Abstract
Because Tyr35β is located at the convergence of the α1β1, α1β2, and α1α2 interfaces in deoxyhemoglobin, it can be argued that mutations at this position may result in large changes in the functional properties of hemoglobin. However, only small mutation-induced changes in functional and structural properties are found for the recombinant hemoglobins βY35F and βY35A. Oxygen equilibrium-binding studies in solution, which measure the overall oxygen affinity (the p50) and the overall cooperativity (the Hill coefficient) of a hemoglobin solution, show that removing the phenolic hydroxyl group of Tyr35β results in small decreases in oxygen affinity and cooperativity. In contrast, removing the entire phenolic ring results in a fourfold increase in oxygen affinity and no significant change in cooperativity. The kinetics of carbon monoxide (CO) combination in solution and the oxygen-binding properties of these variants in deoxy crystals, which measure the oxygen affinity and cooperativity of just the T quaternary structure, show that the ligand affinity of the T quaternary structure decreases in βY35F and increases in βY35A. The kinetics of CO rebinding following flash photolysis, which provides a measure of the dissociation of the liganded hemoglobin tetramer, indicates that the stability of the liganded hemoglobin tetramer is not altered in βY35F or βY35A. X-ray crystal structures of deoxy βY35F and βY35A are highly isomorphous with the structure of wild-type deoxyhemoglobin. The βY35F mutation repositions the carboxyl group of Asp126α1 so that it may form a more favorable interaction with the guanidinium group of Arg141α2. The βY35A mutation results in increased mobility of the Arg141α side chain, implying that the interactions between Asp126α1 and Arg141α2 are weakened. Therefore, the changes in the functional properties of these 35β mutants appear to correlate with subtle structural differences at the C terminus of the α-subunit.
Keywords: Hemoglobin, mutant, ligand-binding kinetics, ligand affinity, X-ray crystallography, single-crystal microspectrophotometry
The allosteric properties of hemoglobin are directly linked to the structures of its subunit-subunit interfaces. In particular, the assembly of the α2β2 hemoglobin tetramer generates an α-α interface (designated α1α2), a β-β interface (designated β1β2), and two types of α-β interfaces (designated α1β1 and α1β2). The α1α2 and β1β2 interfaces form a solvent channel that is almost devoid of direct intersubunit contacts and provides binding sites for a variety of allosteric effectors (Perutz 1989; Perutz et al. 1994). The only direct contacts between like subunits occur at the α1α2 interface, where ionic interactions form between the C-terminal residue of one α-subunit, Arg141α, and residues Asp126α and Lys127α on the opposite α-subunit. In contrast, extensive contacts are formed between unlike subunits at the α1β1 and α1β2 interfaces. These two interfaces, however, have very different characteristics. The α1β2 interface is highly polar and dynamic, undergoing large changes in structure as a function of ligation state, whereas the α1β1 interface is static and less polar. The most direct way to determine the relative importance of residues that contribute interactions to the subunit-subunit interfaces of the hemoglobin tetramer is to perform detailed site-directed mutagenesis studies on these residues. We did this in the case of residue 35β by creating and performing complementary structural and functional studies on the mutants βY35F and βY35A.
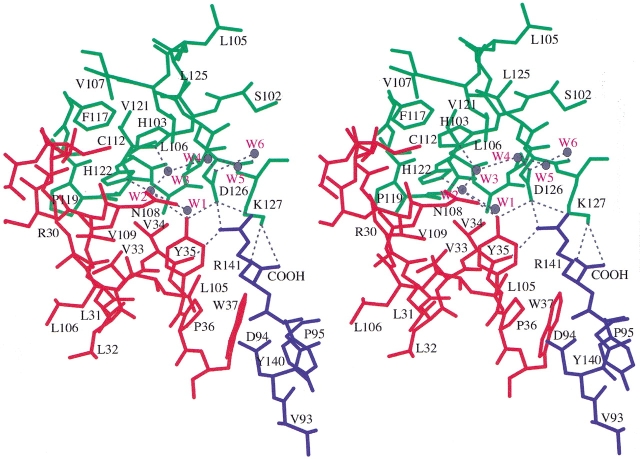
Located at the convergence of the α1β1, α1β2, and α1α2 interfaces, Tyr35β contributes to a complex network of atomic interactions through which it can potentially influence the structure and stability of each of these interfaces (see Fig. 1 ▶). In the case of the α1β1 interface, the phenolic hydroxyl group of Tyr35β1 makes two intersubunit interactions, one direct and one indirect. The direct interaction consists of a hydrogen bond to the side chain carboxylate oxygen, Oδ2, of Asp126α1. The indirect interaction consists of an intersubunit water bridge between Tyr35β1 and the side chain of His122α1 (water molecule W2 in Fig. 1 ▶). The hydroxyl group also interacts with another water molecule, W1, that is part of a network of water molecules (W1 through W6 in Fig. 1 ▶) at the α1β1 interface. In the case of the α2β1 interface (or the symmetry-related α2β1 interface), the side chain of Tyr35β1 makes van der Waals contacts between the edge of its phenolic ring and the guanidinium side chain of Arg141α2. In the case of the α1α2 interface, Tyr35β1 interacts with both Asp126α1 and Arg141α2, and therefore, it can potentially influence the structure and stability of this interface as well. Additionally, Tyr35β1 can indirectly influence the α1α2 interface through van der Waals contacts it makes with Val34β1, because Val34β1 makes van der Waals contacts with Asp126β1 and a hydrogen bond with Arg141α2. Tyr35β1 and Val34β1 are unique in that they are the only residues that contribute atomic interactions to both the α1β1 and α1β2 interfaces.
Fig. 1.
Stereo diagram showing the location Tyr35β? in human deoxyhemoglobin. The α1-, α2-, and β1-subunits are colored green, blue, and red, respectively. Tyr35β1 is located at the convergence of the α1β1, α2β1, and α1α2 interfaces. Hydrogen bonds are indicated by dashed black lines, and water molecules bound within the α1β1 interface are shown in gray with red labels.
Because Tyr35β contributes to a complex network of potentially important intersubunit contacts, a variety of studies on more than one mutation is required to dissect the importance of individual interactions. By analyzing the site-directed mutant βY35F both in solution and in deoxy crystals, we have extended the previously reported findings of Nakatsukasa et al. (1998) for this mutant. A similar series studies of the βY35A mutant increases our understanding of the role that Tyr35β plays in cooperative mechanism of hemoglobin. By studying the two mutants with a variety of techniques, it is possible to assign structure/function relationships to specific atomic interactions.
Results
CO combination kinetics by rapid mixing
Kinetics of carbon monoxide (CO) combination with the deoxygenated hemoglobin molecule were measured at pH 6, 7, and 8 in the presence and absence of inositol hexaphosphate (IHP). The rate constants of these second-order reactions for human hemoglobin A and the 35β variants are presented in Table 1. Under these conditions, the combination of CO with human hemoglobin A is an autocatalytic process that, when fitted to a single exponential function, has an overall rate constant that varies from 0.09 × 106 M−1sec−1 to 0.31 × 106 M−1sec−1 depending on the pH and presence or absence of IHP. At pH 6 and 7, the βY35F substitution slows these reactions slightly without affecting their autocatalytic nature. At pH 8, the kinetic difference between native hemoglobin A and βY35F increases because of a decreased pH dependence of the kinetic properties of the mutant.
Table 1.
CO combination rate constants following rapid mixing (106 M−1 sec−1)
| pH 6.0 | pH 7.0 | pH 8.0 | ||||
| Hemoglobin | −IHP | +IHP | −IHP | +IHP | −IHP | +IHP |
| HbA | 0.18 Aa | 0.10 A | 0.18 A | 0.09 A | 0.31 A | 0.17 A |
| βY35F | 0.15 A | 0.09 A | 0.15 A | 0.08 A | 0.21 A | 0.13 A |
| βY35A | 0.31 A | 0.20 A | 0.34 | 0.19 A | 1.1 0.3 | 0.28 A |
| 30% 70% | ||||||
a Designates autocatalytic nature of reaction.
In contrast, substitution of Tyr35β with alanine doubles the rate at which the deoxygenated tetramer reacts with CO at pH 6 and 7. At pH 7 in the absence of IHP, this mutant fails to show accelerating CO-binding kinetics, and at pH 8 it becomes kinetically heterogeneous in the absence of IHP and fails to show acceleration in its presence.
CO recombination following photodissociation
Kinetics of CO recombination following flash photolysis are typically heterogeneous with two kinetic phases, the rates of which differ by more than an order of magnitude. The slower of the two kinetic phases is associated with a rate constant similar to that measured for the combination of CO with the deoxygenated tetramer following rapid mixing. For βY35F and βY35A, rate constants for the slow kinetic phases (Table 2) agree well with those measured under the same conditions by rapid mixing in the stopped flow apparatus (Table 1). The rapid phase is caused by αβ dimers. The magnitude of this phase is concentration dependent and varies with the presence and concentration of organic phosphates such as IHP. At a concentration of 100 μM IHP, the relative amount of dimers is reduced as a result of preferential binding of IHP to the ligand-saturated hemoglobin tetramer. This pattern is observed in Table 2 for the CO recombination kinetics of wild-type hemoglobin and the two 35β variants at pH 7 in the presence and absence of IHP. The ratio of the magnitudes of the fast and slow kinetic phases offers a measure of the extent to which the ligand-saturated hemoglobin tetramer dissociates into αβ dimers. In this regard, the two variants appear very similar to one another but are slightly more dissociated than wild-type hemoglobin. The data in Table 2 predict values of −7.8 ± 0.1 kcal/mole for HbA, in agreement with the results of Doyle et al. (1992); −7.4 ± 0.1 kcal/mole for βY35F; and −7.3 ± 0.1 kcal/mole for βY35A. A value of −7.7 ± 0.2 kcal/mole determined by the same method was previously reported for βV1M by Doyle et al. (1992).
Table 2.
CO recombination rate constants following flash photolysis at pH 7.0 (106 M−1 sec−1)
| Hemoglobin | −IHP | +IHP |
| HbA | 4.7 (68%) | 4.0 (25%) |
| 0.19 (32%) | 0.09 (75%) | |
| βY35F | 4.5 (72%) | 3.4 (27%) |
| 0.17 (28%) | 0.09 (73%) | |
| βY35A | 4.2 (75%) | 4.4 (27%) |
| 0.40 (25%) | 0.20 (73%) |
Oxygen equilibrium measurements in solution
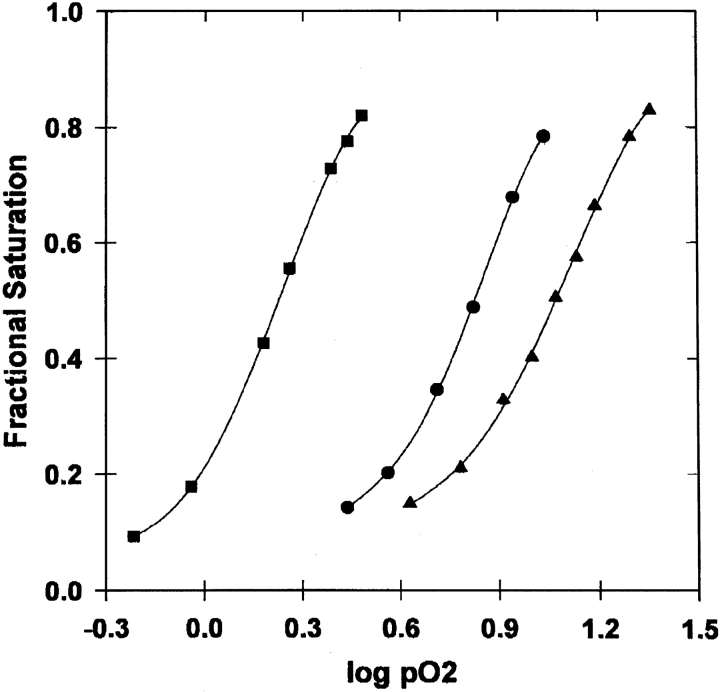
The oxygen-binding curves for the βY35F and βY35A mutants as well as the βV1M variant are shown in Figure 2 ▶. The equilibrium-binding properties are summarized in Table 3. If p50 is taken as an approximation of p-median for the calculation of average free energy of oxygen binding, then the binding of oxygen is made 3.3 kcal/4 mole O2 more favorable by the βY35A substitution and 1.2 kcal/4 mole O2 less favorable by the βY35F substitution. The cooperativity (n-Hill in Table 3) is unchanged by the βY35A mutation. In the case of the βY35F mutation, the Hill coefficient is decreased slightly by 0.4, but this may not be significant because the standard deviation of the difference in n is 0.28 (= 0.2 × [2]1/2 based on the data in Table 3).
Fig. 2.
Oxygen equilibrium curves measured tonometrically. Data are shown for βV1M (circles), βY35A (squares), and βY35F (triangles). Solid lines are nonlinear least-squares fits. The experimental conditions were 100 mM HCl-bis-Tris at pH 7 and 20°C.
Table 3.
Oxygen-binding parameters for Hb solutions
| Hemoglobin | p50 (torr) | n-Hill (max)a |
| βV1M | 6.8 ± 0.8 | 2.8 ± 0.2 |
| βY35F | 11.7 ± 1.0 | 2.4 ± 0.2 |
| βY35A | 1.7 ± 0.2 | 2.7 ± 0.2 |
a The Hill plots of these data are asymmetrical with the maximum Hill coefficients occurring in the second half of the saturation process. This is similar to the findings of Doyle et al. (1992) for the βV1M construct.
The differences between the 35β variants and HbA result from the combined effects of the mutations at 35β and the substitution of methionine for the valine at the β-chain N termini. However, given that residue 35β and the β-chain N termini are not in contact, it is reasonable to assume that the changes resulting from the 35β mutations and the changes resulting from the βV1M substitution are additive. Furthermore, LiCata and Ackers (1995) have studied additivity of double mutations on the dimer-tetramer assembly free energies in deoxy and fully oxygenated states for 24 naturally occurring hemoglobins. They found that although the double mutations are not strictly additive, the magnitude of any nonadditivity is on average only equal to ± 0.2 kcal/mole Hb tetramer. Therefore, it is reasonable to take the effects of 35β substitutions on the properties of βV1M to reflect the changes they would cause in HbA.
The relationships between changes in the free energy of oxygen binding and changes in the free energy of dimer assembly can be analyzed by means of the linkage scheme:
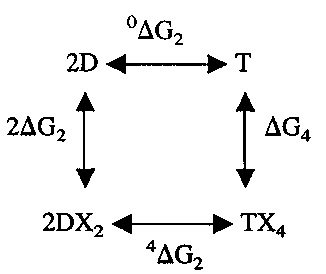 |
CO recombination kinetics show that 4ΔG2 for βY35F and βY35A are only slightly increased compared with HbA and βV1M. The comparison of fully liganded CO and oxyhemoglobins is meaningful, because at pH 7.4 these liganded forms have the same free energy of assembly (Huang and Ackers 1996). The assumption that the oxygen affinity of the αβ dimers is not affected by the modification of 35β is supported by the similarities of the rate constants associated with the rapid phases of the recombination kinetics and by the location of this residue. If this is accepted, then there is a direct relationship between the oxygen affinity of the tetramer and the free energy of association of the deoxygenated αβ dimers. The slight reduction in the oxygen affinity of βY35F indicates little change in the stability of the deoxygenated tetramer of this variant. This is inconsistent with the strong destabilization of the deoxy tetrameric structure in the micromolar range of heme concentration that would be expected from the previous Hb Philly studies (Rieder et al. 1969; Asakura et al. 1976, 1981). This indicates that the reported Hb Philly mutation (Tyr35β → Phe) is incorrect. The same conclusion was reached by Nakatsukasa et al. (1998) as a result of their examination of an independently prepared βY35F variant of HbA. On the other hand, the increased oxygen affinity of βY35A in conjunction with its relatively unchanged 4ΔG2 requires a significant destabilization of its deoxygenated tetramer, i.e., an increase in 0ΔG2. This destabilization of the deoxygenated βY35A is evidenced by the hetergeneity of CO combination at pH 8 in the absence of IHP, which is probably caused by the presence of rapidly reacting deoxygenated αβ dimers.
Equilibrium measurements of oxygen binding to crystals of quaternary-T hemoglobin
Crystals of βY35F and βY35A were grown anaerobically from polyethylene glycol (PEG) solutions. Crystals of deoxyhemoglobin which grow from PEG solutions are orthorhombic with the b crystal axis usually having the shortest dimension. It is for this reason that the spectra are normally taken only with the light polarized parallel to the a or c crystal axes. This also was found to be the case for crystals of βY35F. However, all of the crystals of βY35A examined had grown with their shortest dimension along the a crystal axis. For this variant, spectra were obtained with light polarized parallel to the b or c crystal axes.
Polarized absorption spectra for βY35F and βY35A crystals were determined at several oxygen pressures. Hill plots of the oxygen-binding data are shown in Figure 3 ▶. In Table 4, the p50s and Hill coefficients obtained from the data in Figure 3 ▶ are compared with the same parameters for wild-type hemoglobin. The Hill coefficients for the mutant and wild-type hemoglobins are close to 1 because the crystal lattice restricts the quaternary structure to T-like conformations. The oxygen affinity and Hill coefficient of crystalline βY35F are slightly lower than those of crystalline hemoglobin. Measurements of the fractional saturation of βY35F crystals at a single oxygen pressure as a function of pH indicate no significant Bohr effect (data not shown). The lack of a Bohr effect in crystalline βY35F is consistent with previous studies on wild-type hemoglobin crystals (Rivetti et al. 1993a). As can be seen in panel b of Figure 3 ▶, data were obtained on the βY35A variant only up to 30% oxygen saturation. Above this level of oxygenation, βY35A crystals become unstable and show irreversible increases in oxygen affinity. Again, as seen in Table 4, both the oxygen affinity and Hill coefficient are greater for βY35A crystals than for crystals of HbA. The unusual instability of these crystals with respect to oxygenation may contribute to the increased Hill coefficients.
Fig. 3.
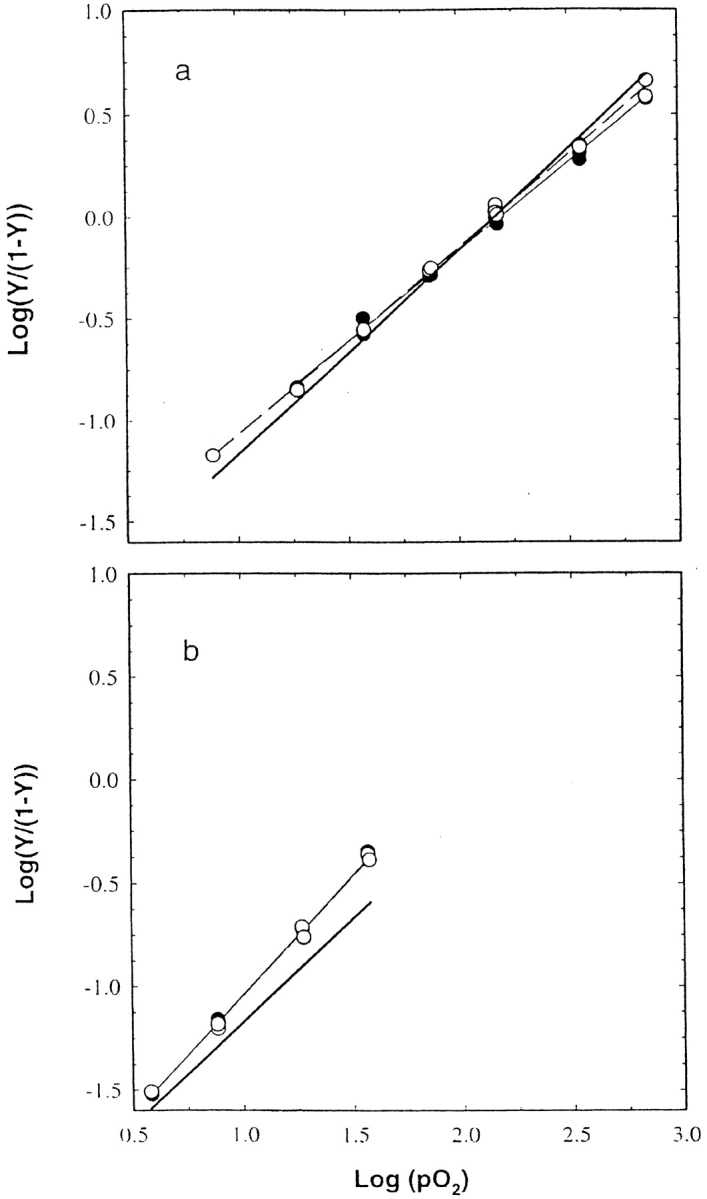
Hill plots of the binding of oxygen to crystals of βY35F and βY35A. The Hb crystals were suspended in a solution containing 54% (w/v) PEG 8000, 1 mM EDTA, 10 mM phosphate at pH 7.24 and 15°C. Polarized absorption spectra were recorded at different oxygen pressures and were fitted to a linear sum of the deoxy, oxy, and oxidized Hb reference spectra to determine the fractional saturation with oxygen and the fractional concentration of oxidized hemes. (a) Data obtained with crystals of βY35F. The p50 and Hill coefficient of these crystals are 157 ± 3.8 torr and 0.88 ± 0.02 for light polarized parallel to the a crystal axis (closed circles and light solid line), and 147.5 ± 2.4 torr and 0.91 ± 0.01 for light polarized parallel to the c crystal axis (open circles and dashed line). (b) Data obtained with crystals of βY35A. The p50 and Hill coefficient of these crystals are 79.3 ± 3.4 torr and 1.16 ± 0.02 for light polarized parallel to the a crystal axis (closed circles and light solid line), and 80.5 ± 3.4 torr and 1.15 ± 0.02 for light polarized parallel to the c crystal axis (open circles and dashed line). The heavy, solid lines in panels a and b represent the properties of the binding of oxygen to crystals of HbA.
Table 4.
Oxygen-binding parameters for Hb crystalsa
| Hemoglobin | p50a (mm Hg) | p50b (mm Hg) | p50c (mm Hg) | na | nb | nc |
| HbAb | 136 ± 1.0 | 133 ± 1.0 | 1.00 ± 0.01 | 1.01 ± 0.01 | ||
| βY35F | 157 ± 3.8 | 148 ± 2.4 | 0.88 ± 0.02 | 0.91 ± 0.01 | ||
| βY35A | 79 ± 3.4 | 80 ± 3.4 | 1.16 ± 0.02 | 1.15 ± 0.02 |
a The standard errors listed refer to a single titration.
b These values are from Mozzarelli et al. (1997).
X-ray crystal structures of deoxy βY35A and βY35F
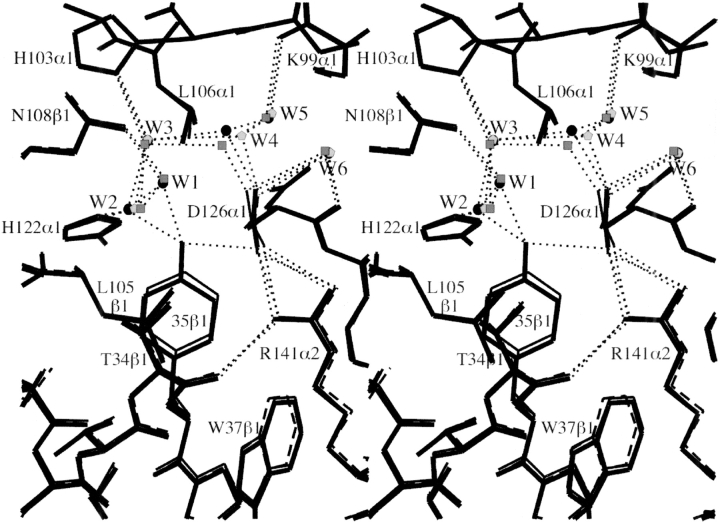
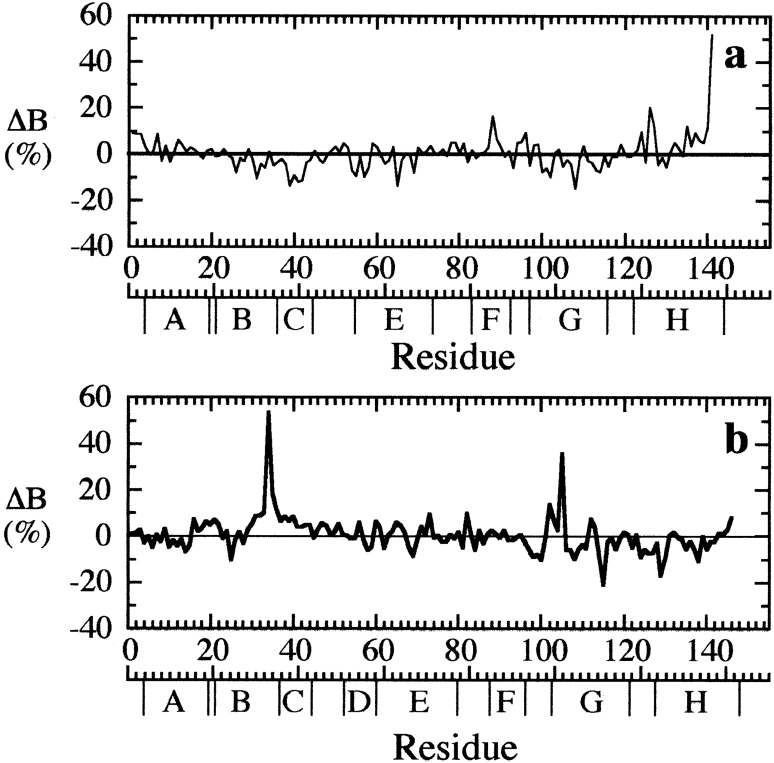
Despite the fact that Tyr35β is clearly an integral part of the dimer-dimer interface, our crystallographic studies show that removal of the phenolic hydroxyl group (βY35F), or even the entire phenolic side chain (βY35A), results in extremely small structural perturbations to the deoxy tetramer. In βY35F, loss of the hydrogen bond between the side chain carboxyl group of Asp126α1 and the hydroxyl group of Tyr35β1 leads to a small shift in the benzene ring of Phe35β1, a slight rotation (∼15°) of the Asp126α1 carboxyl group, and small changes in the associated water molecule network at the α1β1 interface (Fig. 4 ▶). In particular, water W4 shifts by ∼0.8 Å to maintain its interaction with Asp126α1, and the mobility of water molecule W1 increases (as indicated by a 65% increase in its temperature factor), reflecting the loss of its interaction with the hydroxyl group of Tyr35β1. In the case of βY35A, removal of the entire phenolic ring eliminates van der Waals contacts with Thr34β1, Leu105β1, and Arg141α2 as well as the polar interactions associated with the OH group. Creation of the large cavity in βY35A leads to increases in the atomic temperature factors of residues Thr34β1, Leu105β1, and Arg141α2 (Fig. 5 ▶) and the elimination of water molecular W1, but it does not result in significant atomic displacements of the adjacent residues (Fig. 4 ▶). It is important to note that although the structural changes associated with mutations βY35F and βY35A are very small, virtually identical mutation-induced changes are associated with both residue 35β1 and residue 35β2 (data not shown).
Fig. 4.
Stereo diagram showing the environment of residue 35β1 in deoxy HbA (thick bonds), deoxy βY35F (thin bonds), and deoxy βY35A (dashed bonds). Hydrogen bonds are indicated by thin dotted lines, and bound water molecules are indicated for deoxy HbA (circles), deoxy βY35F (squares), and deoxy βY35A (pentagons). The atomic models are overlaid following sieve fit superposition (Kavanaugh et al. 1998) of the α1-subunits.
Fig. 5.
The percent change in average atomic temperature factors (ΔB = 100[BβY35F − BβV1M]/BβV1M) is plotted versus residue number for the α-subunits (a) and β-subunits (b) of βY35A. The ΔB values for the α1- and α2-subunits have been averaged, as have the ΔB values for the β1- and β2-subunits.
Discussion
The relatively mild structural and functional perturbations that result from replacing Tyr35β with either a phenylalanine or an alanine are somewhat surprising because Tyr35β is completely buried at the intersection of the α1β1, α1β2, and α1α2 interfaces in the deoxyhemoglobin tetramer. In fact, hemoglobin Philly (Rieder et al. 1969; Asakura et al. 1976, 1981), a naturally occurring mutant hemoglobin with very high oxygen affinity, decreased cooperativity, and decreased stability, was reported to be a Tyr35β→Phe substitution. Moreover, it was indicated, based on the increased sulphydryl reactivities of cysteines 104α and 112β, that the mutation in hemoglobin Philly destabilized the α1β1 interface, shifting the monomer dimer equilibrium toward monomer formation. However, Nakatsukasa et al. (1998) performed oxygen equilibrium measurements in solution on the recombinant βY35F variant and found this mutant hemoglobin to have a slightly increased p50 and normal cooperativity. Therefore, they concluded that the hemoglobin Philly mutation must be something other than a Tyr35β→Phe substitution. Nagai et al. (1999) further reported that the difference ultraviolet resonance Raman spectrum between the deoxy and CO forms of βY35F is nearly identical to that of HbA, supporting this conclusion. In agreement with these results, we find that the kinetics of CO combination of βY35F in solution and the oxygen affinity of crystalline βY35F indicate that the deoxy quaternary-T structure of this mutant has slightly reduced ligand affinity and is not destabilized significantly. In addition, the CO recombination kinetic data reported above indicate that the βY35F mutation causes little, if any, difference in the stability of the fully oxygenated tetramer.
The fact that the βY35F mutation does not significantly reduce the stability of the deoxy tetramer implies that the Tyr35β1• • • • Asp126α1 hydrogen bond makes no significant contribution to the stability of the deoxy HbA tetramer, or alternatively that loss of this interaction in βY35F is compensated for by other interactions. If the former possibility is true, the Tyr35β1• • • • Asp126α1 hydrogen bond may still be present in isolated αβ dimers, or the interaction of water with Tyr35β and Asp126α is energetically equivalent to the Tyr35β1• • • • Asp126α1 hydrogen bond. If the latter is true, crystallographic analysis of the deoxy βY35F structure indicates a stronger interaction between Asp126α1 and Arg141α2 may compensate for the loss of the Tyr35β1• • • • Asp126α1 hydrogen bond. In the wild-type deoxyhemoglobin tetramer, the side chain of Asp126α1 is buried between the α1-, β1-, and α2-subunits, where its Oδ2 atom makes three intersubunit interactions with other buried polar atoms. Specifically, the Oδ2 atom interacts with the hyroxyl group of Tyr35β1 and the Nη1 and Nη2 atoms of the Arg141α2 guanidinium group. In deoxy βY35F, however, the Oδ2 atom of Asp126α1 only interacts with Arg141α2 because the 35β hydroxyl has been eliminated. Consequently, the interactions between the Oδ2 atom and the Arg141α2 guanidinium group should, in principle, have increased strength in the βY35F mutant. The rotation of Asp126α1 carboxyl group observed in deoxy βY35F crystal structure (Fig. 4 ▶) is consistent with this possibility, because the rotation repositions the Oδ2 atom so that it points more directly at the Arg141α2 guanidinium group. It has been well documented that the stability of the deoxy hemoglobin tetramer is strongly linked to the α1α2 interactions associated with Arg141α (Antonini et al. 1961; Perutz 1970; Perutz and TenEyck 1972; Bonaventura et al. 1974; Kilmartin et al. 1975; Kavanaugh et al. 1995). Therefore, slightly stronger interactions between Asp126α1 and Arg141α2 may be the stereochemical basis for the slightly reduced ligand affinity and cooperativity of the quaternary-T βY35F (as observed in crystals of βY35F) as well as the overall decreases measured in solution.
Although the loss of the hydrogen bond associated with Tyr35β has a very small stabilizing effect on the deoxyhemoglobin tetramer, the loss of the van der Waals interactions associated with the benzene ring of Tyr35β destabilizes the deoxy tetramer and increases the oxygen affinity of the quaternary-T structure. The oxygen-binding isotherm measured in solution (Fig. 2 ▶) clearly shows that βY35A has increased oxygen affinity. Collectively, the oxygen-binding measurements made on crystals (Fig. 3 ▶) and the CO combination (Table 1) and recombination rates (Table 2) measured in solution, indicate that this increase in ligand affinity is in large measure because of changes in the deoxy tetramer. Because the magnitudes of the fast and slow CO-rebinding phases following flash photolysis are very similar for βY35A and HbA (Table 2), it can be concluded that the βY35A mutation has little effect on the stability of the liganded structure. The faster CO combination rates measured in the presence of IHP (Table 2) and lower p50 measured for βY35A crystals (Table 3) both indicate that the quaternary-T structure of the βY35A tetramer has increased oxygen affinity.
The increased oxygen affinity and decreased stability of the deoxy βY35A tetramer correlate with increased mobility of the Arg141α in the deoxy βY35A crystal structure (Fig. 5 ▶). A similar observation has been made for a series of site-directed mutants at position 37β (Kavanaugh et al. 1992b, 1998; ; Rivetti et al. 1993b; Kelly et al. 1994; Kiger et al. 1998; Kwiatkowski et al. 1998; Peterson and Friedman 1998) and for deoxy desArg hemoglobin (Kavanaugh et al. 1995). Presumably the increased mobility at the α-subunit C termini in the βY35A, desArg hemoglobin, and the 37β mutant deoxyhemoglobin structures results in increased oxygen affinity by allowing ligation-induced changes in structure to be more easily accommodated.
Materials and methods
Mutagenesis and mutant hemoglobin assembly
The βY35F and βY35A mutations were created by cassette mutagenesis, and the mutant β-chains were overexpressed in Escherichia coli using the T7 expression system developed by Hernan et al. (1992). Because of differences in N-terminal processing in mammals and bacteria, β-globins produced with this expression system contain the additional substitution βV1M. This replacement that does not significantly affect the structure or the functional properties of hemoglobin (Doyle et al. 1992; Hernan et al. 1992; Kavanaugh et al. 1992a). The bacteria were harvested by centrifugation, and the mutant β-globin chains were purified, reconstituted with heme, and combined with α-globin as described by Hernan et al. (1992). Approximately 110 mg of βY35F hemoglobin and 210 mg βY35A hemoglobin were obtained from 24 L of bacterial culture.
Measurement of CO combination kinetics by rapid mixing
Rapid-mixing CO combination kinetics were performed with an OLIS (On Line Instrument Systems Inc.) stopped-flow apparatus, which is similar to the one first described by Gibson and Milnes (1964). The procedure for measuring the rate of CO combination with deoxygenated hemoglobin was similar to that of Gibson (1959). The time course of the reaction was followed by monitoring the change in absorbance at 420 nm and 435 nm using a cuvette with a 1.7-cm pathlength. Concentrations of CO and hemoglobin (in heme equivalents) were 20 μM and 2 μM, respectively. Dithionite was present at a concentration of ∼2 mM.
Measurements of CO recombination following photodissociation
Measurements of CO recombination following photodissociation were performed as previously described (Doyle et al. 1992). Photolysis was by means of the simultaneous discharge of three photographic strobe units (Sunpak Auto 544) equipped with thyristor quenching devices. These were adjusted to produce a rectangular pulse of light ∼0.5 msec in duration. The flash radiation was filtered through solutions of auramine, which has high extinction coefficients at 420 nm and 435 nm, the wavelengths at which the time course was monitored. This reduced or eliminated interference between the flash and the measurement of changes in absorbance as a function of time. Reactant concentrations were the same as those used in the rapid-mixing experiments.
Measurements of oxygen-binding equilibrium in solution
Oxygen-binding curves were measured by tonometry essentially by the Nagel et al. (1965) modification of the method of Allen et al. (1950). A 500-mL tonometer with an attached 2-mm pathlength cuvette was used. The Hb concentration was 160 μM in heme equivalents. Spectral measurements were performed with a Cary 14 spectrophotometer modified by OLIS for computer control and online data acquisition. Measurements were performed at 20°C in 100 mM HCl–bis-Tris (HCl–bis[2-hydroxyethyl]iminotris[hydroxymethyl]methane) buffer at pH 7. This buffer was prepared by titrating HCl against the base form of bis-Tris and then diluting the resulting solution with deionized water to a final chloride concentration of 0.1 M. Deoxygenation was accomplished by equilibration with oxygen-free nitrogen. Reduction system enzymes (Hayashi et al. 1973) were added to prevent metHb formation.
Microspectrophotometric measurements of oxygen binding to hemoglobin crystals
The small (100 to 150 μm long and 20 μm thick) crystals required for microspectrophotometry were grown from PEG 8000 (Hampton Research) solutions at room temperature as previously described (Rivetti et al. 1993b). Once grown, the crystals were washed first with 20% (w/v) and then 36% (w/v) anaerobic PEG containing 10 mM phosphate at pH 7.2 and 30 mM sodium dithionite. Crystals were then stored at 4°C until used for data collection.
Before spectra were collected, single crystals of either βY35F or βY35A were first resuspended in 36% (w/v) PEG 8000, 10 mM potassium phosphate, 1 mM EDTA, 30 mM dithionite at pH 7.0. Then crystals of βY35F were suspended eight times in a solution containing 54% (w/v) PEG 8000, 10 mM potassium phosphate, 3270 U/ml catalase at pH 7.0, unless otherwise stated, and loaded into the Dvorak-Stotler flow cell (Dvorak and Stotler 1971) in air. Crystals of βY35A crack on exposure to air; therefore, the PEG solution was saturated with nitrogen, and the resuspension and loading into the Dvorak-Stotler cell took place in an anaerobic glove box.
Polarized absorption spectra were recorded with the electric vector of the linearly polarized light parallel to the a and c optical axes for crystals of βY35F and parallel to the b and c axes for crystals of βY35A. Oxygen pressures between 0 and 760 torr were obtained from gas mixtures prepared using a gas-mixing generator, Environics 200. Oxygen-binding curves were determined at 15°C. The fractional saturation with oxygen and the fractional concentration of oxidized hemes were calculated by fitting of the observed spectra to a linear combination of reference spectra from deoxy, oxy, and oxidized hemoglobin crystals plus baseline (Rivetti et al. 1993a). In the case of βY35F crystals, HbA reference spectra were used, whereas in the case of βY35A crystals, reference spectra were obtained by exposing βY35A crystals to a dithionite-containing solution (deoxyhemoglobin spectra), then after removal of dithionite, to a solution containing 5 mM ferricyanide (oxidized reference spectra). Oxyhemoglobin reference spectra were obtained by exposing crystals to an oxygen pressure of 760 torr at 5°C. Under these conditions, the crystals are fully saturated and still birefringent, despite evident crystal cracks. All measurements were performed with a content of oxidized hemes <15%.
X-ray diffraction analysis
Before crystallization, all mutant hemoglobins were stripped of organic and inorganic ions by passing them over a Dintzis column (Riggs 1981), which was modified by the addition of a 1-mm layer of chelating resin (iminodiacetic acid, Sigma #C-7901) to the top of the column. The stripped oxyhemoglobins were frozen and stored in liquid nitrogen until used for crystallization.
Monoclinic crystals (space group P21) of the mutant deoxyhemoglobins were grown in 100-μL batch set-ups at room temperature from solutions of concentrated ammonium sulfate, as described by Perutz (1968) for deoxyhemoglobin A, except that 10 mM ferrous citrate was used as the reducing agent. Crystals of βY35F (unit cell dimensions: a = 63.2 Å, b = 83.7 Å, c = 53.8 Å, and β = 99.4°) and βY35A (unit cell dimensions: a = 63.2 Å, b = 83.7 Å, c = 53.7 Å, and β = 99.3°) were isomorphous with βV1M crystals (unit cell dimensions: a = 63.2 Å, b = 83.7 Å, c = 53.8 Å, and β = 99.4°). All crystallization solutions were thoroughly deoxygenated before their use, and all crystallization work was conducted in a glove bag that was continuously purged with nitrogen.
Single crystals of the βY35F and βY35A mutants were washed briefly in crystallization buffer in which the total salt concentration was increased to 2.6 M (Perutz 1968) and then mounted in quartz capillaries for data collection. Diffraction data were collected on a Rigaku AFC6 diffractometer fitted with a San Diego Multiwire Systems area detector. All diffraction data were scaled and merged according to the procedure of Howard et al. (1985). In each case, degradation because of radiation damage was <15% as determined from a subset of diffraction data that was collected at the beginning and end of data collection. Data were collected out to a resolution of 1.8 Å (48,043 independent reflections) and 1.7 Å (55,581 independent reflections), respectively, for the crystals of the βY35A and βY35F mutants. The Rsymm values for the βY35A and βY35F data sets are 6.1% and 6.3%, respectively. Each mutant data set was split into a working set, consisting of 90% of the data, that was used for refinement, and a test set, consisting of 10% of the data, that was used to calculate Rfree (Brünger 1992a).
Refinement of the deoxy βY35F and βY35A crystal structures consisted of rigid body refinement using X-PLOR (Brünger 1992b) followed by restrained least-squares refinement with PROLSQ (Hendrickson 1985; Sheriff 1987). The initial atomic model for both refinements was the 1.8 Å structure of deoxyhemoglobin βV1M (Kavanaugh et al. 1993) in which Tyr35β was converted to a phenylalanine or an alanine. The standard crystallographic R values for the initial βY35F and βY35A models were 0.177 and 0.174, respectively, for data between 8.0 Å and 1.8 Å resolution with magnitudes >2σ (48,021 reflections for βY35F and 42,698 reflections for βY35A). Twenty cycles of rigid body refinement of the entire tetramer, followed by 20 refinement cycles of the two αβ dimers, and finally 20 refinement cycles of the four individual subunits resulted in an R value of 0.170 for both mutant structures. Following rigid body refinement, the structures were subjected to 15 cycles of restrained least-squares refinement, which included refinement of individual atomic temperature factors. The resulting βY35F and βY35A atomic models have R values of 0.162 and 0.157, respectively, and corresponding Rfree values of 0.218 and 0.224. The final atomic models have excellent stereochemistry with root mean square (rms) deviations from ideal bond lengths of 0.014 Å and 0.016 Å and rms deviations from ideal angles of 1.7° and 1.6° for βY35F and βY35A, respectively. The mutant atomic models were analyzed using the least-squares superposition methods previously described (Kavanaugh et al. 1992b, 1998).
Acknowledgments
This work was supported by National Institutes of Health Program Project Grant PO1 GM58890. Refined coordinates and structure factors for the βY35F and βY35A mutant hemoglobins have been deposited in the Brookhaven Protein Data Bank.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
Bis-tris, bis(2-hydroxyethyl)iminotris(hydroxymethyl)methane
Tris, tris(hydroxymethyl)aminomethane
EDTA, disodium ethylenediaminetetraacetate
PEG, polyethylene glycol
r.m.s., root mean square
IHP, inositol hexaphosphate
HbA, human hemoglobin major component
βV1M, recombinant hemoglobin with the Val 1β → Met mutation
βY35F, recombinant hemoglobin with the Tyr 35β → Phe and Val 1β → Met mutations
βY35A, recombinant hemoglobin with the Tyr 35β → Ala and Val 1β → Met mutations
met-hemoglobin, hemoglobin with iron oxidized to Fe(III).
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/
References
- Allen, D.W., Guthe, K.F., and Wyman, J. 1950. Further studies on the oxygen equilibrium of hemoglobin. J. Biol. Chem. 187 393–410. [PubMed] [Google Scholar]
- Antonini, E., Wyman, J., Zito, R., Rossi-Fanelli, A., and Caputo, A. 1961. Studies on carboxypeptidase digests of human hemoglobin. J. Mol. Biol. 236 PC60–PC63. [PubMed] [Google Scholar]
- Asakura, T., Adachi, K., Wiley, J.S., Fung, L.W., Ho, C., Kilmartin, J.V., and Perutz, M.F. 1976. Structure and function of haemoglobin Philly (Tyr C1 [35] beta replaced by Phe). J. Mol. Biol. 104 185–195. [DOI] [PubMed] [Google Scholar]
- Asakura, T., Adachi, K., Schwartz, E., and Wiley, J. 1981. Molecular stability of Hb Philly (α2β2 35[C1] Tyr → Phe): The relationship of hemoglobin stability to ligand state as defined by heat and mechanical shaking tests. Hemoglobin 5 177–190. [DOI] [PubMed] [Google Scholar]
- Brünger, A.T. 1992a. Free R value: A novel statistical quantity for assessing the accuracy of crystal structures. Nature 355 472–474. [DOI] [PubMed] [Google Scholar]
- Brünger, A.T. 1992b. X-PLOR, version 3.1. Yale University Press, New Haven.
- Doyle, M.L., Lew, G., De Young, A., Kwiatkowski, L., Wierzba, A., Noble, R.W., and Ackers, G.K. 1992. Functional properties of human hemoglobins synthesized from recombinant mutant beta-globins. Biochemistry 31 8629–8639. [DOI] [PubMed] [Google Scholar]
- Dvorak, J.A. and Stotler, W.F. 1971. A controlled-environment culture system for high resolution light microscopy. Exp. Cell Res. 68 144–148. [DOI] [PubMed] [Google Scholar]
- Gibson, Q.H. 1959. Photochemical formation of a quick reacting form of haemoglobin. Biochem. J. 71 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, Q.H. and Milnes, L. 1964. Apparatus for rapid and sensitive spectrophotometry. Biochem. J. 91 161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, A., Suzuki, T., and Shin, M. 1973. An enzymatic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim. Biophys. Acta 310 309–316. [DOI] [PubMed] [Google Scholar]
- Hendrickson, W.A. 1985. Stereochemically restrained refinement of macromolecular structures. Methods Enzymol. 115 252–270. [DOI] [PubMed] [Google Scholar]
- Hernan, R.A., Hui, H.L., Andracki, M.E., Noble, R.W., Sligar, S.G., Walder, J.A., and Walder, R.Y. 1992. Human hemoglobin expression in Escherichia coli: Importance of optimal codon usage. Biochemistry 31 8619–8628. [DOI] [PubMed] [Google Scholar]
- Howard, A.J., Nielsen, C., and Xuong, N.H. 1985. Software for a diffractometer with multiwire area detector. Methods Enzymol. 114 452–472. [DOI] [PubMed] [Google Scholar]
- Huang, Y. and Ackers, G.K. 1996. Transformation of cooperative free energies between ligation systems of hemoglobin: Resolution of the carbon monoxide binding intermediates. Biochemistry 35 704–718. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, J.S., Rogers, P.H., and Arnone, A. 1992a. High-resolution X-ray study of deoxy recombinant human hemoglobins synthesized from beta-globins having mutated amino termini. Biochemistry 31 8640–8647. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, J.S., Rogers, P.H., Case, D.A., and Arnone, A. 1992b. High-resolution X-ray study of deoxyhemoglobin Rothschild 37β Trp → Arg: A mutation that creates an intersubunit chloride-binding site. Biochemistry 31 4111–4121. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, J.S., Moo-Penn, W.F., and Arnone, A. 1993. Accommodation of insertions in helices: The mutation in hemoglobin Catonsville (Pro 37α-Glu-Thr 38α) generates a 310 → α bulge. Biochemistry 32 2509–2513. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, J.S., Chafin, D.R., Arnone, A., Mozzarelli, A., Rivetti, C., Rossi, G.L., Kwiatkowski, L.D., and Noble, R.W. 1995. Structure and oxygen affinity of crystalline desArg141α human hemoglobin A in the T state. J. Mol. Biol. 248 136–150. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, J.S., Weydert, J.A., Rogers, P.H., and Arnone, A. 1998. High-resolution crystal structures of human hemoglobin with mutations at tryptophan 37β: Structural basis for a high-affinity T-state. Biochemistry 37 4358–4373. [DOI] [PubMed] [Google Scholar]
- Kelly, R.M., Hui, H.L., and Noble, R.W. 1994. Chloride acts as a novel negative heterotropic effector of hemoglobin Rothschild (β37 Trp → Arg) in solution. Biochemistry 33 4363–4367. [DOI] [PubMed] [Google Scholar]
- Kiger, L., Klinger, A.L., Kwiatkowski, L.D., De Young, A., Doyle, M.L., Holt, J.M., Noble, R.W., and Ackers, G.K. 1998. Thermodynamic studies on the equilibrium properties of a series of recombinant βW37 hemoglobin mutants. Biochemistry 37 4336–4345. [DOI] [PubMed] [Google Scholar]
- Kilmartin, J.V., Hewitt, J.A., and Wootton, J.F. 1975. Alteration of functional properties associated with the change in quaternary structure in unliganded haemoglobin. J. Mol. Biol. 93 203–218. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski, L.D., Hui, H.L., Wierzba, A., Noble, R.W., Walder, R.Y., Peterson, E.S., Sligar, S.G., and Sanders, K.E. 1998. Preparation and kinetic characterization of a series of βW37 variants of human hemoglobin A: Evidence for high-affinity T quaternary structures. Biochemistry 37 4325–4335. [DOI] [PubMed] [Google Scholar]
- LiCata, V.J. and Ackers, G.K. 1995. Long-range small magnitude nonadditivity of mutational effects in proteins. Biochemistry 34 3133–3139. [DOI] [PubMed] [Google Scholar]
- Mozzarelli, A., Rivetti, C., Rossi, G.L., Eaton, W.A., and Henry, E.R. 1997. Allosteric effectors do not alter the oxygen affinity of hemoglobin crystals. Protein Sci. 6 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, M., Wajcman, H., Lahary, A., Nakatsukasa, T., Nagatomo, S., and Kitagawa, T. 1999. Quaternary structure sensitive tyrosine residues in human hemoglobin: UV resonance raman studies of mutants at α140, β35, and β145 tyrosine. Biochemistry 38 1243–1251. [DOI] [PubMed] [Google Scholar]
- Nagel, R.L., Wittenberg, J.B., and Ranney, H.M. 1965. Oxygen equilibria of the hemoglobin-haptoglobin complex. Biochim. Biophys. Acta 100 286–289. [DOI] [PubMed] [Google Scholar]
- Nakatsukasa, T., Nomura, N., Miyazaki, G., Imai, K., Wada, Y., Ishimori, K., Morishima, I., and Morimoto, H. 1998. The artificial α1β1-contact mutant hemoglobin, Hb Phe-35β, shows only small functional abnormalities. FEBS Lett. 441 93–96. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. 1968. Preparation of haemoglobin crystals. J. Crystal Growth 2 54–56. [Google Scholar]
- ———. 1970. Stereochemistry of cooperative effects in haemoglobin. Nature 228 726–739. [DOI] [PubMed] [Google Scholar]
- ———. 1989. Mechanisms of cooperativity and allosteric regulation in proteins. Q. Rev. Biophys. 22 139–237. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. and TenEyck, L.F. 1972. Stereochemistry of cooperative effects in hemoglobin. Cold Spring Harb. Symp. Quant. Biol. 36 295–310. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F., Shih, D.T., and Williamson, D. 1994. The chloride effect in human haemoglobin: A new kind of allosteric mechanism. J. Mol. Biol. 239 555–560. [DOI] [PubMed] [Google Scholar]
- Peterson, E.S. and Friedman, J.M. 1998. A possible allosteric communication pathway identified through a resonance Raman study of four β37 mutants of human hemoglobin A. Biochemistry 37 4346–4357. [DOI] [PubMed] [Google Scholar]
- Rieder, R.F., Oski, F.A., and Clegg, J.B. 1969. Hemoglobin Philly (β35 Tyrosine → Phenylalanine): Studies in the molecular pathology of hemoglobin. J. Clinical Invest. 48 1627–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs, A. 1981. Preparation of blood hemoglobins of vertebrates. Methods Enzymol. 76 5–29. [DOI] [PubMed] [Google Scholar]
- Rivetti, C., Mozzarelli, A., Rossi, G.L., Henry, E.R., and Eaton, W.A. 1993a. Oxygen binding by single crystals of hemoglobin. Biochemistry 32 2888–2906. [DOI] [PubMed] [Google Scholar]
- Rivetti, C., Mozzarelli, A., Rossi, G.L., Kwiatkowski, L.D., Wierzba, A.M., and Noble, R.W. 1993b. Effect of chloride on oxygen binding to crystals of hemoglobin Rothschild (β37 Trp → Arg) in the T quaternary structure. Biochemistry 32 6411–6418. [DOI] [PubMed] [Google Scholar]
- Sheriff, S. 1987. Addition of symmetry-related contact restraints to PROTIN and PLOSQ. J. Appl. Crystallogr. 20 55–57. [Google Scholar]