Abstract
The HslVU complex is a bacterial two-component ATP-dependent protease, consisting of HslU chaperone and HslV peptidase. Investigation of protein–protein interactions using SPR in Escherichia coli HslVU and the protein substrates demonstrates that HslU and HslV have moderate affinity (Kd = 1 μM) for each other. However, the affinity of HslU for HslV fivefold increased (Kd ~0.2 μM) after binding with the MBP~SulA protein indicating the formation of a “ternary complex” of HslV–HslU–MBP~SulA. The molecular interaction studies also revealed that HslU strongly binds to MBP~SulA with 10−9 M affinity but does not associate with nonstructured casein. Conversely, HslV does not interact with the MBP–SulA whereas it strongly binds with casein (Kd = 0.2 μM) requiring an intact active site of HslV. These findings provide evidence for “substrate-induced” stable HslVU complex formation. Presumably, the binding of HslU to MBP~SulA stimulates a conformational change in HslU to a high-affinity form for HslV.
Keywords: ATP-dependent protease, proteasomal homolog, protein–protein interaction, SPR analysis
Genome sequencing, biochemical, and structural studies have provided a wealth of information regarding different proteasome-related enzyme systems that play an important role in the intracellular protein homeostasis (Gottesman 1996). In bacteria these include two-component systems ClpAP (Katayama-Fujimura et al. 1987; Maurizi et al. 1990), ClpXP (Gottesman et al. 1993), and HslVU (also known as ClpYQ) (Missiakas et al. 1996; Rohrwild et al. 1996). The HslVU protease is considered as a bacterial analog of the eukaryotic 26S proteasome (Rohrwild et al. 1996; Gille et al. 2003), where the HslU component (a member of AAA-ATPase superfamily) (Neuwald et al. 1999) acts as chaperone (unfoldase) and ATPase (Rohrwild et al. 1996; Seong et al. 2000), and the HslV component, the amino-terminal threonine protease (Chuang et al. 1993), confers peptidase activity. HslU and HslV form a 840,000 relative molecular mass complex with HslV dodecamers of two-stacked hexameric rings and two HslU hexamers bound to both ends of the HslV rings (Bochtler et al. 2000; Sousa et al. 2000; Song et al. 2003). Whereas the carboxy-terminal tail of HslU is found buried inside in Escherichia coli HslVU (Bochtler et al. 2000), it distends and intercalates into a cleft between adjacent HslV subunits in the Haemophilus influenzae HslVU complex (Sousa et al. 2000). This insertion was accompanied by a conformational change in the active site of HslV, which suggested an allosteric activation of HslV by the carboxy-terminal tail of HslU (Sousa et al. 2000; Kwon et al. 2003). The HslV activation studies using synthetic peptides comprising the HslU carboxy-terminal sequence supported the role of this peptide in allosteric activation as well as in HslVU complex formation (Ramachandran et al. 2002; Seong et al. 2002).
In an ATP-dependent process whose mechanistic details are unclear, the HslU hexamer recognizes, unfolds, and translocates substrate proteins into the central proteolytic chamber of the HslV dodecamer for degradation (Huang and Goldberg 1997; Kwon et al. 2003). Furthermore, interaction of HslU stimulates the low basal peptidase activity of HslV (Seol et al. 1997). The HslVU protease is involved in the proteolysis of short-lived proteins such as the cell-division inhibitor SulA protein (Cordell et al. 2003), the heat-shock factor σ32 (Kanemori et al. 1997), and the transcription activator RcsA (Torres-Cabassa and Gottesman 1987). More recently, localization of HslV on the cell surface of Helicobacter pylori has provided the evidence for a role of this enzyme in bacteria–host interaction (Du and Ho 2003). In vitro, the HslVU protease can degrade short peptides as well as mainly nonstructured proteins such as casein (Seol et al. 1997; Yoo et al. 1997). The SulA protein has a high susceptibility to precipitate; therefore, its characterization has been expedited by producing the maltose-binding protein–SulA fusion protein (MBP–SulA) (Higashitani et al. 1995, 1997). The fusion protein has been shown to retain the cell division inhibitory activity in vivo and to behave as in vitro substrate for HslVU and Lon (a homo-oligomer ATP-dependent protease) (Sonezaki et al. 1995). It has been demonstrated that the E. coli HslVU and Lon specifically bind with and degrade the SulA “domain” of the MBP–SulA protein (Higashitani et al. 1997; Seong et al. 1999; Kang et al. 2001).
Despite the fact that HslU and HslV are the constituents of the same system, their affinity for each other has been reported as low, and the two components in the complex are loosely associated (Rohrwild et al. 1996, 1997; Yoo et al. 1997; Bochtler et al. 2000). The yeast two-hybrid assays have shown that E. coli HslU and HslV have a weak interaction (Lee et al. 2003). Earlier biochemical studies on HslVU speculated the requirement of additional factor(s) for the formation and stability of the complex (Rohrwild et al. 1996). These observations raised questions, i.e., whether HslU shuttles between states of high and low affinity for HslV and delivers substrate during the catalytic process (Song et al. 2000) and whether there is any role(s) of protein substrates as “inducing factors” in arrangement of the HslVU complex.
We have performed real-time monitored surface plasmon resonance (SPR) analysis in order to determine interactions in the E. coli HslVU system and its protein substrates. We examined (1) the binding of HslU to free HslV and inhibited HslV, (2) the binding of MBP~SulA fusion protein (as a folded protein) and casein (as a model unfolded protein) to HslV and HslU, and (3) the binding of HslU to HslV in the presence of natural substrate MBP~SulA. The protein–protein interaction studies carried out by SPR technology shed light on molecular recognition events that take part during the catalytic process of the E. coli HslVU system. This series of experiments afforded binding affinities along with kinetic constants related to the interactions between the components of HslVU protease and their protein substrates.
Results and Discussion
Interaction of HslU with free HslV and inhibited HslV
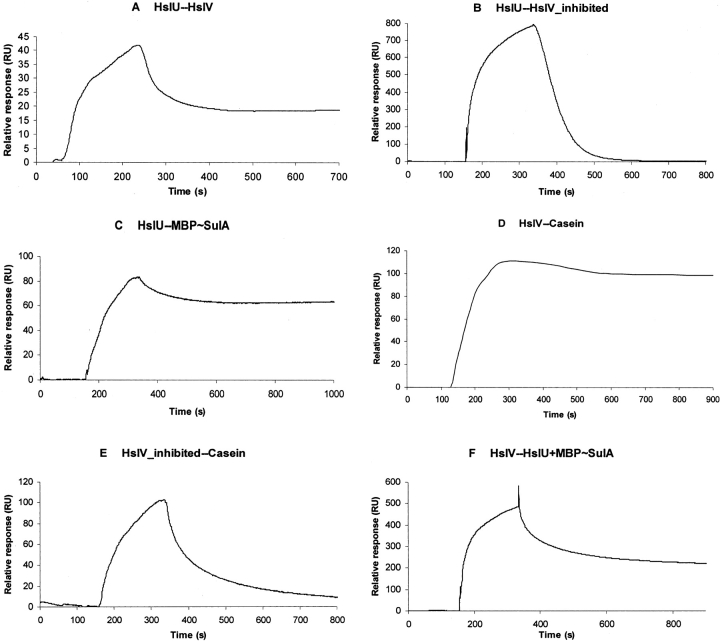
The oligomerization of HslV protomers in the dodecameric form occurs spontaneously; however, ATP binding (but not its hydrolysis) is required for the hexamer formation of HslU, which is a prerequisite for association with HslV (Huang and Goldberg 1997; Rohrwild et al. 1997; Yoo et al. 1997). Therefore, the nonhydrolyzable ATP homolog, ATPγS, was added to the buffer used for immobilization of E. coli HslU on the sensor chip to avoid ADP contamination of samples. SPR analysis showed that the immobilized HslU binds to HslV with Kd = 1.0 μM (Fig. 1A ▶; Table 1). The SPR assays were also performed with immobilized HslV, to check the reproducibility. Very similar Kd values were obtained for both sets of binding experiments. However, binding of HslU to HslV that was inhibited by calpain inhibitor-I is 10–15 times weaker because the dissociation of the complex is much faster as revealed by dissociation curves (Fig. 1B ▶; Table 1).
Figure 1.
Quantitative analysis of protein–protein interactions by surface plasmon resonance. E. coli HslV (free and calpain inhibitor-I inhibited) and HslU were immobilized on sensor chips CM5 (Biacore). Binding curves for HslV (free and inhibited), MBP~SulA, casein, and the mixture of HslU+MBP~SulA were expressed in resonance units (RU) as a function of time. The concentrations of the analytes were as follows: HslV (free and inhibited), 10 μM, MBP~SulA, 1.5 μM, casein, 5 μM; and 1:1 mixture of HslU+MBP~SulA, 1:1 μM. The kinetic parameters are reported in Table 1. Binding curves for (A) HslU and HslV interaction, (B) HslU and inhibited HslV interaction, (C) HslU and MBP~SulA interaction, (D) HslV and casein interaction, (E) inhibited HslV and casein interaction, and (F) HslV and mixture of HslU and MBP~SulA interaction.
Table 1.
Kinetic parameters of protein–protein interactions in the E. coli HslVU and protein substrates MBP–SulA and casein carried out by surface plasmon resonance
| No. | Immobilized protein | Soluble protein | kdiss (sec −1) × 10−3 | kass (M −1 sec−1) × 103 | Kd (μM) |
| 1 | HslU | HslV | 1.0 | 1.0 | 1.0 ± 0.1 |
| 2 | HslU | HslV-inhibited | 16 | 1.1 | 13.0 ± 3.5 |
| 3 | HslV | HslU | 1.4 | 1.1 | 1.1 ± 0.1 |
| 4 | HslU | Casein | N.B. | — | — |
| 5 | HslU | MBP~SulA | 0.15 | 5.0 | 0.03 ± 0.05 |
| 6 | HslV | Casein | 0.31 | 1.5 | 0.2 ± 0.01 |
| 7 | HslV | MBP~SulA | N.B. | — | — |
| 8 | HslV-inhibited | Casein | 7.1 | 0.04 | ~200 ± 50 |
| 9 | HslV-inhibited | MBP~SulA | N.B. | — | — |
| 10 | HslV | HslU+MBP~SulA | 0.7 | 3.0 | 0.2 ± 0.03 |
Binding assays involving HslU were carried out in the presence of 0.1 mM ATPγS. Kinetic data are mean values of three independent experiments and shown by the error range for the Kd values (N.B., no binding).
The crystallographic and peptide activation studies have suggested the role of the carboxy-terminal tails of HslU in HslVU complex formation (Sousa et al. 2000; Ramachandran et al. 2002; Seong et al. 2002). However, the observed HslU affinity for HslV (Kd= 1–1.2 μM) is ~15× more than the binding of synthetic peptide (comprising the HslU carboxy-terminal sequence) to HslV determined by fluorescence spectroscopy measurements (Kd = 17 μM) (Ramachandran et al. 2002).
Interaction of HslU and HslV with the protein substrates MBP~SulA and casein
In order to ascertain the binding affinities of HslU and HslV for their protein substrates, MBP~SulA fusion protein and casein have been used. Freshly purified MBP~SulA and casein samples were applied on HslU and HslV immobilized on separate chips. The bindings assays of HslU and HslV for MBP~SulA and casein revealed intriguing results that showed that HslU binds to MBP~SulA with high affinity (Kd = 30 nM) (Fig. 1C ▶), whereas HslV does not interact with it. On the other side, HslU did not bind with casein while HslV formed a strong complex with it (Kd = 200 nM) (Fig. 1D ▶). The strong affinity of HslU for MBP~SulA (Kd = 30 nM) is comprehensible as the unfolding of structured proteins like MBP~SulA is a necessary step during the catalytic cycle of HslVU (Song et al. 2000). It has been demonstrated that E. coli HslU exclusively interacts with the SulA “domain” of the MBP–SulA fusion protein (Kang et al. 2001); therefore, the recognition and binding of SulA and not the MBP portion are responsible for this effect. On the contrary, the unfolding is not required for the proteolysis of nonstructured casein; therefore, HslV can directly and strongly (Kd = 200 nM) bind with it. The nonaffinity of HslV for MBP~SulA has also been observed by two-hybrid assays that showed that HslV alone did not appear to associate with its natural in vivo substrates SulA, RcsA, and RpoH (Lee et al. 2003).
The binding experiments were also performed between the inhibited HslV and casein, in order to scrutinize the casein-binding site in HslV. An accessible active site of the HslV protease is required for binding to casein as the inhibited HslV showed virtually no binding with a 1000× decrease in binding affinity (Kd ~200 μM) (Fig. 1E ▶; Table 1). This is different from the SPR analysis of 20S proteasome and insulin B-chain interactions (Dorn et al. 1999), which elucidated that the binding of the insulin B-chain was not affected by blocking the proteasome active site with a specific inhibitor, and thus the substrate-binding site in the proteasome was found distinct from the catalytic site.
Interaction of HslV with the mixture HslU+MBP~SulA; formation of the HslVU–substrate complex
After finding the affinities of HslU and HslV for each other and for the protein substrates, we determined the role of protein substrates in HslVU complex formation. For this purpose, the 1:1 mixture of HslU+MBP~SulA was applied to the immobilized HslV (the hexameric form of HslU was considered as a unit with a molecular mass of 300 kDa). The mixture of HslU+MBP~SulA bound with HslV with 5× increased affinity (Kd = 0.2 μM) compared to the binding of HslU to HslV (Kd = 1.0 μM), indicating the formation of a “ternary complex” of HslV–HslU–MBP~SulA (Fig. 1F ▶).
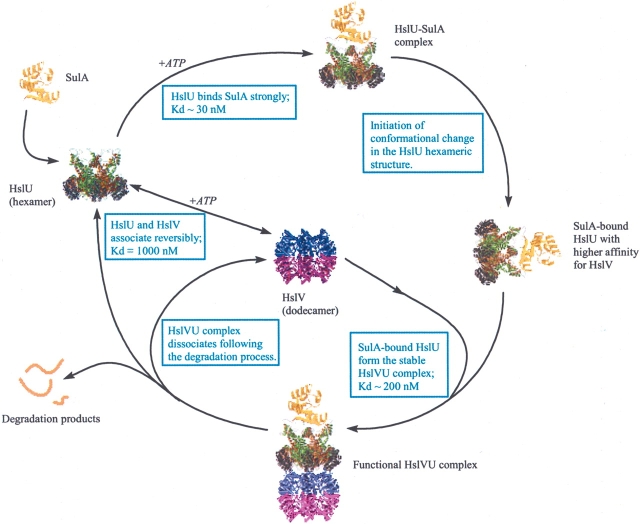
The requirement of additional factor(s) in the formation of stable and functional HslVU complex has been contemplated (Rohrwild et al. 1996). The HslV–HslU binding assays in the presence of folded substrate MBP~SulA showed the formation of a stronger complex and suggested an “inducing role” for MBP~SulA in the assembly of the HslVU complex. As it was determined before that the HslU forms a strong complex with MBP~SulA, it is therefore plausible that interaction with MBP~SulA initiates conformational changes in the HslU structure, which could result in the tighter binding of HslU to its counterpart HslV (Fig. 2 ▶). Compared to the binding affinity of synthetic peptides (comprising the HslU carboxy-terminal sequence) to HslV (Ramachandran et al. 2002), the HslU–MBP~SulA complex formed an 80× stronger association with HslV (Kd = 17μM vs. 0.2 μM) (Table 1). This observation pointed out that C terminus of HslU is not the only structural device that takes part in the docking of HslU to HslV. Binding measurements with complementary methods such as light scattering using other native and in vitro protein substrates would be useful in order to further characterize the “substrate-assisted” HslVU assemblage. As per our experience, the unfolding and aggregation of MBP~SulA protein start shortly after elution from the affinity column; therefore, we attempted to perform SPR assays involving MBP~SulA as soon as possible after purification. It is possible that under in vivo conditions SulA could mediate HslVU complex formation with kinetic parameters stronger than observed in in vitro conditions.
Figure 2.
Schematic illustration of “substrate-induced” HslVU assemblage inferred from SPR data. The affinity of HslU augments for HslV following association with the MBP~SulA and shaping the HslVU–substrate complex. Presumably, after proteolysis and release of degradation products, the HslVU complex dissociates into its components and is ready for another round.
Materials and methods
Protein purification
E. coli HslV and HslU were purified as previously described (Bochtler et al. 1997, 2000). The state of oligomerization in the presence and absence of ATP was assessed by ultracentrifugation and gel filtration (data not shown). As SulA has a high tendency to aggregate, therefore, maltose-binding protein with SulA fusion protein (MBP~SulA) was used as the substrate. The pMal-p2-SulA plasmid and E. coli strain CSH26 were kindly provided by Dr. A. Higashitani (National Institute of Genetics, Japan). MBP~SulA fusion protein was overexpressed and purified as described previously (Higashitani et al. 1997). Casein was purchased from Sigma.
Enzymatic assays
ATPase activity of HslU was determined as described earlier (Lanzetta et al. 1979). Hydrolysis of the fluorogenic peptide carbobenzoxy-Gly-Gly-Leu-7-amido-4-methyl-coumarin by HslV was performed as reported (Rohrwild et al. 1996) with 1 μg of HslV and 2.5 μg of HslU. The FITC-casein was used as a model unstructured protein substrate, and the degradation of FITC-casein was detected by HPLC. The degradation of MBP~SulA fusion protein by HslVU protease was carried out as follows. The reaction mixture (60 μL) contained 4 μg of MBP~SulA, 1 μg of HslV, 2.5 μg of HslU, 0.02% Triton X-100, 1 mM DTT, and 3 mM ATP in 20 mM Tris-HCl (pH 7.5), 5 mM MgCl2 buffer. After 5–7 h of incubation at 37°C, the reaction was stopped by adding 35 μL of 50 mM Tris-HCl (pH 6.8), 0.1 M DTT, 2% SDS, 0.1% bromo-phenol blue, and 10% glycerol, and analyzed on 12% slab gel containing SDS.
Surface plasmon resonance assays
Real-time monitored surface plasmon resonance assays were performed with BIAcore 1000 instrumentation (BIAcore AB). All experiments were conducted at 20°C. The HslU, free HslV, and inhibited HslV (HslV samples were inhibited by the addition of 1 mM calpain inhibitor-I, N-acetyl-Leu-Leu-norleucinal) were immobilized onto carboxylated dextran chips (sensor chip CM5 from BIAcore AB) using the standard amine coupling procedure as recommended by the manufacturer. Immobilization resulted in 9600 resonance units (RU) (HslV), 12,500 RU (HslU), and 5000 RU (HslV-inhibited). Binding assays were performed in 50 mM HEPES (pH 7.4), 150 mM NaCl, 0.05% P20 surfactant (BIAcore), 2 mM CaCl2, and 0.1 mM ATPγS at a flow rate of 20 μL/min. Some experiments were also performed without ATPγS. The soluble analytes were applied at 1–10 μM. Freshly purified MBP–SulA fusion protein samples were used for binding assays in order to avoid aggregation of this protein. After 3 min, dissociation was started by replacing the analyte with buffer. The dissociation curve was monitored for 15 min. The measurements were performed in triplicates using independently prepared sensor chips. Experimental curves (sensograms) were analyzed with BIAevaluation software version 2.1, and kinetic constants were calculated by nonlinear fitting of the association and dissociation curves.
Acknowledgments
We thank Robert Huber and Elisabeth Weyher for helpful discussion and mass spectroscopic analyses, Claudia Hartmann for her contribution to the MBP~SulA purification and MBP~SulA degradation assay, and A. Higashitani (National Institute of Genetics, Japan) for the gift of MBP~SulA fusion protein clone. M.K.A. and R.R. are recipients of research fellowships from the Alexander von Humboldt Foundation, Germany.
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04970405.
References
- Bochtler, M., Ditzel, L., Groll, M., and Huber, R. 1997. Crystal structure of heat shock locus V (HslV) from Escherichia coli. Proc. Natl. Acad. Sci. 94 6070–6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochtler, M., Hartmann, C., Song, H.K., Bourenkov, G.P., Bartunik, H.D., and Huber, R. 2000. The structures of HslU and the ATP-dependent protease HslU–HslV. Nature 403 800–805. [DOI] [PubMed] [Google Scholar]
- Chuang, S.E., Burland, V., Plunkett III, G., Daniels, D.L., and Blattner, F.R. 1993. Sequence analysis of four new heat-shock genes constituting the hslTS/ibpAB and hslVU operons in Escherichia coli. Gene 134 1–6. [DOI] [PubMed] [Google Scholar]
- Cordell, S.C., Robinson, E.J.H., and Lowe, J. 2003. Crystal structure of the SOS cell devision inhibitor SulA and in complex with FtsZ. Proc. Natl. Acad. Sci. 100 7889–7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn, I.T., Eschrich, R., Seemuller, E., Guckenberger, R., and Tampe, R. 1999. High-resolution AFM-imaging and mechanistic analysis of the 20 S proteasome. J. Mol. Biol. 288 1027–1036. [DOI] [PubMed] [Google Scholar]
- Du, R.J. and Ho, B. 2003. Surface localised heat shock protein 20 (HslV) of Helicobacter pylori. Helicobacter 8 257–267. [DOI] [PubMed] [Google Scholar]
- Gille, C., Goede, A., Schlöetelburg, C., Preißner, R., Kloetzel, P.-M., Göbel, U.B., and Frömmel, C. 2003. A comprehensive view on proteasomal sequences: Implications for the evolution of the proteasome. J. Mol. Biol. 326 1437–1448. [DOI] [PubMed] [Google Scholar]
- Gottesman, S. 1996. Proteases and their targets in Escherichia coli. Ann. Rev. Genet. 30 465–506. [DOI] [PubMed] [Google Scholar]
- Gottesman, S., Clark, W.P., De Crecy-Lagard, V., and Maurizi, M.R. 1993. ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. J. Biol. Chem. 268 22618–22626. [PubMed] [Google Scholar]
- Higashitani, A., Higashitani, N., and Horiuchi, K. 1995. A cell division inhibitor SulA of Escherichia coli directly interacts with FtsZ through GTP hydrolysis. Biochem. Biophys. Res. Commun. 209 198–204. [DOI] [PubMed] [Google Scholar]
- Higashitani, A., Ishii, Y., Kato, Y., and Horiuchi, K. 1997. Functional dissection of a cell-division inhibitor, SulA, of Escherichia coli and its negative regulation by Lon. Mol. Gen. Genet. 254 351–357. [DOI] [PubMed] [Google Scholar]
- Huang, H. and Goldberg, A.L. 1997. Proteolytic activity of the ATP-dependent protease HslVU can be uncoupled from ATP hydrolysis. J. Biol. Chem. 272 21364–21372. [DOI] [PubMed] [Google Scholar]
- Kanemori, M., Nishihara, K., Yanagi, H., and Yura, T. 1997. Synergistic roles of HslVU and other ATP-dependent proteases in controlling in vivo turnover of σ32 and abnormal proteins in Escherichia coli. J. Bacteriol. 179 7219–7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, M.S., Lim, B.K., Seong, I.S., Seol, J.H., Tanahashi, N., Tanaka, K., and Chung, C.H. 2001. The ATP-dependent CodWX (HslVU) protease in Bacillus subtilis is an N-terminal serine protease. EMBO J. 20 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama-Fujimura, Y., Gottesman, S., and Maurizi, M.R. 1987. A multiple-component ATP-dependent protease from Escherichia coli. J. Biol. Chem. 262 4477–4485. [PubMed] [Google Scholar]
- Kwon, A.-R., Kessler, B.M., Overkleeft, H.S., and McKay, D.B. 2003. Structure and reactivity of an asymmetric complex between HslV and I-domain deleted HslU, a prokaryotic homolog of the eukaryotic proteasome. J. Mol. Biol. 330 185–195. [DOI] [PubMed] [Google Scholar]
- Lanzetta, P.A., Alvarez, L.J., Reinach, P.S., and Candia, O.A. 1979. An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 100 95–97. [DOI] [PubMed] [Google Scholar]
- Lee, Y.Y., Chang, C.F., Kuo, C.L., Chen, M.C., Yu, C.H., Lin, P.I., and Wu, W.F. 2003. Subunit oligomerization and substrate recognition of the Escherichia coli ClpYQ (HslUV) protease implicated by in vivo protein–protein interactions in the yeast two-hybrid system. J. Bacteriol. 185 2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurizi, M.R., Clark, W.P., Katayama, Y., Rudikoff, S., Pumphery, J., Bowers, B., and Gottesman, S. 1990. Sequence and structure of ClP, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J. Biol. Chem. 265 12536–12545. [PubMed] [Google Scholar]
- Missiakas, D., Schwager, F., Betton, J.M., Georgopoulos, C., and Raina, S. 1996. Identification and characterization of HslV HslU (ClpQ ClpY) proteins involved in overall proteolysis of misfolded proteins in Escherichia coli. EMBO J. 15 6899–6909. [PMC free article] [PubMed] [Google Scholar]
- Neuwald, A.F., Aravind, L., Spouge, J.L., and Koonin, E.V. 1999. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9 27–43. [PubMed] [Google Scholar]
- Ramachandran, R., Hartmann, C., Song, H.K., Huber, R., and Bochtler, M. 2002. Functional interactions of HslV (ClpQ) with the ATPase HslU (ClpY). Proc. Natl. Acad. Sci. 99 7396–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrwild, M., Coux, O., Huang, H.-C., Moerschell, R.P., Yoo, S.J., Seol, J.H., Chung, C.H., and Goldberg, A.L. 1996. HslV–HslU: A novel ATP-dependent protease complex in Escherichia coli related to the eukaryotic proteasome. Proc. Natl. Acad. Sci. 93 5808–5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrwild, M., Pfeifer, G., Santarius, U., Muller, S.A., Huang, H.-C., Engel, A., Baumeister, W., and Goldberg, A.L. 1997. The ATP-dependent HslVU protease from Escherichia coli is a four-ring structure resembling the proteasome. Nat. Struct. Biol. 4 133–139. [DOI] [PubMed] [Google Scholar]
- Seol, J.H., Yoo, S.J., Shin, D.H., Shim, Y.K., Kang, M.S., Goldberg, A.L., and Chung, C.H. 1997. The heat-shock protein HslVU from Escherichia coli is a protein-activated ATPase as well as an ATP-dependent proteinase. Eur. J. Biochem. 247 1143–1150. [DOI] [PubMed] [Google Scholar]
- Seong, I.S., Oh, J.Y., Yoo, S.J., Seol, J.H., and Chung, C.H. 1999. ATP-dependent degradation of SulA, a cell division inhibitor, by the HslVU protease in Escherichia coli. FEBS Lett. 456 211–214. [DOI] [PubMed] [Google Scholar]
- Seong, I.S., Oh, J.Y., Lee, J.W., Tanaka, K., and Chung, C.H. 2000. The HslU ATPase acts as a molecular chaperone in prevention of aggregation of SulA, an inhibitor of cell division in Escherichia coli. FEBS Lett. 477 224–229. [DOI] [PubMed] [Google Scholar]
- Seong, I.S., Kang, M.S., Choi, M.K., Lee, J.W., Koh, O.J., Wang, J., Eom, S.H., and Chung, C.H. 2002. The C-terminal tails of HslU ATPase act as a molecular switch for activation of HslV peptidase. J. Biol. Chem. 277 25976–25982. [DOI] [PubMed] [Google Scholar]
- Sonezaki, S., Ishii, Y., Okita, K., Sugino, T., Kondo, A., and Kato, Y. 1995. Overproduction and purification of SulA fusion protein in Escherichia coli and its degradation by Lon protease in vitro. Appl. Microbiol. Biotechnol. 43 304–309. [DOI] [PubMed] [Google Scholar]
- Song, H.K., Hartmann, C., Ramachandran, R., Bochtler, M., Behrendt, R., Moroder, L., and Huber, R. 2000. Mutational studies on HslU and its docking mode with HslV. Proc. Natl. Acad. Sci. 97 14103–14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, H.K., Bochtler, M., Azim, M.K., Hartmann, C., Huber, R., and Ramachandran, R. 2003. Isolation and characterization of prokaryotic proteasome homolog HslVU (ClpQY) from Thermotoga maritima and the crystal structure of HslV. Biophys. Chem. 100 437–452. [DOI] [PubMed] [Google Scholar]
- Sousa, M.C., Trame, C.B., Tsuruta, H., Wilbanks, S.M., Reddy, V.S., and McKay, D.B. 2000. Crystal and solution structures of an HslVU protease–chaperone complex. Cell 103 633–643. [DOI] [PubMed] [Google Scholar]
- Torres-Cabassa, A.S. and Gottesman, S. 1987. Capsule synthesis in Escherichia coli K-12 is regulated by proteolysis. J. Bacteriol. 169 981–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, S.J., Seol, J.H., Seong, I.S., Kang, M.S., and Chung, C.H. 1997. ATP binding, but not its hydrolysis, is required for assembly and proteolytic activity of the HslVU protease in Escherichia coli. Biochem. Biophys. Res. Commun. 238 581–585. [DOI] [PubMed] [Google Scholar]