequence of each mutant was confirmed by dideoxynucleotide sequencing. All numbering of nucleotides and amino acid residues in the FGFR2 sequence refer to the sequence GenBank accession no. X52832 (29).
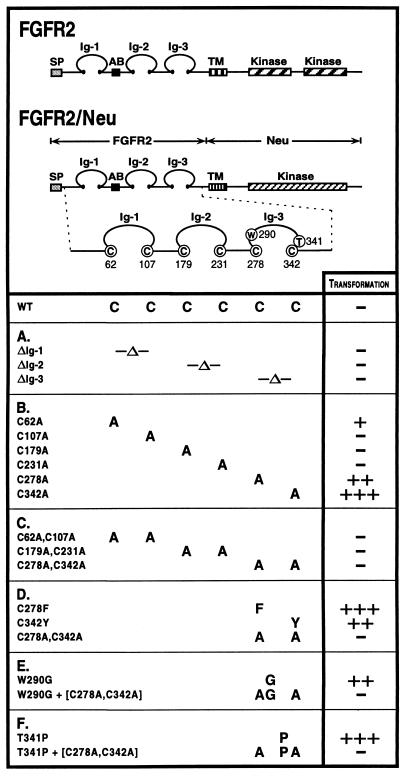
Figure 1.
Structure and transforming activity of FGFR2 constructs. FGFR2 is shown in comparison with FGFR2/Neu chimeric receptors, which contain the extracellular domain of FGFR2 fused to the transmembrane and intracellular domains of Neu. The six cysteine residues in the FGFR2 extracellular domain involved in intramolecular disulfide bonding are Cys-62, Cys-107, Cys-179, Cys-231, Cys-278, and Cys-342. (A) Deletions of the Ig domains remove the sequence between the disulfide bonded cysteines and replace those cysteine residues with alanine. (B) Single mutations of Cys → Ala in the extracellular domain. (C) Pair-wise mutations of Cys → Ala affecting each disulfide bond in Ig-1, Ig-2 and Ig-3. (D) Craniosynostosis mutations affecting Cys-278 and Cys-342. (E) The noncysteine craniosynostosis mutation W290G. (F) The noncysteine craniosynostosis mutation T341P. Transforming activity for each chimeric receptor, shown at right, was measured by focus formation assays in NIH 3T3 cells. Results presented are the average of at least three experiments. The results are given as a percentage of the transformation efficiency of oncogenic Neu and were reported as follows: −, 0–5%; +, 5–10%; ++, 10–20%; +++, >20%.
Transformation Assays.
NIH 3T3 cells were used in transformation assays as described (8, 30). Data reported for focus assays are the average of three to five experiments for each construct, expressed as a percentage of the transformation efficiency obtained with pSV2neuNT, encoding p185neu with the mutation V664E (31).
Dimerization and Kinase Assays.
COS-1 cells were transfected with each construct as described (8). Cell lysates were analyzed by SDS/PAGE on a 4–12% gradient gel under reducing or nonreducing conditions as described elsewhere (30). Proteins were then transferred to nitrocellulose membrane and incubated with FGFR2 antisera (polyclonal FGFR2/bek C-17, Santa Cruz Biotechnology) or 4G10 (anti-phosphotyrosine mAb, Upstate Biotechnology) and detected by enhanced chemiluminescence (ECL, Amersham).
For kinase assays, lysates were immunoprecipitated with FGFR2 antisera (polyclonal FGFR2/bek C-17, Santa Cruz Biotechnology) followed by incubation with protein A-Sepharose (Sigma). Autophosphorylation was assayed as described (11, 30) and 32P-labeled proteins were resolved by SDS/PAGE under nonreducing conditions on a 4–12% gradient gel and detected by autoradiography.
Molecular Modeling.
The molecular model of the wild-type Ig-3 domain of FGFR2 was constructed by replacing the side chains of telokin, a myosin light chain homolog, with the side chains of FGFR2. Coordinates for telokin were taken from the crystal structure (Brookhaven Protein Data Bank entry 1TLK). Insertions and deletions were modeled by extracting backbone coordinates from fragments containing a similar sequence to FGFR2 from the Brookhaven Protein Data Bank. Homology replacements and energy minimizations were performed as described (32).
RESULTS
Mutations Affecting the Disulfide Bonds of the FGFR2 Extracellular Domain.
The three Ig domains of the FGFR2 extracellular domain are stabilized by three disulfide bonds formed by Cys-62 and Cys-107 (Ig-1 domain), Cys-179 and Cys-231 (Ig-2 domain), and Cys-278 and Cys-342 (Ig-3 domain). There are no other cysteine residues in the FGFR2 extracellular domain except for Cys-9, which is removed during signal peptide cleavage. This minimal number of cysteine residues makes FGFR2 an ideal model system for probing the importance of disulfide bond formation in receptor activation. Chimeric FGFR2/Neu receptors (Fig. 1), containing the FGFR2 extracellular domain fused to the transmembrane and kinase domains of Neu, allow the extent of activation to be assayed through focus formation using the intracellular domain of Neu as a reporter, as described (8, 11).
To examine whether specific deletion of a single Ig domain would result in receptor activation, deletions of each of the individual Ig domains were constructed as FGFR2/Neu chimeras and assayed for transformation of NIH 3T3 cells. None of the deletion mutants was transforming (Fig. 1A), indicating that merely removing any of the Ig domains is not sufficient to activate the receptor.
To address whether activation of the receptor can be accomplished by mutations affecting individual cysteine residues in the Ig domains, chimeric receptors containing each of the single mutations C62A, C107A, C179A, C231A, C278A, and C342A were constructed (Fig. 1B). Although the C107A mutant was inactive, the C62A mutant exhibited modest activation, indicating that activating mutations might occur in the Ig-1 domain. Furthermore, mutations affecting the cysteine residues that stabilize the Ig-3 domain, C278A and C342A, efficiently transformed NIH 3T3 cells when assayed as chimeric FGFR2/Neu receptors.
The cysteine residues of the extracellular domain were also mutated in pairs to remove both partners of each disulfide bond (Fig. 1C). The double mutants (C62A, C107A) and (C179A, C231A), affecting the Ig-1 and Ig-2 domains, respectively, were inactive in transformation assays. In contrast to the results described above for the single mutants C278A and C342A, the double mutant (C278A, C342A), affecting the Ig-3 disulfide bond, also lacked transforming activity. These results indicate that elimination of both partners of any one disulfide bond does not lead to receptor activation.
Cellular Transformation by Chimeric Receptors with Noncysteine Mutations in the Ig-3 Domain.
Various mutations have been identified in craniosynostosis patients that do not result in unpaired cysteines (Table 1 C and D). To evaluate the mechanism by which these mutations affect receptor activity, we constructed mutated receptors containing both cysteine and noncysteine mutations in the extracellular domain by using the constructs described in Fig. 1. As a control, we included the single mutants, C278F and C342Y, that have been identified in patients with Crouzon and Pfeiffer syndromes. These mutations result in an unpaired cysteine in the extracellular domain. When assayed as chimeric FGFR2/Neu receptors (Fig. 1D), these constructs induce transformation of NIH 3T3 cells (Fig. 2).
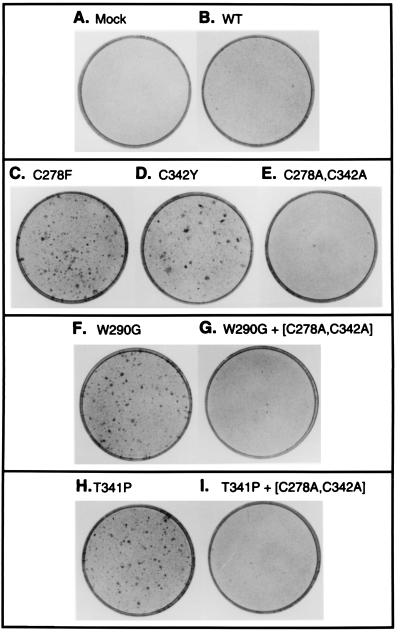
Figure 2.
Transformation of NIH 3T3 cells by FGFR2/Neu chimeras. Representative plates from transformation assays described in Fig. 1 were fixed and stained with Giemsa. (A) Mock-transfected cells. (B) Wild type. (C) C278F. (D) C342Y. (E) (C278A, C342A). (F) W290G. (G) (W290G, C278A, C342A). (H) T341P. (I) (T341P, C278A, C342A).
Among the noncysteine craniosynostosis mutations shown in Table 1C, we chose the mutations W290G and T341P identified in patients with Crouzon or Pfeiffer syndrome, respectively, for further analysis. When the single mutants W290G and T341P were assayed as chimeric FGFR2/Neu receptors (Fig. 1 E and F), both resulted in NIH 3T3 transformation comparable to the single mutants, C278F and C342Y (Fig. 2).
Cellular Transformation by Noncysteine Mutants Depends on Cys-278 and Cys-342.
As described above, single mutations affecting either partner of the Ig-3 disulfide bond, such as C278A and C278F or C342A and C342Y, resulted in efficient receptor activation, whereas simultaneous mutation of both residues to Ala, creating the double mutant (C278A, C342A), resulted in a nontransforming derivative (Fig. 1D). This suggested that transformation by each of the single mutants C278F and C342Y arises from retention of a single unpaired cysteine residue. The large number of amino acid substitutions observed at residues 278 and 342 in craniosynostosis patients (Table 1A) also suggests that it is the loss of the native disulfide bond, leaving an unpaired cysteine, rather than the introduction of any specific amino acid in place of Cys-278 or Cys-342, which is significant for receptor activation.
We next combined the double mutant (C278A, C342A) with each of the noncysteine craniosynostosis mutants examined in this study, W290G and T341P. Whereas each of the single mutants W290G and T341P was transforming, as described above, transforming activity was completely lost when these single mutants were combined with the double mutant (C278A, C342A) (Figs. 1 E and F and 2). The inactivity of the resulting triple mutants (W290G, C278A, C342A) and also (T341P, C278A, C342A), clearly indicates a requirement for the Ig-3 cysteine residues in these craniosynostosis syndromes that do not directly create or destroy a cysteine residue.
Aberrant Dimerization and Activation of Mutant Receptors.
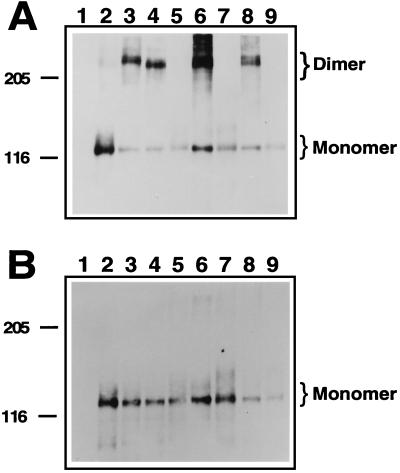
COS-1 cells were transfected with full-length FGFR2 constructs described in Fig. 1. Fig. 3A shows the mutant receptors analyzed under nonreducing conditions. Both of the single cysteine mutants, C278F and C342Y, formed dimers of approximately 220 kDa (Fig. 3A, lanes 3 and 4), in contrast to either wild-type FGFR2 or the double mutant (C278A, C342A), which exist primarily in the monomeric form (Fig. 3A, lanes 2 and 5). Both of the noncysteine mutants, W290G and T341P, also exhibited dimer formation (Fig. 3A, lanes 6 and 8) at comparable levels to the single cysteine mutants. However, when each of the mutants W290G and T341P was combined with Cys → Ala substitutions at residues 278 and 342, the resulting triple mutants (W290G, C278A, C342A) and (T341P, C278A, C342A) exhibited FGFR2 receptors that were exclusively monomeric (Fig. 3A, lanes 7 and 9). Under reducing conditions, receptors were detected primarily as monomeric species, as shown in Fig. 3B.
Figure 3.
Dimerization of activated FGFR2 mutants. COS-1 cells transiently transfected with wild-type or mutant FGFR2 expression constructs were lysed and cellular proteins were resolved on a SDS/PAGE gel. Proteins were transferred to nitrocellulose and subjected to Western blotting using antisera indicated below. (A) Samples were resolved on a 4–12% gradient gel under nonreducing conditions, incubated with anti-FGFR2 antiserum, and visualized by using enhanced chemiluminescence. Monomeric FGFR2 with an apparent molecular mass of 110 kDa and dimeric FGFR2 of approximately 220 kDa are indicated. Molecular mass markers in kDa are indicated. (B) Equivalent samples from A were resolved under reducing conditions on a 4–12% SDS/PAGE gel and visualized as described above. Lanes: 1, mock-transfected cells; 2, wild type; 3, C278F; 4, C342Y; 5, (C278A, C342A); 6, W290G; 7, (W290G, C278A, C342A); 8, T341P; 9, (T341P, C278A, C342A).
Activated Receptors Show Increased Levels of Kinase Activity and Phosphotyrosine Incorporation.
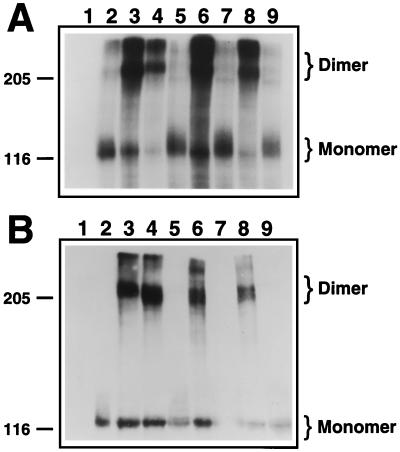
To determine whether the increased dimer formation observed for the mutant receptors correlated with increased kinase activity, immunoprecipitates were subjected to in vitro kinase assays. All of the receptors that formed dimers, as shown in Fig. 3A, also exhibited significant phosphorylation of the dimeric form of the receptor, demonstrating activation of the receptor (Fig. 4A).
Figure 4.
In vitro kinase assay of FGFR2 receptors. Constructs encoding FGFR2 wild-type or mutant receptors were transiently transfected into COS-1 cells. The cells were lysed, material was immunoprecipitated with FGFR2 antiserum, and an in vitro autophosphorylation reaction was performed in the presence of radiolabeled ATP. (A) Radiolabeled proteins were resolved on a SDS/PAGE 4–12% gradient gel and detected by autoradiography. (B) Identical aliquots were electrophoresed on a SDS/PAGE 4–12% gradient gel, after which proteins were transferred to nitrocellulose, subjected to immunoblotting using the anti-phosphotyrosine antiserum 4G10, and visualized via enhanced chemiluminescence. Monomeric and dimeric species of FGFR2 are indicated. Molecular mass markers in kDa are also indicated. Lanes: 1, mock-transfected cells; 2, wild type; 3, C278F; 4, C342Y; 5, (C278A, C342A); 6, W290G; 7, (W290G, C278A, C342A); 8, T341P; 9, (T341P, C278A, C342A).
Additionally, cellular lysates containing mutant receptors were resolved under nonreducing conditions and subjected to Western blot analysis using a phosphotyrosine-specific antibody (4G10). The same mutants that exhibited dimerization as shown in Fig. 3A and that exhibited kinase activation (Fig. 4A) also exhibited significant incorporation of phosphotyrosine (Fig. 4B). Significantly, the kinase activity and phosphotyrosine incorporation of the two noncysteine mutants examined herein, W290G and T341P, were reduced to background levels when each of these single mutants was combined with the Cys → Ala mutations affecting the Ig-3 disulfide bond, creating the triple mutants (W290G, C278A, C342A) and (T341P, C278A, C342A). These results demonstrate a requirement for the Ig-3 cysteine residues in FGFR2 activation by mutations that do not directly create or destroy a cysteine residue.
Molecular Modeling of the Ig-3 Domain.
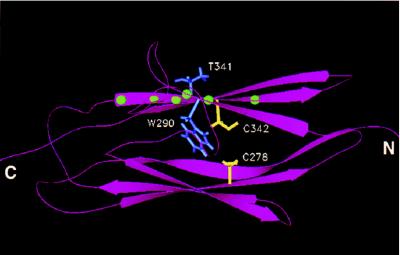
We used molecular modeling to construct a three-dimensional representation of the Ig-3 domain of FGFR2 based on the crystallographic coordinates of telokin, a myosin light chain kinase homolog, an approach that has been used previously (33). As shown in Fig. 5, both W290 and T341 lie close to the disulfide-bonded cysteines, and it is apparent that these craniosynostosis mutations are in a position to disrupt the formation of the disulfide bond. The mutation W290G involves the substitution of a large hydrophobic residue by glycine, which likely causes conformational changes that would disrupt the disulfide bond. Similarly, the T341P mutation would alter the β-strand containing Cys-342, which would be expected to disrupt its bonding with Cys-278. From this analysis, it is apparent that the noncysteine craniosynostosis mutations function through disruption of the Ig-3 disulfide bond, creating free cysteine residues that can form intermolecular disulfide bonds resulting in receptor dimerization and activation.
Figure 5.
Molecular modeling of Ig-3 domain of FGFR2. Molecular modeling was used to create a representation of Ig-3 of wild-type FGFR2 based on the crystallographic coordinates of the myosin light chain kinase homolog telokin. A ribbon diagram of the modeled structure is shown indicating the position of the Ig-3 cysteine residues (shown in yellow) relative to the amino acid side chains of W290 and T341 (shown in blue). The mutations W290G and T341P were examined in this study. Balls (shown in green) on the ribbon diagram indicate the positions of other noncysteine craniosynostosis mutations in the Ig-3 domain (Table 1C).
DISCUSSION
FGFR2 Activation by Noncysteine Mutations in the Ig-3 Domain Is Dependent on Cys-278 and Cys-342.
We have previously used FGFR2/Neu chimeric receptors as a measure of the extent of extracellular domain activation in FGFR2. In these chimeras, activation of the extracellular domain of FGFR2 leads to dimerization of the receptor and activation of the Neu kinase domain (11). By using similar chimeric receptors, we show herein that the mutations W290G and T341P, in the extracellular domain of FGFR2, resulted in activation of the receptor. The extent of this activation was comparable to the activation observed for the Crouzon/Pfeiffer syndrome mutations, C278F and C342Y, which are representative of those craniosynostosis syndrome mutations that involve the loss of a cysteine residue (Table 1A). In addition to receptor activation, both the noncysteine mutations W290G and T341P show the same levels of receptor dimerization and activation of the kinase domain as the cysteine mutations C278F and C342Y. Additionally, the dimerization observed for both of these mutants was reducible, indicating that the dimerization was accomplished by disulfide bond formation. Because the levels of receptor activation and the clinical phenotypes observed for the cysteine and noncysteine mutants are identical, a common mechanism for activation between the two mutants is suggested.
For both of the noncysteine mutants characterized in this study, cellular transformation by chimeric receptors containing the mutations in the extracellular domain of FGFR2 was dependent on the presence of Cys-278 and Cys-342, residues that normally form a disulfide bond that stabilizes the Ig-3 domain. Additionally, for both noncysteine activating mutants, dimerization and kinase activation was completely abolished when both Cys-278 and Cys-342 were removed by mutation. In addition, molecular modeling of the FGFR2 Ig-3 domain reveals that the noncysteine activating mutations have the potential to disrupt the disulfide bond.
Activating Regions in the Ig-3 Domain of FGFR2.
In the Ig-3 domain, noncysteine mutations that give rise to craniosynostosis syndromes cluster around two regions that lie structurally close to the disulfide bond and center around W290 and T341. The craniosynostosis mutations found in the structural region around W290 includes the point mutations W290G examined herein, as well as W290R, Q289P, and the deletion ΔH287–Q289. Side chains in this region all lie in close proximity to the disulfide-bonded cysteines. All of the side chain substitutions from the craniosynostosis mutations in this region are structurally profound and would likely alter the local structure of this region.
The other activating region in the FGFR2 Ig-3 domain centers around Thr-341. Craniosynostosis mutations in this region include G338R, G338E, Y340H, T341P, A344P, and A344G, and the deletion ΔG345–P361, all of which lie on the β-strand containing Cys-342. Some of these mutations dramatically alter the size of the side chain, likely forcing the β-strand to rotate, and moving the Cys-342 side chain into a nonbonding position relative to Cys-278. The remaining craniosynostosis mutations substitute either a proline or a glycine into the β-strand. These side chains are generally disruptive to elements of secondary structure and would disrupt the β-strand, again moving Cys-342 out of bonding range with Cys-278.
FGFR2 Activation by Cysteine Mutations in the Ig-1 Domain.
The only Cys → Ala mutation in either the Ig-1 or Ig-2 domains that resulted in receptor activation was C62A, which would leave Cys-107 unpaired and potentially available for aberrant dimerization. These results correlate with the lack of observed craniosynostosis mutations in the Ig-2 domain and suggest that these cysteine residues are inaccessible for intermolecular bonding. Indeed, the lone craniosynostosis mutation identified to date in either the Ig-1 or Ig-2 domains is the Y105C mutation (22), which creates a free cysteine residue right next to Cys-107. Although there has been only one observed craniosynostosis mutations in Ig-1, our data raise the possibility that other mutations in this region will be linked to human skeletal disorders in the future.
Future Directions and Non-Ig-3 Mutations.
Although FGFR2 activation by point mutations in the extracellular domain appears to result primarily from inappropriate disulfide bond formation, activation of the receptor can lead to many different phenotypes with multiple syndromes even resulting from the same mutation (Table 1). Additionally, noncraniosynostosis syndromes can also result from cysteine-dependent activation of FGFR2. Two recently reported mutations (24), S372C and Y375C, cause Beare–Stevenson cutis gyrata syndrome, a lethal skeletal and skin disorder. Although we have not examined these mutations in this work, we would predict that they also involve aberrant intermolecular disulfide bonding. The explanation for why various clinical syndromes are observed for similar mutations remains conjectural but may reflect different degrees of receptor activation, altered receptor trafficking, or altered interactions with downstream signaling intermediates. There also exists a family of mutations in the extracellular domain of FGFRs that offers no apparent mechanism for activation (Table 1D). Within the linker domain between Ig-2 and Ig-3, there exists the tripeptide RSP that is conserved throughout the FGFR family and that is associated with many developmental syndromes. Within this region, mutations in FGFR1 cause Pfeiffer syndrome (P252R), and mutations in FGFR2 cause Apert syndrome (S252W and P253R), whereas mutations in FGFR3 cause nonsyndromic craniosynostosis (P250R) (13, 16, 34, 35). Because these mutations do not involve cysteine residues, nor do they lie in close proximity to a disulfide bond, the mechanism of their activation remains unresolved.
Parallels with Activating Mutations in Other Receptor Tyrosine Kinases.
In addition to the FGFRs, other receptor tyrosine kinases exhibit constitutive activation resulting from extracellular mutations. RET, the erythropoietin receptor, and the epidermal growth factor receptor are activated by mutations that create unpaired cysteine residues leading to disulfide-linked dimers and ligand-independent signaling (36–39). Moreover, the c-fms receptor is partially activated by noncysteine mutations in one of its Ig domains (40). Numerous other receptor families exhibit multiple Ig-like domains, such as the neurotrophin receptor family, the Eph-like receptor family, or the Tie receptor family; it seems likely that similar noncysteine mutations, resulting in receptor activation by the mechanism proposed herein for FGFR2, will be identified in the Ig-like domains of these other receptor tyrosine kinases.
Acknowledgments
We thank Herbert Yue, Charlotte Bell, and Pedro Ramos for technical assistance; and Laura Castrejon for editorial assistance. This work was supported by National Institutes of Health Grant DE 12581 and by Grant 1RB-0318 from the University of California Breast Cancer Research Program. S.C.R. and K.C.H. gratefully acknowledge support from National Institutes of Health Training Grant GM 07313.
ABBREVIATIONS
- FGFR
fibroblast growth factor receptor
- Ig-3
third immunoglobulin-like domain
References
- 1.Johnson D E, Williams L T. Adv Cancer Res. 1993;60:1–41. doi: 10.1016/s0065-230x(08)60821-0. [DOI] [PubMed] [Google Scholar]
- 2.Partanen J, Vainikka S, Alitalo K. Philos Trans R Soc London Ser B. 1993;340:297–303. doi: 10.1098/rstb.1993.0071. [DOI] [PubMed] [Google Scholar]
- 3.Ullrich A, Schlessinger J. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 4.Spivak-Kroizman T, Lemmon M A, Dikic I, Ladbury J E, Pinchasi D, Huang J, Jaye M, Crumley G, Schlessinger J, Lax I. Cell. 1994;79:1015–1024. doi: 10.1016/0092-8674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadi M, Dikic I, Sorokin A, Burgess W H, Jaye M, Schlessinger J. Mol Cell Biol. 1996;16:977–989. doi: 10.1128/mcb.16.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muenke M, Schell U. Trends Genet. 1995;11:308–313. doi: 10.1016/s0168-9525(00)89088-5. [DOI] [PubMed] [Google Scholar]
- 7.Webster M K, Donoghue D J. Trends Genet. 1997;13:178–182. doi: 10.1016/s0168-9525(97)01131-1. [DOI] [PubMed] [Google Scholar]
- 8.Webster M K, Donoghue D J. EMBO J. 1996;15:520–527. [PMC free article] [PubMed] [Google Scholar]
- 9.Naski M C, Wang Q, Xu J, Ornitz D M. Nat Genet. 1996;13:233–237. doi: 10.1038/ng0696-233. [DOI] [PubMed] [Google Scholar]
- 10.Webster M K, D’Avis P Y, Robertson S C, Donoghue D J. Mol Cell Biol. 1996;16:4081–4087. doi: 10.1128/mcb.16.8.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galvin B D, Hart K C, Meyer A N, Webster M K, Donoghue D J. Proc Natl Acad Sci USA. 1996;93:7894–7899. doi: 10.1073/pnas.93.15.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neilson K M, Friesel R. J Biol Chem. 1996;271:25049–25057. doi: 10.1074/jbc.271.40.25049. [DOI] [PubMed] [Google Scholar]
- 13.Wilkie A O M, Morriss-Kay G M, Jones E Y, Heath J K. Curr Biol. 1995;5:500–507. doi: 10.1016/s0960-9822(95)00102-3. [DOI] [PubMed] [Google Scholar]
- 14.Meyers G A, Day D, Goldberg R, Daentl D L, Przylepa K A, Abrams L J, Graham J M, Feingold M, Moeschler J B, Rawnsley E, et al. Am J Hum Genet. 1996;58:491–498. [PMC free article] [PubMed] [Google Scholar]
- 15.Oldridge M, Wilkie A O M, Slaney S F, Poole M D, Pulleyn L J, Turland P, Hockley A D, Wake M J C, Goldin J H, Winter R M, et al. Hum Mol Genet. 1995;4:1077–1082. doi: 10.1093/hmg/4.6.1077. [DOI] [PubMed] [Google Scholar]
- 16.Park W-J, Meyers G A, Li X, Theda C, Day D, Orlow S J, Jones M C, Jabs E W. Hum Mol Gen. 1995;4:1229–1233. doi: 10.1093/hmg/4.7.1229. [DOI] [PubMed] [Google Scholar]
- 17.Schell U, Hehr A, Feldman G J, Robin N H, Zackai E H, de Die-Smulders C, Viskochil D H, Stewart J M, Wolff G, Ohashi H, et al. Hum Mol Gen. 1995;4:323–328. doi: 10.1093/hmg/4.3.323. [DOI] [PubMed] [Google Scholar]
- 18.Reardon W, Winter R M, Rutland R, Pulleyn L J, Jones B M, Malcolm S. Nat Genet. 1994;8:98–103. doi: 10.1038/ng0994-98. [DOI] [PubMed] [Google Scholar]
- 19.Rutland P, Pulleyn L J, Reardon W, Baraitser M, Hayward R, Malcolm S, Winter R M, Oldridge M, Slaney S F, Poole M D, Wilkie A O M. Nat Genet. 1995;9:173–176. doi: 10.1038/ng0295-173. [DOI] [PubMed] [Google Scholar]
- 20.Gorry M C, Preston R A, White G J, Zhang Y Z, Singhal B K, Losken H W, Parker M G, Nwokoro N A, Post J C, Ehrlich G D. Hum Mol Gen. 1995;4:1387–1390. doi: 10.1093/hmg/4.8.1387. [DOI] [PubMed] [Google Scholar]
- 21.Steinberger D, Mulliken J B, Muller U. Hum Gen. 1995;96:113–115. doi: 10.1007/BF00214198. [DOI] [PubMed] [Google Scholar]
- 22.Pulleyn L J, Reardon W, Wilkes D, Rutland P, Jones B M, Hayward R, Hall C M, Brueton L, Chun N, Lammer E, et al. Eur J Hum Genet. 1996;4:283–291. doi: 10.1159/000472215. [DOI] [PubMed] [Google Scholar]
- 23.Jabs E W, Li X, Scott A F, Meyers G, Chen W, Eccles M, Mao J, Charnas L R, Jackson C E, Jaye M. Nat Genet. 1994;8:275–279. doi: 10.1038/ng1194-275. [DOI] [PubMed] [Google Scholar]
- 24.Przylepa K A, Paznekas W, Zhang M, Golabi M, Bias W, Bamshad M J, Carey J C, Hall B D, Stevenson R, Orlow S, et al. Nat Genet. 1996;13:492–494. doi: 10.1038/ng0896-492. [DOI] [PubMed] [Google Scholar]
- 25.Lajeunie E, Hong M A, Bonaventure J, Munnich A, Le Merrer M, Renier D. Nat Genet. 1995;9:108. doi: 10.1038/ng0295-108. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Park W-J, Pyeritz R E, Jabs E W. Nat Genet. 1995;9:232–233. doi: 10.1038/ng0395-232. [DOI] [PubMed] [Google Scholar]
- 27.Wilkie A O M, Slaney S F, Oldridge M, Poole M D, Ashworth G J, Hockley A D, Hayward R D, David D J, Pulleyn L J, Rutland P, et al. Nat Genet. 1995;9:165–172. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]
- 28.Cohen M. In: Craniosynostosis Diagnosis, Evaluation, and Management. Cohen M, editor. New York: Raven; 1986. pp. 413–590. [Google Scholar]
- 29.Dionne C A, Crumley G, Bellot F, Kaplow J M, Searfoss G, Ruta M, Burgess W H, Jaye M, Schlessinger J. EMBO J. 1990;9:2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L I, Webster M K, Meyer A N, Donoghue D J. J Cell Biol. 1997;137:619–631. doi: 10.1083/jcb.137.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bargmann C I, Hung M-C, Weinberg R A. Cell. 1986;45:649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- 32.Robertson S C, Donoghue D J. Mol Cell Biol. 1996;16:3472–3479. doi: 10.1128/mcb.16.7.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray T E, Eisenstein M, Shimon T, Givol D, Yayon A. Biochemistry. 1995;34:10325–10333. doi: 10.1021/bi00033a002. [DOI] [PubMed] [Google Scholar]
- 34.Bellus G A, Gaudenz K, Zackai E H, Clarke L A, Szabo J, Francomano C A, Muenke M. Nat Genet. 1996;14:174–176. doi: 10.1038/ng1096-174. [DOI] [PubMed] [Google Scholar]
- 35.Muenke M, Schell U, Hehf A, Robin N H, Losken H W, Schinzel A, Pulleyn L J, Rutland P, Reardon W, Malcolm S, Winter R M. Nat Genet. 1994;8:269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- 36.Santoro M, Carlomagno F, Romano A, Bottaro D P, Dathan N A, Greico M, Fusco A, Vecchio G, Matoskova B, Kraus M H, Di Fiore P P. Science. 1995;267:381–383. doi: 10.1126/science.7824936. [DOI] [PubMed] [Google Scholar]
- 37.Sorokin A, Lemmon M A, Ullrich A, Schlessinger J. J Biol Chem. 1994;269:9752–9759. [PubMed] [Google Scholar]
- 38.Watowich S S, Yoshimura a, Longmore G D, Hilton D J, Yoshimura Y, Lodish H F. Proc Natl Acad Sci USA. 1992;89:2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshimura A, Longmore G, Lodish H F. Nature (London) 1990;348:647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- 40.Carlberg K, Rohrschneider L. Mol Biol Cell. 1994;5:81–95. doi: 10.1091/mbc.5.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]