Abstract
Receptor–ligand interactions between synthetic peptides and normal human erythrocytes were studied to determine Plasmodium falciparum merozoite surface protein-3 (MSP-3) FC27 strain regions that specifically bind to membrane surface receptors on human erythrocytes. Three MSP-3 protein high activity binding peptides (HABPs) were identified; their binding to erythrocytes became saturable, had nanomolar affinity constants, and became sensitive on being treated with neuraminidase and trypsin but were resistant to chymotrypsin treatment. All of them specifically recognized 45-, 55-, and 72-kDa erythrocyte membrane proteins. They all presented α-helix structural elements. All HABPs inhibited in vitro P. falciparum merozoite invasion of erythrocytes by ~55%–85%, suggesting that MSP-3 protein’s role in the invasion process probably functions by using mechanisms similar to those described for other MSP family antigens.
Keywords: P. falciparum, merozoite surface protein 3, erythrocyte, invasion inhibition
The avalanche of sequences and data generated by elucidating the Plasmodium falciparum genome has led to a great amount of information being made available regarding the sequences of thousands of proteins; the current challenge lies in identifying each piece to enable understanding their function (Gardner et al. 2002). Our efforts in this respect have been aimed at deepening our knowledge of antigens localized on merozoite membrane, surface proteins, and proteins forming part of organelles, since they play a potential role in initial recognition. Merozoite membrane components have also been the object of extensive studies and it is hoped that they represent perfect targets for defense mechanisms due to their localization (Holder 1994). Elucidating the biological function and the additional data available concerning all these proteins is of great importance in developing potential targets as candidates for an anti-malarial vaccine.
These proteins are often referred to as “surface proteins” but their relationship with the merozoite membrane has still to be completely defined. Some authors have suggested that merozoite surface protein-3 (MSP-3) is situated on the merozoite surface, is associated with merozoite surface molecules, is comprised of a group of proteins forming an integral part of the merozoite membrane, is secreted into the parasitophorous vacuole, and undergoes proteolytic processing there in different P. falciparum isolates (McColl et al. 1994; Oeuvray et al. 1994a,b). This protein is also known as secreted polymorphic antigen associated with the merozoite (SPAM).
The protein’s N terminus consists of regions that are polymorphic among different strains; the protein’s C-terminal domain is conserved among various parasite isolates (Huber et al. 1997; McColl and Anders 1997; Escalante et al. 1998). Comparable with the MSP-6 sequence of amino acids with which it presents 85% similarity to the MSP-3C-terminal region, it also shares a specific ILGWEFGGG-(AV)-P sequence pattern and a glutamine-acid-rich region (Trucco et al. 2001).
MSP-3 has ~48-kDa molecular weight fragments as precursors of variable molecular weight. One of this protein’s important structural characteristics is that it has a domain composed of three blocks of tandem-repeat heptads having AXXAXXX consensus sequences. Sera obtained against recombinant protein and synthetic peptides from MSP-3 conserved and variable regions have shown these antibodies’ differential reactivity to the parasite’s antigen, allowing an alanin heptapeptide repeat domain to be identified as an antigenic diversity site among MSP-3 polypeptides (McColl and Anders 1997).
Despite the diversity within and flanking the heptad domain the AXXAXXX motif is highly conserved, as are other features of the sequence predicting the formation of an α-helical secondary structure. Further analysis of the molecule has revealed a repetitive structure resulting in three charged helices, providing evidence for a coil–coil structure within the molecule (Mulhern et al. 1995). MSP-3 also contains a glutamine-acid rich domain and a putative leucine zipper sequence in the C-terminal domain, when the MSP-3 gene is disrupted by homologous recombination, expressing a truncated form of MSP-3 lacking the putative leucine zipper but retaining the glutamine-acid-rich domain. P. falciparum merozoites lacking MSP-3 and ABRA on their surface present reduced erythrocyte invasion (Mills et al. 2002).
Plasmodium MSP3 may be an important target for antibody-mediated MSP3 protective immunity. Its importance has been recognized, since antibodies directed against it could prevent merozoite invasion in an antibody-dependent cell inhibition (ADCI) assay (Oeuvray et al. 1994a; Singh et al. 2004).
The immunogenicity and protective efficacy of various antigen-adjuvant formulations derived either from P. falciparum MSP-3 or glutamate-rich protein (GLURP) were evaluated in Saimiri sciureus monkeys. Some of the S. sciureus monkeys immunized with MSP-3 were able to fully or partially control parasitaemia upon experimental P. falciparum blood-stage infection (Carvalho et al. 2004; Soe et al. 2004; Theisen et al. 2004).
The protective potential of a fusion protein derived from GLURP genetically coupled to P. falciparum MSP3 has also been evaluated; parasite growth inhibition has been shown in studies using mouse anti-GLURP-MSP3 IgG antibodies in a monocyte-dependent manner. This shows once again that MSP3 could be a valuable strategy for future P. falciparum vaccine development (Theisen et al. 2004).
This study defines erythrocyte binding regions for P. falciparum MSP-3 protein FC27 strain (accession no. AAC09378) (McColl et al. 1994) that could be functionally significant at the moment of invasion. The results show that peptides bound specifically to erythrocytes, peptides 31193 (21KSFINITLSLFLLHLYIYI40), 31202 (201YQKANQAVLKAKEASSYDYI220), and 31209 (341VKEAAESIMKTLAGLIKGNNY360), were found in the protein. It is shown that peptide binding was saturable, having between 120–270 nM affinity constants, and the effect of enzyme treatment on each of these high binding ability peptides’ target cell binding was determined, showing that their receptors were sensitive to neuraminidase and trypsin and that, independently of chymotrypsin treatment, all peptides had α-helix structural elements. The possible functional role of peptides at the moment of invasion is also discussed, bearing in mind that all of them inhibited in vitro merozoite invasion of erythrocytes at 200 and 100 μM.
Materials and methods
Peptide synthesis
Sequential 20-mer peptides, corresponding to the complete P. falciparum FC27 strain MSP-3 protein amino-acid sequence (GenBank accession no. AAC09378) (McColl et al. 1994), were synthesized by the solid phase multiple peptide system (Merrifield 1963; Houghten 1985); t-Boc amino acids (Bachem) and MBHA resin (0.7 meq/g) were used. Peptides were cleaved by the Low-High HF technique (Tam et al. 1983), purified by RP-HPLC, lyophilized, and analyzed by MALDI-TOF mass spectrometry. Tyrosine was added to the C terminus of those peptides that did not contain this amino acid in their sequences to enable 125I-labeling. Synthesized peptides are shown in one-letter code in Figure 1, A and B ▶.
Figure 1.
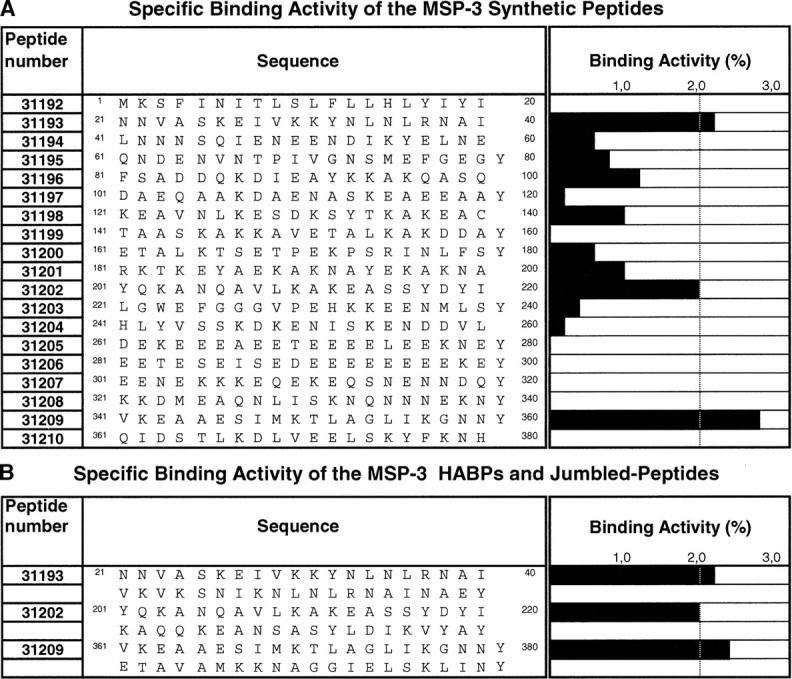
Erythrocyte binding assays using PfMSP-3 peptides. (A) Each of the black bars represents the slope of the specific binding graph, which is named specific binding activity. Peptides with 2% were considered as having high specific erythrocyte binding (HABPs). (B) Specific binding activity for HABP peptide analogs; original and jumbled peptide sequences are shown to the right, and the bars on the left represent specific binding. The numbers in the first column represent FIDIC’s internal code number for those peptides synthesized.
Radiolabeling
The peptides were 125I-labeled according to previously described methodology (Yamamura et al. 1978; Urquiza et al. 1996; Curtidor et al. 2001; Rodriguez et al. 2003; Ocampo et al. 2004). Briefly, 3.2 μLNa125I (100mCi/mL) were oxidized with 12.5 μL chloramine-T (2.25 μg/μL) and added to 5 μg peptide for 5 min at room temperature. The reaction was stopped by adding 15 μL sodium bisulphite (2.25 μg/μL) and 50 μL NaI (0.16 M). The radiolabeled peptide was then separated on a Sephadex G-10 column (Pharmacia).
Binding assay
Human erythrocytes (2 × 108 cells/μL), obtained from healthy donors, were washed in HBS buffer until the buffy coat was removed and then incubated with different radiolabeled-peptide concentrations (10–200 nM), in the absence (total binding)or the presence(nonspecific binding) of 40 μM unlabeled peptide. The sample reached 200 μL final volume with HBS and was incubated for 90 min at room temperature (Urquiza et al. 1996; Curtidor et al. 2001; Rodriguez et al. 2003; Ocampo et al. 2004). The cells were then washed five times with HBS and bound cell radiolabeled peptide was quantified in an automatic γ counter (4/200 plus ICN Biomedicals, Inc). The binding assays were performed in triplicate.
Jumbled-peptide binding assay
HABP sequences determined in the binding assay were used in synthesizing the same peptides, but now in jumbled order (i.e., the same amino-acid composition as HABPs but having random sequence) and then tested in binding assays (Ocampo et al. 2004). The assays were carried out in triplicate in conditions identical to those described in the binding assay section. Synthesized peptides are shown in one-letter code in Figure 1B ▶.
Saturation assays
An erythrocyte binding assay was used to ascertain saturation with all HABPs. The following modifications were introduced: 1.5 × 108 cells were used at 255 μL final volume and the radiolabeled peptide concentration was between 0 and 800 nM. The unlabeled peptide concentration was 40 μM. Cells were washed with HBS and a × counter was used for measuring cell-bound radiolabeled peptide (Yamamura et al. 1978; Weiland and Molinoff 1981; Hulme 1993; Urquiza et al. 1996; Curtidor et al. 2001; Rodriguez et al. 2003; Ocampo et al. 2004).
Enzyme treatment
Erythrocytes (5%) suspended in HBS buffer were treated with 150 μU/mL neuraminidase (ICN 9001-67-6) at 37°C for 1 h, washed five times with HBS buffer, and centrifuged at 1000g for 5 min. Erythrocytes (5%) were similarly treated with trypsin (Sigma T-1005) or chymotrypsin (Sigma C-4129) in TBS buffer (5 mM Tris-HCl, 140 mM NaCl [pH 7.4]) at final 0.75 g/mL concentration. After incubation at 37°C for 1 h, they were washed five times with HBS buffer to which 0.1mM PMSF had been added. Following enzyme treatment, these erythrocytes were tested in HABP binding assays in previously reported conditions. HABP binding was compared between enzyme-treated and -untreated RBCs (Orlandi et al. 1992; Duraisingh et al. 2003).
Cross-linking assays
Radiolabeled HABPs were cross-linked to erythrocyte membranes in the presence or absence of unlabeled peptide for identifying specific erythrocyte binding sites. The cross-linking binding test was performed by using a final 1% cell concentration, followed by incubation with the radiolabeled peptide in the presence or absence of 40 μM unlabeled peptide for 90 min at room temperature. After incubation, cells were washed with HBS and the bound peptide was cross-linked to 10 μM BS3, Bis (sulfosuccinimidyl suberate) (PIERCE), for 20 min at 4°C. The reaction was stopped with 20 nM Tris-HCl (pH 7.4) and washed again with HBS. Then cells were treated with lysis buffer (5 mM Tris-HCl, 7 mM NaCl, 1 mM EDTA, 0.1 mM PMSF). The obtained membrane proteins were solubilized in Laemmli buffer and separated by SDS/PAGE (12% [w/v] polyacrylamide gels). The gels were exposed on BioRad Imaging Screen K (BioRad Molecular Imager FX; BioRad Quantity One, Quantitation Software) for 2 d to determine which proteins had become cross-linked to the radiolabeled peptides. The apparent molecular weight was determined by using molecular weight markers (New England BioLabs).
Merozoite invasion inhibition assay
Sorbitol synchronized P. falciparum (FCB-2 strain) cultures were incubated until the late schizont stage at final 0.5% parasitaemia and 5% haematocrite in RPMI 1640+10% O + plasma (Trager and Jenson 1978; Lambros and Vanderberg 1979). The cultures were seeded in 96-well cell culture plates (Nunc), in the presence of test peptides at 20 and 100 μM concentrations. Each peptide was tested in triplicate. After incubation for 18 h at 37°C in a 5%O2/5% CO2/90% N2 atmosphere, the supernatant was recovered and the cells stained with 15 μg/mL hydroethydine, incubated at 37°C for 30min andwashed three times with PBS. The suspensions were analyzed by CellQuest software using a FACsort (Becton Dickinson immunocytometry system) in Log FL2 data mode (Wyatt et al. 1991). Infected erythrocytes were treated with EGTA and chloroquine; uninfected erythrocytes were used as controls.
CD measurement
CD assays were performed at room temperature on nitrogen-flushed cells using a Jasco J-810 spectropolarimeter. Spectra were recorded in 190–260-nm wavelength intervals using a 1-cm path-length rectangular cell. Each spectrum was obtained from averaging three scans taken at a 20 nm/min scan rate with 1 nm spectral bandwidth (corrected for baseline) using Jasco software. TFE titration involved dissolving the lyophilized purified peptides in the appropriate solvent: (1) 0.1 mM sodium phosphate buffer (pH 6.07) and varying concentrations of TFE or (2) 0%–30% aqueous TFE. Typical peptide concentration was 0.1 mM. The results were expressed as mean residue ellipticity [Q], the units being degrees × cm2 × dmol − 1 according to the [Q]=Ql /(100 lcn ) function where Ql is measured ellipticity, l is optical path-length, c is peptide concentration, and n is the number of amino-acid residues in the sequence (Adler et al. 1973; Johnson 1990).
Results
MSP-3 peptides specifically bound to human erythrocytes
Binding assays were used for determining specific erythrocyte binding activity for 19 synthetic peptides covering the total length of the MSP3 protein (McColl et al. 1994). Peptide binding activity was defined as being the amount (in picomoles) of peptide that bound specifically to erythrocytes per added peptide (in picomoles). HABPs were defined as being those peptides showing activity ≥2%, according to previously established criteria (Urquiza et al. 1996; Curtidor et al. 2001; Rodriguez et al. 2003; Ocampo et al. 2004).
As in other studies, three types of RBC binding behavior were found for MSP-3: high specific binding peptides (i.e., HABP-31209), peptides that did not bind to RBCs (i.e., 31192), and high nonspecific binding peptides where peptides bound to RBCs but there was no inhibition with the same nonradiolabeled peptide (i.e., 31201) (Vera Bravo et al. 2000; Garcia et al. 2002).
Three erythrocyte HABPs were found in MSP-3 peptides: 31193 (21KSFINITLSLFLLHLYIYI40) in the N-terminal, 31202 (201YQKANQAVLKAKEASSYD YI220) in the central, and 31209 (341VKEAAESIMKTL AGLIKGNNY360) in the C-terminal domains (Fig. 1A ▶).
Analogs containing “jumbled” sequences were synthesized to investigate whether MSP-3 HABP binding was due solely to their amino-acid composition or their specific sequence. These jumbled peptides had the same amino-acid composition as the high binding ones but in a random sequence (33008, 33010, and 33011, respectively). These peptides’ specific binding was less than that presented by the original HABPs (Fig. 1B ▶).
Binding constants for erythrocyte HABPs
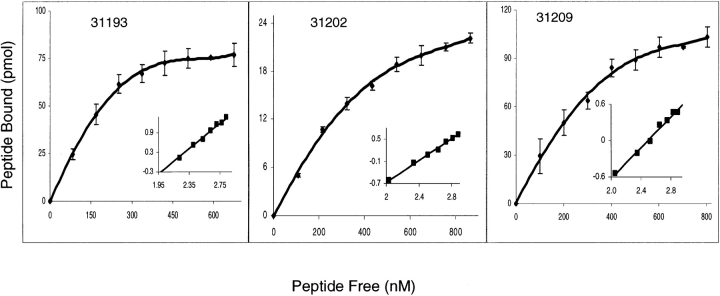
Affinity constants, number of binding sites per cell, and Hill coefficients were determined for HABPs by saturation assays and Hill analysis. The affinity constants (Kd) determined were 140 nM Kd for peptide 31193 (21KSFINITLSLFLLHLYIYI40), 260 nM for peptide 31202 (201YQKANQAVLKAKEASSYDYI220), and 215 nM for peptide 31209 (341VKEAAESIMKTLAGLIKG NNY360) (Fig. 2 ▶). Hill coefficients were 1.5, 1.3, and 1.1, respectively, suggesting positive cooperativity. The number of binding sites per cell was found to be 145,000, 90,000, and 150,000, respectively (Table 1).
Figure 2.
Saturation curves for 31193, 31202, and 31209 HABPs. Increasing quantities of labeled peptide were added in the presence or the absence of unlabeled peptide. The curve represents the specific binding. In the Hill plot (inset), the abscissa is log F and the ordinate is log (B/Bmax − B), where F is free peptide, B is bound peptide, and Bmax is the maximum amount of bound peptide.
Table 1.
MSP-3 protein HABP constants
| Peptide | Kd (nM) | Hill Coef. | Sites/cell |
| 31193 | 140 | 1.2 | 145,000 |
| 31202 | 260 | 1.3 | 90,000 |
| 31209 | 215 | 1.1 | 150,000 |
Affinity constants (Kd), Hill coefficients (nH), and number of binding sites per cell are shown for MSP-3 HABPs. Affinity constants and the number of binding sites were determined by analyzing saturation curves. Hill analysis was performed from the saturation data.
Enzyme treatment
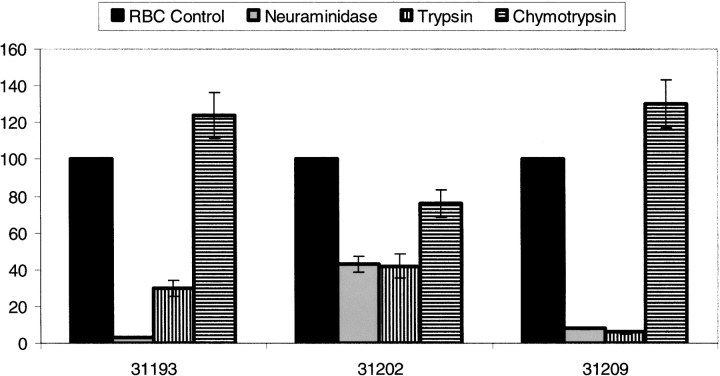
RBCs were enzymatically treated and tested in binding assays with HABPS to determine the probable nature of receptors on RBCs. Figure 3 ▶ shows the changes in HABP specific binding when assayed for binding to enzyme-treated RBCs compared to untreated RBCs specific binding. Specific binding was not modified for all HABPs when RBCs were treated with neuraminidase; on the contrary, when RBCs were treated with trypsin and chymotrypsin their specific binding became notably reduced in all peptides, especially in peptides 31193 and 31209.
Figure 3.
MSP-3 peptide binding to enzyme-treated erythrocytes. Peptide binding was compared between enzyme-treated RBC and untreated RBC. The bars represent the percentage of specific binding activity obtained when radiolabeled HABPs were incubated with erythrocytes treated with chymotrypsin, trypsin, and neuraminidase with respect to untreated erythrocytes.
Cross-linking assays
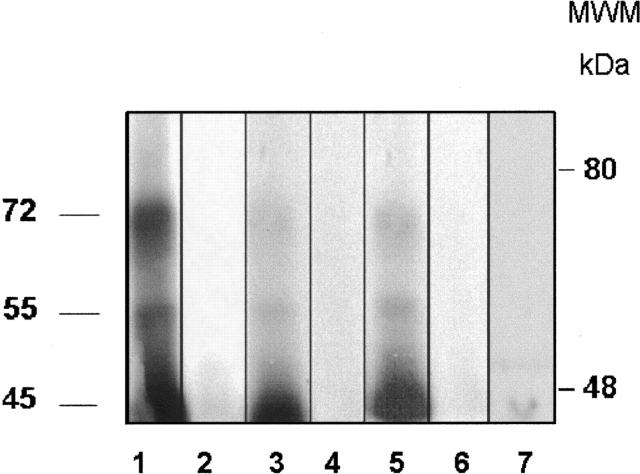
HABPs 31193, 31202, and 31209 recognized erythrocyte membrane protein having apparent 45-, 55-, and 72-kDa molecular weight bands in binding and erythrocyte cross-linking assays when erythrocyte membranes and HABPs were cross-linked to BS3(Bis(sulfosuccinimidyl) suberate) followed by separation in SDS/PAGE. Radiolabeled peptide interaction with this protein was inhibited when the binding was performed in the presence of an excess of unlabeled peptide, indicating that it was a specific interaction. Peptides 31193, 31202, and 31209 are shown; scrambled peptide 33010 did not present any recognition (Fig. 4 ▶).
Figure 4.
Autoradiograph from HABP cross-linking assays. Erythrocyte membrane proteins were cross-linked with radiolabeled peptides 31193 (lanes 1,2), 31202 (lanes 3,4), and 31209 (lanes 5,6). (Lanes 1,3,5) Total binding (i.e., cross-linking in the absence of unlabeled peptide). (Lanes 2,4,6) Inhibited binding (i.e., cross-linking in the presence of unlabeled peptide). (Lane 7) Total binding of scrambled peptide 33010.
Merozoite invasion inhibition assays
The possible role of MSP-3 HBAPs in in vitro merozoite invasion was investigated. The peptides were added to cultures at the schizont stage before merozoites became liberated from infected erythrocytes. The effect of each P. falciparum MSP-3 C27 strain peptide on merozoite invasion was tested in in vitro cultures. Table 2 displays the effects of high binding affinity peptides on parasites in RBC invasion. Peptides 31202 and 31209 inhibited parasite invasion (59% ± 4%, 55% ± 1%, respectively). Peptide 31193 showed a higher inhibitory effect on parasite invasion at 200 μM (85% ± 2%). A significant effect was found in invasion assays for all peptides at 200 μM and 100 μM. By comparison, the two low binding affinity peptides tested and controls did not have any effect on parasite inhibition invasion.
Table 2.
Inhibition of parasite invasion to erythrocytes by MSP-3 peptides
| % Inhibition invasion | ||
| Peptide | 200 μMa | 100μMa |
| 31193 | 85 ± 2 | 8 ± 6 |
| 31202 | 59 ± 4 | 38 ± 1 |
| 31209 | 56 ± .1 | 2 ± 1 |
| 33010 | 6 ± 1 | 2 ± 1 |
| 31199 | 3 ± 1 | 2 ± 1 |
| Control chloroquine | 100 ± 1 | |
| Control EGTA | 100 ± 1 | |
Invasion and development inhibition assays. The assays were performed as described in Materials and Methods at 100 and 200 μM concentrations. 31193, 31202, and 31209 high binding affinity peptides are shown as well as nonbinding peptide 31199 and scrambled peptide 33010. The percentage of merozoite invasion inhibition or intraerythocyte development inhibition is shown with its respective standard deviation. Chloroquine was used as control for the inhibition assays.
aMean ± standard deviation of three experiments.
Circular dichroism analysis
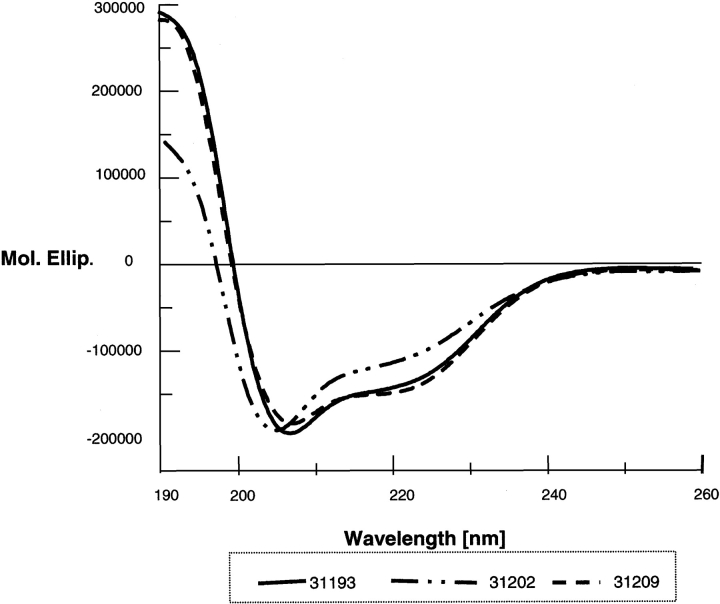
High binding peptides did not present homology among themselves; structural analysis, approximated by circular dichroism (CD), showed that there were some probable structural elements (Fig. 5 ▶), though all MSP-3 HABPs displayed α-helix-like features according to two 208- and 222-nm minimum values and 190-nm maximum ellipticity (Fig. 4 ▶).
Figure 5.
Circular dichroism analysis of MSP-3 HABPs. α-Helix-like structural elements were present in all HABPs; as maximum value was presented at 190 nm and minimum values at 208 and 222 nm, this suggested helical structural elements.
Discussion
Interactions between merozoites and erythrocytes during initial contact are mediated by merozoite surface proteins or the complexes that they form. It has been suggested that these interactions are molecularly mediated among specific receptors on the erythrocyte membrane and merozoite ligands. Studies have shown that merozoites use surface proteins that are involved in the recognition and invasion process, such as MSP-1, MSP-2, MSP-4, MSP-5, and MSP-8 (Smythe et al. 1988; Holder et al. 1994; Marshall et al. 1997; Ocampo et al. 2000; Black et al. 2001). A better understanding of the complex process of P. falciparum merozoite invasion requires that the numerous potential parasite ligands become identified and characterized. Studying interactions between peptides and RBC receptors was carried out as part of our approach using the conceptual framework of the simple bimolecular interaction model, employing MSP-3 protein sequences and normal erythrocytes.
Nineteen nonoverlapped, 20-amino-acid-long peptides covering the complete MSP-3 FC27 strain sequence were characterized; specific MSP-3 normal RBC binding sequences were identified by binding assays. The binding assay results’ profile revealed three erythrocyte HABPs in MSP-3: 31193 (21KSFINI TLSLFLLHLYIYI40) in the N-terminal, 31202 (201YQKANQAVLKAKEASSYDYI220) in the central, and 31209 (341VKEAAESIMKTLAGLIKGNNY360) in the C-terminal region (Fig. 1A ▶).
All these HABPs showed saturable binding, having a finite number of binding sites per cell. The affinity constants suggested that these are important sequences in parasite recognition of and binding to the host cell, due to their high affinity (nanomolar).
Determining physicochemical constants revealed that high affinity binding between HABPs and erythrocyte surface proteins presented positive cooperativity (i.e., a simple receptor–ligand interaction in which the binding of a first ligand favored the entry of other ligands of the same nature).
The number of binding sites per cell is correlated to binding activity and found in the range of numbers reported for erythrocyte surface proteins.
Analyzing binding sequences showed that these high binding peptides had more than 60% charged amino-acid composition respecting nonpolar amino acids. Charge-dependent binding assays were thus done with jumbled peptides to discard, finding that they did not bind to the RBC or did so in much lesser percentages with respect to the original peptide, showing the binding was sequence dependent.
Another assay employed different enzyme treatments for establishing the possible nature of HABP receptors. Treated erythrocytes were used for determining specific binding to modified erythrocytes, finding that neuraminidase treatment led to binding becoming drastically reduced for al lHABPs. Trypsin treatment presented the same behavior, bearing in mind that neuraminidase removes sialic acid and trypsin removes sialoglycoprotein surface components. These treatments drastically affected all HABPs’ binding, suggesting the glycoprotein nature of the receptor site.
Regarding the nature of the possible receptor for these HABPs on the erythrocyte surface, studies with SDS-PAGE and autoradiography have identified 45-, 55-, and 72-kDa proteins that are specifically inhibited in the presence of the same nonradiolabeled peptide. The results shown here showed that HABPs were binding to the same receptor on erythrocyte membrane, but with different affinity.
Taking into account that glycophorins do not contain a homogeneous population due to their different stages of aggregation and the possible alterations that they undergo during the erythrocyte’s life cycle, our results lead us to suggest once more that HABP receptors are glycoproteic. Another possibility is that the receptor could be a protein associated with glycophorins. Analysis of cross-linking revealed three (45-, 55-, and 72-kDa) receptor proteins; their binding to these molecules was inhibited when an excess of nonradiolabeled peptide was present. This provides evidence of a specific interaction; however, further studies are required for a precise definition of receptor molecules.
Previous studies have revealed a 27-amino-acid region (184–210) corresponding to the 3D7 strain from the C-terminal part that has been identified as being a target for a protective antibody response in hyperimmune sera samples (Oeuvray et al. 1994a). This sequence shares part of the sequence for high binding peptide 31202, suggesting that these sequences present similar antigenic determinants.
 |
Other studies have shown that heptapeptide AXXAXXX domain structure sequence shapes predict secondary α-helical structure formation (Mulhern et al. 1995). CD analysis (performed for obtaining general information about the three HABPs’ structure) has also shown α-helical structural elements. These HABP peptides’ amino-acid sequences did not present homology, leading to it being thought that this could be a highly structured protein.
When HABPs were tested in in vitro invasion inhibition assays in P. falciparum cultures, it was seen that all peptides were able to inhibit the invasion process by 55% to 85% at 200 μM concentration. Peptide 31202 presented a significant effect (38%) on merozoite invasion inhibition at 50 μM, making it an interesting target in developing tools for inhibiting P. falciparum merozoite interaction with erythrocytes.
It has been widely demonstrated how merozoite surface membrane antigens play a very important role in the invasion process since sequences against these antigens are able to inhibit it. This work has shown that the MSP-3 protein could be mediating or participating in recognition processes and that some of its sequences have been able to inhibit the invasion process in in vitro assays, suggesting a role for this protein in recognizing and inhibiting invasion.
Promising results have been obtained by identifying specific RBC binding sequences, since they present biological activity in themselves or inhibit parasite invasion to some degree. Our studies have involved the use of this innovative methodology to allow high specific binding sequences to be identified; as these have been very precisely modified they can make a nonimmunogenic sequence into an immunogenic one and induce protection in experimental challenge in Aotus monkeys. They thus represent an excellent tool for identifying possible candidates to be used in developing a multistage, multi-component anti-malarial vaccine (Espejo et al. 2004).
Acknowledgments
This research project was supported by the President of the Republic of Colombia’s office and the Colombian Ministry of Public Health. We thank Jason Garry for patiently reading the manuscript.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.041304505.
References
- Adler, A.J., Greenfield, N.J., and Fasman, G.D. 1973. Circular dichroism and optical rotatory dispersion of proteins and polypeptides. Methods Enzymol. 27 675–735. [DOI] [PubMed] [Google Scholar]
- Black, C.G., Wu, T., Wang, L., Hibbs, A.R., and Coppel, R.L. 2001. Merozoite surface protein 8 of Plasmodium falciparum contains two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 114 217–226. [DOI] [PubMed] [Google Scholar]
- Carvalho, L.J., Oliveira, S.G., Theisen, M., Alves, F.A., Andrade, M.C., Zanini, G.M., Brigido, M.C., Oeuvray, C., Povoa, M.M., Muniz, J.A., et al. 2004. Immunization of Saimiri sciureus monkeys with Plasmodium falciparum merozoite surface protein-3 and glutamate-rich protein suggests that protection is related to antibody levels. Scand. J. Immunol. 59 363–372. [DOI] [PubMed] [Google Scholar]
- Curtidor, H., Urquiza, M., Suarez, J.E., Rodriguez, L.E., Ocampo, M., Puentes, A., Garcia, J.E., Vera, R., Lopez, R., Ramirez, L.E., et al. 2001. Plasmodium falciparum acid basic repeat antigen (ABRA) peptides: Erythrocyte binding and biological activity. Vaccine 19 4496–4504. [DOI] [PubMed] [Google Scholar]
- Duraisingh, M.T., Maier, A.G., Triglia, T., and Cowman, A.F. 2003. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc. Natl. Acad. Sci. 100 4796–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante, A.A., Lal, A.A., and Ayala, F.J. 1998. Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics 149 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo, F., Bermudez, A., Torres, E., Urquiza, M., Rodriguez, R., Lopez, Y., and Patarroyo, M.E. 2004. Shortening and modifying the 1513 MSP-1 peptide’s α-helical region induces protection against malaria. Biochem. Biophys. Res. Commun. 315 418–427. [DOI] [PubMed] [Google Scholar]
- Garcia, J.E., Puentes, A., Suarez, J., Lopez, R., Vera, R., Rodriguez, L.E., Ocampo, M., Curtidor, H., Guzman, F., Urquiza, M., et al. 2002. Hepatitis C virus (HCV) E1 and E2 protein regions that specifically bind to HepG2 cells. J. Hepatol. 36 254–262. [DOI] [PubMed] [Google Scholar]
- Gardner, M.J., Hall, N., Fung, E., White, O., Berriman, M., Hyman, R.W., Carlton, J.M., Pain, A., Nelson, K.E., Bowman, S., et al. 2002. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419 498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder, A.A. 1994. Proteins on the surface of the malaria parasite and cell invasion. Parasitology 108 (Suppl.) S5–S18. [DOI] [PubMed] [Google Scholar]
- Holder, A.A., Blackman, M.J., Borre, M., Burghaus, P.A., Chappel, J.A., Keen, J.K., Ling, I.T., Ogun, S.A., Owen, C.A., and Sinha, K.A. 1994. Malaria parasites and erythrocyte invasion. Biochem. Soc. Trans. 22 291–295. [DOI] [PubMed] [Google Scholar]
- Houghten, R.A. 1985. General method for the rapid solid-phase synthesis of large numbers of peptides: Specificity of antigen–antibody interaction at the level of individual amino acids. Proc. Natl. Acad. Sci. 82 5131–5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber, W., Felger, I., Matile, H., Lipps, H.J., Steiger, S., and Beck, H.P. 1997. Limited sequence polymorphism in the Plasmodium falciparum merozoite surface protein 3. Mol. Biochem. Parasitol. 87 231–234. [DOI] [PubMed] [Google Scholar]
- Hulme, E.C. 1993. Receptor–ligand interactions. A practical approach. Oxford University Press, New York.
- Johnson Jr., W.C. 1990. Protein secondary structure and circular dichroism: A practical guide. Proteins 7 205–214. [DOI] [PubMed] [Google Scholar]
- Lambros, C. and Vanderberg, J.P. 1979. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 65 418–420. [PubMed] [Google Scholar]
- Marshall, V.M., Silva, A., Foley, M., Cranmer, S., Wang, L., McColl, D.J., Kemp, D.J., and Coppel, R.L. 1997. A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect. Immun. 65 4460–4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl, D.J. and Anders, R.F. 1997. Conservation of structural motifs and antigenic diversity in the Plasmodium falciparum merozoite surface protein-3 (MSP-3). Mol. Biochem. Parasitol. 90 21–31. [DOI] [PubMed] [Google Scholar]
- McColl, D.J., Silva, A., Foley, M., Kun, J.F., Favaloro, J.M., Thompson, J.K., Marshall, V.M., Coppel, R.L., Kemp, D.J., and Anders, R.F. 1994. Molecular variation in a novel polymorphic antigen associated with Plasmodium falciparum merozoites.Mol. Biochem. Parasitol. 68 53–67. [DOI] [PubMed] [Google Scholar]
- Merrifield, R.B. 1963. Solid phase peptide synthesis. 1. The synthesis of tetrapeptide. J. Am. Chem. Soc. 85 2149–2154. [Google Scholar]
- Mills, K.E., Pearce, J.A., Crabb, B.S., and Cowman, A.F. 2002. Truncation of merozoite surface protein 3 disrupts its trafficking and that of acidic-basic repeat protein to the surface of Plasmodium falciparum merozoites. Mol. Microbiol. 43 1401–1411. [DOI] [PubMed] [Google Scholar]
- Mulhern, T.D., Howlett, G.J., Reid, G.E., Simpson, R.J., McColl, D.J., Anders, R.F., and Norton, R.S. 1995. Solution structure of a polypeptide containing four heptad repeat units from a merozoite surface antigen of Plasmodium falciparum. Biochemistry 34 3479–3491. [DOI] [PubMed] [Google Scholar]
- Ocampo, M., Urquiza, M., Guzman, F., Rodriguez, L.E., Suarez, J., Curtidor, H., Rosas, J., Diaz, M., and Patarroyo, M.E. 2000. Two MSA 2 peptides that bind to human red blood cells are relevant to Plasmodium falciparum merozoite invasion. J. Pept. Res. 55 216–223. [DOI] [PubMed] [Google Scholar]
- Ocampo, M., Curtidor, H., Vera, R., Valbuena, J.J., Rodriguez, L.E., Puentes, A., Lopez, R., Garcia, J.E., Tovar, D., Pacheco, P., et al. 2004. MAEBL Plasmodium falciparum protein peptides bind specifically to erythrocytes and inhibit in vitro merozoite invasion. Biochem. Biophys. Res. Commun. 315 319–329. [DOI] [PubMed] [Google Scholar]
- Oeuvray, C., Bouharoun-Tayoun, H., Gras-Masse, H., Bottius, E., Kaidoh, T., Aikawa, M., Filgueira, M.C., Tartar, A., and Druilhe, P. 1994a. Merozoite surface protein-3: A malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84 1594–1602. [PubMed] [Google Scholar]
- Oeuvray, C., Bouharoun-Tayoun, H., Grass-Masse, H., Lepers, J.P., Ralamboranto, L., Tartar, A., and Druilhe, P. 1994b. A novel merozoite surface antigen of Plasmodium falciparum (MSP-3) identified by cellular-antibody cooperative mechanism antigenicity and biological activity of antibodies. Mem. Inst. Oswaldo Cruz 89 (Suppl 2.) 77–180. [DOI] [PubMed] [Google Scholar]
- Orlandi, P.A., Klotz, F.W., and Haynes, J.D. 1992. A malaria invasion receptor, the 175-kilodalton erythrocyte binding antigen of Plasmodium falciparum recognizes the terminal Neu5Ac(α 2–3)Gal- sequences of glycophorin A. J. Cell. Biol. 116 901–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, L.E., Ocampo, M., Vera, R., Puentes, A., Lopez, R., Garcia, J., Curtidor, H., Valbuena, J., Suarez, J., Rosas, J., et al. 2003. Plasmodium falciparum EBA-140 kDa protein peptides that bind to human red blood cells. J. Pept. Res. 62 175–184. [DOI] [PubMed] [Google Scholar]
- Singh, S., Soe, S., Mejia, J.P., Roussilhon, C., Theisen, M., Corradin, G., and Druilhe, P. 2004. Identification of a conserved region of Plasmodium falciparum MSP3 targeted by biologically active antibodies to improve vaccine design. J. Infect. Dis. 190 1010–1018. [DOI] [PubMed] [Google Scholar]
- Smythe, J.A., Coppel, R.L., Brown, G.V., Ramasamy, R., Kemp, D.J., and Anders, R.F. 1988. Identification of two integral membrane proteins of Plasmodium falciparum. Proc. Natl. Acad. Sci. 85 5195–5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soe, S., Theisen, M., Roussilhon, C., Aye, K.S., and Druilhe, P. 2004. Association between protection against clinical malaria and antibodies to merozoite surface antigens in an area of hyperendemicity in Myanmar: Complementarity between responses to merozoite surface protein 3 and the 220-kilodalton glutamate-rich protein. Infect. Immun. 72 247–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam, J.P., Heath, W.F., and Merrifield, R.B. 1983. SN 1 and SN 2 mechanisms for the deprotection of synthetic peptides by hydrogen fluoride. Studies to minimize the tyrosine alkylation side reaction. Int. J. Pept. Protein Res. 21 57–65. [DOI] [PubMed] [Google Scholar]
- Theisen, M., Soe, S., Brunstedt, K., Follmann, F., Bredmose, L., Israelsen, H., Madsen, S.M., and Druilhe, P. 2004. A Plasmodium falciparum GLURP-MSP3 chimeric protein; expression in Lactococcus lactis, immunogenicity and induction of biologically active antibodies. Vaccine 22 1188–1198. [DOI] [PubMed] [Google Scholar]
- Trager, W. and Jenson, J.B. 1978. Cultivation of malarial parasites. Nature 273 621–622. [DOI] [PubMed] [Google Scholar]
- Trucco, C., Fernandez-Reyes, D., Howell, S., Stafford, W.H., Scott-Finnigan, T.J., Grainger, M., Ogun, S.A., Taylor, W.R., and Holder, A.A. 2001. The merozoite surface protein 6 gene codes for a 36 kDaprotein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol. Biochem. Parasitol. 112 91–101. [DOI] [PubMed] [Google Scholar]
- Urquiza, M., Rodriguez, L.E., Suarez, J.E., Guzman, F., Ocampo, M., Curtidor, H., Segura, C., Trujillo, E., and Patarroyo, M.E. 1996. Identification of Plasmodium falciparum MSP-1 peptides able to bind to human red blood cells. Parasite Immunol. 18 515–526. [DOI] [PubMed] [Google Scholar]
- Vera Bravo, R., Marin, V., Garcia, J., Urquiza, M., Torres, E., Trujillo, M., Rosas, J., and Patarroyo, M.E. 2000. Amino terminal peptides of the ring infected erythrocyte surface antigen of Plasmodium falciparum bind specifically to erythrocytes. Vaccine 18 1289–1293. [DOI] [PubMed] [Google Scholar]
- Weiland, G.A. and Molinoff, P.B. 1981. Quantitative analysis of drugreceptor interactions: I. Determination of kinetic and equilibrium properties. Life Sci. 29 313–330. [DOI] [PubMed] [Google Scholar]
- Wyatt, C.R., Goff, W., and Davis, W.C. 1991. A flow cytometric method for assessing viability of intraerythrocytic hemoparasites. J. Immunol. Methods 140 23–30. [DOI] [PubMed] [Google Scholar]
- Yamamura, H.I., Enna, S.J., and Kuhar, M.J. 1978. Neurotransmitter receptor binding. Raven Press, New York.