Abstract
The interaction of the native Alzheimer’s peptide C-terminal fragment Aβ (29–42), and two mutants (G33A and G37A) with neutral lipid bilayers made of POPC and POPE in a 9:1 molar ratio was investigated by solid-state NMR. This fragment and the lipid composition were selected because they represent the minimum requirement for the fusogenic activity of the Alzheimer’s peptide. The chemical shifts of alanine methyl isotropic carbon were determined by MAS NMR, and they clearly demonstrated that the major form of the peptide equilibrated in membrane is not in a helical conformation. 2H NMR, performed with acyl chain deuterated POPC, demonstrated that there is no perturbation of the acyl chain’s dynamics and of the lipid phase transition temperature. 2H NMR, performed with alanine methyl-deuterated peptide demonstrated that the peptide itself has a limited mobility below and above the lipid phase transition temperature (molecular order parameter equal to 0.94). MAS 31P NMR revealed a specific interaction with POPE polar head as seen by the enhancement of POPE phosphorus nuclei T2 relaxation. All these results are in favor of a β-sheet oligomeric association of the peptide at the bilayer interface, preferentially recruiting phosphatidyl ethanolamine polar heads.
Keywords: Alzheimer, membrane fusion, POPE, 31P NMR relaxation times, lipid dynamics, 2H NMR
Alzheimer’s disease (AD) is a neurodegenerative disease leading to a progressive loss of memory and a cognitive decline. It is affecting an increasing number of people in the world (~40 million in 2003) (Antzutkin 2004), and although it is one of the most studied pathologies, it is not yet fully understood at the molecular level. One of the AD features is extracellular amyloid deposits within patients’ brains (Kumar-Singh et al. 2002). The major component of these plaques is the amyloid β-peptide (Aβ). This peptide is generated through the proteolytic cleavage of the β-amyloid precursor protein (APP), a glycoprotein of 695–770 amino acids (Evin and Weidemann 2002). After hydrolysis of APP at its N-terminus by a β-secretase, the Aβ peptide is released by γ-secretase as a 39–43 residues peptide (Hardy and Selkoe 2002). Missense mutations within genes of APP and/or presenilins (PS1 and PS2) lead to an increased production and accumulation of Aβ (1–42), which is more hydrophobic and has a larger propensity to aggregate than the Aβ (1–40).
Several studies have demonstrated that Aβ (1–40) and Aβ (1–42) are neurotoxic (Pillot et al. 1999; Butterfield and Kanski 2002). This property can be linked to experimental data showing, on the one hand, the interactions of Aβ peptides with lipids and cholesterol (Terzi et al. 1997; Waschuk et al. 2001; Eckert et al. 2003) and, on the other hand, the destabilizing effects of membranes by amyloid peptides, such as the fusion of small unilamellar vesicles (Pillot et al. 1996), changes in membrane fluidity, and permeability (Xiaocui et al. 2002), lipids peroxidation (Butterfield and Kanski 2002), or formation of a calcium–permeable ion-channel (Lin et al. 2001).
β-Amyloid peptides have an extracellular hydrophilic N-terminus (residues 1–28) and a membrane-inserted C-terminus region (residues 29–40 or 29–42). Destabilizing and fusogenic properties are likely to be due to the carboxy terminus of Aβ (1–40) and Aβ (1–42) (Mingeot-Leclercq et al. 2002). Based on energetic considerations, Mingeot-Leclercq et al. have proposed that Aβ (29–42) is a tilted peptide: it induces membrane permeabilization and fusion, and it could penetrate membranes as an α-helix whose axis forms an angle of 70° with the bilayer plane (Mingeot-Leclercq et al. 2002). Several solid-state NMR experiments performed on Aβ peptides in regard to fibril formation revealed a β-sheet structure (Petkova et al. 2002; Tycko 2003). Fewer NMR experiments have been addressed to the Aβ (1–40) and Aβ (1–42) peptide structures in interaction with membranes (Terzi et al. 1997; Bokvist et al. 2004).
The present study aimed specifically at providing a better understanding on Aβ (29–42)–membrane interactions at equilibrium. Specific questions were addressed using a combination of solid-state NMR approaches: (1) What is the major stable secondary structure of this peptide in a membrane environment, (2) where is it located in the membrane and what are the stabilizing interactions, (3) what is the peptide’s and lipid’s dynamics in this complex, and (4) what are the lipid perturbations which could explain its activity of fusion peptide. These NMR techniques were: (1) determination of peptide’s carbon chemical shifts as an indication of the secondary structures (Wishart and Sykes 1994; Saito et al. 2000), (2) 2H NMR on labeled peptides and labeled phospholipids providing a direct measure of order parameters, i.e., magnitudes of motion of labeled C-D bonds (Davis 1983), (3) 31P NMR isotropic chemical shift and chemical shift anisotropy determinations related to the lipid’s head group orientation and dynamics (Seelig et al. 1977; Bokvist et al. 2004) and relaxation time analyses, which are more directly linked to head group dynamics (Dufourc et al. 1992).
The lipid environment which we selected was made of palmitoyl-oleoyl phosphatidylcholine (POPC) and palmitoyl-oleoyl phosphatidylethanolamine (POPE) (9:1 molar ratio). Indeed, it was observed previously that 10 mol % of POPE was necessary and sufficient to observe Aβ-induced fusion of liposomes (Mingeot-Leclercq et al. 2003).
Results and Discussion
The interaction between the N-terminally acetylated Aβ (29–42) peptide and zwitterionic membranes was characterized by using a complete set of NMR experiments with respect to the structure and dynamics of the peptide backbone and to the peptide location in the membrane bilayers.
In the first part, the study is focused on two 13CβH3 or CβD3-Ala residues, which were introduced into two different Aβ (29–42) peptides at positions evenly distributed along the Aβ primary structure—i.e., Gly33 → Ala and Gly37 → Ala. By using the carbon 13C-labeled peptides and high spinning frequency MAS NMR, isotropic chemical shifts were used to probe the local secondary structure of the Aβ peptide. The quadrupolar splittings of CD3 segment of the deuterated peptides were used to establish the local backbone dynamics in the absence and in the presence of zwitterionic membrane.
In the second part, the effects of Aβ (29–42) on the lipid molecular structure and membrane supramolecular organization were monitored with static 2H NMR and Magic Angle Spinning 31P NMR.
13C MAS NMR experiments: Structural implications of isotropic chemical shifts
Relations between chemical shifts and structure have been largely based on empirical rules, but the use of quantum chemical calculations is becoming increasingly important. Recently, significant advances in establishing the relationships between chemical shifts and protein backbone conformations have been made, driven by the large number of assignments now available, by empirical and semiempirical analysis of shifts in proteins of known structure, and by ab initio calculations on protein fragments (Saito et al. 2000; Sun et al. 2002). It is thus now well-recognized that 13C chemical shifts of the backbone Cα and carbonyl carbons as well as side-chain Cβ signals of any amino acid residues in polypeptides and proteins are significantly displaced depending upon their local peptide conformations, defined by the torsion angles Φ and Ψ. Therefore, the local conformation of a peptide chain can be conveniently determined from its 13C chemical shift, with reference to accumulated data base. At least six kinds of different local conformations can be distinguished for the Ala residues as summarized in Saito et al. For instance, the resonance frequency 3–13C Ala residues is equal to 14.9 ppm for an α-helical local conformation while it is 18.9 ppm for a β-sheet secondary structure (Saito et al. 2000).
Chemical shifts and high spinning frequency MAS experiments have been used to obtain isotropic chemical shift of specifically 13C labeled Aβ (29–42) peptides.
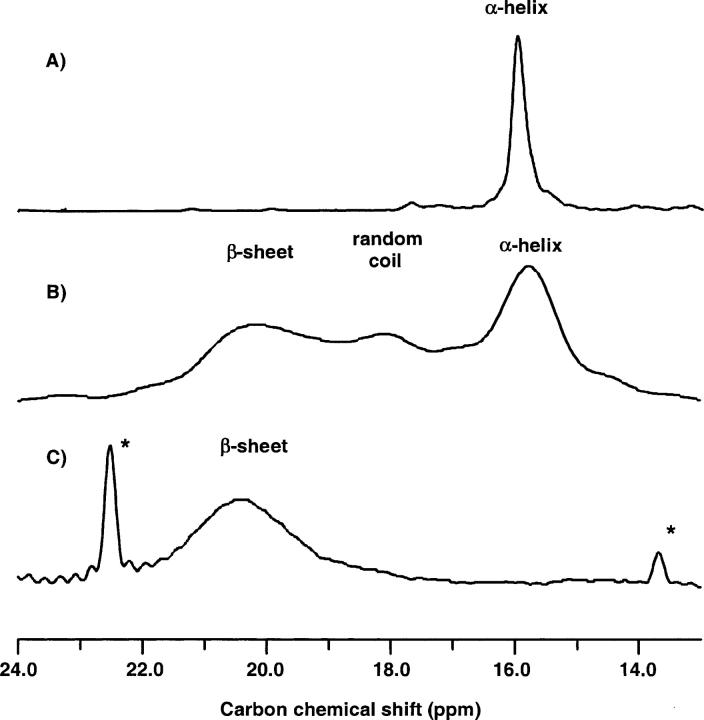
Figure 1 ▶ shows carbon MAS NMR spectra centered around the methyl region of Aβ (29–42) G33A(13Cβ) diluted in HFP (1,1,1,3,3,3-hexafluoro-2-propanol) solvent at 303 K (Fig. 1A ▶) and 218 K (Fig. 1B ▶). The 13C chemical shifts of the Aβ (29–42) peptides were calibrated with respect to the 13CO signal of an external 13C-labeled glycine carbonyl (176.03 ppm, according to Saito et al. 2000) in all three spectra. As discussed above, isotropic chemical shifts are sensitive markers for secondary structure, and a chemical shift typical of α-helices is observed in HFP solution at room temperature. Similarly, the spectrum of a lyophilized powder of pure peptide displays a unique resonance around 19 ppm, typical of a β-sheet conformation, as expected for this amyloid peptide fragment (data not shown). The α-carbon and CO carbon natural abundance resonances were also observed and had chemical shifts in accordance with the expected secondary structures (i.e., α for HFP solutions and β for lyophilized powders). When freezing the HFP solution under magic-angle spinning conditions in the spectrometer different conformations were observed, as indicated by the spectrum of Figure 1B ▶ with a broad resonance, and three resolved maxima, one at 16 ppm (α-helix), one at 20.5 ppm (β-sheet), and one at 18 ppm (random coil). This indicates that, upon freezing, part of the peptide retains its initial α-helix conformation, while part is shifted to other conformations, namely random coil and β-sheet. Figure 1B ▶ illustrates very clearly the capacity of MAS 13C NMR to probe the local secondary structure around the labeled carbon atom. When 5 mol % of the peptide was incorporated into the POPC/POPE (9:1) bilayers at 303 K, the MAS spectrum clearly showed that it is mostly in a β-sheet conformation (Fig. 1C ▶). The same results were obtained at 2.5 mol % of peptide, and with the Gly → Ala mutant at positions 33 and 37. The spectra were also shown to be stable for 2 h after liposome preparation (minimum time of equilibration before the acquisition of data) and for 1 week, something important to stress, since slow equilibria have been described for amyloid peptide–membrane systems (Bokvist et al. 2004). These results do not demonstrate that the secondary structure is a β-sheet all along the peptide; however, the proposal seems reasonable, since the two positions analyzed are at one-third and two-thirds of the sequence.
Figure 1.
13C NMR spectra of Aβ (29–42), mutant G33 → A33(13Cβ), obtained by MAS NMR at 8.2-kHz spinning speed. (A) spectrum of an HFP solution at 303 K of the peptide, where it exists mainly in α-helical conformation; (B) spectrum of the same HFP solution, in the frozen state at 218 K; (C) spectrum of the peptide inserted into POPC/POPE 9:1 lipid bilayers at 303 K, where it is mainly in a β-sheet conformation. Peaks labeled with a * are due to natural abundance lipid resonances.
One question arises while observing the same spectra for the peptide in water in the presence and in the absence of lipid bilayers: Are we looking at a β-sheet conformation of the peptide inserted into the membranes, or merely at a membrane-expulsed structure. We thus carefully checked that, after density gradient centrifugation a single band containing both the peptide and the lipids was observed on the sucrose gradient. This was shown to be the case, both for the 2.5 mol % and the 5 mol % conditions, and after 1 day and 3 days of equilibration.
Therefore, both G → A mutants of Aβ (29–42) do exist in a stable β-sheet conformation in POPC/POPE 9:1 lipid bilayers in the liquid crystalline phase.
Static 2H NMR experiments: Dynamical behavior of Aβ (29–42) in the absence and the presence of lipids
Dynamical information is complementary to structural information. For instance, where a monomeric tilted peptide in a fluid membrane is expected to be highly disordered, i.e., to have a small molecular order parameter, a large aggregate such as a β-amyloid fiber should have a much higher degree of structural order. We observed a high degree of structural order as demonstrated by the narrow 13C and 15N NMR lines detected under MAS conditions. Indeed, sharp resonances in solid-state NMR were shown to be characteristic of well-structured, homogeneous peptides (Tycko 2004).
2H static NMR is an ideal probe of the local dynamics of peptides in lipids (Davis 1983). Static 2H NMR spectra have been recorded for the β-CD3 of G33 → A33 and G37 → A37 peptides, in a crystalline state, and in the presence of membranes, both below (218 K) and above (298 K), the gel to liquid–crystalline phase transition of the lipid mixture (τm); the gel to liquid crystalline phase transition temperatures are equal to 271 K (POPC) and 298 K (POPE) for pure lipids and to 277 K for a 1:1 mixture of POPC and POPE (Epand and Bottega 1988). A 9:1 POPC:POPE mixture is thus expected to have a gel-to-liquid crystalline phase transition temperature close to 271 K, and was actually found to occur between 271 and 272 K by 2H NMR.
The characteristic parameter of these spectra is the quadrupole splitting (ΔνQ) as defined by the separation of the most intense peaks. Alanine CD3 ΔνQ provides a direct measure of the Cα–Cβ segment’s order parameter (SC—C = 1/2 〈3cos2θ - 1〉), which is directly related to the magnitude of motion of this bond. The results were identical for the two labeled positions 33 and 37. In the crystalline state, and in lipids below τm, a mean quadrupolar splitting of 39.5 ± 0.4 kHz was found, which is close to the rigid limit value for such a CD3 quadrupolar splitting (S = 1). These groups perform fast threefold hopping motions and symmetric rotations about the C—C bond axis, which scales the rigid value of quadrupolar splitting by a factor of 1/3.
Above τm, the CD3 quadrupolar splittings in lipid membranes was slightly smaller. A motion averaged quadrupolar splitting equal to 37.4 ± 0.4 kHz was obtained corresponding to a bond vector order parameter S of 0.94 ± 0.01. A small but significant difference was observed between lyophilized powder (or peptide/lipid complex in the gel phase) and peptide/lipid complex in the fluid phase, confirming again that the peptide is in contact with the membrane since its dynamics is sensitive to the lipid phase transition. On the other hand, an order parameter of 0.94 for the peptide chain (at the level of Alanine 33 and 37) is very high and close to the rigid limit value, which is again in favor of a β-sheet aggregate rather than a monomer freely diffusing in the bilayer. It may be stressed that, within the signal to noise resolution limits of this experiment, one single quadrupolar splitting is observed which shows that the population of peptides is homogeneous in terms of conformation and dynamics.
We now turn to the questions of the peptide’s localization within the bilayer, and of its capacity to induce lipid perturbations, using solid-state NMR on lipid’s spins, 2H and 31P.
2H and 31P NMR analyses of the lipid dynamics
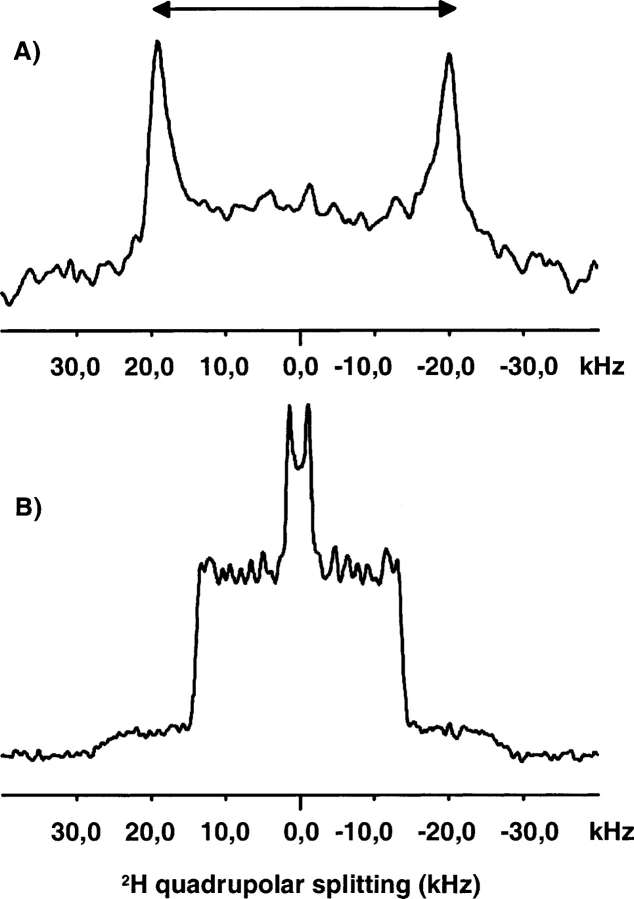
In a first set of experiments, POPC deuterated on its sn1-palmitoyl acyl chain (POPC-d31) was used to investigate the effects of Aβ (29–42) on chain order, mobility, and gel to liquid crystalline phase transition of the lipid bilayer. 2H NMR is widely used to probe the conformational disorder of liquid crystalline lipid bilayers (Davis 1983). For instance, addition of cholesterol or amphiphilic drugs that penetrate into the lipid membrane leads to dramatic changes of the quadrupolar splitting of acyl chains. In the presence of Aβ (29–40), no change in the quadrupole splittings or in the shape of the spectra was observed (Fig. 2B ▶). Furthermore, the phase transition of POPC/POPE (9:1) lipid mixture MLVs occurs between 271 K and 273 K, both in the presence and in the absence of Aβ (29–42), irrespective of the peptide/lipid molar ratio, i.e., for 2.5 mol % and 5 mol %. The unaffected phase transition temperature and quadrupolar splittings of the mixture in the presence of Aβ (29–42) peptide indicates that the peptide does not penetrate deeply into the hydrophobic core of the bilayer, but rather, binds superficially at the interface.
Figure 2.
Static 2H NMR spectra of POPC/POPE (9:1) + 5 mol % Aβ (29–42) 33G → 33A mutant, at 303 K. (A) spectrum obtained using Ala(CβD3) deuterium-labeled peptide; (B) spectrum obtained using POPC-d31 perdeuterated on its palmitoyl chain.
31P NMR was then used to monitor changes in the phase behavior of the phospholipid bilayer upon interaction with Aβ (29–42) (Ghosh 1988).
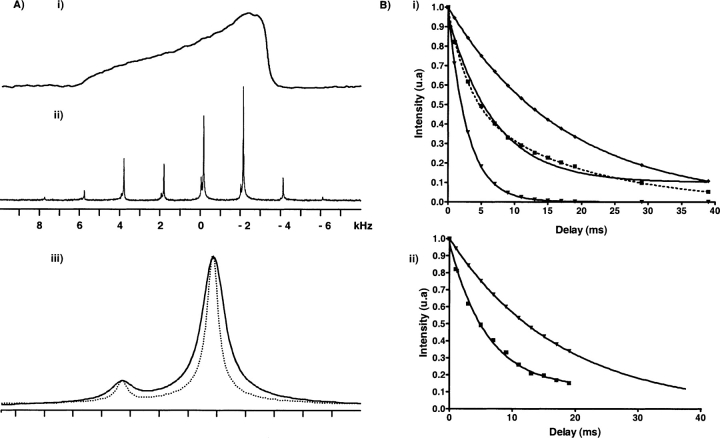
A typical static 31P NMR spectrum from a POPC:POPE mixture (9:1 molar ratio) is shown in Fig. 3Ai ▶). It is dominated by the chemical shift anisotropy of the phosphate from both PC and PE, partially averaged by fast axial rotation of lipid molecules. The spectrum is a superposition of two powder spectra originating from the phosphorus nuclei of POPC and POPE. The maximum chemical shielding anisotropy was 47 ppm. It was identical with and without Aβ (29–42). On this spectrum, it was, however, not possible to distinguish between the CSAs contributions of PC and PE headgroups.
Figure 3.
31P NMR spectra of Aβ (29–42) in complex with POPC/POPE (9:1) lipid bilayers, at 293 K. (Ai) Static 31P powder spectrum; (Aii) 31P MAS spectrum at a spinning frequency of 2 kHz; (Aiii) expansion of the 31P isotropic signal at a spinning frequency of 8 kHz, in the absence (dotted line) and in the presence (continuous line) of 5 mol % of Aβ (29–42) 37G•A mutant peptide (scale 1 ppm = 202 Hz per tic). (B) Variations of the signal intensity versus echo delay. The intensities have been normalized with respect to their value at 20 μsec echo delay. (Bi) PC resonance; the experimental data (squares) were fitted by a mono-exponential decay (solid line) and by a bi-exponential decay (dotted line). Note that the bi-exponential fitting is clearly required. Theoretical mono-exponential decays corresponding to each T2 component are also shown below (T2 = 2.92 msec, • and above (T2 = 18 msec, ♦) the experimental decay. (Bii) PE resonances; a single mono-exponential decay was sufficient to fit PE decay curves in the POPC:POPE 9:1 mixture, both in the absence (18 msec, •) and in the presence (5.9 msec, ▪) of peptide.
Mechanical rotation of the MLV suspensions at the magic angle (54.7°) partially averages the effective CSA and results in higher resolution spectra in which the phosphate resonances from all individual lipids are well resolved due to their different isotropic chemical shifts.
The NMR spectrum obtained for a pure POPC:POPE mixture at 2 kHz sample rotation is represented in Figure 3Aii ▶. This spectrum is composed of a pair of central resonances flanked by a series of spinning sidebands separated by multiples of the spinning frequency. The narrow line-widths reflect long transverse relaxation time T2 (18 msec) and high lipid mobility in the liquid crystalline bilayers at 293 K. The relevant isotropic 31P NMR chemical shift difference between PC and PE without peptides is 0.63 ppm. At this spinning frequency, 31P chemical shift anisotropy parameters of both lipid components are reflected in the intensities of the resulting spinning sidebands pattern. By using the standard Hertzfeld-Berger’s method we found both CSA to be axially symmetric, as expected for fast axially diffusing lipids, with a span (i.e., σ// − σ⊥) equal to 47 ± 0.3 ppm and 37.5 ± 0.6 ppm for PC and PE, respectively. At higher spinning frequencies, these sidebands disappear, while the central resonances remain unchanged in position. At this speed, a quantitative analysis can be carried out by integration of the individual resonance with an intensity ratio of 9:1 reflecting the chemical composition of the bilayer (Fig. 3Aiii ▶, dotted line).
In the presence of peptide, the wide line 31P spectra showed no deviation from the powder distribution (data not shown). This confirms that the lipid system remains in the liquid crystalline lamellar phase upon addition of Aβ (29–42). In particular, there is no evidence of isotropic or HII resonances, showing that the interaction with the peptide does not induce nonbilayer structures in these conditions.
Under MAS conditions, changes in the individual PC and PE lipid species within the bilayer caused by Aβ (29–42) can be monitored simultaneously. The individual 31P NMR resonances are not affected by the addition of peptide as observed by Bokvist et al. (2004) on Aβ (1–40): the addition of peptide to PC neutral bilayer does not lead to any change in 31P chemical shift associated with change in local electrostatic environment. However, in our case, the interaction with Aβ (29–42) leads to a significant and specific broadening of the PC and PE 31P resonances as observed in Figure 3Aiii ▶ (continuous line).
This nonclassical behavior has been explored further by analyzing T2 relaxation times of phosphorus nuclei of the two different polar heads. 31P T2s of each resonance were extracted from the variation of the relative intensity versus echo time τ recorded with a classical spin echo experiment (π/2-τ-π-τ-Acq). For the PE 31P resonance, the intensity decay could be fitted by a single exponential and the transverse relaxation time (5.9 msec) was clearly shorter than for PE in a pure POPC:POPE mixture (18 msec) as illustrated in Figure 3Bii ▶. By contrast, a two-exponential decays model was required for PC phosphorus resonance. Figure 3Bi ▶ compares mono- and bi-exponential decays for the PC resonance and clearly shows that the mono-exponential fitting is not sufficient.
These results show that the entire population of PE is affected in the presence of Aβ (29–42), whereas two PC populations can be distinguished, one characterized by a T2 typical of pure lipids (T2 = 18 msec), and the other having a much shorter T2 (T2 = 2.9 msec).
The same results were observed with 2.5 mol % of peptides, the respective T2s being 18 msec for unaffected POPC, 3 msec for POPC bound to Aβ, and 6.1 msec for POPE. Therefore, in both cases (5 mol % and 2.5 mol %), the peptide is able to recruit the entire POPE population (respectively 2 and 4 POPE molecule per peptide) while leaving part of POPC molecules unaffected. The peptide interacting and noninteracting POPC molecules are on slow exchange at the 31P NMR time scale (10−4 sec).
These results demonstrate a pronounced tendency of Aβ (29–42) to interact with PE head groups more than with PC head groups, in accordance with the necessity to add PE lipids to induce vesicle fusion.
Molecular modeling
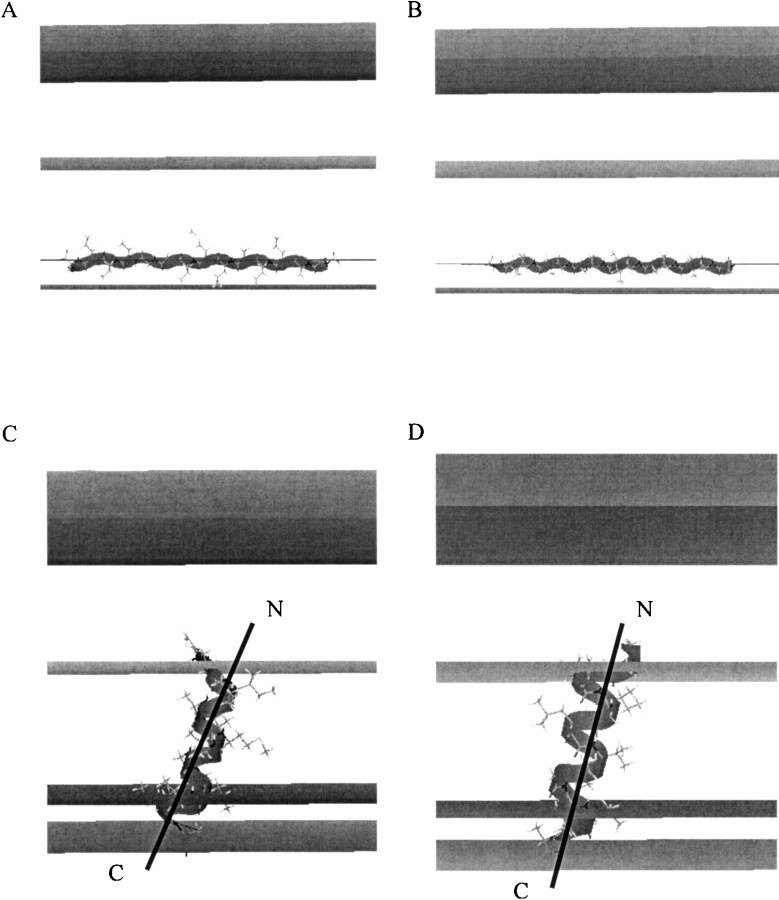
The IMPALA procedure was used to check the influence of the peptide conformation on its insertion in a lipid bilayer (Lins et al. 2001). Briefly, this method simulates the interaction between a peptide and a simplified model membrane using a Monte Carlo minimization procedure coupled to an angular dynamics.
Figure 4 ▶ shows the most stable position in the model bilayer of Aβ (29–42) peptides mutants G33 → A33 and G37 → A37, in α-helical or β-sheet conformation. The Aβ (29–42) G33 → A33 and G37 → A37 mutants in a helical conformation present an angle with the bilayer plane of 79.4° and 86.4°, respectively, whereas the wild-type peptide penetrates membranes with an angle of 66°. This could explain the partial loss of the fusogenic properties of the mutants peptides in agreement with the work of Martin et al. in 1994 (Martin et al. 1994). The Aβ (29–42) peptides in a β-sheet conformation are parallel to the lipid/water interface. This supports our NMR results that indicates that mutants peptides are adsorbed at the bilayer interface, and is in good agreement with the literature (Demeester et al. 2000; Bokvist et al. 2004).
Figure 4.
Best configurations of Aβ (29–42) peptides in ribbon representation after Monte Carlo simulation. Middle plane = bilayer center (z = 0); first plane = lipid acyl chain/polar head interface at 13.5 Å from the center; second plane = lipid/water interface (z = 18 Å). (A,C) Aβ (29–42) G33 → A33 peptides as a β-sheet and an α-helix, respectively. (B,D) Aβ (29–42) G37 → A37 peptides as a β-sheet and an α-helix, respectively.
Conclusions
In the present study, the steady-state interaction of the Alzheimer peptide C-terminal fragment (Aβ 29–42), and two mutants 33,37-(G → A) with neutral lipid bilayers made of POPC and POPE in a 9-to-1 ratio was investigated by solid-state NMR. This peptide fragment and this lipid composition were selected because they are the minimum required for the fusogenic activity of the Alzheimer peptide. It has been postulated that the fusogenic activity could be due to the propensity of this peptide to be “tilted,” i.e., to insert in bilayers as an oblique α-helix, neither perpendicular nor parallel to the bilayer plane as many peptides do (Pillot et al. 1996; Mingeot-Leclercq et al. 2002). Isotropic carbon chemical shifts clearly demonstrate that the peptide equilibrated in the membrane is not helical. 2H NMR demonstrates that there is no perturbation of the acyl chain dynamics and of the lipid phase transition temperature, and that the peptide itself has a limited mobility even in the fluid phase. Moreover, 31P NMR reveals a specific interaction of the peptide with the POPE polar head as seen by the enhancement of the T2 relaxation associated with a restricted mobility. All these results and molecular modeling support the conclusion that when the peptide has been inserted in the membrane, its steady-state structure is an oligomeric association of β-sheet peptides located at the bilayer interface, preferentially recruiting PE lipids. The selective recruitment of PE by Aβ aggregates further suggests the idea that the inaccessibility of this crucial lipid could be an aspect of the cellular disorders induced in the Alzheimer’s disease. It is in complete agreement with the observation that Aβ 40/42 binding is controlled by lipid head group interactions and leads to the production of interfacial packing defects (Waschuk et al. 2001).
Experimental demonstration of the structure and location of tilted peptides in membranes is a difficult task, although several evidences do exist in the literature (Bradshaw et al. 2000; Han et al. 2001). One limitation results from the reasonable hypothesis that the tilted state of the peptide fragment actually responsible for the fusogenic activity is transient and is an intermediate form toward a stable membrane-inserted conformation as it could be for the Alzheimer peptide. In such a case, most of the available methods such as solid-state NMR, neutron diffraction, or polarized ATR-IR (Martin et al. 2003) will detect the major membrane-inserted conformation after stabilization. Our results demonstrate firmly that after fusogenic events have occurred, the Aβ peptide is not a tilted peptide but merely a major β aggregated structure.
Our strategy, combining 13C isotropic chemical shift for conformational analyses, 2H NMR on labeled peptides, and on lipids for dynamical analyses and MAS 31P NMR including relaxation time measurements for the detection of peptide–lipid-specific interactions provided a clear view on peptide’s and lipid’s structure and dynamics in POPC/POPE mixture. It could now be applied on the entire Alzheimer peptides Aβ (1–40) and Aβ (1–42) and in different experimental conditions: it has been shown that cholesterol is an important factor affecting the membrane insertion of Aβ (1–40), which drastically increases the helical content of Aβ (1–40) in DMPC, DPPC, and DPPC + sphingomyelin monolayers and bilayers (Ji et al. 2002). The role of gangliosides is increasingly stressed on Alzheimer fibrils formation and pathogenicity (Kakio et al. 2001; Tashima et al. 2004). In particular, it has been recently proposed that asia-loGM1 lipids may stabilize the peptide in a monomeric form, thus preventing the formation of fibrils (Mandal and Pettegrew 2004). If lipid binding states, different from the occurrence of the β-sheet at the bilayer surface are found in some lipid mixtures relevant for the peptide’s activity, other solid-state NMR approaches will be required to refine the peptide’s structure and orientation, both using MAS and distance determinations (Tycko 2004) and using anisotropic constraints in oriented lipid bilayers (Mo et al. 2004).
Materials and methods
Materials
β-Amyloid peptides 29–42, Aβ (29–42), GAIIGLMVGGVVIA, and the two labeled mutants G33A and G37A were synthesized by Polypeptides Laboratories. The mutants were synthesized either in 13C labeled form or in 2H labeled form, both labels being introduced on the alanine methyl group, using labeled alanine during peptide synthesis. All peptides were subsequently N-terminally acetylated by treatment with acetic anhydride–pyridine overnight at room temperature. Their molecular weights were controlled by mass spectrometry.
1-Palmitoyl(d31)-2-Oleoyl-sn-glycero-3-phosphocholine (POPC-d31) and 1-Palmitoyl(d31)-2-Oleoyl-sn-glycero-3-phosphatidyleth-anolamine (POPE) were purchased from Avanti Polar Lipids. Deuterium-depleted water was obtained from Aldrich. Salts and solvents were of analytical grade.
Proteo-liposomes preparation
Briefly, the peptide was dissolved in 1,1,1,3,3,3-hexafluoro-2-pro-panol (HFP) then dried under a stream of nitrogen to obtain a film. HFP lipid solutions were added to the peptide film in a POPC/ POPE/peptide molar ratio of 86:9:5 (5 mol % of peptide), or 87.25: 10.25:2.5 (2.5 mol % of peptide), and the mixture was evaporated overnight under high vacuum. Subsequently, the dry peptide/lipid film was resuspended with an excess of deionized water under extensive vortexing to produce proteoliposomes. It was then lyophilized and rehydrated with deuterium-depleted water for NMR experiments. Peptide sample homogeneity and final peptide/lipids ratio were checked by sucrose density gradient centrifugation, by the modified Lowry’s procedure and by phosphorus analysis, respectively.
Fusogenic activity of Aβ (29–42) and its ala mutants
Each experiment was carried out with Small Unilamellar Vesicles (SUVs) of POPC/POPE: 9/1. The vesicles were prepared in Tris buffer pH 7.4 ( Tris 10 mM, NaCl 150 mM, EDTA 0.1 mM, NaN3 1 mM). The final phospholipid concentration was checked by phosphorus assay, and liposomes were used within the day following their preparation. The fusion assays were performed as described earlier (Van Bambeke et al. 1995), using a mixture of labeled and unlabeled vesicles (final concentration in lipids: 5 μM), by recording the increase in fluorescence due to the de-quenching of octadecylrhodamine B chloride (R18) upon dilution in nonlabeled liposome. Labeled and unlabeled liposomes were mixed at a ratio of 1:4. Peptides were added and fluorescence was immediately recorded at room temperature for 25 min, using an excitation wavelength at 560 nm and an emission wavelength at 590 nm (Perkin-Elmer LS-30, Perkin-Elmer Ltd.). Fusion experiments were achieved with four different peptides/lipid ratio of each N-acetylated peptide. All labeled mutants and N-acetylated peptides induced the fusion of lipidic phases, although with fusogenic properties that were about four times less efficient then the wild-type peptide.
Homogeneity of proteo-liposomes
Sucrose gradients were 0%–30% sucrose. The samples (15% sucrose) were added in the tube and the linear gradient was made over. The tubes were centrifuged in a SW41 Ti rotor (Beckman ultracentrifuge) for 20 h at 103,847g. Fractions of 1 mL were collected for protein and lipid assays. To minimize the amount of sucrose in the samples (which interferes with protein and lipid assays), 100 μL of each fraction was diluted into 900 μL of Milli-Q water and centrifuged in a TLA 100.4 rotor in a Beckman ultracentrifuge at 104,300g for 1 h. The pellets were then diluted in 300 μL of Milli-Q water. Phospholipid content was determined by phosphorus assay, and peptide amount using standard Lowry protocol.
Solid-state NMR experiments
The NMR experiments were performed with a Bruker DMX narrow bore spectrometer operating at 1H Larmor frequency of 500.13 MHz.
13C and 31P spectra were obtained with a doubly-tuned DOTY XC5-MAS probe equipped with a 5-mm spinning module. The 1H RF field strength for heteronuclear two-pulse phase modulation (TPPM) decoupling was equal to 66 kHz. 13C cross polarization (CP) MAS spectra were acquired using a 1H excitation pulse length of 5 μsec and a CP spin-lock field strength of about 50 kHz. The CP contact time was 2 msec; the relaxation delay was equal to 3 sec. 31P MAS spectra were acquired using a hahn echo pulse sequence, and echo delays were varied from 0.1 to 40 msec during T2 measurement experiments. 2H NMR spectra were recorded on a Bruker 7 mm probe with a solenoid coil oriented at 90° with respect to the magnetic field. Quadrupolar splittings were recorded by using a standard quadrupolar echo sequence. Deuterium π/2 pulses were equal to 5 μsec, the refocusing delay and the repetition time were set to 30 μsec and 1 sec, respectively.
Typically, the NMR spectra were obtained on 2 mg of labeled peptide, 23.2 mg of phospholipids, and 25 μL of deuterium-depleted H2O.
Molecular modeling
Construction of the two helical peptides and two β-sheet peptides was carried out as previously described (Brasseur et al. 1992) with the Hyperchem program. Side-chain conformations were optimized by Polak-Ribiere conjugate gradient.
The IMPALA procedure allows the study of interactions between a peptide and a modeled membrane using simple restraint functions designed to mimic properties of the membrane as described elsewhere (Lins et al. 2001). Briefly, The interface is described by a C(z) function. C(z) can be considered as the water concentration with a value of 1 outside the membrane and 0 in the membrane core. C(z) varies between 1 and 0 in the region corresponding to the lipid/water interface (z ∈ [13.5–18] Å). Two restraints are used to mimic membrane properties, mainly the hydrophobic effect (named Eint) and the lipidic perturbation (named Elip), depending upon the accessible surface of the peptide atoms. Modification of the internal structure was performed using an angular dynamic procedure of 6000 steps with a maximal torsion angle speed of 1.5°/step.
Acknowledgments
The authors from IPBS thank CNRS, University Paul Sabatier, Région Midi-Pyrénées and the EU (structural funds) for financing the NMR spectrometers. The COST action d22 “Protein–lipid interactions” is acknowledged for initiating of the present collaboration between R. Brasseur’s and A. Milon’s groups. R.B. is Research Director at the National Funds for Scientific Research of Belgium (FNRS). A.T. is Research Director at INSERM (France). This work was supported by the “Ministère de la Région Wallonne” contract no. 114830 (βA4). The work of S.R. was supported by the “Ministère de la Région Wallonne” contract no. 0215223 (NANOSENS).
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.041291405.
References
- Antzutkin, O.N. 2004. Amyloidosis of Alzheimer’s Aβ peptides: Solid-state nuclear magnetic resonance, electron paramagnetic resonance, transmission electron microscopy, scanning transmission electron microscopy and atomic force microscopy studies. Magn. Reson. Chem. 42 231–246. [DOI] [PubMed] [Google Scholar]
- Bokvist, M., Lindstrom, F., Watts, A., and Grobner, G. 2004. Two types of Alzheimer’s β-amyloid (1–40) peptide membrane interactions: Aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J. Mol. Biol. 335 1039–1049. [DOI] [PubMed] [Google Scholar]
- Bradshaw, J.P., Darkes, M.J., Harroun, T.A., Katsaras, J., and Epand, R.M. 2000. Oblique membrane insertion of viral fusion peptide probed by neutron diffraction. Biochemistry 39 6581–6585. [DOI] [PubMed] [Google Scholar]
- Brasseur, R., Lins, L., Vanloo, B., Ruysschaert, J.M., and Rosseneu, M. 1992. Molecular modeling of the amphipathic helices of the plasma apolipoproteins. Proteins 13 246–257. [DOI] [PubMed] [Google Scholar]
- Butterfield, D.A. and Kanski, J. 2002. Methionine residue 35 is critical for the oxidative stress and neurotoxic properties of Alzheimer’s amyloid β-peptide 1–42. Peptides 23 1299–1309. [DOI] [PubMed] [Google Scholar]
- Davis, J.H. 1983. The description of membrane lipid conformation, order and dynamics by 2H-NMR. Biochim. Biophys. Acta 737 117–171. [DOI] [PubMed] [Google Scholar]
- Demeester, N., Baier, G., Enzinger, C., Goethals, M., Vandekerckhove, J., Rosseneu, M., and Labeur, C. 2000. Apoptosis induced in neuronal cells by C-terminal amyloid β-fragments is correlated with their aggregation properties in phospholipid membranes. Mol. Membr. Biol. 17 219–228. [DOI] [PubMed] [Google Scholar]
- Dufourc, E.J., Mayer, C., Stohrer, J., Althoff, G., and Kothe, G. 1992. Dynamics of phosphate head groups in biomembranes. Comprehensive analysis using phosphorus-31 nuclear magnetic resonance lineshape and relaxation time measurements. Biophys. J. 61 42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert, G.P., Kirsch, C., Leutz, S., Wood, W.G., and Muller, W.E. 2003. Cholesterol modulates amyloid β–peptide’s membrane interactions. Pharmaco-psychiatry 36 S136–143. [DOI] [PubMed] [Google Scholar]
- Epand, R.M. and Bottega, R. 1988. Determination of the phase behaviour of phosphatidylethanolamine admixed with other lipids and the effects of calcium chloride: Implications for protein kinase C regulation. Biochim. Bio-phys. Acta 944 144–154. [DOI] [PubMed] [Google Scholar]
- Evin, G. and Weidemann, A. 2002. Biogenesis and metabolism of Alzheimer’s disease Aβ amyloid peptides. Peptides 23 1285–1297. [DOI] [PubMed] [Google Scholar]
- Ghosh, R. 1988. 31P and 2H NMR studies of structure and motion in bilayers of phosphatidylcholine and phosphatidylethanolamine. Biochemistry 27 7750–7758. [DOI] [PubMed] [Google Scholar]
- Han, X., Bushweller, J.H., Cafiso, D.S., and Tamm, L.K. 2001. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat. Struct. Biol. 8 715–720. [DOI] [PubMed] [Google Scholar]
- Hardy, J. and Selkoe, D.J. 2002. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 297 353–356. [DOI] [PubMed] [Google Scholar]
- Ji, S.R., Wu, Y., and Sui, S.F. 2002. Cholesterol is an important factor affecting the membrane insertion of β-amyloid peptide (A β 1–40), which may potentially inhibit the fibril formation. J. Biol. Chem. 277 6273–6279. [DOI] [PubMed] [Google Scholar]
- Kakio, A., Nishimoto, S.I., Yanagisawa, K., Kozutsumi, Y., and Matsuzaki, K. 2001. Cholesterol-dependent formation of GM1 ganglioside-bound amyloid β-protein, an endogenous seed for Alzheimer amyloid. J. Biol. Chem. 276 24985–24990. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh, S., Cras, P., Wang, R., Kros, J.M., van Swieten, J., Lubke, U., Ceuterick, C., Serneels, S., Vennekens, K., Timmermans, J.P., et al. 2002. Dense-core senile plaques in the Flemish variant of Alzheimer’s disease are vasocentric. Am. J. Pathol. 161 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, H., Bhatia, R., and Lal, R. 2001. Amyloid β protein forms ion channels: Implications for Alzheimer’s disease pathophysiology. FASEB J. 15 2433–2444. [DOI] [PubMed] [Google Scholar]
- Lins, L., Charloteaux, B., Thomas, A., and Brasseur, R. 2001. Computational study of lipid-destabilizing protein fragments: Towards a comprehensive view of tilted peptides. Proteins 44 435–447. [DOI] [PubMed] [Google Scholar]
- Mandal, P.K. and Pettegrew, J.W. 2004. Alzheimer’s disease: NMR studies of asialo (GM1) and trisialo (GT1b) ganglioside interactions with Aβ (1–40) peptide in a membrane mimic environment. Neurochem. Res. 29 447–453. [DOI] [PubMed] [Google Scholar]
- Martin, I., Dubois, M.C., Defrise-Quertain, F., Saermark, T., Burny, A., Brasseur, R., and Ruysschaert, J.M. 1994. Correlation between fusogenicity of synthetic modified peptides corresponding to the NH2-terminal extremity of simian immunodeficiency virus gp32 and their mode of insertion into the lipid bilayer: An infrared spectroscopy study. J. Virol. 68 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, I., Goormaghtigh, E., and Ruysschaert, J.M. 2003. Attenuated total reflection IR spectroscopy as a tool to investigate the orientation and tertiary structure changes in fusion proteins. Biochim. Biophys. Acta 1614 97–103. [DOI] [PubMed] [Google Scholar]
- Mingeot-Leclercq, M.P., Lins, L., Bensliman, M., Van Bambeke, F., Van Der Smissen, P., Peuvot, J., Schanck, A., and Brasseur, R. 2002. Membrane destabilization induced by β-amyloid peptide 29–42: Importance of the amino-terminus. Chem. Phys. Lipids 120 57–74. [DOI] [PubMed] [Google Scholar]
- Mingeot-Leclercq, M.P., Lins, L., Bensliman, M., Thomas, A., Van Bambeke, F., Peuvot, J., Schanck, A., and Brasseur, R. 2003. Piracetam inhibits the lipid-destabilising effect of the amyloid peptide Aβ C-terminal fragment. Biochim. Biophys. Acta 1609 28–38. [DOI] [PubMed] [Google Scholar]
- Mo, Y., Cross, T.A., and Nerdal, W. 2004. Structural restraints and heterogeneous orientation of the gramicidin A channel closed state in lipid bilayers. Biophys. J. 86 2837–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova, A.T., Ishii, Y., Balbach, J.J., Antzutkin, O.N., Leapman, R.D., Delaglio, F., and Tycko, R. 2002. A structural model for Alzheimer’s β-amyloid fibrils based on experimental constraints from solid state NMR. Proc. Natl. Acad. Sci. 99 16742–16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillot, T., Goethals, M., Vanloo, B., Talussot, C., Brasseur, R., Vandekerck-hove, J., Rosseneu, M., and Lins, L. 1996. Fusogenic properties of the C-terminal domain of the Alzheimer β-amyloid peptide. J. Biol. Chem. 271 28757–28765. [DOI] [PubMed] [Google Scholar]
- Pillot, T., Drouet, B., Queille, S., Labeur, C., Vandekerchkhove, J., Rosseneu, M., Pincon-Raymond, M., and Chambaz, J. 1999. The nonfibrillar amyloid β-peptide induces apoptotic neuronal cell death: Involvement of its C-terminal fusogenic domain. J. Neurochem. 73 1626–1634. [DOI] [PubMed] [Google Scholar]
- Saito, H., Tuzi, S., Yamaguchi, S., Tanio, M., and Naito, A. 2000. Conformation and backbone dynamics of bacteriorhodopsin revealed by (13)C-NMR. Biochim. Biophys. Acta 1460 39–48. [DOI] [PubMed] [Google Scholar]
- Seelig, J., Gally, G.U., and Wohlgemuth, R. 1977. Orientation and flexibility of the choline head group in phosphatidylcholine bilayers. Biochim. Biophys. Acta 467 109–119. [DOI] [PubMed] [Google Scholar]
- Sun, H., Sanders, L.K., and Oldfield, E. 2002. Carbon-13 NMR shielding in the twenty common amino acids: Comparisons with experimental results in proteins. J. Am. Chem. Soc. 124 5486–5495. [DOI] [PubMed] [Google Scholar]
- Tashima, Y., Oe, R., Lee, S., Sugihara, G., Chambers, E.J., Takahashi, M., and Yamada, T. 2004. The effect of cholesterol and monosialoganglioside (GM1) on the release and aggregation of amyloid β-peptide from liposomes prepared from brain membrane-like lipids. J. Biol. Chem. 279 17587–17595. [DOI] [PubMed] [Google Scholar]
- Terzi, E., Holzemann, G., and Seelig, J. 1997. Interaction of Alzheimer β-amyloid peptide(1–40) with lipid membranes. Biochemistry 36 14845–14852. [DOI] [PubMed] [Google Scholar]
- Tycko, R. 2003. Insights into the amyloid folding problem from solid-state NMR. Biochemistry 42 3151–3159. [DOI] [PubMed] [Google Scholar]
- ———. 2004. Progress towards a molecular-level structural understanding of amyloid fibrils. Curr. Opin. Struct. Biol. 14 96–103. [DOI] [PubMed] [Google Scholar]
- Van Bambeke, F., Tulkens, P.M., Brasseur, R., and Mingeot-Leclercq, M.P. 1995. Aminoglycoside antibiotics induce aggregation but not fusion of negatively-charged liposomes. Eur. J. Pharmacol. 289 321–333. [DOI] [PubMed] [Google Scholar]
- Waschuk, S.A., Elton, E.A., Darabie, A.A., Fraser, P.E., and McLaurin, J.A. 2001. Cellular membrane composition defines A β–lipid interactions. J. Biol. Chem. 276 33561–33568. [DOI] [PubMed] [Google Scholar]
- Wishart, D.S. and Sykes, B.D. 1994. The 13C chemical-shift index: A simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR 4 171–180. [DOI] [PubMed] [Google Scholar]
- Xiaocui, M., Sha, Y., Lin, K., and Nie, S. 2002. The effect of fibrillar A β 1–40 on membrane fluidity and permeability. Protein Pept. Lett. 9 173–178. [DOI] [PubMed] [Google Scholar]