Abstract
During evolution of land plants, a specific motif occurred in the N-terminal domain of the chloroplast-localized small heat shock protein, Hsp21: a sequence with highly conserved methionines, which is predicted to form an amphipathic α-helix with the methionines situated along one side. The functional role of these conserved methionines is not understood. We have found previously that treatment, which causes methionine sulfoxidation in Hsp21, also leads to structural changes and loss of chaperone-like activity. Here, mutants of Arabidopsis thaliana Hsp21 protein were created by site-directed mutagenesis, whereby conserved methionines were substituted by oxidation-resistant leucines. Mutants lacking the only cysteine in Hsp21 were also created. Protein analyses by nondenaturing electrophoresis, size exclusion chromatography, and circular dichroism proved that sulfoxidation of the four highly conserved methionines (M49, M52, M55, and M59) is responsible for the oxidation-induced conformational changes in the Hsp21 oligomer. In contrast, the chaperone-like activity was not ultimately dependent on the methionines, because it was retained after methionine-to-leucine substitution. The functional role of the conserved methionines in Hsp21 may be to offer a possibility for redox control of chaperone-like activity and oligomeric structure dynamics.
Keywords: Chaperone-like activity, methionine sulfoxidation, redox-response, small heat shock protein
The small heat shock proteins (sHsps) belong to an eukaryotic heat shock protein family in which all members contain a conserved C-terminal domain of ∼100 amino acids and a variable N-terminal domain (Caspers et al. 1995; de Jong et al. 1998). Different roles have been suggested for sHsps, such as regulation of cytoskeleton protein dynamics (Lavoie et al. 1993; Wang and Spector 1996) and regulation of apoptosis (Mehlen et al. 1997). All sHsps are known to show a non-ATP-dependent, chaperone-like activity in vitro, which was first shown for αB-crystallin (Horwitz 1992). The sHsps protect other proteins from aggregation at elevated temperatures, by binding their molten globule forms to their outer surface (Lindner et al. 1997, 1998). When the temperature decreases, the protected proteins are released, probably assisted by ATP-dependent Hsp70 chaperones (Lee and Vierling 2000).
Interestingly, the variable N-terminal domain of the chloroplast-localized sHsp, Hsp21, contains a highly conserved methionine-rich sequence (Waters et al. 1996). Secondary structure predictions indicate that this methionine-rich sequence can form an amphipathic α-helix with all the methionines exposed on one side (Chen and Vierling 1991). The conservation of the methionines in this domain among divergent plant species indicates that it is of functional importance, but the actual function of Hsp21 in the chloroplast, as well as the role of the highly conserved methionines, is not understood.
Like other sHsps, Hsp21 is an oligomeric protein, but the actual number of subunits per oligomer is not known. The apparent molecular mass can be estimated by gel filtration or native PAGE to be approximately 400 kD. We have found previously that the Arabidopsis thaliana Hsp21 oligomer undergoes conformational changes in response to oxidation, coinciding with and probably being caused by sulfoxidation of the methionine residues in the conserved amphipathic helix (Gustavsson et al. 1999). This oxidation-induced conformational change of Hsp21 occurs concomitantly with a loss of the chaperone-like activity and a decrease in α-helical secondary structure (Härndahl et al. 2001).
All amino acids that occur naturally in proteins can be modified oxidatively, but the two most readily oxidized are the sulfur-containing cysteine and methionine (Berlett and Stadtman 1997). Cysteine oxidation, which can lead to a variety of products such as the cysteic acid or formation of disulfide bridges with another cysteine residue, is the best characterized of the two. Because of the normally reducing intracellular environment, disulfide bridges are not as common, and their formation in response to, for example, oxidative stress often leads to loss of protein function, which can be regained by reduction of the disulfide bridge. Oxidation of methionine to the methionine sulfoxide leads to a drastic decrease in hydrophobicity and to a more rigid structure of the side chain. There are numerous reports about how methionine sulfoxidation, like disulfide bridge formation between cysteines, leads to loss of protein function (Gao et al. 1998; Johnson and Travis 1979; Vogt 1995). Similar to the example of disulfide bridge formation, methionine sulfoxidation is a reversible reaction. The enzyme responsible for reduction of methionine sulfoxides, the peptide methionine sulfoxide reductase (MsrA), is found in various organisms ranging from bacteria to plants and mammals (Moskovitz et al. 1995, 1996; Sadanandom et al. 2000). In this paper we have applied a site-directed mutagenesis approach to assess the importance of the conserved methionine residues in Hsp21 and the involvement of methionine sulfoxidation in the oxidation-induced structural changes and chaperone-like activity of the Arabidopsis thaliana Hsp21 oligomer.
Mutant Hsp21 protein with methionines substituted by leucines was therefore prepared. Methionine and leucine are hydrophobic and resemble each other structurally. Also, in substitution mutations during evolution, methionine is most frequently replaced by leucine. However, the leucine side chain contains no sulphur atom and is not readily oxidized. To investigate the possible contribution of cysteine oxidation in oxidation-induced structural changes, the single cysteine residue (C151) in Arabidopsis thaliana was replaced by alanine. The resulting set of mutant proteins with different substitutions was evaluated and showed that methionines substitution clearly abolished the oxidation-induced conformational changes of the Hsp21 oligomer, whereas cysteine substitution gave no effect in this respect. The chaperone-like activity of Hsp21 was largely retained in leucine-containing Hsp21 mutants. The data obtained in this study indicate that the methionine-rich Hsp21, which evolved during land-plant evolution (Waters and Vierling 1999), presumably coevolved with a highly reducing environment in the chloroplast.
Results
In Arabidopsis thaliana Hsp21, the predicted amphipathic α-helix contains four methionines (M49, M52, M55, and M59) that are highly conserved in most land plants (M49, M52, and M59: conserved in 10 out of 10 species examined; M55: nine out of 10 [data not shown]) and two methionines (M62 and M67) that are less well conserved (M62: two out of 10; M67: five out of 10). Another methionine residue is found at position 35 in the N-terminal part of the Arabidopsis thaliana Hsp21 sequence and is conserved in only two of the 10 species examined. There are also two additional highly conserved methionines, M97 and M101 (both 10 out of 10), which are not located in the N-terminal domain but in the structurally ordered C-terminal domain. The two conserved methionines in this C-terminal domain are less prone to methionine sulfoxidation, although all methionines in Hsp21 can be oxidized into methionine sulfoxides upon treatment with hydrogen peroxide as detected by mass spectrometry (Gustavsson et al. 1999). In the structure that was resolved for Methanococcus jannaschii Hsp16 (Kim et al. 1998), another sHsp-like chaperone, the N-terminal region was disordered and therefore not seen in the structure. This indicates that the N-terminal region is flexible. Thus in the case of Hsp21, the amphipathic α-helix in the N-terminal region may act as a flexible arm and respond to redox changes conducted via methionine sulfoxidation of the six methionines, thereby affecting the conformation of the Hsp21 oligomer.
To evaluate how the methionines in this flexible part of Hsp21 are involved in the conformational changes in Hsp21 observed in response to oxidation and how the methionines affect the chaperone-like activity, mutants with either the four most highly conserved or all six methionines in the amphipathic α-helix were substituted for leucines by site-directed mutagenesis. These mutants, Hsp21-M(49,52,55, 59)L and Hsp21-M(49,52,55,59,62,67)L, are referred to as -4M, and -6M. Two other mutants were made with the only cysteine in Hsp21 replaced by alanine: Hsp21-C151A and Hsp21-M(49,52,55,59,62,67)L,C151A, referred to as -C and -6M-C. The mutations were verified at the level of the expressed proteins using MALDI/TOF mass spectrometry on peptides obtained by proteolysis and at the DNA level by sequencing of the coding regions of the plasmids (data not shown). The DNA sequencing verified that no unwanted mutations had occurred during the PCR reactions.
Methionines required for conformational change in response to oxidation
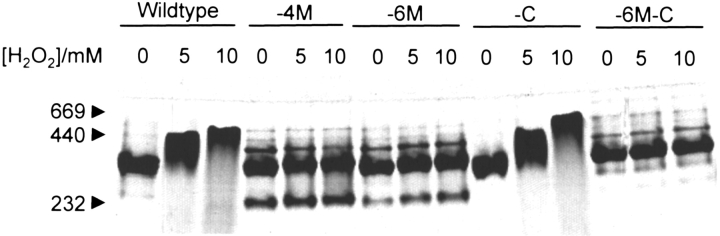
To evaluate if the oxidation-induced conformational change reported previously for Hsp21 (Gustavsson et al. 1999) can occur in the mutants, samples of purified wild-type and mutant Hsp21 protein were oxidized with 5 or 10 mM hydrogen peroxide and subjected to nondenaturing PAGE as shown in Figure 1 ▶. For wild-type Hsp21, the conformational change is seen as a shift of the 400 kD band into an upper band at 450 kD in response to oxidation with either 5 or 10 mM hydrogen peroxide. For the mutants in which four or six methionines are replaced by leucines (-4M, -6M), there is no shift into an upper band. The Hsp21 oligomer forms a major band at 400 kD. Additional weak extra bands can also be seen, which could be misfolded oligomers or degraded oligomers, but the main band still accounts for some 95% of the protein for the -6M mutant. The mutant with the one cysteine removed (-C) behaves like the wild type, showing a shift of the 400 kD band into an upper band at 450 kD in response to oxidation with either 5 or 10 mM hydrogen peroxide. Thus, the shift into the upper band indicating a change in oligomeric conformation of Hsp21 is because of sulfoxidation of the conserved methionines, not cysteine oxidation. The mutant in which the six methionines and the cysteine were replaced by leucines and alanine, respectively, (-6M-C) behaved like the -4M and -6M mutants.
Fig. 1.
Nondenaturing PAGE of wild-type and mutant Hsp21 showing oxidation-dependent structural changes of the Hsp21 oligomer. The mutants with methionines substituted by leucine, Hsp21-M(49,52,55,59)L and Hsp21-M(49,52,55,59,62,67)L, are referred to as -4M, and -6M. The mutants with cysteine replaced by alanine, Hsp21-C151A and Hsp21-M(49, 52,55,59,62,67)L,C151A, are referred to as -C and -6M-C.
To further distinguish between the wild-type and mutant forms of Hsp21, oxidation and subsequent size exclusion chromatography (SEC) analyses were performed in ammonium bicarbonate, a buffer known to create conditions that cause disassembly of the oxidized form of the Hsp21 oligomer into smaller oligomers of approximately 100 kD (Härndahl et al. 2001). Figure 2 ▶ shows size exclusion chromatograms for (Fig. 2A ▶) nonoxidized and (Fig. 2B ▶) oxidized samples of wild-type and the mutant forms of Hsp21. As seen for the wild-type Hsp21 oligomer, a change in apparent molecular weight occurred upon oxidation from 400 kD to smaller forms, predominantly one of 100 kD (indicated with asterisk), with part of the 400 kD peak still remaining. However, the mutants in which methionines were replaced by leucines (-4M, -6M, and -6M-C) all appeared unaffected by the oxidation. In case of the -C mutant, the 400 kD peak was broadened after oxidation but without the appearance of the 100 kD form. Thus, it appears that it is cysteine oxidation in the wild-type Hsp21 that makes the oligomer fall apart, but methionine sulfoxidation in the amphipathic helix is the prerequisite for any conformational change.
Fig. 2.
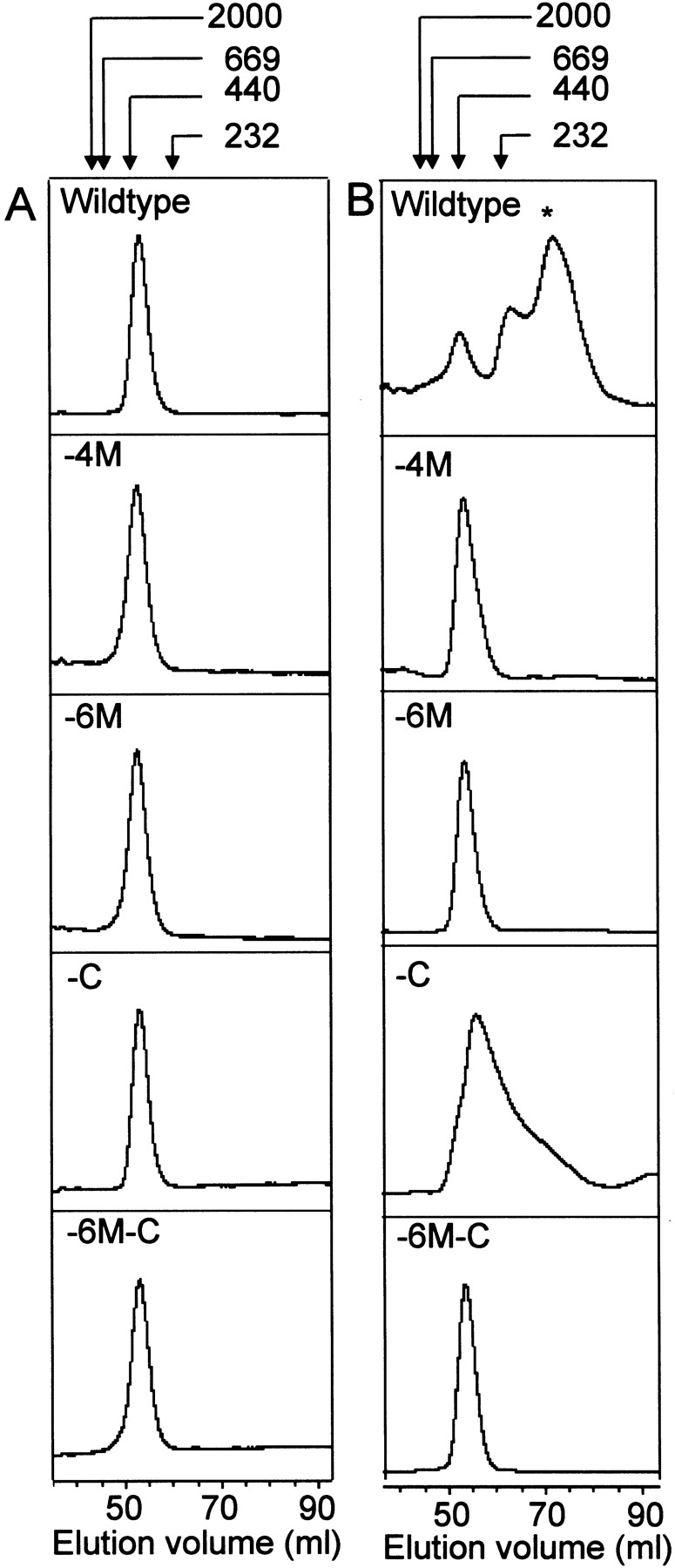
Size-exclusion chromatography of wild-type and mutant Hsp21 showing oxidation-dependent structural changes of the Hsp21 oligomer. Chromatograms of (A) control samples and (B) oxidized (5mM H2O2) samples. The asterisk in the upper right panel indicates the 100 kD form of Hsp21 after oxidation in ammonium bicarbonate buffer. The mutants with methionines substituted by leucine, Hsp21-M(49,52,55,59)L and Hsp21-M(49,52,55,59,62,67)L, are referred to as -4M, and -6M. The mutants with cysteine replaced by alanine, Hsp21-C151A and Hsp21-M(49,52,55,59, 62,67)L,C151A, are referred to as -C and -6M-C.
Importance of cystein-151 for high-temperature-induced aggregation
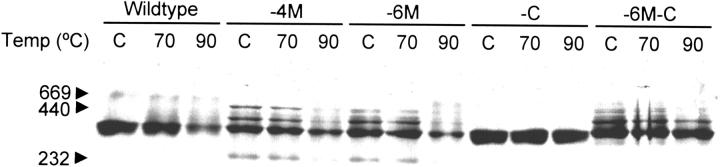
Previously we observed that temperature-induced aggregation of wild-type Hsp21 is prevented by the reductant DTT, indicating that there is an oxidative event contributing to the temperature-induced aggregation events that occur during heat denaturation at very high temperatures (> 70°C) (Härndahl et al. 1999). To study differences in high-temperature-induced aggregation between wild-type and mutant Hsp21, protein samples were incubated at 70°C or 90°C and analyzed on nondenaturing PAGE (Fig. 3 ▶). The 70°C sample of wild-type Hsp21 oligomer resembled the control, whereas in the 90°C sample, the band appeared weaker because of formation of aggregates that do not enter the gel. A similar pattern was observed for the -4M and -6M mutants with somewhat less aggregation. However, both mutants in which the cysteine had been replaced (-C, and -6M-C) appeared unaffected by heat treatment at 90°C. These data show that cysteine-151, one in each monomer, is crucial for the aggregation of the Hsp21 oligomer that occurs at very high temperatures.
Fig. 3.
High-temperature-induced aggregation of wild-type and mutant Hsp21 oligomers. Nondenaturing PAGE after incubation of wild-type and mutant Hsp21 at temperatures indicated in the figure for 1 h. The mutants with methionines substituted by leucine, Hsp21-M(49,52,55,59)L and Hsp21-M(49,52,55,59,62,67)L, are referred to as -4M, and -6M. The mutants with cysteine replaced by alanine, Hsp21-C151A and Hsp21-M(49,52,55,59,62,67)L,C151A, are referred to as -C and -6M-C.
Methionine but not cysteine oxidation causes loss of α-helical CD signal
Previously, methionine sulfoxidation in wild-type Hsp21 was found by CD spectroscopy to lead to a partial unfolding and loss of α-helical secondary structure, which did not occur in the Hsp21 -6M mutant (Härndahl et al. 2001). Hence, the Hsp21 -4M and -C mutants were also screened by CD to record their response to oxidation in terms of the 222 nm signal (indicated by arrows in Fig. 4 ▶) from the methionine-rich amphipathic α-helix. An oxidation-induced decrease in 222 nm signal was seen for wild-type Hsp21 (Fig. 4A ▶) and in the Hsp21 mutant in which the cysteine was replaced (Fig. 4C ▶). However, such a decrease in the 222 nm CD signal was totally lacking in the Hsp21 mutant without the four most conserved (-4M) methionines in the amphipathic α-helix (Fig. 4B ▶). Thus, methionine sulfoxidation of the four most conserved methionine residues (M49, M52, M55, and M59) is responsible for the conformational change which causes loss of α-helical secondary structure with no contribution of cysteine oxidation. Oxidation of only the two semiconserved methionines, M62 and M67, in the -4M mutant is obviously not sufficient to cause loss in the α-helical secondary structure.
Fig. 4.
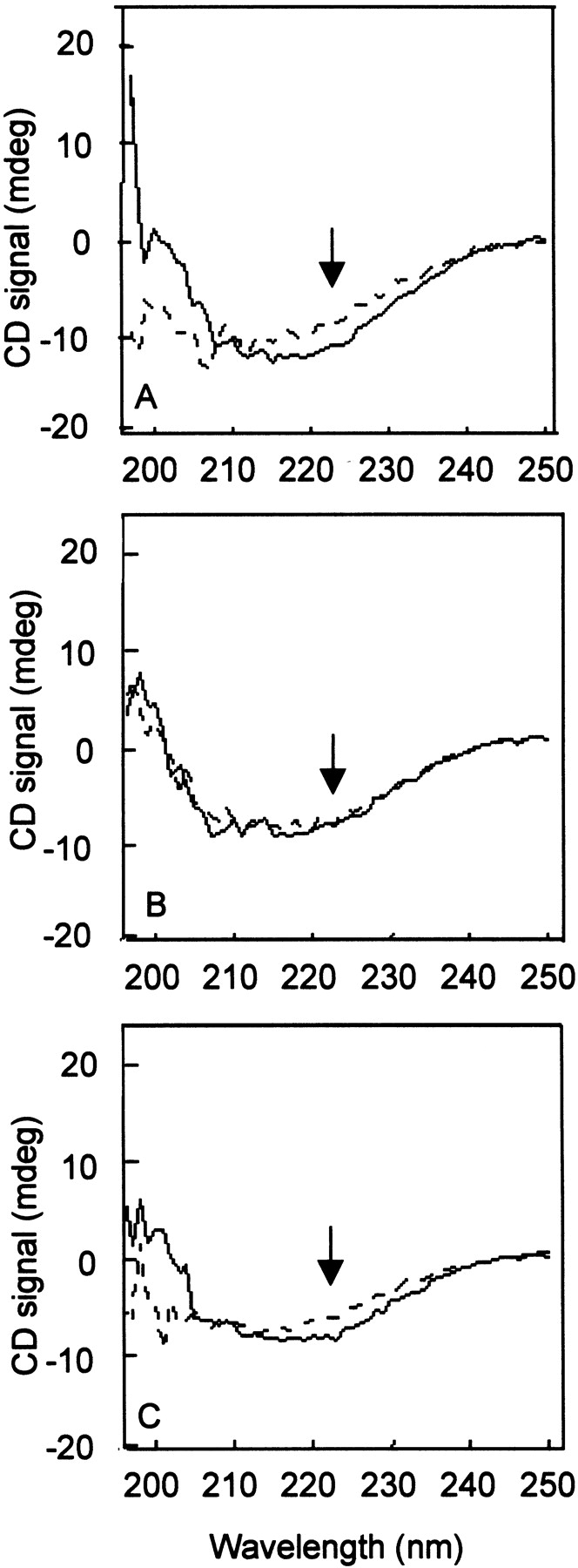
CD spectra showing oxidation-induced loss of α-helical signal. Solid line: control protein samples. Dashed line: protein samples oxidized with 5 mM hydrogen peroxide. (A) Wild-type Hsp21. (B) Hsp21 mutant with four methionines substituted by leucine, Hsp21-M(49,52,55,59)L, is referred to as -4M; C Hsp21 mutant with cysteine replaced by alanine, Hsp21-C151A, is referred to as -C. The arrows point out the CD signal at 222 nm, indicative of α-helical secondary structure. The change in CD upon oxidation is significant, giving upon oxidation of the Hsp21 wild type, on average, a 20.2% decrease at 222 nm in four independent experiments with a standard deviation of 2.2%. For the mutant Hsp21 proteins with either four or six methionines replaced by leucines, there is no change at 222 nm upon oxidation.
Effects of mutations on the chaperone-like activity of Hsp21 and its sensitivity to oxidation
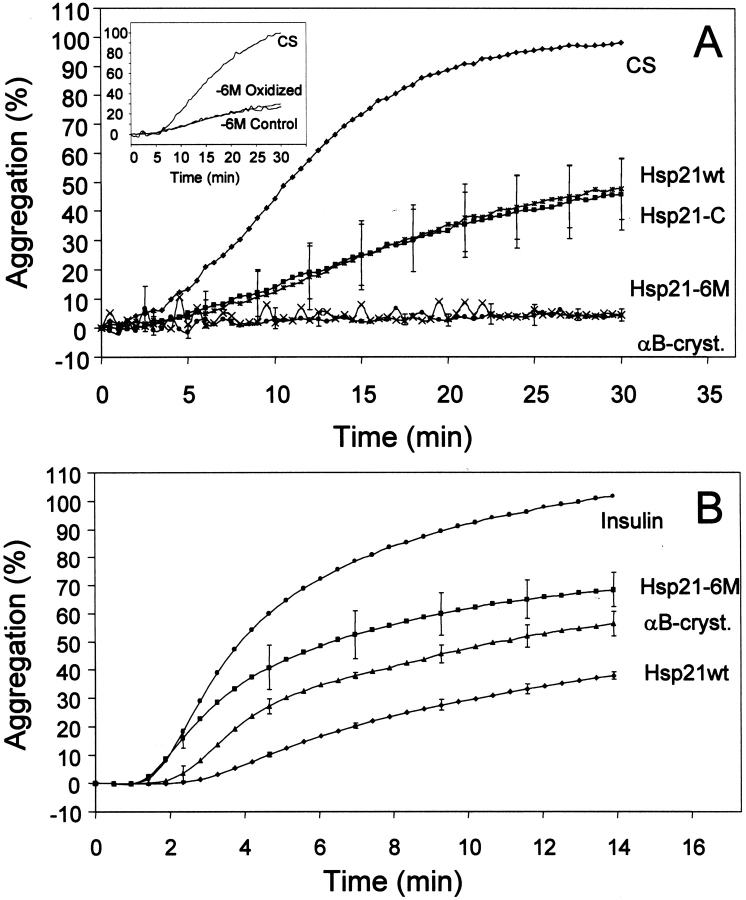
To investigate if replacement of methionines by leucines affected the chaperone-like activity of Hsp21, aggregation assays were performed with two different substrate proteins, citrate synthase and insulin, both commonly used in light-scattering based assays of chaperone-like activity (Bova et al. 1999). Denaturation is induced by high temperature (43°C) in case of the temperature-sensitive citrate synthase (Buchner et al. 1998; Ehrnsperger et al. 1997) and by the reductant DTT in case of insulin, which becomes prone to aggregation upon reduction causing breakage of a critical disulfide bridge (Farahbakhsh et al. 1995). As shown in Figure 5A ▶, the -6M mutant showed even better chaperone-like activity than wild-type Hsp21. Furthermore, as shown in the insert, the chaperone-like activity of -6M was unaffected by oxidation. In the insulin assay (Fig. 5B ▶), the -6M mutant has less chaperone-like activity than wild-type Hsp21, although it still shows good chaperone-like activity. In both assays, the mutant with four methionines replaced by leucine (-4M) behaved in a way similar to -6M (data not shown). The mutant from which the six methionines and the cysteine had been removed (-6M-C) showed very little chaperone-like activity (data not shown) for reasons we cannot explain at this point. It is possible that this mutant has a less stable tertiary structure (see below).
Fig. 5.
Light-scattering measurements of the chaperone-like activity of wild-type and mutant Hsp21 with citrate synthase (A) or insulin (B) as substrates. Aggregation of citrate synthase and insulin with no chaperone added is indicated in the figure as a negative control, and the suppression of aggregation by the chaperone-like activity of αB-crystalline (Horwitz 1992; Lindner et al. 1997) is indicated in the figure as a positive control. sHsps do not show any increase in light scattering without citrate synthase and insulin (data not shown). Standard deviation bars are indicated for incubations with wild-type and mutant Hsp21. Hsp21 mutant with six methionines substituted by leucine, Hsp21-M(49,52,55,59,62,67)L, is referred to as -6M; Hsp21 mutant with cysteine replaced by alanine, Hsp21-C151A, is referred to as -C. The insert in panel A shows the chaperone-like activity of control or oxidized -6M mutant Hsp21.
Conformational stability of wild-type and mutant Hsp21
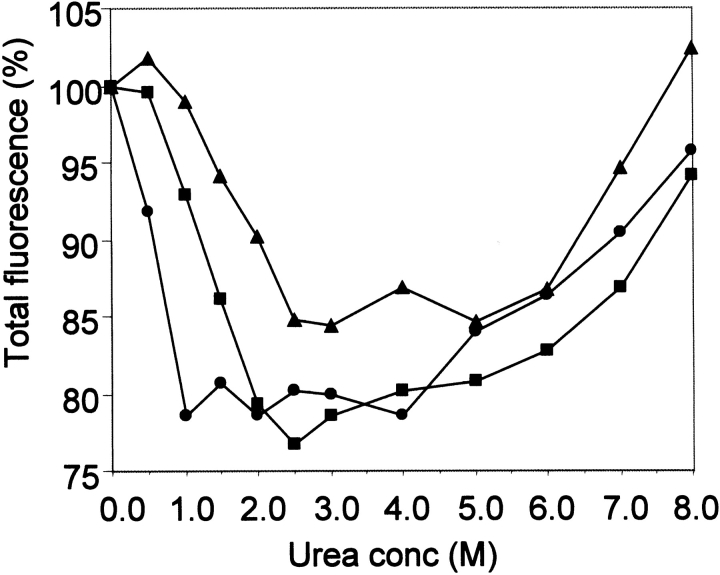
Site-directed mutagenesis may affect the conformational stability of a protein, which can be evaluated by determining the tryptophan fluorescence changes in increasing concentrations of a denaturant like urea. By comparing the behavior of Hsp21 in urea gradient electrophoresis (Fig. 6A ▶) with the total tryptophan fluorescence (calculated by integration of fluorescence between 315 and 420 nm) at increasing urea concentrations (Fig. 6B ▶), we could analyze the conformational stability of the Hsp21 oligomer. In a first phase at very low urea concentrations (Fig. 6A ▶ [I]), Hsp21 is still intact as an oligomer. When it reaches a certain urea concentration, the oligomer starts to disassemble, which is accompanied by a rapid decrease in fluorescence intensity (Fig. 6B ▶). Early during this second phase (II) at intermediate urea concentrations, fluorescence becomes red shifted (peak maximum shifted from 340 to 358 nm) as unfolding of the Hsp21 monomer starts (data not shown). At higher urea concentrations (III), the red-shifted fluorescence increases continuously during the gradual unfolding.
Fig. 6.
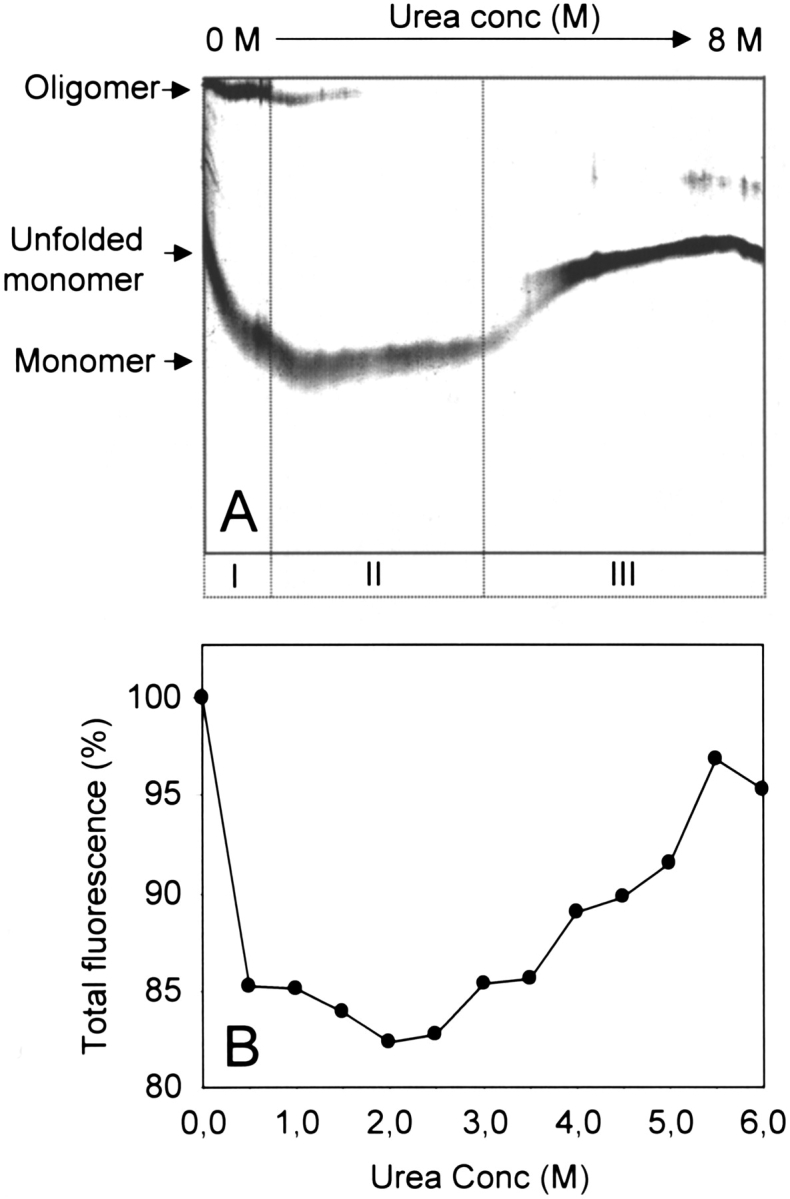
Conformational stability of Hsp21 oligomer studied by urea gradient electrophoresis and tryptophan fluorescence. (A) Urea gradient polyacrylamide gel showing the intact wild-type pea (Pisum sativum) Hsp21 oligomer (I) and how the oligomer disassembles into monomers at higher urea concentrations (II). At even higher urea concentrations (III), unfolding of the Hsp21 monomers leads to a decrease in electrophoretic mobility. (B) The total fluorescence (calculated by integration of fluorescence between 315 and 420 nm) of wild-type pea (P. sativum) Hsp21 is presented for increasing urea concentrations.
To assess the conformational stability of the mutant forms of Hsp21, tryptophan fluorescence spectra were recorded in different urea concentration for all the mutant Hsp21 proteins. In Figure 7 ▶, total fluorescence is presented, showing that conformational stability is higher in the Hsp21 mutants with four or all six methionines replaced by leucine (squares and triangles, respectively) compared to wild-type Hsp21 (circles). In the mutant Hsp21 proteins, the initial fluorescence level characteristic of oligomeric Hsp21 was completely retained in 0.5 M urea, which was the approximate midpoint of the fluorescence decrease for wild-type Hsp21 protein. This indicates a more stable oligomeric conformation for the methionine-less mutant forms compared to wild-type Hsp21. Furthermore, in both the mutants, the red shift was delayed until higher urea concentrations: The red shift of the fluorescence peak maximum from 340 to 358 nm did not occur until 2.5 M urea, whereas in wild-type Hsp21, the red shift started at 1 M urea (data not shown). Fluorescence from the mutant from which cystein had been removed (-C) was similar to wild-type Hsp21, whereas the -6M-C mutant deviated from all the other Hsp21 proteins, showing a higher fluorescence intensity in the absence of urea, which indicated an altered tryptophan environment, and a fluorescence increase in 0.5 M urea (data not shown). The additive effect of methionine and cystein replacement thus results in an altered tertiary structure of the -6M-C mutant protein, which could also explain its poor chaperone-like activity, which was discussed above.
Fig. 7.
Tryptophan fluorescence of wild-type and mutant Hsp21 in increasing urea concentration. The total fluorescence (calculated by integration of fluorescence between 315 and 420 nm) is presented for increasing urea concentrations. Data were normalized by setting the fluorescence value in 0 M urea for each protein to 100%. Filled circles: wild-type Hsp21. Filled squares: Hsp21 mutant with four methionines substituted by leucine, Hsp21-M(49,52,55,59)L, is referred to as -4M. Filled triangles: Hsp21 mutant with six methionines substituted by leucine, Hsp21-M(49,52,55,59,62,67)L, is referred to as -6M.
Discussion
Hsp21 mutants with the four most conserved of the six methionines in the amphipathic α-helix replaced by leucines lacked response to oxidation in terms of conformational change in the Hsp21 oligomer as judged by nondenaturing PAGE and size exclusion chromatography (Figs. 1, 2 ▶ ▶). These results are consistent with the finding that loss of α-helical secondary structure upon oxidation (Fig. 4 ▶), did not occur unless the four most conserved methionine residues (M49, M52, M55, and M59) were present. Thus, the redox-response of the Hsp21 oligomer, reported here as a conformational change in the Hsp21 oligomer and loss of α-helical secondary structure, which previously was shown to correlate well with methionine sulfoxidation (Gustavsson et al. 1999), indeed depend on sulfoxidation of the four most conserved methionine residues with no involvement of cysteine oxidation.
Chaperone-like activity was unaffected by oxidation when the six methionines were replaced by leucines in the -6M Hsp21 mutant (Fig. 5A ▶, insert). Remarkably, in the citrate synthase assay, the -6M Hsp21 mutant also showed even better chaperone-like activity than wild-type Hsp21 (Fig. 5A ▶). In the insulin assay, however, its relative activity compared to the wild-type Hsp21 was not as good (Fig. 5B ▶). In both assays the chaperone-like activity of the -6M Hsp21 mutant was of the same order of magnitude as the well-established chaperone-like activity of αB-crystallin (Horwitz 1992; Lindner et al. 1997, 1998; van Boekel et al. 1999), but in the insulin assay, the chaperone-like activity of wild-type Hsp21 was even better than that of αB-crystallin. The presence of DTT in the insulin assay may resemble the reducing conditions which prevail in the chloroplast, to which Hsp21 probably has adapted to function optimally. In several reports on proteins, which contain functionally important methionines, substitution leads to loss in efficiency (Yuan and Vogel 1999). Here, an apparent improvement of the chaperone-like activity was obtained for the -6M Hsp21 mutant in the DTT-less CS assay in which there is probably a "background" methionine sulfoxidation in the wild-type Hsp21. In none of the assays did replacement of methionines by leucines in Hsp21 cause a major decrease in chaperone-like activity.
The mutant-lacking C151 behaved similarly to wild-type Hsp21 except at unphysiological denaturating temperatures (70°C and 90°C) in which it was less aggregation-prone, indicating that such temperature-induced aggregation involves cysteine oxidation. The C151 may reside in the interphase between subunits in the Hsp21 oligomer as judged by sequence alignment with the Methanococcus jannaschii Hsp16 structure (Kim et al. 1998). This could explain why wild-type Hsp21 but not C151A yielded 100 kD tetramers after methionine sulfoxidation (Fig. 2 ▶). But under physiological conditions, oxidation of cystein 151 is probably less important. The cysteine residue C151 in Arabidopsis thaliana Hsp21 is nonconserved, and other plant species contain either several cystein residues (e.g., pea [Pisum sativum]) or no cysteines at all. In Hsp25 disulfide bond formation did not affect secondary structure, degree of oligomerization, or chaperone activity (Zavialov et al. 1998).
Even if the leucine-substituted form of Hsp21 shows functional chaperone-activity in vitro, the methionines may be important for other in vivo functions of Hsp21. When the methionine side chain undergoes oxidation, which the leucine side chain is unable to, its hydrophobicity is markedly decreased, together with a decrease in structural flexibility. If the methionine-rich region of Hsp21 is involved in the chaperone-like activity, oxidation of the methionines would most likely affect the binding of the substrate proteins. The methionine sulfoxide-containing conformationally changed form of Hsp21, which has lost chaperone-like activity (Härndahl et al. 2001), may fulfill another, yet unknown role in the cell. It may, for example, bind to components other than the reduced form of Hsp21, for which chaperone-like activity might be the main functional role. Such redox-dependent regulation of Hsp21 would be an alternative pathway to regulate its function, parallel to, for example, phosphorylation of other sHsps. Phosphorylated forms of the mammalian Hsp27 show no chaperone-like activity (Rogalla et al. 1999), but bind cytoskeletal elements (Zhu et al. 1994).
Replacement of methionines by leucines did not decrease, but rather increased the conformational stability of Hsp21 oligomer stability (Fig. 7 ▶). The midpoint of the urea-induced oligomer disassembly (fluorescence decrease) was clearly affected in the mutants: 0.5 M urea for wild-type Hsp21, but approximately 1.5 M urea for both Hsp21 mutants (-4M and -6M). Also, the fluorescence red shift typical for unfolding occurred at much lower urea concentration in the wild-type Hsp21 (1 M urea) compared to the mutants (2.5 M urea). These results indicate that substitution of leucines by methionines during evolution decreased conformational stability. The oxidizable methionines in Hsp21 were maybe allowed during land plant evolution (Waters and Vierling 1999) only since the chloroplast represented a safe reducing environment in which methionines in Hsp21 can be kept in a reduced state. Therefore, one should expect land plant evolution to coincide with the evolution of factors that contribute to the reduced environment: NADPH-producing Photosystem II, and redox-controlling systems like thioredoxin, glutaredoxin, and ascorbate peroxidase.
Thus, methionines may also have evolved in Hsp21 to allow regulation of different functions of Hsp21. In a reducing environment, the chaperone function might be the main task for the large oligomeric form of Hsp21, whereas during oxidative stress, the smaller 100 kD forms caused by oxidation-induced disassembly of Hsp21 may have another function. Of the four methionines conserved among angiosperms (M49, M52, M55, and M59), only one is present in the moss Funario hygrometrica (Waters and Vierling 1999), although secondary predictions indicate an α-helical secondary structure. Thus, the possibility for redox regulation of chaperone-like activity as in Hsp21 may be missing in evolutionary "early" organisms. In this context it should be mentioned that another type of regulation, which is fairly well characterized — phosphorylation of the D1-protein in Photosystem II — is a late evolutionary event that occurs in seed plants but not in mosses, liverworts, and ferns (Pursiheimo et al. 1998). Transgenic Arabidopsis plants expressing the leucine-substituted form of Hsp21 could shed further light on whether the leucine-substituted form of Hsp21 is fully functional or if regulation of Hsp21 by methionine sulfoxidation is needed and is of physiological importance.
Materials and methods
Homology comparison of Hsp21 sequences from different land plant species
Multiple alignment of Hsp21 sequences from 10 different land plant species (Arabidopsis thaliana, soybean, petunia, tobacco, tomato, pea, maize, rice, wheat, and barley) was made using the ClustalW software available online at http://www.expasy.ch/cgi-bin/blast.pl.
Site-directed mutagenesis
Mutagenesis of the expression vector pAZ376, encoding the mature form of Arabidopsis thaliana Hsp21, was performed using the QuickChange Site-Directed Mutagenesis Kit (Stratagene). For the mutagenesis of methionines 49, 52, and 55 to leucines, the primer 5′-GGAAACAGTGGTGACTCCTGCGACGCTGTTGACAATC TATGATACC-3′ and its corresponding antisense primer were used, leading to plasmid pAZ376-M(49,52,55)L. Annealing temperature was 50°C. Using pAZ376-M(49,52,55)L as template and primer 5′-CGCTGTTGACAATCTATGGGACCTGTCCTACAA GC-3′ and its corresponding antisense primer, a mutant with methionines 49, 52, 55, and 59 mutated to leucines was made, leading to plasmid pAZ376-M(49,52,55,59)L. Annealing temperature was 55°C. Using pAZ376-M(49,52,55,59)L as template and primer 5′-GGGACCTGTCCGACAAGCTCCTGTGAGACGGA CAGAGTCC-3′ and its corresponding antisense primer, a mutant with methionines 49, 52, 55, 59, 62, and 67 mutated to leucines was made, leading to plasmid pAZ376-M(49,52,55,59,62,67)L. Annealing temperature was 50°C. For the mutagenesis of cysteine 151 to alanine in pAZ376 and pAZ376-M(49,52,55,59,62,67)L, primer 5′-CGAGGGTCTGTTGCGACTCTTTCTGTTCTAG-3′ and its corresponding antisense primer were used, leading to plasmids pAZ376-C151A and pAZ376-M(49,52,55,59,62,67)L, C151A. Annealing temperature was 52°C. Underlined nucleotides show the mutated codons.
Expression and purification of mutants
The plasmids pAZ376-M(49,52,55,59)L, pAZ376-M(49,52,55,59, 62,67)L, pAZ376-M(49,52,55,59,62,67)L,C151A, and pAZ376-C151A were transformed into competent Escherichia coli BL21 (DE3) cells and grown at 37°C until midlog phase in Luria-Bertani (LB) broth containing 0.2 mg/mL of ampicillin. Isopropyl β-D-thiogalactopyranoside (IPTG) was then added to a final concentration of 0.5 mM, and incubation was continued for 4 h. Pure mutant Hsp21 was prepared essentially as described earlier (Härndahl et al. 1998) using only size exclusion chromatography in the final purification step.
Size exclusion chromatography
Size exclusion chromatography was performed using a Pharmacia HiLoad/Superdex 200 HR 16/60 column at 4°C. Equilibration and elution was performed with 0.1 M ammonium bicarbonate at pH 7.8 or 25 mM Tris-HCl at pH 7.0 at a flow rate of 0.75 mL/min.
Oxidation or heat treatment of purified Hsp21
Purified recombinant wild-type and mutant Hsp21 were oxidized at a concentration of 0.4 mg protein/mL for 2 h at 37°C with 5.0 mM H2O2 in the different buffer/salt combinations indicated in the figure legends. Oxidation was stopped by the addition of 20 mM DTT. Heat treatment was performed by incubation at 70°C or 90°C for 1 h.
Electrophoresis
Nondenaturing PAGE (3 %–27% gradient of acrylamide) was run at 100 V for 0.5 h and 150 V overnight at 15°C. Nondenaturing PAGE samples were precipitated with acetone (200 μL sample to 1 mL freeze-cold acetone) and resuspended in sample buffer (0.25 M Tris/HCl, 40% [v/v] glycerol) at pH 7.8. Then, 20 μg protein/lane was loaded on the gel. Proteins were detected by Coomassie Brilliant Blue staining. Urea gradient electrophoresis was performed as described previously (Härndahl et al. 1998). Briefly, a horizontal urea concentration gradient (0–8M) was casted perpendicular to the direction of migration, superimposed on a horizontal 15%–11% polyacrylamide gradient to compensate for the urea effect on the electrophoretic behavior of proteins that does not involve unfolding. On the top surface of the gel, 200 μg of pea (P. sativum) Hsp21 was loaded, and electrophoresis was run in a Tris–borate buffer system (pH 8.6 without SDS at 20°C) for 20 min at 10 mA and then for 3 h at 20 mA.
CD and fluorescence spectroscopy
Oxidation-induced changes in the CD spectra of wild-type and mutant Hsp21 protein were recorded as described previously (Härndahl et al. 2001). Conformational stability of wild-type and mutant Hsp21 protein was determined by recording tryptophan fluorescence spectra in increasing urea concentration, using a FluoroMax-2 fluorimeter (Instruments SA) with an excitation wavelength of 295 nm, an excitation slit width of 3 nm, and an emission slit width of 3 nm. Spectra were collected in a 10-mm pathlength quartz cell (Hellma). The sample buffer consisted of 10 mM potassium phosphate at pH 7.0, and protein concentration was 0.05 mg/mL.
Light-scattering assays for chaperone-like activity assays
Thermal aggregation of citrate synthase was induced by incubation at 43°C, and aggregation of insulin was induced by the addition of DTT as previously described (Härndahl et al. 2001). The ability of Hsp21 and the Hsp21 mutants to suppress aggregation was recorded by light scattering measurements.
Acknowledgments
This work was supported by grants from the Swedish Natural Sciences Research Council, the Crafoord Foundation, and the Carl Tryggers Foundation. B.P.A.K was supported by the Netherlands Organisation for Scientific Research (NWO). We acknowledge Ellen Tufvesson and Ulrika Härndahl for initiating analyses of Hsp21 by urea gradient electrophoresis and tryptophan fluorescence.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
CD, circular dichroism
DTT, dithiothreitol
H2O2, hydrogen peroxide
MALDI/TOF, matrix assisted laser desorption ionization/time of flight
PAGE, polyacrylamide gel electrophoresis
SDS, sodium lauryl sulfate
sHsp, small heat shock protein
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.11301.
References
- Berlett, B.S. and Stadtman, E.R. 1997. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 272 20313–20316. [DOI] [PubMed] [Google Scholar]
- Bova, M.P., Yaron, O., Huang, Q., Ding, L., Haley, D.A., Stewart, P.L., and Horwitz, J. 1999. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc. Natl. Acad. Sci. 96 6137–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner, J., Grallert, H., and Jakob, U. 1998. Analysis of chaperone function using citrate synthase as nonnative substrate protein. Methods Enzymol. 290 323–338. [DOI] [PubMed] [Google Scholar]
- Caspers, G.J., Leunissen, J.A.M., and De Jong, W.W. 1995. The expanding small heat-shock protein family, and structure predictions of the conserved "alpha-crystallin domain". J. Mol. Evol. 40 238–248. [DOI] [PubMed] [Google Scholar]
- Chen, Q. and Vierling, E. 1991. Analysis of conserved domains identifies a unique structural feature of a chloroplast heat shock protein. Mol. Gen. Genet. 226 425–431. [DOI] [PubMed] [Google Scholar]
- de Jong, W.W., Caspers, G.J., and Leunissen, J.A. 1998. Genealogy of the alpha-crystallin–small heat-shock protein superfamily. Int. J. Biol. Macromol. 22 151–162. [DOI] [PubMed] [Google Scholar]
- Ehrnsperger, M., Graeber, S., Gaestel, M., and Buchner, J. 1997. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 16 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahbakhsh, Z.T., Huang, Q.L., Ding, L.L., Altenbach, C., Steinhoff, H.J., Horwitz, J., and Hubbell, W.L. 1995. Interaction of alpha-crystallin with spin-labeled peptides. Biochemistry 34 509–516. [DOI] [PubMed] [Google Scholar]
- Gao, J., Yin, D., Yao, Y., Williams, T.D., and Squier, T.C. 1998. Progressive decline in the ability of calmodulin isolated from aged brain to activate the plasma membrane Ca-ATPase. Biochemistry 37 9536–9548. [DOI] [PubMed] [Google Scholar]
- Gustavsson, N., Härndahl, U., Emanuelsson, A., Roepstorff, P., and Sundby, C. 1999. Methionine sulfoxidation of the chloroplast small heat shock protein and conformational changes in the oligomer. Protein Sci. 8 2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, J. 1992. Alpha crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. 89 10449–10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härndahl, U., Tufvesson, E., and Sundby, C. 1998. The chloroplast small heat shock protein-purification and characterization of pea recombinant protein. Protein Expr. Purif. 14 87–96. [DOI] [PubMed] [Google Scholar]
- Härndahl, U., Buffoni-Hall, R., Osteryoung, K.W., Vierling, E., Bornman, J.F., and Sundby, C. 1999. The chloroplast heat shock protein undergoes oxidation dependent conformational changes and may protect plants against oxidative stress. Cell Stress Chaperon. 4 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härndahl, U., Kokke, B.P.A., Gustavsson, N., Linse, S., Berggren, K., Tjerneld, F., Boelens, W.C., and Sundby, C. 2001. The chaperone-like activity of a small heat shock protein is lost after sulfoxidation of conserved methionines in a surface-exposed amphipathic a-helix. Biochim. Biophys. Acta 1545 227–237. [DOI] [PubMed] [Google Scholar]
- Johnson, D. and Travis, J. 1979. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J. Biol. Chem. 254 4022–4026. [PubMed] [Google Scholar]
- Kim, K.K., Kim, R., and Kim, S.H. 1998. Crystal structure of a small heat-shock protein. Nature 394 595–599. [DOI] [PubMed] [Google Scholar]
- Lavoie, J.N., Gingras Breton, G., Tanguay, R.M., and Landry, J. 1993. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J. Biol. Chem. 268 3420–3429. [PubMed] [Google Scholar]
- Lee, G.J. and Vierling, E. 2000. A small heat shock protein cooperates with heat shock protein 70 systems to reactivate a heat-denatured protein. Plant Physiol. 122 189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner, R.A., Kapur, A., and Carver, J.A. 1997. The interaction of the molecular chaperone, alpha-crystallin, with molten globule states of bovine alpha-lactalbumin. J. Biol. Chem. 272 27722–27729. [DOI] [PubMed] [Google Scholar]
- Lindner, R.A., Kapur, A., Mariani, M., Titmuss, S.J., and Carver, J.A. 1998. Structural alterations of alpha-crystallin during its chaperone action. Eur. J. Biochem. 258 170–183. [DOI] [PubMed] [Google Scholar]
- Mehlen, P., Mehlen, A., Godet, J., and Arrigo Andre, P. 1997. hsp27 as a switch between differentiation and apoptosis in murine embryonic stem cells. J. Biol. Chem. 272 31657–31665. [DOI] [PubMed] [Google Scholar]
- Moskovitz, J., Rahman, M.A., Strassman, J., Yancey, S.O., Kushner, S.R., Brot, N., and Weissbach, H. 1995. Escherichia coli peptide methionine sulfoxide reductase gene: Regulation of expression and role in protecting against oxidative damage. J. Bacteriol. 177 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskovitz, J., Weissbach, H., and Brot, N. 1996. Cloning and expression of a mammalian gene involved in the reduction of methionine sulfoxide residues in proteins. Proc. Natl. Acad. Sci. 93 2095–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursiheimo, S., Rintamaki, E., Baena-Gonzalez, E., and Aro, E.M. 1998. Thylakoid protein phosphorylation in evolutionally divergent species with oxygenic photosynthesis. FEBS Lett. 423 178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalla, T., Ehrnsperger, M., Preville, X., Kotlyarov, A., Lutsch, G., Ducasse, C., Paul, C., Wieske, M., Arrigo, A.P., Buchner, J., et al. 1999. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 274 18947–18956. [DOI] [PubMed] [Google Scholar]
- Sadanandom, A., Poghosyan, Z., Fairbairn, D.J., and Murphy, D.J. 2000. Differential regulation of plastidial and cytosolic isoforms of peptide methionine sulfoxide reductase in Arabidopsis. Plant Physiol. 123 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Boekel, M.A., de Lange, F., de Grip, W.J., and de Jong, W.W. 1999. Eye lens alphaA- and alphaB-crystallin: Complex stability versus chaperone-like activity. Biochim. Biophys. Acta 1434 114–123. [DOI] [PubMed] [Google Scholar]
- Vogt, W. 1995. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic. Biol. Med. 18 93–105. [DOI] [PubMed] [Google Scholar]
- Wang, K. and Spector, A. 1996. Alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur. J. Biochem. 242 56–66. [DOI] [PubMed] [Google Scholar]
- Waters, E.R. and Vierling, E. 1999. Chloroplast small heat shock proteins: Evidence for atypical evolution of an organelle-localized protein. Proc. Natl. Acad. Sci. 96 14394–14399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters, E.R., Lee, G.J., and Vierling, E. 1996. Evolution, structure and function of the small heat shock proteins in plants. J. Exp. Bot. 47 325–338. [Google Scholar]
- Yuan, T. and Vogel H.J. 1999. Substitution of the methionine residues of calmodulin with the unnatural amino acid analogs ethionine and norleucine: Biochemical and spectroscopic studies. Protein Sci. 8 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavialov, A., Benndorf, R., Ehrnsperger, M., Zav́yalov, V., Dudich, I., Buchner, J., and Gaestel, M. 1998. The effect of the intersubunit disulfide bond on the structural and functional properties of the small heat shock protein Hsp25. Int. J. Biol. Macromol. 22 163–173. [DOI] [PubMed] [Google Scholar]
- Zhu, Y., O'Neill, S., Saklatvala, J., Tassi, L., and Mendelsohn, M.E. 1994. Phosphorylated HSP27 associates with the activation-dependent cytoskeleton in human platelets. Blood 84 3715–3723. [PubMed] [Google Scholar]