Abstract
Objective
Total plasma adiponectin and high molecular weight (HMW) polymeric adiponectin are strongly positively correlated with insulin sensitivity. However we have recently reported paradoxical hyperadiponectinemia in patients with severe insulin resistance due to genetically defective insulin receptors. This implies either that the insulin receptor has a critical physiological role in controlling adiponectin production and/or clearance, or that constitutive insulin receptor dysfunction influences adiponectin levels through developmental effects. The aim of the current study was to distinguish between these possibilities using a human model of reversible antibody-mediated insulin receptor dysfunction, and to refine the previous observations by determining adiponectin complex distribution.
Research Design and Methods
Cross-sectional and longitudinal determination of fasting plasma adiponectin and adiponectin complex distribution in patients with extreme insulin resistance due to insulin receptor mutations, anti-insulin receptor antibodies (type B insulin resistance), or of undefined cause.
Results
Despite extreme insulin resistance, patients with type B insulin resistance (all female; mean age 42 years (range 12-54)) had dramatically elevated total plasma adiponectin compared to the general population (mean 43.0 mg/l (range 31.3-54.2) vs mean 8.9 mg/l (range 1.5-28.5 for B.M.I.<25 kg/m2)), which was accounted for largely by HMW polymers. Hyperadiponectinaemia resolved in parallel with reduction of insulin receptor antibodies and clinical resolution of insulin resistance.
Conclusions
While the well established inverse relationship between plasma insulin and adiponectin levels may, in part, reflect positive effects of adiponectin on insulin sensitivity, these data suggest that the magnitude of the effect of insulin action on adiponectin levels may have been underestimated.
Introduction
The ability of white adipose tissue to elaborate molecules with endocrine actions of relevance to fuel metabolism is well established (1). Most abundant of these adipose tissue-derived factors is adiponectin, a multimeric protein with homology to complement factor 1q (1). Adiponectin has excited considerable interest as a marker of insulin resistance because of the strong correlation between its plasma levels and insulin sensitivity (1), and because low plasma adiponectin is predictive of future type 2 diabetes (2). Furthermore, based on the elevation of adiponectin seen upon treatment with thiazolidinediones (3), the insulin-sensitizing effect of either infusion or transgenic overexpression of adiponectin in insulin resistant rodents (4-6), and the significant association between genetic variants in the adiponectin gene and type 2 diabetes risk in human populations (1), it has been suggested that defects in adiponectin production and/or action may be an aetiological factor in a significant proportion of human insulin resistance. Correcting suppressed adiponectin in insulin resistance has thus become an attractive therapeutic strategy.
However we have recently reported paradoxical hyperadiponectinaemia in patients with insulin receptor loss-of-function mutations, and have suggested that this arises either from abnormal adipose tissue development, or from loss of insulin action in mature adipose tissue (7). In this study we sought to discriminate between these possibilities by studying a group of patients with acquired loss of insulin receptor function and extreme insulin resistance due to insulin receptor blocking antibodies (type B insulin resistance).
The proportion of adiponectin accounted for by HMW adiponectin multimers, or the absolute concentration of HMW multimers, correlate better with insulin sensitivity in normal and type 2 diabetic populations than total plasma adiponectin (1), and human mutations in the adiponectin gene which are associated with type 2 diabetes produce mutant species which show impaired multimerisation (8). This led us also to refine the previous findings by determining adiponectin complex distribution in patients with either congenital or acquired insulin receptor dysfunction, or idiopathic severe insulin resistance.
Research Design and Methods
Subjects with severe insulin resistance were recruited with informed consent in line with procedures approved either by the local research ethics committee in Cambridge, U.K., or by the institutional review board of the NIDDK. Type B insulin resistance was diagnosed on the basis of clinical and biochemical evidence of severe hyperinsulinaemia with detectable insulin receptor binding antibodies. Anti-insulin receptor antibody titres were determined by immunoprecipitation of insulin receptor preparations using patients’ sera followed by Western blotting, using one of two closely related protocols (9; 10).
Venous blood was drawn in the fasting state and plasma immediately extracted and stored at -20°C. Leptin and adiponectin assays have been described previously (7). Adiponectin complex distribution was determined by separating 20 μl of human serum over a Superdex 200 10/300 GL column (GE Healthcare Bio-Sciences Corp.) using an AKTA FPLC system (GE Healthcare Bio-Sciences Corp). The column was equilibrated in phosphate-buffered saline, pH 7.4 and 0.215 ml fractions collected. Samples (40 μl) were collected over the entire elution of adiponectin and incubated with 10 μl of 5X Laemmli sample buffer before electrophoresis on a Criterion precast 26-well gel (Bio-Rad). Immunoblotting using 1:500 polyclonal anti-adiponectin (N-terminal) antibody followed by incubation with IR-Dye 800-coupled goat anti-rabbit secondary antibody (Rockland) was undertaken. The fluorescence signal at 30 KDa was quantified using the LI-COR Odyssey infrared imaging system in conjunction with Odyssey v1.2 software (LI-COR Biotechnology, Lincoln, NE). Samples were from 6 normal control subjects (3 male, 3 female), 4 patients with insulin receptor (INSR) mutations (1 male: 16 years, INSR P193L homozygote; 3 female: 14 years, INSR P193L homozygote; 41 years, INSR F382V homozygote; 28 years, INSR K460E/Q672X compound heterozygote; metabolic parameters for all described previously (7)), initial samples from patients 1,2 and 4 with type B insulin resistance (Tables 1,2), and 3 female patients with idiopathic severe insulin resistance (mean age 34.3 years, mean fasting blood glucose 197 mg/dl, mean fasting insulin 279 pmol/l, mean total adiponectin 2.8 mg/l).
Table 1. Characteristics of the patients with type B insulin resistance studied.
SLE = systemic lupus erythematosis; MCTD = mixed connective tissue disease
| Patient | Gender | Age, years | Ethnic Origin | Associated Autoimmune Disease | Duration of severe insulin resistance prior to diagnosis of type B insulin resistance |
|---|---|---|---|---|---|
| 1 | F | 52 | Peruvian | MCTD | 6 months |
| 2 | F | 12 | African-American | SLE | 3 months |
| 3 | F | 50 | African-American | none | 16 months |
| 4 | F | 54 | African-American | none | 6 months (10 years of type 2 diabetes) |
| 5 | F | 44 | African-American | SLE | 4 years |
| 6 | F | 37 | Indian | SLE | 7 months |
| 7 | F | 20 | African-American | MCTD | 6 months |
Table 2. Treatment, clinical and biochemical parameters of type B insulin resistant patients during therapy.
Reference ranges for leptin and adiponectin are the 5th-95th centiles from gender and BMI-matched non diabetic controls
| Patient | Time from Diagnosis, months | BMI, kg/m2 | Fasting blood glucose, mg/dl | Fasting plasma insulin, pmol/l | HbA1c, % | Leptin, μg/l | Adiponectin*, mg/l | Diabetes Treatment | Immunosuppression | anti-insulin receptor Ab titre | Serum creatinine (mg/dL)/CrCL# (ml/min) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 20.4 | 244 | 11952 | 10.2 | <0.1 | 51.1 | metformin, 4000iu insulin/day | nil | +++ | 0.4/149 |
| 6 | 21.8 | 66 | 17682 | 8.6 | 1.2 | 21.9 | 2350iu insulin/day | nil | ++ | 0.3/152 | |
| 11 | 23.4 | 78 | 11681 | 8.7 | 2.3 | 16.3 | 1250iu insulin/day | nil | + | 0.4/157 | |
| 17 | 23.8 | 55 | 14015 | 8 | 9.8 | 15.6 | 1250iu insulin/day | nil | - | 0.4/143 | |
| 35 | 30.1 | 81 | 82 | 5.7 | 55.7 | 6.2 | nil | nil | - | 0.6/113 | |
| 2 | 0 | 19.1 | 85 | 490 | 5.1 | 35.6 | 42 | rosiglitazone, metformin, 450iu insulin/day | 60mg prednisone/day | +++ | 0.5/152 |
| 3 | 21.8 | 70 | 212 | 7.6 | 18.6 | 60 | rosiglitazone, metformin, 450iu insulin/day | 5mg prednisone/day | + | 0.5/134 | |
| 3 | 0 | 23 | 378 | 6876 | 8.7 | 6 | 31.3 | 1000iu insulin/day | plasmapheresis, rituximab, cyclophosphamide | +++ | 0.5/NA |
| 10 | 24.9 | 69 | 249 | 7.3 | 48.4 | 21.8 | nil | 20mg prednisone/day | + | 0.7/166 | |
| 4 | 0 | 31.1 | 237 | 17029 | 12.1 | <0.1 | 54.2 | 1400iu insulin/day | nil | +++ | 0.6/NA |
| 5 | 0 | 25.6 | 108 | 2979 | 8.4 | 8.5 | 36.2 | 1200iu insulin/day | 20mg prednisone/day | ++ | 1.4/76 |
| 6 | 0 | 16.8 | 63 | 12,500 | 12.1 | 0.4 | 32.0 | 2800iu insulin/day | nil | ++ | 0.3/NA |
| 7 | 0 | 20.7 | 321 | 42852 | 11.9 | 1.7 | 54.4 | 19,000iu insulin/day | nil | +++ | 0.6/97 |
Normal ranges for adiponectin from a control population of 515 healthy female controls are 4.4-17.7 mg/l for B.M.I. <25 kg/m2, 3.5-15.5 mg/l for B.M.I. 25-30 kg/m2, and 2.6-14.9 mg/l for B.M.I. 30-35 kg/m2.
CrCL = creatinine clearance, determined from 24 hour urine collection.
Normative adiponectin and leptin data were derived from the MRC Ely Study cohort, representative of an ethnically homogeneous Europid population in Eastern England (11). Those with diabetes on the basis of fasting blood glucose or of oral glucose tolerance testing were excluded from analysis. Complete data (fasting insulin, leptin and adiponectin) were available for 872 non-diabetic participants (357 male, 515 female), and were used to generate sex and BMI-specific reference ranges for adiponectin. Samples from patients with type 1 diabetes were obtained as part of the Oxford Regional Prospective Study of type 1 diabetes (12).
Results
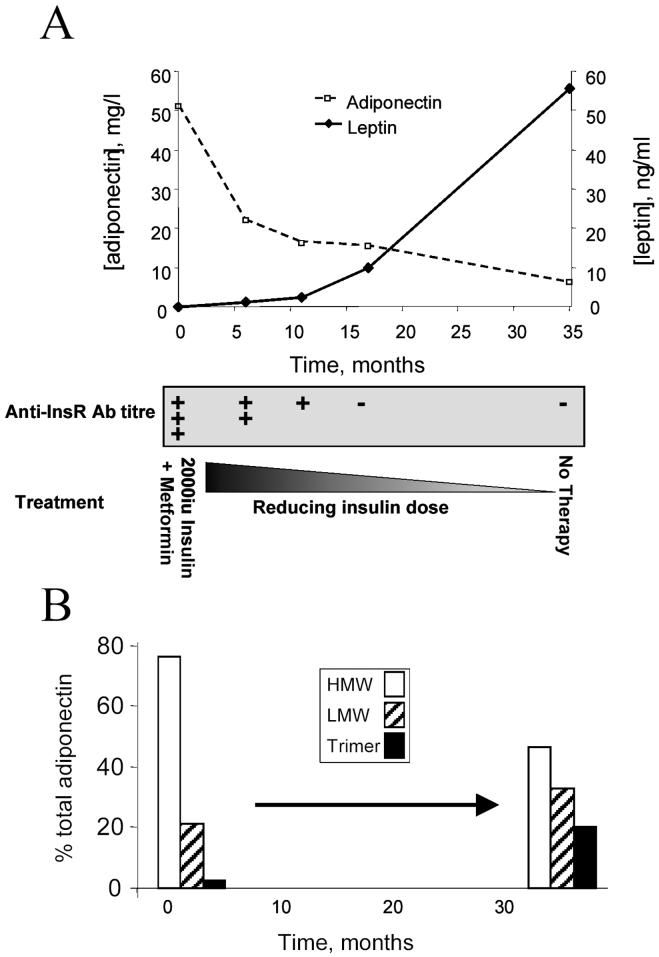
The characteristics of patients with type B insulin resistance were in keeping with the cumulative published experience of type B IR (9) (table 1). Despite marked fasting hyperinsulinaemia at presentation with extremely high requirements for exogenous insulin and oral insulin sensitizing agents, all patients had total plasma adiponectin well above the sex and BMI-specific reference range, with low or normal leptin. Indeed, plasma adiponectin values of all patients at the height of their insulin resistance were significantly above the highest plasma adiponectin recorded in the 872 non-diabetic control subjects (28.5 mg/l). Clinical improvement with resolution of insulin resistance and reduction of anti insulin receptor antibody titre was associated with decreases of plasma adiponectin to within the normal range, increases in BMI, improved glycaemia and insulinaemia, and reduction or cessation of treatment for diabetes. Patient 2 showed an increase in adiponectin over three months of therapy, but although the insulin receptor antibody titre waned over this period, glycaemic control deteriorated despite unchanged insulin and metformin doses, suggesting worsening insulin resistance. Data from patient 1, who received no immunomodulatory therapy, and in whom insulin resistance and anti insulin receptor antibodies abated spontaneously over 3 years, demonstrate the marked and reciprocal responses of leptin and adiponectin to insulin sensitization (Fig. 1A). Importantly, only patient 5 had evidence of renal disease (SLE nephritis), and her measured creatinine clearance of 76 ml/min was only modestly depressed. No correlation was seen in other patients between changes in creatinine clearance and plasma adiponectin (Table 2).
Figure 1.
Longitudinal course of patient 1 during spontaneous resolution of type B insulin resistance. A) total plasma adiponectin B) adiponectin complex distribution determined before and after resolution of insulin resistance.
Peak adiponectin levels corresponded not only to extreme insulin resistance, but also to poor glycaemic control, as evidenced by high HbA1c values (Table 2). However in 10 female subjects with type 1 diabetes (mean age 17.0 years (range 13.1-20.6)) and similar HbA1c levels (mean 11.3% (range 9.2-13.2)) we found adiponectin levels to be much lower than in type B insulin resistant patients, with no overlap between groups (mean 9.4 mg/l (range 3.5-13.7) vs 43.0 mg/l (31.3-54.2)), suggesting that hyperglycaemia and relative insulin deficiency per se do not explain the observation. Although this control group was younger than most of the type B patients studied, published experience suggests that in adult type 1 diabetic patients, too, the observed increase in plasma adiponectin is modest compared to healthy controls and does not approach the magnitude of the difference in the type B patients (13).
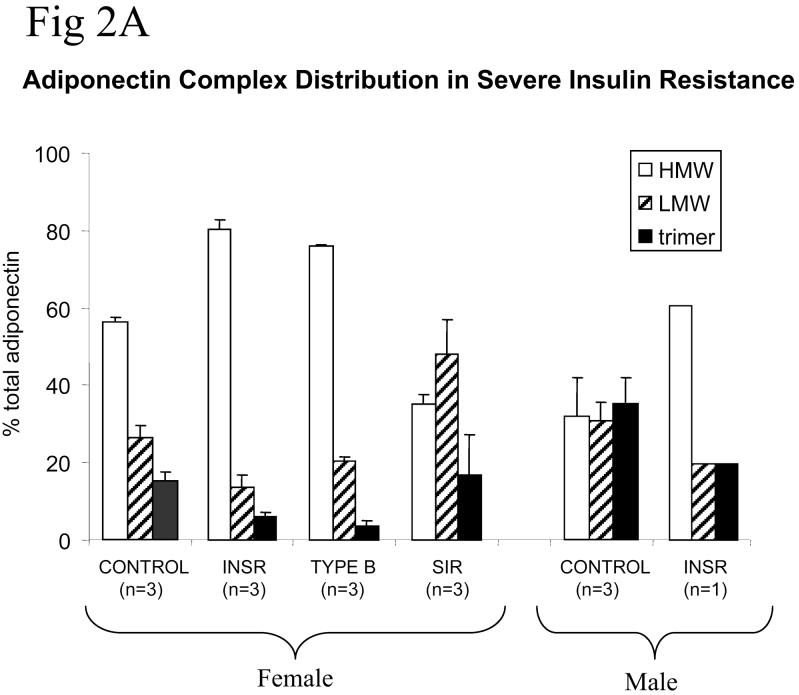
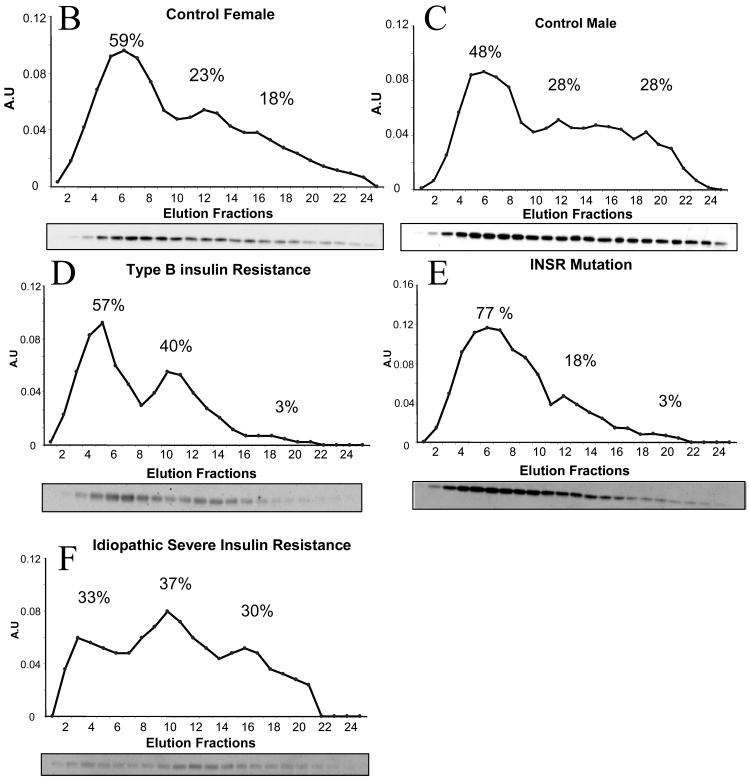
To determine whether insulin receptor dysfunction leads to changes in the distribution of higher order multimers of adiponectin, FPLC was undertaken of plasma from normal volunteers, and from patients with insulin receptor mutations, type B insulin resistance, or idiopathic severe insulin resistance. The high total adiponectin in genetic and antibody-mediated insulin receptor dysfunction was accounted for largely by HMW adiponectin (figure 2), in marked contrast to patients with idiopathic severe insulin resistance, who had a preponderance of lower molecular weight oligomers (figure 2). Furthermore, comparison of complex distribution before and after clinical resolution of type B insulin resistance in one patient revealed a marked shift in profile towards lower molecular weight species with restitution of insulin receptor function (Fig. 1B). Representative elution profiles of adiponectin are also shown (Fig 2B-F), demonstrating the ability of the technique used to detect trimers, hexamers and HMW species.
Figure 2.
A) Adiponectin complex distribution from patients with severe insulin resistance and controls. CONTROL = healthy volunteers (3 female (mean age 35 years, mean BMI 20 kg/m2, mean fasting blood glucose 91 mg/dl, mean fasting insulin 40.3 pmol/l, mean total adiponectin 7.8 mg/l); 3 male (mean age 35 years, mean BMI 22.7 kg/m2, mean fasting blood glucose 90 mg/dl, mean fasting insulin 40.3 pmol/l, mean total adiponectin 5.0 mg/l); INSR = patients with loss-of-function insulin receptor mutations; Type B = patients with type B insulin resistance; SIR = patients with severe insulin resistance of undefined genetic aetiology. Error bars represent standard deviations. B)-F) Representative FPLC elution profiles with Western blots for each patient group studied.
Discussion
Plasma adiponectin consistently correlates positively with insulin sensitivity in normal human populations (1), and we have previously established that this relationship also holds in almost all patients with severe insulin resistance, in whom adiponectin is extremely low (7). Striking exceptions have proved to be patients with loss-of-function mutations in the insulin receptor, in whom plasma adiponectin is not only an order of magnitude higher than seen in other states of severe insulin resistance, but is also significantly higher than in the normal population (7). We suggested that this discordance between high plasma adiponectin and extreme insulin resistance may be accounted for either by direct effects in adipocytes of the loss of insulin receptor function, or by the effects on adipose tissue development of severely impaired insulin receptor function in utero and beyond (7). Using type B insulin resistance as a model of acquired and reversible insulin receptor dysfunction in adult life, we now have established that dramatic hyperadiponectinaemia with loss of insulin receptor function is not dependent on receptor dysfunction during development, and moreover is reversible on restitution of receptor function. Our finding that the hyperadiponectinaemia in states of insulin receptor dysfunction is accounted for by a large preponderance of HMW multimers further accentuates the dissociation between plasma adiponectin and insulin sensitivity, as HMW adiponectin has been shown to correlate better with insulin sensitivity than total plasma adiponectin (1). In contrast, the patients studied with idiopathic severe insulin resistance showed the same unexplained leftward shift in complex distribution that is seen in highly prevalent but milder insulin resistance.
The robust association of hypoadiponectinaemia with the earliest detectable stages of insulin resistance has raised the possibility that hypoadiponectinaemia may play a causal role in prevalent forms of insulin resistance. However the causal link in humans has yet to be established, and hyperadiponectinaemia in states of extreme insulin resistance due to insulin receptor dysfunction demonstrates that they may be entirely dissociated in some settings. Nevertheless this observation is not at odds with the notion of an aetiological role for hypoadiponectinaemia in other, more common, forms of insulin resistance: indeed, hypersecetion of adiponectin by adipocytes with a very proximal defect in insulin action could be regarded as an extreme compensatory response aimed at systemic insulin sensitization.
The mechanistic basis for this radical dissociation between insulin sensitivity and circulating adiponectin is unclear. It may in principle be accounted for by increased adiponectin secretion from adipocytes, by reduced clearance of circulating adiponectin, or by a combination of these. Several lines of evidence suggest that an effect on secretion is dominant: the 60% increase in plasma adiponectin reported in mice with adipose-specific deletion of the insulin receptor provides evidence that there is an adipocyte-specific role of the insulin receptor in determining plasma adiponectin levels in vivo (14), while the shift in complex distribution towards HMW forms reported here, allied to previous demonstration that higher order adiponectin multimers do not interconvert in the circulation in vivo (15), is also suggestive of a change in adipocyte secretory activity.
Hypersecretion of adiponectin from adipocytes with reduced or absent insulin receptor function could be attributable 1) to loss of direct suppression of adiponectin synthesis and secretion at a transcriptional or post transcriptional level by insulin receptor activation, or 2) to a more indirect effect mediated by changes in cellular metabolic or redox status in the absence or reduction of functional insulin receptor. A direct suppressive effect of insulin on adipocyte synthesis and secretion of adiponectin is generally not supported by studies in vitro and ex vivo to date (16-19), however chronic insulin action in the context of a more physiological hormonal, nutritional and cellular milieu may elicit a different response. A longitudinal study of the development of insulin resistance in rhesus monkeys found no change in adipose tissue adiponectin mRNA despite low plasma adiponectin, suggesting that post transcriptional events may predominate (17).
Hypersecretion of adiponectin could instead be related to the unrestrained catabolic mode of adipocytes with hypofunctional insulin receptors, consistent with the modestly elevated adiponectin seen in poorly controlled type 1 diabetes (13). However the most physiological insulinopaenic catabolic state is fasting, and neither medium term fasting nor anorexia nervosa have consistently been shown to produce elevations in adiponectin (e.g. (20; 21)). A further possibility is that lack of insulin receptor function leads to elevated adiponectin through a reduction in intracellular reactive oxygen species (ROS), which have been shown to suppress adiponectin expression in vitro and ex vivo (22; 23), and to be modulated by insulin receptor activity (24).
This report attests to the utility of using human disease models such as T1DM (pure insulin deficiency) and INSR mutations or type B insulin resistance (isolated loss of INSR function with severe hyperinsulinaemia) to make novel observations of relevance to the biology of human insulin action in vivo.
Acknowledgements
SOR is supported by the Wellcome Trust, and JL and NJW by the UK Medical Research Council
References
- 1.Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–1545. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 2.Lindsay RS, Funahashi T, Hanson RL, Matsuzawa Y, Tanaka S, Tataranni PA, Knowler WC, Krakoff J. Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet. 2002;360:57–58. doi: 10.1016/S0140-6736(02)09335-2. [DOI] [PubMed] [Google Scholar]
- 3.Yu JG, Javorschi S, Hevener AL, Kruszynska YT, Norman RA, Sinha M, Olefsky JM. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 4.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 6.Shklyaev S, Aslanidi G, Tennant M, Prima V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek EW, Scarpace PJ, Zolotukhin S. Sustained peripheral expression of transgene adiponectin offsets the development of diet-induced obesity in rats. Proc Natl Acad Sci U S A. 2003;100:14217–14222. doi: 10.1073/pnas.2333912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semple RK, Soos MA, Luan J, Mitchell CS, Wilson JC, Gurnell M, Cochran EK, Gorden P, Chatterjee VK, Wareham NJ, O’Rahilly S. Elevated plasma adiponectin in humans with genetically defective insulin receptors. J Clin Endocrinol Metab. 2006;91:3219–3223. doi: 10.1210/jc.2006-0166. [DOI] [PubMed] [Google Scholar]
- 8.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 9.Arioglu E, Andewelt A, Diabo C, Bell M, Taylor SI, Gorden P. Clinical course of the syndrome of autoantibodies to the insulin receptor (type B insulin resistance): a 28-year perspective. Medicine (Baltimore) 2002;81:87–100. doi: 10.1097/00005792-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Coll AP, Morganstein D, Jayne D, Soos MA, O’Rahilly S, Burke J. Successful treatment of Type B insulin resistance in a patient with otherwise quiescent systemic lupus erythematosus. Diabet Med. 2005;22:814–815. doi: 10.1111/j.1464-5491.2005.01529.x. [DOI] [PubMed] [Google Scholar]
- 11.Wareham NJ, Byrne CD, Williams R, Day NE, Hales CN. Fasting proinsulin concentrations predict the development of type 2 diabetes. Diabetes Care. 1999;22:262–270. doi: 10.2337/diacare.22.2.262. [DOI] [PubMed] [Google Scholar]
- 12.Schultz CJ, Konopelska-Bahu T, Dalton RN, Carroll TA, Stratton I, Gale EA, Neil A, Dunger DB, Oxford Regional Prospective Study Group Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Diabetes Care. 1999;22:495–502. doi: 10.2337/diacare.22.3.495. [DOI] [PubMed] [Google Scholar]
- 13.Frystyk J, Tarnow L, Hansen TK, Parving HH, Flyvbjerg A. Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia. 2005;48:1911–1918. doi: 10.1007/s00125-005-1850-z. [DOI] [PubMed] [Google Scholar]
- 14.Bluher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 15.Peake PW, Kriketos AD, Campbell LV, Shen Y, Charlesworth JA. The metabolism of isoforms of human adiponectin: studies in human subjects and in experimental animals. Eur J Endocrinol. 2005;153:409–417. doi: 10.1530/eje.1.01978. [DOI] [PubMed] [Google Scholar]
- 16.Pereira RI, Draznin B. Inhibition of the phosphatidylinositol 3′-kinase signaling pathway leads to decreased insulin-stimulated adiponectin secretion from 3T3-L1 adipocytes. Metabolism. 2005;54:1636–1643. doi: 10.1016/j.metabol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 18.Bogan JS, Lodish HF. Two compartments for insulin-stimulated exocytosis in 3T3-L1 adipocytes defined by endogenous ACRP30 and GLUT4. J Cell Biol. 1999;146:609–620. doi: 10.1083/jcb.146.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke M, Ewart MA, Santy LC, Prekeris R, Gould GW. ACRP30 is secreted from 3T3-L1 adipocytes via a Rab11-dependent pathway. Biochem Biophys Res Commun. 2006;342:1361–1367. doi: 10.1016/j.bbrc.2006.02.102. [DOI] [PubMed] [Google Scholar]
- 20.Merl V, Peters A, Oltmanns KM, Kern W, Born J, Fehm HL, Schultes B. Serum adiponectin concentrations during a 72-hour fast in over- and normal-weight humans. Int J Obes (Lond) 2005;29:998–1001. doi: 10.1038/sj.ijo.0802971. [DOI] [PubMed] [Google Scholar]
- 21.Tagami T, Satoh N, Usui T, Yamada K, Shimatsu A, Kuzuya H. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2004;89:1833–1837. doi: 10.1210/jc.2003-031260. [DOI] [PubMed] [Google Scholar]
- 22.Soares AF, Guichardant M, Cozzone D, Bernoud-Hubac N, Bouzaidi-Tiali N, Lagarde M, Geloen A. Effects of oxidative stress on adiponectin secretion and lactate production in 3T3-L1 adipocytes. Free Radic Biol Med. 2005;38:882–889. doi: 10.1016/j.freeradbiomed.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park HS, Jin DK, Shin SM, Jang MK, Longo N, Park JW, Bae DS, Bae YS. Impaired generation of reactive oxygen species in leprechaunism through downregulation of Nox4. Diabetes. 2005;54:3175–3181. doi: 10.2337/diabetes.54.11.3175. [DOI] [PubMed] [Google Scholar]