Abstract
Angiogenin (Ang), an inducer of neovascularization, is secreted by several types of human tumor cells and appears critical for their growth. The murine anti-Ang monoclonal antibody (mAb) 26–2F neutralizes the activities of Ang and dramatically prevents the establishment and metastatic dissemination of human tumor cell xenografts in athymic mice. However, for use clinically, the well-documented problem of the human anti-globulin antibody response known to occur with murine antibodies requires resolution. As a result, chimeric as well as totally humanized antibodies are currently being evaluated as therapeutic agents for the treatment of several pathological conditions, including malignancy. Therefore, we have constructed a chimeric mouse/human antibody based on the structure of mAb 26–2F. Complementary DNAs from the light and heavy chain variable regions of mAb 26–2F were cloned, sequenced, and genetically engineered by PCR for subcloning into expression vectors that contain human constant region sequences. Transfection of these vectors into nonproducing mouse myeloma cells resulted in the secretion of fully assembled tetrameric molecules. The chimeric antibody (cAb 26–2F) binds to Ang and inhibits its ribonucleolytic and angiogenic activities as potently as mAb 26–2F. Furthermore, the capacities of cAb 26–2F and its murine counterpart to suppress the formation of human breast cancer tumors in athymic mice are indistinguishable. Thus cAb 26–2F, with its retained neutralization capability and likely decreased immunogenicity, may be of use clinically for the treatment of human cancer and related disorders where pathological angiogenesis is a component.
Angiogenesis, a multifaceted process by which new blood vessels form, occurs in many physiological and pathological situations, including cancer. Indeed, the critical contribution of angiogenesis to the growth, invasiveness, and metastatic dissemination of tumor cells is now well documented (reviewed in refs. 1 and 2). Mediators that affect angiogenesis are thus appropriate molecular targets against which to direct anticancer therapeutic strategies. One of these, angiogenin (Ang), a unique member of the ribonuclease superfamily of proteins, is a potent inducer of neovascularization and is serving as the focus of ongoing investigations into its structure/function relationships and clinical applications (reviewed in ref. 3).
Because Ang was originally isolated from medium conditioned by a human tumor cell line (4) and subsequently shown to be expressed by several histologically distinct types of human tumors (5), inhibitors of its functions have been developed to evaluate their antitumor effects. One of these, the murine monoclonal antibody (mAb) 26–2F, neutralizes the ribonucleolytic, angiogenic, and mitogenic activities of human Ang (6, 7). It is an IgG1κ with a binding affinity of 1.6 nM that recognizes a discontinuous epitope in Ang involving Trp-89 and residues in the segment 38–41, located in two adjacent loops of the Ang 3-dimensional structure (6, 8). Although not directly cytotoxic to tumor cells in vitro, mAb 26–2F is extremely effective in interfering with their establishment and metastatic spread in athymic mice (9–11). Thus, Ang antagonists should be of major clinical utility for the treatment of cancer.
The use of murine antibodies in patients is problematic, owing to their decreased serum half-lives and induction of human anti-mouse antibody immune responses, directed mainly against mouse Ig constant (C) regions (12–15). The latter is of particular concern in the case of antiangiogenesis therapies, where chronic administration of therapeutic agents may be required. To minimize this problem, chimeric antibodies have been genetically engineered in which murine heavy (H) and light (L) chain variable (V) domains are combined with human C regions, thereby replacing ≈70% of the murine antibody molecule with human sequences (16, 17). Several of these chimerized antibodies are under evaluation in patients for a variety of diseases (18–20). Therefore, as a first step toward producing an anti-Ang antibody amenable to clinical testing, a mouse/human chimeric analogue of mAb 26–2F has been constructed. Here we describe the cloning and sequencing of the VL and VH domains of mAb 26–2F and their expression together with human C regions as a fully assembled chimeric mAb (cAb 26–2F). cAb 26–2F is very similar if not identical to its murine counterpart in binding affinity, Ang neutralization capacity, and, importantly, in its antitumor activity against human breast cancer xenografts in athymic mice.
MATERIALS AND METHODS
Mice.
Female athymic mice were obtained at 5 weeks of age from the isolator bred colony of Charles River Breeding Laboratories and maintained under specific pathogen-free conditions in a temperature- and humidity-controlled environment. Experiments were begun 1 week later.
Monoclonal Igs.
The mAb 26–2F (6) was purified from ascites fluid by affinity chromatography using GammaBind Plus Sepharose (Pharmacia). Ascites fluid (80 ml) was diluted 1:1 with PBS, centrifuged, and the supernatant filtered through a glass fiber filter and 0.2 μm cellulose nitrate filter. After a further dilution with PBS to 400 ml, the antibodies were adsorbed onto the gel, washed with PBS, and eluted with 0.1 M glycine⋅HCl into tubes containing an appropriate amount of 1 M Tris⋅HCl for neutralization. Following dialysis against 0.9% NaCl, the antibodies were quantified by enzyme-linked immunoadsorbent assay (ELISA), and stored at −70°C. The chimerized analogue of mAb 26–2F (cAb 26–2F, see below) obtained from each of the transfectoma cell types was purified from ascites fluid as described above. MOPC 31C, a nonspecific IgG1κ-secreting mouse hybridoma (CCL 130, American Type Culture Collection) was propagated, and IgG purified from ascites as described (9).
Cell Lines.
The murine nonproducing myeloma cell lines P3X63-Ag8.653 (P3X) (CRL 1580) and Sp2/0 (CRL 1581) were obtained from the American Type Culture Collection. The estrogen-sensitive MCF-7 and estrogen-insensitive MDA-MB-435 human breast cancer cell lines were supplied by Marc E. Lippman (Georgetown University Medical Center) and Isaiah J. Fidler (University of Texas M.D. Anderson Cancer Center), respectively. We have determined that both cell lines secrete Ang in vitro. All cells were maintained in DMEM supplemented with 2 mM l-glutamine, 10% heat-inactivated fetal bovine serum, and antibiotics (growth medium).
Isolation of mAb 26–2F VL and VH Region cDNAs.
Polyadenylated RNA was prepared from mAb 26–2F-producing hybridoma cells using the PoliATtract System 1000 mRNA isolation kit (Promega). The procedure followed for VL and VH cDNA isolation was essentially that of Coloma et al. (21) with minor modifications. For VH first-strand cDNA synthesis, the reaction used the CH1 antisense primer MγC.CH1 AS and avian myeloblastosis virus reverse transcriptase (Promega). VH cDNA amplification was performed using MγC.CH1 AS as the antisense primer and a set of three universal sense primers complementary to the N termini of most VH leader sequences (MHALT1.RV, MHALT2.RV, and MHALT3.RV). The VL domain-encoding cDNA was obtained using the Pharmacia Mouse ScFv Module/Recombinant Phage Antibody system. VL cDNA amplification was performed using Taq DNA polymerase (Promega), the C region MCk AS.XBA antisense primer, and five universal sense primers complementary to the N terminus of VL leader sequences (MLALT1.RV, MLALT2.RV, MLALT3.RV, MLALT4.RV, and MLALT.5).
PCR amplifications of both VH and VL cDNAs were carried out for 30 cycles in a MicroCycler thermal controller (Eppendorf) under the following conditions: 1 min denaturing (94°C), 2 min annealing (55°C), 2 min extension (72°C) followed by a final extension step of 7 min (72°C). The products were analyzed by electrophoresis in a 1.5% TAE agarose gel stained with ethidium bromide. The amplified cDNAs were then electrophoresed on a 2% low melting agarose gel in 0.5× TAE and eluted using a Magic PCR Preps DNA Purification kit (Promega).
Subcloning and Sequencing.
Each V domain-encoding cDNA was ligated into a pT7Blue T vector (Novagen) using T4–DNA ligase (Promega). The ligation mixture was used for transformation of NovaBlue competent cells (Novagen). Plasmid DNA minipreps were analyzed by 1.5% agarose gel electrophoresis after digestion with appropriate restriction enzymes. Several clones containing inserts of the expected size were sequenced in both directions using a Sequenase 2.0 sequencing kit (United States Biochemicals).
V Domain cDNA Engineering.
To clone VL and VH cDNAs into their appropriate expression vectors, they were each subjected to further PCR reactions using the following primers: H chain sense primer: MHALT2.RV (21) hybridizing to the N terminus of the H chain leader sequence and containing the EcoRV restriction site for cloning into the H chain expression vector. H chain antisense primer (H-P2 antisense): CTAGCTAGCTGAGGAGACGGTGACTGAGGTTCCT hybridizing to the J region and containing a NheI site for cloning into the CH1 region of the H chain expression vector. L chain sense primer (L-P2 sense): GGGGATATCCACCATGGAGACAGACACACTCCTGCTATGGGTCCTGCT corresponding to oligonucleotide MLALT1.RV (21), containing a 10 nucleotide extension at the 3′ end and hybridizing to the N terminus of the L chain leader sequence. An EcoRV site is present for cloning in the L chain expression vector. L chain antisense primer (L-P2 antisense): AGCCGTCGACTTACGTTTCAGCTCCAGCTTGGTCCCAG hybridizing to the J region and containing a splicing signal sequence as well as a SalI site for cloning into the intronic sequence of the L chain expression vector.
The amplified products were gel purified and cloned into pT7Blue T vectors for sequencing as described above. For both modified VL and VH domains, the cDNAs from two identical clones were excised with either EcoRV and SalI (for VL) or with EcoRV and NheI (for VH) for cloning into expression vectors.
Construction of Chimeric Genes.
The L and H chain expression vectors (pAG4622 and pAH4604, respectively) were constructed (21) and kindly provided by Sherie L. Morrison (University of California, Los Angeles). The pAG4622 vector contains the genomic sequence encoding the C-region domain of the human κ L chain and the gpt (22) selectable marker. The pAH4604 vector contains the hisD (23) selectable marker in addition to sequences encoding the human H chain γ1 C-region domain. The promoter region in each vector is derived from the anti-dansyl mAb 27–44 (21). For each VL and VH domain, cDNA fragments obtained from two identical clones were appropriately digested and ligated into their respective expression vectors. The ligated products were used to transform HB101 competent cells (Promega) and the recombinant vectors were isolated using the Wizard Plus Maxipreps DNA purification system (Promega). Prior to transfection, they were linearized with the PvuI isoschizomer BspCI restriction enzyme (Stratagene) and gel purified.
Transfection and Selection.
The chimeric H and L chain expression plasmids were cotransfected into SP2/0 or P3X nonproducing myeloma cells by electroporation as described (21). Following transfection, the cells were kept on ice for 10 min, diluted in growth medium, and placed into 96-well tissue culture plates (1 × 104 cells per well). The cells were refed 48 hr later with growth medium containing histidinol (Sigma) at a final concentration of 5 or 10 mM for SP2/0 or P3X cells, respectively. After ≈14 days, supernatants from growing colonies were screened by ELISA for the presence of chimeric antibodies.
Two selected stable transfectants, P4–5 and S13–1, obtained from the transfection of P3X or SP2/0 cells, respectively, were subcloned twice by limiting dilution. To obtain sufficient material for further analysis, cAb 26–2F from each cell source was purified from ascites fluid as described above.
Immunoassays.
Screening ELISA. Chimeric antibody producing transfectomas were detected by a modification of the screening ELISA described in Fett et al. (6). Briefly, affinity-purified goat anti-human IgG Fc (γ-chain specific) and goat anti-human κ chain (each at 10 μg/ml, Organon Teknika–Cappel), or human Ang (1 μg/ml) was coated onto 96-well plates. Following blocking of the wells with 0.5% ovalbumin, 50 μl of culture supernatant diluted 1:1 with 0.25% ovalbumin was added. After a 2-hr incubation at room temperature, the plates were washed and alkaline phosphatase-labeled goat anti-human IgG (Kirkegaard & Perry Laboratories) was added to each well, followed 1 hr later by addition of p-nitrophenyl phosphate to the washed plates. The reaction was stopped with 3 N NaOH and absorptivities were measured on a Dynatech MR600 ELISA plate reader at 405 nm with a turbidity reference of 630 nm.
Radioimmunoassay for binding affinity.
A competition radioimmunoassay for binding affinity (6) with the following modifications was used to determine IC50s, the concentration of unlabeled Ang at which the binding of its iodinated derivative is decreased by 50%. Plates were coated (10 μg/ml in borate coating buffer, 50 μl per well) with either goat anti-mouse IgG Fc (γ-chain specific, Organon Teknika–Cappel) for capture of mAb 26–2F or goat anti-human IgG Fc (see above) for capture of the chimeric antibody. Radioactivity was determined using a Micromedic 4/600plus Gamma Counter.
Concentration Determinations.
Ig concentrations were determined spectroscopically, assuming that a 1 mg/ml solution has an absorbance of 1.43 at 280 nm.
tRNA Assay.
Formation of perchloric acid soluble fragments from yeast tRNA was measured as described (24).
Angiogenesis Assay.
The chicken chorioallantoic membrane (CAM) assay was used according to Fett et al. (6).
Western Blot Analysis.
The general procedures for SDS/10% PAGE, transfer, and Western blotting have been described (25). Samples were boiled in a buffer containing 5% 2-mercaptoethanol before loading onto the gel. For detection of human components, goat anti-human IgG Fc and κ chain antibodies were used. Ig chains were visualized with alkaline phosphatase-labeled rabbit anti-goat IgG and nitroblue tetrazolium as substrate.
Antitumor Activity in Vitro.
Direct cytotoxicity of cAb 26–2F toward MDA-MB-435 and MCF-7 cells was examined using a described [3H]thymidine assay (9).
Antitumor Activity in Vivo.
This was assessed by a modification of the orthotopic model of human breast cancer tumor growth in athymic mice described by Price et al. (26). Tumor cells (MDA-MB-435 or MCF-7) were harvested by standard trypsinization procedures, washed in Hanks’ buffered salt solution, and counted by trypan blue exclusion hemacytometry. Viable cells (MDA-MB-435, 5 × 105 in 10 μl, or MCF-7, 1 × 106 in 20 μl) were injected into the surgically exposed mammary fat pad using a manual repeating dispenser (Hamilton). For MCF-7 cells a pellet of 17β-estradiol (0.72 mg per pellet, 60-day release; Innovative Research of America) was placed 1 cm from the site of tumor cell injection as the source of standard estrogen supplementation. The incision was closed with an autoclip and local subcutaneous treatment was begun within 30 min as described in the legend to Fig. 4. Tumor growth was monitored by caliper measurements.
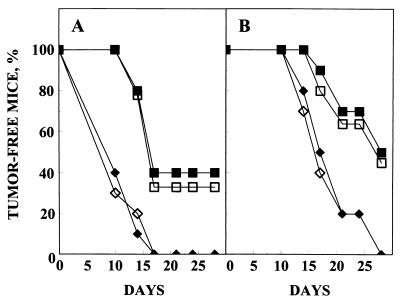
Figure 4.
Prevention of MDA-MB-435 (A) and MCF-7 (B) tumor formation by mAb 26–2F or cAb 26–2F. Tumor cells [5 × 105 (A) or 1 × 106 (B) per mouse] were injected into the surgically exposed mammary fat pad on day 0. For MCF-7 cells, a 17β-estradiol pellet was implanted in each mouse as a source of exogenous estrogen. Within 30 min of tumor cell injection the mice were treated with local subcutaneous injections of either PBS (♦) or Igs [mAb 26–2F (▪), cAb 26–2F (□), MOPC 31C (⋄); 240 μg/dose (A) and (B)]. Mice were then treated locally with 120 μg/dose (A) or 240 μg/dose (B) 6 times per week until sacrifice on day 28. n = 10 for all groups.
RESULTS AND DISCUSSION
Isolation of cDNAs Encoding mAb 26–2F V Domains.
Polyadenylated RNA was isolated from mAb 26–2F-producing hybridoma cells. cDNA sequences encoding the mAb 26–2F variable domains (VL and VH) were amplified by PCR using gene-specific primers designed to hybridize to the leader sequence of each domain (5′ primers) and to the C region N-terminal coding sequences positioned immediately downstream of the V–J region (3′ primers). Using this strategy, no amino acid substitutions that could effect chimeric antibody activity are introduced into the VL or VH cDNAs.
VL and VH amplified cDNAs were then cloned into pT7Blue T vectors and recombinant plasmids, isolated from independent clones, were sequenced. For each type of cDNA, at least two clones were identical. The nucleotide and deduced amino acid sequences for the VL and VH domains of mAb 26–2F are shown in Fig. 1. According to the classification of Kabat et al. (27), the DNA sequences encode VH IIID and Vk III V regions, each including three complementarity-determining regions and four framework regions. The deduced amino acid sequence of the first 16 N-terminal amino acids of each V domain is identical to that obtained by Edman degradation of the protein (data not shown).
Figure 1.
Nucleotide and deduced amino acid sequences for mAb 26–2F VL (Upper) and VH (Lower) domains. The sequences were interpreted according to Kabat et al. (27). Underlined amino acids comprise the three complementarity-determining regions. Portions of the leader sequence are not necessarily correct because they correspond to the PCR primers.
Construction and Expression of Chimeric Antibody Genes.
VH and VL cDNAs were modified at their 3′ end by removing the N-terminal sequence of the murine C region and adding a splicing signal sequence at the VL 3′ end. The resulting VH and VL cDNA-containing plasmids, prepared from independent clones, were digested with EcoRV and XbaI, gel purified, and amplified by PCR using primers complementary to the signal peptides (sense primers) and to the 3′ end (antisense primers) of each VH and VL domain. The gel-purified PCR products were cloned into pT7Blue T vectors and independent clones were sequenced. The sequence analyses confirmed that the expected DNA assembly had been achieved. For each modified VH and VL domain, the cDNA from two identical independent clones were excised with either EcoRV and SalI (for VL) or EcoRV and NheI (for VH) and gel purified. The VL and VH cDNA products were ligated into their respective expression vectors. Several clones, isolated from HB101 competent cell transformation, were analyzed with appropriate restriction enzymes. Recombinant vectors were isolated in duplicate from two distinct clones, each of which derived from independent VL- or VH-containing plasmid clones. Prior to transfection, the recombinant vectors were linearized with PvuI and gel purified.
Combinations of chimeric H and L chain-containing vectors were cotransfected into either P3X or SP2/0 cells by electroporation. Cells were grown in 96-well plates and selected for the presence of the hisD marker by including histidinol in the growth medium. Transfection efficiencies for both cell lines under these conditions were greater than 1 in 104. At approximately 2 weeks after transfection supernatants from surviving cells were assayed by ELISA. These indicated that the vast majority of transfectomas produced human Ig chimeras that bound to Ang; cells secreting only chimeric L chain genes were detected in a small percentage of wells. Two chimeric antibody producing master wells designated S13–1 and P4–5, obtained from the transfection of SP2/0 and P3X cells, respectively, were selected as stable transfectants and subcloned twice by limiting dilution.
Purification and Structural Characterization of cAb 26–2F.
S13–1 or P4–5 transfectoma cells were injected into pristane-primed athymic mice to generate ascites fluid. Antibody was then subsequently isolated by protein G-Sepharose affinity chromatography. The total yield of purified cAb 26–2F from either transfectoma source was ≈3 mg per mouse.
Purified S13–1- and P4–5-derived chimeric antibodies were first subjected to 10 cycles of Edman sequence analysis. L and H chain N-terminal amino acids of both chimeric antibodies were identical (data not shown) and correspond to those of the original mAb 26–2F.
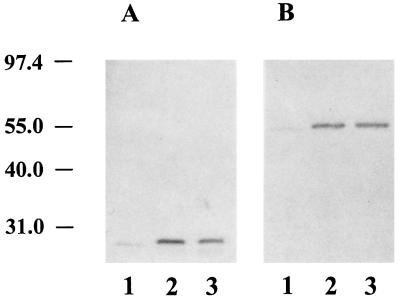
Western blot analysis using reagents specific for human κ and γ1 C region determinants showed that cAb 26–2F from either transfectoma cell source contained reduced chimeric L and H chains of the expected molecular weights (≈25,000 and 55,000, respectively) (Fig. 2). Under nonreducing conditions, cAb 26–2F derived from either S13–1 or P4–5 migrated to a position corresponding to 160,000 daltons (data not shown), thus indicating that the chimeric L and H chains were correctly assembled into complete H2L2 molecules.
Figure 2.
Western blot analysis of cAb 26–2F. Reduced proteins (400 ng) were separated by SDS/10% PAGE and transferred to nitrocellulose sheets. These were incubated with either goat anti-human κ chain (A) or goat anti-human IgG Fc-specific (B) antibodies followed by treatment with alkaline phosphatase-labeled rabbit anti-goat IgG and nitroblue tetrazolium. Lane 1, mAb 26–2F; lane 2, cAb 26–2F from S13–1; lane 3, cAb 26–2F from P4–5. Molecular weight standards (×10−3) are at left.
The IC50s for S13–1- and P4–5-derived cAb 26–2F are 2.1 × 10−9 M and 2.4 × 10−9 M, respectively, values that are essentially indistinguishable, within the error of the assay, to that obtained for mAb 26–2F (1.6 × 10−9 M).
Functional Characterization of cAb 26–2F.
A comparison of the capacity of cAb 26–2F with its murine counterpart to inhibit the angiogenic activity of Ang on the CAM is shown in Table 1. Statistical analysis by the χ2 test indicates that cAb 26–2F purified from either S13–1 (group III, P = 0.8038) or P4–5 (group IV, P = 0.7594) is as potent as mAb 26–2F (group II, P = 0.9556) in inhibiting the biologic activity of an equimolar amount of Ang, which alone is highly active (group I, P = 0.0009). The control MOPC 31C is not inhibitory (group V, P = 0.0004). The Igs alone are inactive on the CAM (groups VI–IX, P > 0.05).
Table 1.
Effect of cAb 26-2F derived from S13-1 or P4-5 myeloma cells on the activity of human Ang in the CAM assay
| mAb
|
MOPC 31C | Assay results* | P† | Status | ||||
|---|---|---|---|---|---|---|---|---|
| Group | Ang | 26-2F | S13-1 | P4-5 | ||||
| I | + | − | − | − | − | 25/45 (56) | 0.0009 | Active |
| II | + | + | − | − | − | 10/45 (22) | 0.9556 | Inactive |
| III | + | − | + | − | − | 11/46 (24) | 0.8038 | Inactive |
| IV | + | − | − | + | − | 11/45 (24) | 0.7594 | Inactive |
| V | + | − | − | − | + | 26/45 (58) | 0.0004 | Active |
| VI | − | + | − | − | − | 9/42 (21) | 0.9718 | Inactive |
| VII | − | − | + | − | − | 7/45 (16) | 0.4492 | Inactive |
| VIII | − | − | − | + | − | 13/42 (31) | 0.3258 | Inactive |
| IX | − | − | − | − | + | 15/45 (33) | 0.2154 | Inactive |
Combined data represent three sets of assays. Each individual assay employed between 15 and 19 eggs. Amount applied per egg was 10 ng of Ang and 100 ng of IgGs.
Results are expressed as the ratio of positive to total surviving eggs; the percentage of positive eggs is given in parentheses.
Significance was calculated from χ2 values of data recorded at 48 ± 2 hr based on comparison with water controls tested simultaneously (10 positive eggs/46 total surviving eggs, 22% positive). To be designated active samples must have a value of P < 0.05.
To this point, the structural and functional data taken together indicated that, as expected, the chimeric antibodies derived from transfection of either SP2/0 or P3X myeloma cells were identical. However, in the course of these studies it was observed that cells derived from clone S13–1 proliferated at a greater rate and, in general, maintained a higher percentage of viable cells in culture. In addition, S13–1 adapted easily to growth in protein-free medium, whereas P4–5 cells died under these conditions, an important consideration when large-scale production necessary for clinical trials is contemplated. For the above reasons, the remaining data to be reported were generated with cAb 26–2F derived solely from clone S13–1, which will serve as the future source of the chimeric antibody.
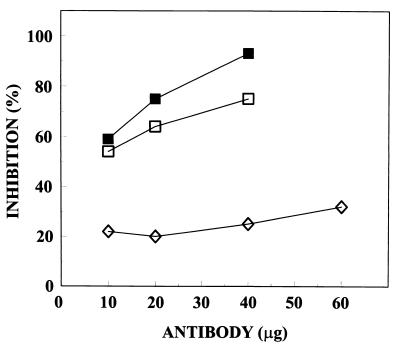
The capacity of cAb 26–2F to inhibit tRNA degradation by Ang was determined by measuring the rate of formation of perchloric acid-soluble fragments. The inhibition curves obtained with mAb 26–2F, cAb 26–2F, and the control MOPC 31C are shown in Fig. 3. At 10 μg, the two antibodies are equally inhibitory, whereas at higher concentrations cAb 26–2F is only slightly less active.
Figure 3.
Inhibition of the ribonucleolytic activity of Ang by mAb 26–2F (▪), cAb 26–2F (□), or control MOPC 31C (⋄). Ang was preincubated with the indicated amounts of Igs and assays were performed in 33 mM Hepes/33 mM NaCl, pH 6.8, at 37°C according to Shapiro et al. (24).
The antitumor activity of cAb 26–2F was subsequently examined using modifications of an orthotopic tumor cell model (26). The results depicted in Fig. 4 indicate that cAb 26–2F is as effective as mAb 26–2F in preventing the formation of tumors of human breast cancer origin. Whereas all PBS- and control MOPC 31C-treated mice develop MDA-MB-435 (Fig. 4A) or MCF-7 (Fig. 4B) tumors by days 17 and 28, respectively, the chimeric and murine antibodies completely prevent the appearance of tumors in ≈40% (MDA-MB-435) and ≈50% (MCF-7) of the treated mice. Because cAb 26–2F does not interfere with thymidine uptake and, by inference, killing of tumor cells in vitro (data not shown), the antitumor effects observed most likely result from the inactivation of tumor-secreted Ang and subsequent interruption of the angiogenic process.
In summary, we have constructed a recombinant chimeric mouse/human anti-Ang antibody, cAb 26–2F, in which the VL and VH regions of mAb 26–2F were inserted into expression vectors containing C regions of human κ chains and γ1 H chains. The resultant chimera retains the properties of the original mAb, including potent activity against human tumor cell xenografts. As a consequence, cAb 26–2F should provide a powerful immunotherapeutic for the treatment of human cancer and other conditions where inhibition of pathological angiogenesis is desired.
Acknowledgments
We thank Drs. Chris Burgess and Richard Martinelli of Chiron Diagnostics for valuable assistance in the initial planning stage of this study. We are especially indebted to Dr. Sherie Morrison for supplying the expression vectors and for guidance on their use. We also thank Drs. Marc Lippman and Isaiah Fidler for supplying tumor cell lines and Drs. Daniel Strydom and Guo-Fu Hu for performing protein sequence analysis. The excellent technical assistance of Najat Ziyadeh, Justin Steele, and Risa Robinson is gratefully acknowledged. This work was supported by grants to J.W.F from the National Institutes of Health (R01CA60046) and the Department of the Army (DAMD17-96-1-6025). R.P. was the recipient of a Fulbright Foreign Travel Scholarship.
ABBREVIATIONS
- Ang
angiogenin
- cAb
chimeric antibody
- CAM
chorioallantoic membrane
- H and L
Ig heavy and light chains, respectively
- V and C
Ig variable and constant regions, respectively
Footnotes
References
- 1.Folkman J. J Natl Cancer Inst. 1989;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 2.Blood C H, Zetter B R. Biochim Biophys Acta. 1990;1032:89–118. doi: 10.1016/0304-419x(90)90014-r. [DOI] [PubMed] [Google Scholar]
- 3.Riordan J F. In: Ribonucleases: Structures and Functions. D’Alessio G, Riordan J F, editors. New York: Academic; 1997. pp. 445–489. [Google Scholar]
- 4.Fett J W, Strydom D J, Lobb L L, Alderman E M, Bethune J L, Riordan J F, Vallee B L. Biochemistry. 1985;24:5480–5486. doi: 10.1021/bi00341a030. [DOI] [PubMed] [Google Scholar]
- 5.Rybak S M, Fett J W, Yao Q-Z, Vallee B L. Biochem Biophys Res Commun. 1987;146:1240–1248. doi: 10.1016/0006-291x(87)90781-9. [DOI] [PubMed] [Google Scholar]
- 6.Fett J W, Olson K A, Rybak S M. Biochemistry. 1994;33:5421–5427. doi: 10.1021/bi00184a010. [DOI] [PubMed] [Google Scholar]
- 7.Hu G-F, Riordan J F, Vallee B L. Proc Natl Acad Sci USA. 1997;94:2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acharya K R, Shapiro R, Allen S C, Riordan J F, Vallee B L. Proc Natl Acad Sci USA. 1994;91:2915–2929. doi: 10.1073/pnas.91.8.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olson K A, French T C, Vallee B L, Fett J W. Cancer Res. 1994;54:4576–4579. [PubMed] [Google Scholar]
- 10.Olson K A, Fett J W, French T C, Key M E, Vallee B L. Proc Natl Acad Sci USA. 1995;92:442–446. doi: 10.1073/pnas.92.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson K A, Fett J W. Proc Am Assoc Cancer Res. 1996;37:57A. (abstr.). [Google Scholar]
- 12.LoBuglio, A. F., Saleh, M., Peterson, L., Wheeler, R., Carrano, R., Huster, W. & Khazaeli, M. B. (1986) Hybridoma 5, Suppl. 1, S151–S161. [PubMed]
- 13.Hammond E H, Wittwer C T, Greenwood J, Knape W A, Yowell R L, Menlove R L, Craven C, Renlund D G, Bristow M R, DeWitt C W, O’Connell J B. Transplantation. 1990;50:776–790. doi: 10.1097/00007890-199011000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Levy R, Miller R. Annu Rev Med. 1983;34:107–127. doi: 10.1146/annurev.me.34.020183.000543. [DOI] [PubMed] [Google Scholar]
- 15.Schroff R, Foon K, Beatty S, Oldham R, Morgan A. Cancer Res. 1985;45:879–889. [PubMed] [Google Scholar]
- 16.Morrison S L, Johnson M J, Herzenberg L A, Oi V T. Proc Natl Acad Sci USA. 1984;81:6851–6855. doi: 10.1073/pnas.81.21.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuberger M S, Williams G T, Mitchell E B, Jouhal S S, Flanagan J G, Rabbitts T H. Nature (London) 1985;314:268–270. doi: 10.1038/314268a0. [DOI] [PubMed] [Google Scholar]
- 18.LoBuglio A F, Wheeler R H, Trang J, Haynes A, Rogers K, Harvey E B, Sun L, Ghrayeb J, Khazaeli M B. Proc Natl Acad Sci USA. 1989;86:4220–4224. doi: 10.1073/pnas.86.11.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Targan S R, Hanauer S B, van Deventer S J H, Mayer L, Present D H, Braakman T, DeWoody K L, Schaible T F, Rutgeerts P J. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 20.Khazaeli M B, Conry R M, LoBuglio A. J Immunother. 1994;15:42–52. doi: 10.1097/00002371-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Coloma M J, Hastins A, Wims L A, Morrison S L. J Immunol Methods. 1992;152:89–104. doi: 10.1016/0022-1759(92)90092-8. [DOI] [PubMed] [Google Scholar]
- 22.Mulligan R C, Berg P. Proc Natl Acad Sci USA. 1981;78:2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman S C, Mulligan R C. Proc Natl Acad Sci USA. 1988;85:8047–8051. doi: 10.1073/pnas.85.21.8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro R, Weremowicz S, Riordan J F, Vallee B L. Proc Natl Acad Sci USA. 1987;84:8783–8787. doi: 10.1073/pnas.84.24.8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurachi K, Rybak S M, Fett J W, Shapiro R, Strydom D J, Olson K A, Riordan J F, Davie E W, Vallee B L. Biochemistry. 1988;27:6557–6562. doi: 10.1021/bi00417a054. [DOI] [PubMed] [Google Scholar]
- 26.Price J E, Polyzos A, Zhang R D, Daniels L M. Cancer Res. 1990;50:717–721. [PubMed] [Google Scholar]
- 27.Kabat E A, Wu T T, Perry H M, Gottesman K S, Foeller C. Sequences of Proteins of Immunological Interest. Bethesda: U. S. Dept. Health and Human Services; 1991. DHHS Publ. No. (NIH) 91–3242, 5th Ed. [Google Scholar]