Abstract
The overproduction of eukaryotic membrane proteins is a major impediment in their structural and functional characterization. Here we have used the nisin-inducible expression system of Lactococcus lactis for the overproduction of 11 mitochondrial transport proteins from yeast. They were expressed at high levels in a functional state in the cytoplasmic membrane. The results also show that the level of expression is influenced by the N-terminal regions of the transporters. Expression levels were improved >10-fold either by replacing or truncating these regions or by adding lactococcal signal peptides. The observed expression levels are now compatible with a realistic exploration of crystallization conditions. The lactococcal expression system may be used for the high-throughput functional characterization of eukaryotic membrane proteins and structural genomics.
Keywords: eukaryotic membrane proteins, mitochondrial carriers, nisin-inducible expression system, Lactococcus lactis, transport
Eukaryotic integral membrane proteins account for ~25% of open reading frames in genomes (Wallin and von Heijne 1998). Many of them are targets for drug development, and they have vital roles in signal transduction, transport, energy conversion, ion conductance, and cell maintenance. The natural levels of most membrane proteins are low, and their overexpression is a prerequisite for functional and structural studies. Many membrane proteins in sequenced genomes have no assigned function, and so far, the structures of only 17 distinct eukaryotic membrane proteins have been determined, all but one of them naturally abundant (http://blanco.biomol.uci.edu/Membrane_Proteins_xtal.html). Many expression systems for membrane proteins have been explored using yeast, insect, or mammalian hosts (Lundstrom 2005), but bacterial overexpression of eukaryotic membrane proteins remains a challenge. Problems arise from fundamental differences in the biogenesis of membrane proteins in eukaryotic and prokaryotic cells (Biochim. Biophys. Acta 1610: 1–153). Another difficulty is that the production of large amounts of membrane proteins often impairs the growth and viability of the expression host. Even when high expression levels are achieved, the expressed membrane proteins are often found in inclusion bodies in a misfolded inactive state (Miroux and Walker 1996; Korepanova et al. 2005).
The Gram-positive lactic acid bacterium Lactococcus lactis has several characteristics that help with large-scale overproduction of proteins (Kunji et al. 2003). The cells grow rapidly to high densities without aeration. Multiple amino acid auxotrophic strains are available that can be used for the incorporation of seleno-methionine for solving phases in X-ray diffraction data or for specific labeling used in NMR studies. A strong and tightly regulated promoter system, based on nisin-controlled expression, allows highly reproducible expression even when the proteins are toxic to the cell (de Ruyter et al. 1996; Kunji et al. 2003). Transport and binding assays for the characterization of the membrane protein can be performed with whole cells, because ligands, inhibitors, and ionophores can act directly on the cytoplasmic membrane in which the membrane proteins are expressed.
In this paper, the effectiveness of L. lactis for the overproduction of eukaryotic membrane proteins has been demonstrated with mitochondrial transporters or carriers. These proteins are found in the inner membranes of mitochondria; they have no prokaryotic homologs, and they transport metabolites and cofactors across the inner membrane (for recent reviews, see Kunji 2004 and Palmieri 2004). Each carrier contains six trans-membrane α-helices, with the N and C termini in the intermembrane space, and the sequence consists of a tripartite sequence repeat of ~100 amino acids containing a signature motif (Saraste and Walker 1982). In the structure of the mitochondrial ADP/ATP carrier in detergent (Pebay-Peyroula et al. 2003) and the membrane (Kunji and Harding 2003), the three repeats form an α-helical bundle with pseudo threefold symmetry. Like most mitochondrial proteins, the carriers are encoded by the nuclear genome, translated in the cytosol, and imported into mitochondria subsequently via the TOM complex in the outer mitochondrial membrane. In the intermembrane space, they enter a unique targeting pathway consisting of chaperones TIM9/10 and the insertion machinery TIM54/22/12 (Rassow et al. 1999; Rehling et al. 2003a,b).
Although the carriers have been overproduced in Escherichia coli in large amounts, the overexpressed proteins are found in inclusion bodies, misfolded, and nonfunctional, and they do not enter the cytoplasmic membrane in significant amounts (Fiermonte et al. 1993). Purification and refolding strategies have been developed, and they have been used successfully in the identification of novel carriers (Palmieri et al. 1996; Palmieri 2004). However, the efficiency of in vitro refolding is very low, and the amounts obtained are incompatible with crystallization trials. Mitochondrial carriers have also been overproduced in yeast mitochondria (Palmieri et al. 1999a), but their purification from carriers with overlapping substrate specificities is difficult (Kunji 2004; Palmieri 2004). Their intrinsic instability in detergents and their susceptibility to proteolysis are additional complications.
Here we show that 11 different carriers were targeted to the cytoplasmic membrane of L. lactis and the proteins were active. The levels of functional expression were improved by rational design. These procedures provide a new high-throughput route for the identification of novel carriers and other unknown membrane proteins.
Results
Expression of wild-type yeast mitochondrial carriers
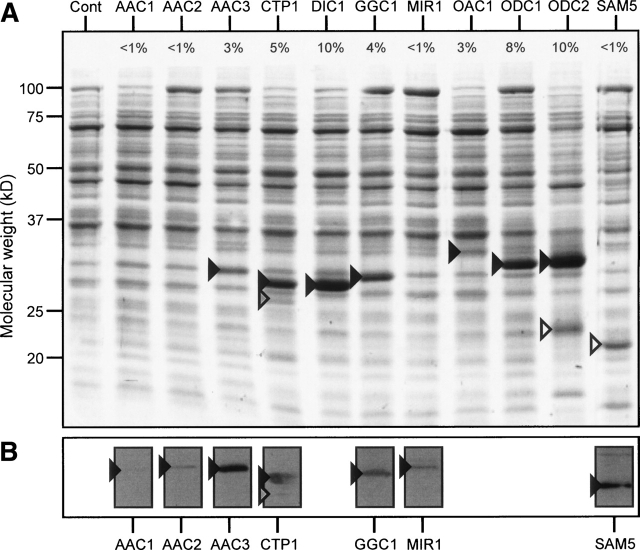
Eleven well-characterized mitochondrial carriers from Saccharomyces cerevisiae were chosen for expression in L. lactis. They are three isoforms of the ADP/ATP carrier AAC1, AAC2, and AAC3 (O’Malley et al. 1982; Lawson and Douglas 1988; Kolarov et al. 1990), the dicarboxylate carrier DIC1 (Palmieri et al. 1996), the GDP/GTP carrier GGC1 (Vozza et al. 2004), the phosphate carrier MIR1 (Guerin et al. 1990), the oxaloacetate carrier OAC1 (Palmieri et al. 1999b), the oxodicarboxylate carriers ODC1 and ODC2 (Palmieri et al. 2001), and the S-adenosyl methionine carrier SAM5 (Marobbio et al. 2003). By fractionation the overexpressed carriers were found exclusively in the cytoplasmic membrane up to levels of 10% of total membrane protein (1 mg/L) (Fig. 1A ▶). In contrast, AAC1, AAC2, MIR1, and SAM5 were expressed poorly, and were detected as a faint band in Western blots (Fig. 1B ▶). No proteolytic fragments were detected, and the addition of protease inhibitors during the isolation of the membranes was not required (Fig. 1 ▶). The only exception was CTP1, where a minor degradation product was observed (<5% of total). The N terminus was intact, but a part of the C-terminal region was missing (Fig. 1B ▶).
Figure 1.
The expression of yeast mitochondrial carriers in lactococcal membranes. (A) SDS-polyacrylamide gel stained with Coomassie blue and (B) Western blots labeled with specific antibodies. ~10 μg membrane protein was loaded in each lane. The control membranes (Cont) were isolated from a strain containing the expression vector pNZ8048 lacking insert. The identities of the expressed carriers were confirmed by the specific antibodies in the Western blots or by N-terminal sequencing. The numbers indicate the expression level of the carrier compared to total membrane protein. Black arrows indicate the position of the expressed mitochondrial carriers the gray arrow a proteolytic fragment of CTP1 as determined by N-terminal sequencing, and white arrows lactococcal membrane proteins that are upregulated by expression of mitochondrial carriers as identified by N-terminal sequencing and mass spectroscopy.
In mitochondria, the carriers exchange one substrate molecule in the mitochondrial matrix for another in the cytosol. Lactococcal membrane vesicles were fused with liposomes in the presence of nonradioactive substrate, and transport activities were measured by the uptake of radiolabeled substrates into the vesicles in exchange for incorporated unlabeled substrates. High transport rates were observed for AAC3, CTP1, DIC1, GGC1, and ODC1 (Fig. 2 ▶) and much lower rates for AAC1, AAC2, OAC1, and ODC2, but still significantly above background. The transport activity of MIR1 was barely detectable (Supplemental Material). Uptake of substrates was observed only when vesicles were loaded with the relevant substrate, implying that transport is coupled tightly to the presence of the incorporated substrate. In control experiments with membranes lacking overexpressed carriers, no significant uptake was observed for these substrates (Fig. 2 ▶), so endogenous transport activities for these substrates are lacking in lactococcal membranes.
Figure 2.
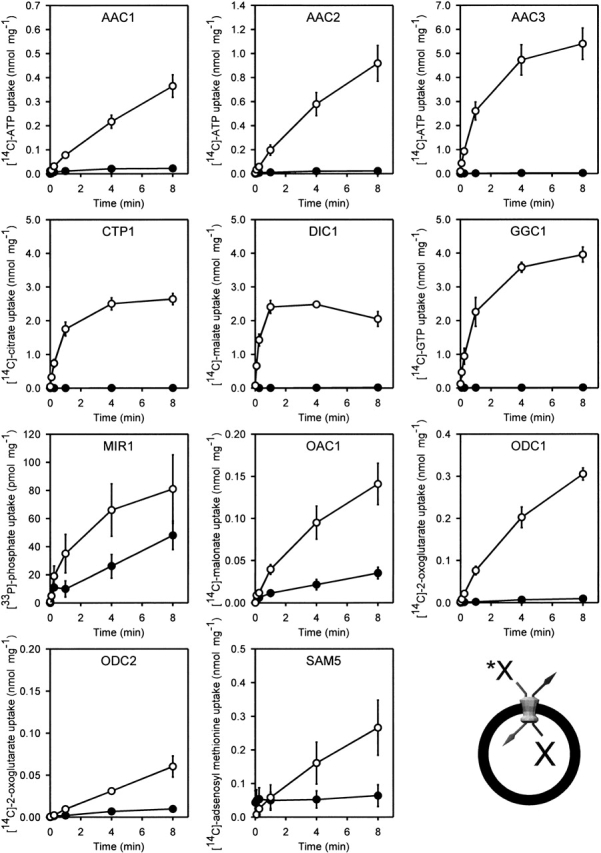
The activity of yeast mitochondrial carriers in transport assays. Lactococcal membranes from the control strain (filled circles) and strains expressing the mitochondrial carriers (open circles) were fused to liposomes in the presence of 5 mM of the relevant unlabelled substrate (X in diagram). Transport was initiated by diluting these vesicles in a buffer containing the same, but radiolabeled, substrate to a final concentration of 1.34 μM (*X in diagram). At different time intervals, vesicles were separated by filtration from the external buffer and the levels of accumulated radioactive substrate were determined by scintillation counting. The accumulated substrate levels were corrected for the total amount of membrane protein. The uptake data are the average of three independent experiments starting from membrane vesicles with the standard error of the mean (SEM).
The expression levels of the three yeast ADP/ATP carriers differed greatly, and in comparison with AAC3, the initial transport rates of AAC2 and AAC1 were 12.2% and 1.5%, respectively. The N-terminal regions of these three isoforms vary both in length and composition, whereas the rest of their sequences are 76% identical (Supplemental Material). The AAC3 is expressed in the lactococcal membrane with its N and C termini to the outside of the cell (Kunji et al. 2003) in accordance with the “positive inside rule” (von Heijne 1986). The requirement to take the N terminus to the outside of the cell may be a limitation in the insertion of membrane proteins in bacteria (Dalbey et al. 1995; Whitley et al. 1995). As all mitochondrial carriers have the same membrane topology, the composition and length in the N-terminal regions could influence overexpression to different degrees.
Therefore, residues 1–21 of the poorly expressed AAC2 were exchanged for residues 1–10 of the well-expressed AAC3 (Fig. 3A ▶). The resulting hybrid protein had an initial transport rate that was six times higher than that of AAC2 (Fig. 3B ▶).
Figure 3.
Replacement of the N-terminal region of the yeast ADP/ATP carrier AAC2 with the equivalent region of AAC3. (A) Schematic representation of the N-terminal region and the first transmembrane α-helix of AAC2 (top), AAC3 (middle), and the hybrid AAC2 with the AAC3 N-terminal region (bottom). The assignment of secondary structure is based on the structure of the bovine ADP/ATP carrier (Pebay-Peyroula et al. 2003). Western blot (B) and transport activity (C) of the control strain and strains expressing AAC3, AAC2, and the hybrid of the AAC2 with the N-terminal region of AAC3. The black arrows indicate the molecular weight of the expressed carriers and hybrid. The uptake data are the average of three independent experiments starting from membrane vesicles with the standard error of the mean (SEM).
Expression of N-terminally truncated mitochondrial carriers
The compositions and lengths of the region preceding the first transmembrane α-helix are not conserved between carriers with the same function. Also, secondary structure predictions and the bovine ADP/ATP structure (Pebay-Peyroula et al. 2003) show that these regions do not contribute directly to the core structure. To determine the effect of the N-terminal region of AAC2 on protein expression and activity, a series of N-terminally truncated versions of the carrier was made (Fig. 4A ▶). The protein expression levels of Δ2–6 AAC2, Δ2–19 AAC2, Δ2–21 AAC2, and Δ2–24 AAC2 were considerably higher than the level of wild-type AAC2 (Fig. 4B ▶). The transport activities of Δ2–19 AAC2 and Δ2–21-AAC2 were >40-fold and 20-fold higher than the wild-type protein (Fig. 4C ▶). Deletion of residues 1–19 increased the specific activity fourfold, when the transport rates are corrected for the differences in expression levels (Supplemental Material), so the truncation improved both the quantity and quality of the expressed material. The Δ2–19 AAC2 deletion removes two lysine residues that could be an obstacle for the translocation of the N-terminal region (von Heijne 1986; Whitley et al. 1995). However, analysis of all the N-terminal regions of all of the carriers expressed showed no correlation between the number of positively charged residues in the N-terminal region and the functional expression level. Deletions beyond residue 21 decreased the specific activity, even though the truncated mutant was expressed at high level. As this change affects the first transmembrane α-helix, it would be expected to have severe consequences for the structural integrity of the protein.
Figure 4.

N-terminal truncation series of the yeast ADP/ATP carrier AAC2. (A) Schematic representation of the N-terminal truncations of AAC2. The numbers in the strain names indicate the residues that have been removed. The assignment of secondary structure is based on the structure of the bovine ADP/ATP carrier (Pebay-Peyroula et al. 2003). Western blot (B) and initial uptake rates (C) of the various truncation mutants and AAC2. The arrowheads indicate the molecular weight of the expressed truncation mutants and AAC2. The initial uptake rates, measured after 15 sec, are the average of three independent experiments carried out in triplicate with the standard error of the mean (SEM).
The poorly expressed phosphate (MIR1) and S-adenosyl-methionine (SAM5) carriers were also truncated up to three residues before the start of the predicted first transmembrane α-helix. Truncation of residues 2–14 in the former and residue 2 in the latter increased the initial uptake rates twofold, compared to the wild-type proteins (Supplemental Material).
Expression of mitochondrial carriers with N-terminal signal peptides
Usually mitochondrial carriers are imported into the inner membrane via the TOM/TIM insertion machinery. However, L. lactis lacks this pathway, but endogenous membrane proteins are inserted into membranes by the Sec translocase (Koivula et al. 1991). Therefore, three different signal peptides were fused to the N terminus of AAC1. A similar approach has been used successfully for the overproduction of G-protein coupled receptors in E. coli and in Pichia pastoris (Grisshammer et al. 1993; Weiss et al. 1995). The type II signal peptide from the oligopeptide binding protein OppA (spOppA) and the type I signal peptides from the cell wall PI type proteinase PrtP (spPrtP) and the secreted protein Usp45 (spUsp45) were employed (Fig. 5A ▶). All three of them are used in the export of soluble proteins, and they contain endogenous cleavage sites for the signal peptidase as predicted by SignalP (Bendtsen et al. 2004). The expression levels and transport activities of the signal peptide–AAC1 fusion proteins were more than five times higher than those of the wild-type protein (Fig. 5B ▶). However, the spPrtP and spUsp45 signal sequences were removed partially, and the spOppA signal sequence was not removed at all, as confirmed by N-terminal sequencing. An unsuccessful attempt was made to improve their cleavage efficiency by introducing more residues of OppA following the signal peptidase cleavage site (data not shown). It is not clear whether the increased transport activities of the spPrtP and spUsp45-AAC1 fusions originate from the cleaved or noncleaved fraction of the protein, but the product of the spOppA–AAC1 fusion is active.
Figure 5.

Lactococcal N-terminal signal peptides fused to the yeast ADP/ATP carrier AAC1. (A) Schematic representation of the lactococcal signal peptides from OppA, PrtP, and Usp45 fused to the N terminus of AAC1. The cleavage sites of the endogenous signal peptidases as indicated by a pair of scissors were predicted with SignalP (Bendtsen et al. 2004). Western blot (B) and transport activity (C) of the fusion proteins in comparison to the wild-type AAC1. The black arrow indicates the molecular weight of the full-length signal peptide-AAC1 fusion, and the white arrow, the mature protein after the signal peptide has been removed by endogenous signal peptidases. The initial uptake rates, measured after 1 min, are the average of three independent experiments carried out in triplicate with the standard error of the mean (SEM).
Signal peptides were also added before the N termini of AAC3 and the poorly expressing AAC2, SAM5, and MIR1 carriers. The transport activity increased 17-fold for spPrtP-AAC2, 14-fold for spOppA-AAC2, threefold for spUsp45-AAC2, and twofold for spPrtP-SAM5 and spUsp45-SAM5 compared to the wild-type proteins. The fusions spOppA-MIR1, spOppA-SAM5, spOppA-AAC3, and the spUsp45-AAC3 fusions had similar activities to the wild-type protein, but the rate of transport was threefold lower for the spPrtP-AAC3 fusion (Supplemental Material).
Discussion
By using their predicted topologies and their known directions of insertion as a starting point, we have redesigned the functional expression of mitochondrial carriers. Enhanced overproduction levels were reached that are compatible with structural studies and are comparable to levels obtained by homologous expression in yeast mitochondria. These strategies could also be used for the overproduction of other important eukaryotic membrane proteins, such as G-protein coupled receptors, which have an N terminus-out topology also. The methods presented here are amenable to high-throughput approaches for the identification of membrane proteins of unknown function. The expression system is extremely reliable and reproducible, and active membrane proteins were produced in all examples that were tried. Without exception, the membrane proteins were targeted to the cytoplasmic membrane of L. lactis, and inclusion bodies did not form. Therefore, whole cells or isolated membrane vesicles could be used for activity assays without the need to refold, purify, and reconstitute the proteins into membranes. For the rapid screening of substrates, whole cells can be used, but a sufficient level of an exchangeable substrate in the cytoplasm is required (Kunji et al. 2003). As demonstrated, simple fusions of isolated membrane vesicles with liposomes were used to measure transport activities under controlled substrate concentrations. In cases where the expression levels were hardly detectable, activity could still be measured because the assays are very sensitive. Finally, the lactococcal membranes contained no endogenous transport activities for any of the substrates that were studied, which is a great advantage in experiments to identify new activities. As most of the substrates are nucleotides or important intermediates in energy-generating pathways, it is possible either that L. lactis lacks dedicated transporters or that these substrates are carried by ABC transporters, which are inactive in membrane vesicles because they require ATP. Recently, the lactococcal expression system was used to identify a novel type of adenine nucleotide carrier from the mitosome of the human parasite Entamoeba histolytica (Chan et al. 2005).
Several observations indicate that the length and composition of the N-terminal region preceding the first transmembrane α-helix is a limiting factor in functional expression. The direction of insertion of proteins into L. lactis membranes differs fundamentally from the biogenesis in yeast, where the proteins are produced in the cytosol and inserted into the inner membranes of mitochondria via the TOM/TIM machinery (Rassow et al. 1999). Therefore, during insertion the termini never cross the inner membrane. Lactococcus synthesized the mitochondrial carriers in the cytoplasm and used the Sec pathway to insert them into the membrane, with their N termini being transported to the outside of the cell (Kunji et al. 2003). This is an unusual situation in bacteria where the vast majority of membrane proteins have their N terminus in the cytoplasm (Arai et al. 2003; Daley et al. 2005). Since the expression levels could be enhanced by modifications that are thought to improve targeting and insertion, it is likely that the bacterium has a system of chaperones and proteolytic enzymes to deal with membrane proteins with difficult topologies. A closer examination of this support system could reveal why Lactococcus has these intriguing properties.
Materials and methods
Enzymes and chemicals
Restriction enzymes were purchased from New England Biolabs. The substrates ATP and 2-oxoglutarate were obtained from Sigma, citric acid from BDH, and malonic acid from Fluka. The [8-14C]-ATP, [1,5-14C]-citrate, and 33P-phosphate were from Amersham Biosciences. The [8–14H]-GTP was from Moravek. The [1-14C]-2-oxoglutarate and S-[methyl-14C]-ade-nosyl-L-methionine were from Perkin Elmer Life Sciences and [14C, U]-malate from American Radiolabeled Chemicals. PIPES, malonate were from Fluka. The antibodies directed against specific peptides were produced by Agrisera using the following amino acid residue sequences: for MIR1 KKFFI DNLGYDTASRYK, for SAM5 KVKSRPYISKLYSQ, for GGC1 NHTKITSGQELNRV, for CTP1DDKQSATPKYHN NGR, and for AAC1, AAC2, and AAC3 YPLDTVRRR MMMT.
DNA techniques
The aac1, aac2, aac3, ctp1, oac1, dic1, odc1, odc2, ggc1, sam5, and mir1 genes from S. cerevisiae were amplified by PCR using the KOD HiFi DNA polymerase (Novagen) to introduce a NcoI- and XbaI-compatible site at the 5′ and 3′ ends of the genes, respectively. The DNA fragments were restricted and ligated into the pNZ8048 vector (de Ruyter et al. 1996), previously restricted by NcoI and XbaI. The signal sequences were derived from the codons of the following lactococcal genes: 1–25 of oppA, 1–33 of prtP, and 1–27 of the usp45. The ligation mixes were electroporated into electrocompetent L. lactis strain NZ9000 (Kunji et al. 2003). All constructs were confirmed by DNA sequencing (Oswel sequencing or Cytomyx).
Expression in L. lactis
L. lactis cells were grown at 30°C in M17 medium (Difco), supplemented with 1% glucose and 5 μg/mL of chloramphenicol, until the OD600 reached 0.5, after which expression was induced by nisin A by adding a 1000-fold dilution of the spent medium of the nisin-producing strain NZ9700. Cells were washed in PIPES buffer (10 mM PIPES; Fluka), 50 mM NaCl at pH 7.0) and membrane vesicles were prepared by mechanical disruption at 30 kpsi (Constant Cell Disruption System). Whole cells were removed by centrifugation at 9700g for 2 × 10 min at 4°C (Sorvall) and membranes were collected by ultracentrifugation at 140,000g at 4°C for 30 min (Beckman), washed once in PIPES buffer, and resuspended at a final protein concentration of ~5 mg/mL. The membrane proteins were separated by SDS-PAGE and stained by Coomassie Brilliant Blue. For Western blotting the proteins were transferred to PVDF membranes and labeled with specific peptide antibodies. For quantification of expression levels, the intensities of the bands in Western blots were determined in the linear range by using the Image Master 2D Elite software.
Transport assays
Liposomes were prepared by mixing E. coli polar lipid extracts and egg yolk phosphatidylcholine (both form Avanti Polar Lipids Inc.) in a 3:1 ratio (w/w) in PIPES buffer to a final concentration of 20 mg/mL. To prepare membrane vesicles loaded with substrate, membranes (1 mg of protein) were mixed with liposomes in a ratio of 1:5 protein: lipid (w/w) in 1 mL PIPES buffer with 5 mM substrate (pH 7). The mixture was frozen in liquid nitrogen and slowly thawed seven times before being stored in liquid nitrogen. Prior to the transport assays, the liposome-vesicle fusions were extruded through a 1-μm membranes (Whatman) and collected by centrifugation 300,000g at 4°C for 30 min (Beckmann MLA 130). The pellet was resuspended in 200 μL PIPES buffer with 5 mM substrate (pH 7). To remove the external substrate, the suspension was applied to a 3.5-mL bed volume Sephadex G-75 gel filtration column, previously equilibrated with PIPES buffer at 4°C. The fused membranes (1 mL) were collected and kept on ice for immediate use in transport assays.
Transport in fused membrane vesicles was initiated by diluting 100 μL of membranes into 300 μL PIPES buffer in the presence of radioactively labeled substrate (final concentration 1.34 μM) at 30°C with constant stirring. At regular time intervals the transport was quenched by the addition of 4 mL ice-cold PIPES buffer, immediately followed by filtration over cellulose nitrate filters (0.45-μm pore size). The filters were washed once with 2 mL of ice-cold PIPES buffer, transferred to a scintillation vial, after which 2 mL of Ultima Gold AB scintillation liquid (Packard Bioscience) was added for counting in a Packard TriCarb 2100 TR-liquid scintillation analyzer.
Electronic supplemental material
Supplementary Figure 1 ▶, alignment of the amino acid sequences of the three ADP/ATP carriers from S. cerevisiae AAC1, AAC2, and AAC3; Supplementary Figure 2 ▶, the normalized specific activity of ADP/ATP carrier AAC2 and the N-terminal truncation mutants; Supplementary Figure 3 ▶, expression and transport activity of the different versions of yeast phosphate carrier MIR1; Supplementary Figure 4 ▶, expression and transport activity of the different versions of yeast S-adenosyl-methionine carrier SAM5; Supplementary Figure 5 ▶, transport activity of the different versions of yeast ADP/ATP carrier AAC2; Supplementary Figure 6, transport activity of the different versions of yeast ADP/ATP carrier AAC3.
Acknowledgments
This work was supported by the Medical Research Council UK. D.-J.S. was supported by an HFSPO long-term fellowship; M.M., by an EC Marie Curie Fellowship (MCFI-2002-01203) and EMBO long-term fellowship (ALTF 43-2002). We thank Chris Tate, Peter Rosenthal, and John Walker for many valuable comments on the manuscript. The laboratory is a member of the EC consortium eMeP.
Abbreviations
NMR. nuclear magnetic resonance
TOM, translo-case of the outer membrane
TIM, translocase of the inner membrane.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051689905.
Supplemental material: see www.proteinscience.org
References
- Arai, M., Ikeda, M., and Shimizu, T. 2003. Comprehensive analysis of transmembrane topologies in prokaryotic genomes. Gene 304: 77–86. [DOI] [PubMed] [Google Scholar]
- Bendtsen, J.D., Nielsen, H., von Heijne, G., and Brunak, S. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340: 783–795. [DOI] [PubMed] [Google Scholar]
- Chan, K.W., Slotboom, D.J., Cox, S., Embley, T.M., Fabre, O., van der Giezen, M., Harding, M., Horner, D.S., Kunji, E.R., Leon-Avila, G., et al. 2005. A novel ADP/ATP transporter in the mitosome of the microaerophilic human parasite Entamoeba histolytica. Curr. Biol. 15: 737–742. [DOI] [PubMed] [Google Scholar]
- Dalbey, R.E., Kuhn, A., and von Heijne, G. 1995. Directionality in protein translocation across membranes: The N-tail phenomenon. Trends Cell Biol. 5: 380–383. [DOI] [PubMed] [Google Scholar]
- Daley, D.O., Rapp, M., Granseth, E., Melen, K., Drew, D., and von Heijne, G. 2005. Global topology analysis of the Escherichia coli inner membrane proteome. Science 308: 1321–1323. [DOI] [PubMed] [Google Scholar]
- de Ruyter, P.G., Kuipers, O.P., and de Vos, W.M. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62: 3662–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiermonte, G., Walker, J.E., and Palmieri, F. 1993. Abundant bacterial expression and reconstitution of an intrinsic membrane-transport protein from bovine mitochondria. Biochem. J. 294 (Pt. 1): 293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisshammer, R., Duckworth, R., and Henderson, R. 1993. Expression of a rat neurotensin receptor in Escherichia coli. Biochem. J. 295 (Pt. 2) 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin, B., Bukusoglu, C., Rakotomanana, F., and Wohlrab, H. 1990. Mitochondrial phosphate transport. N-ethylmaleimide insensitivity correlates with absence of beef heart-like Cys42 from the Saccharomyces cerevisiae phosphate transport protein. J. Biol. Chem. 265: 19736–19741. [PubMed] [Google Scholar]
- Koivula, T., Palva, I., and Hemila, H. 1991. Nucleotide sequence of the secY gene from Lactococcus lactis and identification of conserved regions by comparison of four SecY proteins. FEBS Lett. 288: 114–118. [DOI] [PubMed] [Google Scholar]
- Kolarov, J., Kolarova, N., and Nelson, N. 1990. A third ADP/ATP translocator gene in yeast. J. Biol. Chem. 265: 12711–12716. [PubMed] [Google Scholar]
- Korepanova, A., Gao, F.P., Hua, Y., Qin, H., Nakamoto, R.K., and Cross, T.A. 2005. Cloning and expression of multiple integral membrane proteins from Mycobacterium tuberculosis in Escherichia coli. Protein Sci. 14: 148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunji, E.R.S. 2004. The role and structure of mitochondrial carriers. FEBS Lett. 564: 239–244. [DOI] [PubMed] [Google Scholar]
- Kunji, E.R.S. and Harding, M. 2003. Projection structure of the atractylo-side-inhibited mitochondrial ADP/ATP carrier of Saccharomyces cerevisiae. J. Biol. Chem. 278: 36985–36988. [DOI] [PubMed] [Google Scholar]
- Kunji, E.R.S., Slotboom, D.J., and Poolman, B. 2003. Lactococcus lactis as host for overproduction of functional membrane proteins. Biochim. Biophys. Acta 1610: 97–108. [DOI] [PubMed] [Google Scholar]
- Lawson, J.E. and Douglas, M.G. 1988. Separate genes encode functionally equivalent ADP/ATP carrier proteins in Saccharomyces cerevisiae. Isolation and analysis of AAC2. J. Biol. Chem. 263: 14812–14818. [PubMed] [Google Scholar]
- Lundstrom, K. 2005. Structural genomics of GPCRs. Trends Biotechnol. 23: 103–108. [DOI] [PubMed] [Google Scholar]
- Marobbio, C.M., Agrimi, G., Lasorsa, F.M., and Palmieri, F. 2003. Identification and functional reconstitution of yeast mitochondrial carrier for S-adenosylmethionine. EMBO J. 22: 5975–5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroux, B. and Walker, J.E. 1996. Over-production of proteins in Escherichia coli: Mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260: 289–298. [DOI] [PubMed] [Google Scholar]
- O’Malley, K., Pratt, P., Robertson, J., Lilly, M., and Douglas, M.G. 1982. Selection of the nuclear gene for the mitochondrial adenine nucleotide translocator by genetic complementation of the op1 mutation in yeast. J. Biol. Chem. 257: 2097–2103. [PubMed] [Google Scholar]
- Palmieri, F. 2004. The mitochondrial transporter family (SLC25): Physiological and pathological implications. Pflugers Arch. 447: 689–709. [DOI] [PubMed] [Google Scholar]
- Palmieri, L., Palmieri, F., Runswick, M.J., and Walker, J.E. 1996. Identification by bacterial expression and functional reconstitution of the yeast genomic sequence encoding the mitochondrial dicarboxylate carrier protein. FEBS Lett. 399: 299–302. [DOI] [PubMed] [Google Scholar]
- Palmieri, L., Lasorsa, F.M., Iacobazzi, V., Runswick, M.J., Palmieri, F., and Walker, J.E. 1999a. Identification of the mitochondrial carnitine carrier in Saccharomyces cerevisiae. FEBS Lett. 462: 472–476. [DOI] [PubMed] [Google Scholar]
- Palmieri, L., Vozza, A., Agrimi, G., De Marco, V., Runswick, M.J., Palmieri, F., and Walker, J.E. 1999b. Identification of the yeast mitochondrial transporter for oxaloacetate and sulfate. J. Biol. Chem. 274: 22184–22190. [DOI] [PubMed] [Google Scholar]
- Palmieri, L., Agrimi, G., Runswick, M.J., Fearnley, I.M., Palmieri, F., and Walker, J.E. 2001. Identification in Saccharomyces cerevisiae of two isoforms of a novel mitochondrial transporter for 2-oxoadipate and 2-oxoglutarate. J. Biol. Chem. 276: 1916–1922. [DOI] [PubMed] [Google Scholar]
- Pebay-Peyroula, E., Dahout-Gonzalez, C., Kahn, R., Trezeguet, V., Lauquin, G.J., and Brandolin, G. 2003. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 426: 39–44. [DOI] [PubMed] [Google Scholar]
- Rassow, J., Dekker, P.J., van Wilpe, S., Meijer, M., and Soll, J. 1999. The preprotein translocase of the mitochondrial inner membrane: Function and evolution. J. Mol. Biol. 286: 105–120. [DOI] [PubMed] [Google Scholar]
- Rehling, P., Model, K., Brandner, K., Kovermann, P., Sickmann, A., Meyer, H.E., Kuhlbrandt, W., Wagner, R., Truscott, K.N., and Pfanner, N. 2003a. Protein insertion into the mitochondrial inner membrane by a twin-pore translocase. Science 299: 1747–1751. [DOI] [PubMed] [Google Scholar]
- Rehling, P., Pfanner, N., and Meisinger, C. 2003b. Insertion of hydrophobic membrane proteins into the inner mitochondrial membrane—A guided tour. J. Mol. Biol. 326: 639–657. [DOI] [PubMed] [Google Scholar]
- Saraste, M. and Walker, J.E. 1982. Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett. 144: 250–254. [DOI] [PubMed] [Google Scholar]
- von Heijne, G. 1986. The distribution of positively charged residues in bacterial inner membrane proteins correlates with the transmembrane topology. EMBO J. 5: 3021–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vozza, A., Blanco, E., Palmieri, L., and Palmieri, F. 2004. Identification of the mitochondrial GTP/GDP transporter in Saccharomyces cerevisiae. J. Biol. Chem. 279: 20850–20857. [DOI] [PubMed] [Google Scholar]
- Wallin, E. and von Heijne, G. 1998. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 7: 1029–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, H.M., Haase, W., Michel, H., and Reilander, H. 1995. Expression of functional mouse 5-HT5A serotonin receptor in the methylotrophic yeast Pichia pastoris: Pharmacological characterization and localization. FEBS Lett. 377: 451–456. [DOI] [PubMed] [Google Scholar]
- Whitley, P., Gafvelin, G., and von Heijne, G. 1995. SecA-independent translocation of the periplasmic N-terminal tail of an Escherichia coli inner membrane protein. Position-specific effects on translocation of positively charged residues and construction of a protein with a C-terminal translocation signal. J. Biol. Chem. 270: 29831–29835. [DOI] [PubMed] [Google Scholar]