Abstract
A highly sensitive assay combining immunomagnetic enrichment with multiparameter flow cytometric and immunocytochemical analysis has been developed to detect, enumerate, and characterize carcinoma cells in the blood. The assay can detect one epithelial cell or less in 1 ml of blood. Peripheral blood (10–20 ml) from 30 patients with carcinoma of the breast, from 3 patients with prostate cancer, and from 13 controls was examined by flow cytometry for the presence of circulating epithelial cells defined as nucleic acid+, CD45−, and cytokeratin+. Highly significant differences in the number of circulating epithelial cells were found between normal controls and patients with cancer including 17 with organ-confined disease. To determine whether the circulating epithelial cells in the cancer patients were neoplastic cells, cytospin preparations were made after immunomagnetic enrichment and were analyzed. Epithelial cells from patients with breast cancer generally stained with mAbs against cytokeratin and 3 of 5 for mucin-1. In contrast, no cells that stained for these antigens were observed in the blood from normal controls. The morphology of the stained cells was consistent with that of neoplastic cells. Of 8 patients with breast cancer followed for 1–10 months, there was a good correlation between changes in the level of tumor cells in the blood with both treatment with chemotherapy and clinical status. The present assay may be helpful in early detection, in monitoring disease, and in prognostication.
Evidence is accumulating that primary cancers begin shedding neoplastic cells into the circulation at an early stage (1–4); however, the natural history of these cells, their ability to establish metastases, and their bearing on future relapses are unclear. For instance, circulating tumor cells have been detected by PCR in a variety of patients with a good prognosis who are unlikely to develop metastatic disease (5–8). In addition, immunocytochemistry has detected cancer cells in the bone marrow in a proportion of patients with clinically localized disease (9–11). If tumor cell shedding is, in fact, an early event in tumorigenesis, it may be possible to detect cancer cells in the bloodstream before the primary tumor is large enough to be detected by standard screening examinations.
To explore this possibility, we have developed a cellular assay that is more sensitive than PCR and that allows precise enumeration and characterization of circulating carcinoma cells. In model studies, the sensitivity of the technique is below 1 epithelial cell/ml of blood regardless of the number of leukocytes present and the recovery is between 75 and 100%. The assay was used to study the blood of 30 patients with breast cancer, 3 with prostate cancer, and 13 control individuals. An excess of circulating epithelial cells was found in virtually all of the cancer patients unless they were being treated with chemotherapy. In addition, 8 patients with breast cancer undergoing chemotherapy were followed for 1–10 months to determine whether the level of blood tumor cells correlated with clinical studies. The malignant nature of the cells was demonstrated by their cytology and immunophenotype.
METHODS
Collection of Blood Specimens.
With informed consent, 10–20 ml of blood was drawn from controls and patients with a primary diagnosis of breast or prostate cancer into Vacutainer tubes (Becton Dickinson) containing EDTA as anticoagulant. The samples were processed for flow cytometry within 24 h after collection and within 4 h for preparation of cytospins. Patient age, sex, date of diagnosis, therapeutic interventions, clinical status, and biopsy report were retrieved from the patients’ charts. The protocol was approved by the institutional review boards of the collaborating institutions.
Cell Lines.
The breast carcinoma cell lines BT474 and SKBr3 and the prostate carcinoma cell line LNCaP.FGC were used to evaluate the reagents for immunocytochemical detection and to determine sensitivity of the assay. Maintenance medium for the cell lines was RPMI 1640 containing 10% fetal calf serum supplemented with vitamins, nonessential amino acids, and glutamine.
Sample Preparation for Flow Cytometric Analysis.
To enable the enumeration of epithelial cells present in 20 ml of peripheral blood at frequencies below 1 cell/ml of blood by flow cytometry, the following requirements are necessary: (i) The sample volume has to be reduced without a significant loss of epithelial cells to pass the sample through the flow cytometer in a reasonable time period (sample flow rate = 60 μl/min); (ii) to discriminate epithelial cells from other events, the frequency of nucleated nonepithelial cells has to be reduced by a factor of 1,000 or more (events are dots on the plot after flow cytometric analysis; a dot is not necessarily a cell but can be cellular debris or clumps consisting of iron, nuclei, and proteins); and (iii) the reagent combination used for identification has to be optimized such that no other events appear in the region typical for epithelial cells. These requirements have been accomplished by using an immunomagnetic sample preparation procedure developed at Immunicon, Huntingdon Valley, PA. mAbs against the epithelial cell adhesion molecule (EPCAM) are broadly reactive with tissues of epithelial cell origin (12, 13). The GA73.3 EPCAM antibody (kindly provided by D. Herlyn, Wistar Institute, Philadelphia) was coupled to ferrofluids (14). The characteristics of the EPCAM ferrofluid were chosen such that it maintained colloidal properties, did not react with blood components, and still could be separated in an open field magnetic configuration (15). Blood was incubated with the EPCAM-coated ferrofluid for 15 min, and the vessel containing the blood was placed in a magnetic field and allowed to separate for 10 min. After the blood was aspirated and discarded, the vessel was taken out of the magnetic separator and the collected fraction was resuspended from the walls of the vessel with a cell membrane permeabilization solution and placed in the magnetic separator for 5 min. The solution was aspirated and discarded, and the cells were resuspended in a solution containing phycoerythrin-conjugated anti-cytokeratin (CAM5.2 mAb) and peridinin chlorophyll protein-labeled CD45 for 15 min. After incubation, buffer was added and the cell suspension was magnetically separated for 5 min. After discarding the nonseparated suspension, the collected cells were resuspended in 0.5 ml of a buffer containing a nucleic acid dye. Eighty-five percent of the 0.5-ml sample was aspirated and analyzed on a FACScan or FACSCalibur flow cytometer (Becton Dickinson). Data were acquired in listmode by using a threshold on the fluorescence of the nucleic acid dye. Criteria for multiparameter data analysis included size defined by forward light scatter, granularity defined by orthogonal light scatter, and staining with the phycoerythrin-labeled cytokeratin mAb but not with the peridinin chlorophyll protein-labeled CD45 mAb. Reagents for flow cytometry kindly were provided by Becton Dickinson, San Jose, CA.
Immunocytochemistry.
For some patients, when events were found by flow cytometry, the immunomagnetic sample preparation was repeated and cytospin preparations were made. In brief, primary mAbs recognizing cytokeratins 5, 6, 8, and 18 (CK, 5D3, and LP34, NovoCastra, Newcastle, U.K.), Muc-1 glycoprotein (Muc-1, Ma695 NovoCastra) or prostate-specific membrane antigen (clone J591, a generous gift from Neal Bander, Cornell Medical Center) was added to the slides after blocking nonspecific binding sites with 5% BSA for 30 min. The samples were incubated for 20 min at room temperature, washed twice in PBS for 5 min, and then exposed to secondary rabbit anti-mouse Ig (Z0259, Dako) for another 20 min. After two more washes, the samples were incubated with alkaline-phosphatase-anti-alkaline-phosphatase (APAAP) mouse Ig complexes for 15 min. Finally, the enzyme-substrate [NewFuchsin (Dako)] was added, resulting in the development of red precipitates. The nucleus was counterstained with hematoxyline. The data were recorded by using a Kodak digital camera attached to a light microscope and were stored on CD for later reference.
RESULTS
The basis of the present method is that carcinomas (ectoderm origin) differ from leukocytes (mesoderm origin) in their gene expression, and, therefore, each of these two cell populations has tissue-specific molecules on its surface or intracellularly. Therefore, the assay consists of using a series of mAbs that recognize these tissue-specific molecules. This has been accomplished by using a two-step procedure: The first step involves an immunomagnetic sample preparation in which blood is mixed with a preparation of colloidal iron coated with mAbs specific for epithelial cells. After separation in a magnetic field, a sample volume reduction as well as a 104-fold enrichment of epithelial cells is obtained. This step is essential in obtaining the sensitivity and low background required. The second step consists of tagging the elements in the obtained cell suspension. This involves using a second mAb specific for another molecule on epithelial cells (cytokeratin), a third mAb against a pan leukocyte antigen (CD45), and a nucleic acid dye to allow exclusion of residual red blood cells and other nonnucleated events. The sample then is analyzed by flow cytometry, and all events staining with the nucleic acid dye are analyzed for CD45 and cytokeratin staining as well as forward and orthogonal light scatter characteristics.
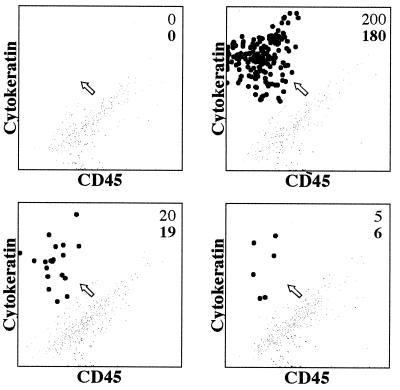
Fig. 1 shows the analysis of a normal blood sample to which varying numbers of a breast carcinoma cell line (SKBr3) were added. As can be seen, the carcinoma cells can be distinguished from the other blood cells and the recovery was between 75 and 100%. The recovery of breast carcinoma cells was consistent over a target frequency range, between 50 and 4500 SKBr3 cells “spiked” into 5 ml of blood from normal donors. Regression analysis of the spiked number of cells plotted vs. the recovered number of cells yielded a correlation coefficient of r2 = 0.96 and with a slope of 0.76, indicating consistent recovery of 76% in this experiment (data not shown). The linearity of the recovery shows that the number of the tumor cells detected reflects the actual peripheral blood tumor cell load. However, as shown in Fig. 1, when very small numbers of breast carcinoma (SKBr3) cells were used for spiking, namely, ≤20 cells/2.5 ml, the recovery of the SKBr3 cells was between 95 and 100%. Such results suggest that the limiting factor for sensitivity is the volume of blood used. However, to use this level of sensitivity by flow cytometry for the diagnosis of cancer, it will be necessary that control bloods routinely display no events, a condition that we have not yet achieved (see below).
Figure 1.
Flow cytometric analysis of mixtures of blood and breast carcinoma cells. Various numbers of SKBr3 breast carcinoma cells (top figure in the upper righthand corner of each dot plot) were added to 2.5 ml of normal peripheral blood. The tumor cells were enriched by using ferrofluid-coated with anti-EPCAM and then stained with a combination of anti-cytokeratin (CAM5.2 mAb conjugated to phycoerythrin) shown on the y axis and anti-CD45 mAb conjugated to peridinin chlorophyll protein (x axis). Only nucleated cells were recorded based on the nucleic acid staining. The cluster of tumor cells (black dots) is identified readily based on its high cytokeratin expression and CD45 negativity (CD45+ are the gray dots). An arrow points to the putative epithelial cell cluster. The number of the recovered tumor cells also is shown (bottom figure in the upper righthand corner of each dot plot).
We next examined blood from patients with breast cancer and from controls for the presence of epithelial cells by using flow cytometry. The data obtained from flow cytometric experiments were analyzed by two individuals in a blinded fashion. One analysis was performed with a computer algorithm created in paint-a-gate pro (Becton Dickinson) and applied to the data files of all patient samples (LT), and a second analysis used individualized gating for each patient (ER). The results indicate that there is no significant difference by paired t test (n = 21, P = 0.84) in inter-observer determinations in the number of circulating epithelial cells. Thus, in the future, an algorithm will be used for the analysis.
Table 1 summarizes the results of studying 13 controls and 30 patients with breast cancer. In control individuals, the number of epithelial cells ranged from 0 to 5 (mean 1.5 ± 1.8). In contrast, there was an average of 15.9 ± 17.4 epithelial cells in the bloods of 14 patients with organ-confined carcinoma of the breast (patients classified as Tx NoMo), 47.4 ± 52.3 in those with nodal involvement, and 122 ± 140 in those with distant metastases. The difference between the control group and patients with carcinoma of the breast with or without metastasis was highly significant [P < 0.001 by multiparameter analysis (Kruskal–Wallis)]. The difference between the organ confined and the distant metastatic group was 0.009 (t test). The number of epithelial cells in patients with organ-confined breast cancer was above the cut-off point (mean value plus 3 SD in the control group = 6.9) in 12 of 14 cases. Moreover, no individual in the control group had more than 5 events classified as epithelial cells, and only 2 of the 14 patients with organ-confined breast cancer had <7 such events. The difference between the control group and patients with organ-confined carcinoma of the prostate was also significant (t test <0.01).
Table 1.
Summary of clinical data
| Blood donors | Donors, Δ | Donors with circulating epithelial cells, n | Average number of epithelial cells per blood sample (±SD) |
|---|---|---|---|
| Controls (healthy individuals) | 13 | 7 | 1.5 ± 1.8 |
| Breast cancer patients | |||
| No detectable spread | 14 | 13 | 15.9 ± 17.4 |
| Spread to local lymph nodes only | 5 | 5 | 47.4 ± 52.3 |
| Distant metastases | 11 | 11 | 122 ± 140 |
| Total patients | 30 | 29 | 56.9 ± 98.2 |
| Prostate cancer patients | |||
| No detectable spread | 3 | 3 | 16 ± 4 |
Flow cytometry was used to analyze the positive events obtained from 20 ml of blood from control individuals; from women with breast carcinoma, or from men with prostate cancer. The numbers of epithelial cells in the blood of the controls are statistically different by t test (P ≤ 0.01) and by Kruskal–Wallis nonparametric analysis (P < 0.001) from each of the three groups of the breast cancer patients and the prostate cancer patients. The data in this table were used to establish a preliminary cut-off value for positive samples. This value was determined by averaging the number of circulating epithelial cells in the normal controls (n = 13) and then adding three times the SD. The average (n = 13) was 1.5 and the SD is 1.8. Cut-off: 1.5 + 5.4 = 6.9. There is no statistical difference between male and female controls.
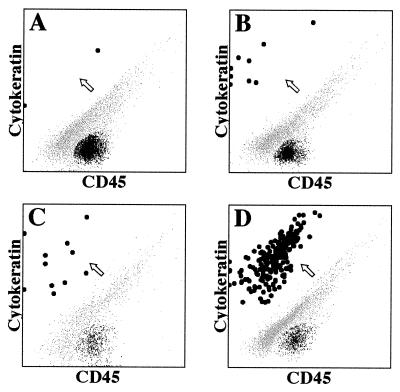
Fig. 2 shows representative examples of the data from Table 1. We chose 10-ml blood samples from: a control, which shows 2 epithelial cells (Fig. 2A); a patient with organ-confined breast carcinoma (9 cells) (Fig. 2B); and a patient with metastatic breast carcinoma (a very large tumor load) (Fig. 2D). Data obtained from a patient with organ-confined prostate carcinoma are also shown (Fig. 2C). As can be seen, there is a clear delineation between the events representing putative epithelial cells (large black dots) and those representing residual leukocytes (black) and debris (gray) that nonspecifically stain with both phycoerythrin and peridinin chlorophyll protein.
Figure 2.
Flow cytometric analyses of blood samples from patients and controls. These are representative examples of the data from the 46 patients and controls (Table 1). Shown are 10-ml blood samples from a control that shows two epithelial cells (A), from a patient with organ-confined breast carcinoma (9 cells, B) or organ-confined prostate carcinoma (11 cells, C), and from a patient with metastatic breast carcinoma (a very large tumor load, D). An arrow points to the cluster of putative epithelial cells (large black dots). Leukocytes are small black dots, and debris is shown as small gray dots.
To examine whether the cells identified as epithelial cells by flow cytometry could be classified as tumor cells, blood samples from some of the normal controls and from patients with cancer in which epithelial cells were detected by flow cytometry were subjected to the immunomagnetic sample preparation followed by a cytospin. This procedure allows individual cells to be studied for morphology and additional markers, either surface or intracellular.
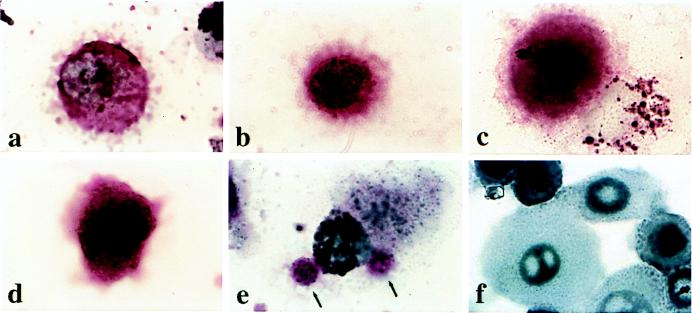
Fig. 3 shows representative cells from a cytospin immunostained with anti-mucin-1 (Fig. 3A) or anti-cytokeratin (Fig. 3B) from a patient with metastatic breast cancer. Fig. 3 C and D show anti-cytokeratin-stained cells from a patient with organ-confined breast cancer (C) and organ-confined prostate cancer (D). The cells tended to be large, brightly stained, and with a high nuclear-to-cytoplasmic ratio in contrast to normal epithelial cells (F). In general, the staining was more intense, and the nuclear and plasma membranes were more distinct in advanced compared with early tumors. Fig. 3E shows a white cell with two brightly stained round objects consistent with apoptotic tumor cell bodies. No cells are shown from the controls because none of the six normal individuals studied with intact putative epithelial cells (i.e., positive events by FACS analysis) had recognizable epithelial cells on the cytospins. Possibly, the events observed on flow cytometry of blood samples from these individuals are artifactual, or the normal epithelial cells are particularly prone to apoptosis and are fragmented in the cytospin.
Figure 3.
Expression of cytokeratin and Muc-1 glycoprotein in circulating breast and prostate carcinoma cells and normal epithelial cells. Circulating tumor cells (a-e) were isolated by using the ferrofluid purification followed by cytospinning and staining of the slide. (a) A cell stained with anti-mucin-1 from a patient with metastatic breast cancer. (b) Same patient but a different cell stained with anti-cytokeratins 5, 6, 8, and 18. (c) A cell stained with anti-cytokeratin from a patient with clinically organ-confined breast tumor. (d) A cell stained with anti-cytokeratin from a patient with clinically organ-confined prostate cancer. (e) Same patient as in a showing two stained bodies, probably apoptotic tumor cell bodies (arrows), stained with anti-cytokeratin and attached to a macrophage. (f) Normal epithelium obtained from human trypinized foreskin (uncultured) and stained with anti-mucin-1. All slides were subjected to an alkaline-phosphatase-anti-alkaline-phosphatase procedure that caused the development of a red precipitate. The nuclei were counterstained with hematoxylin. All images were photographed at ×1,000.
To ensure that there was no subjectivity in the analysis of the cytospins, one of us (J.U.) examined 19 slides in a blinded fashion. The slides represented controls, patients with organ-confined or metastatic cancer or another sample from the same patient stained with different mAbs, and repeats of the same slide to determine whether there was a significant intraobserver error. The results indicate that all four of breast cancer patients and one of three prostate cancer patients were correctly categorized. Of particular importance, in no instance did the observer categorize blood samples from the six controls as having carcinoma cells, i.e., there were no false-positives. Also, there were no intraobserver errors when repeat slides were seen.
To ensure that the ferrofluid did not distort the architecture of the blood epithelial cells, experiments were performed using the same blood sample from a patient with metastatic breast cancer processed by the ferrofluid method or by sucrose gradients as used by others (8, 9, 11, 16–18). The results showed no difference in morphology (data not shown).
We interpret the immunophenotypic and cytologic evidence to indicate that the events recorded by flow cytometry in the blood of cancer patients represent carcinoma cells. Thus, flow cytometry can distinguish the bloods from control individuals vs. cancer patients with a high degree of accuracy.
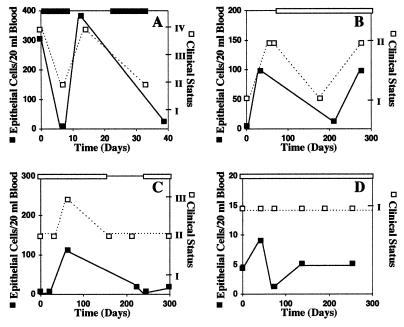
Analysis of blood from cancer patients by flow cytometry measures total circulating carcinoma cells, so it was of interest to determine whether our assay could reflect the patients’ clinical status over time. For this purpose, blood samples from eight patients with breast cancer were examined for 1–10 months at various intervals. The blood level of epithelial cells and the clinical status were plotted against time. In all eight patients, the two curves were in parallel. Fig. 4 shows four representative examples of the results. In a patient with life threatening disease (Fig. 4A), high dose chemotherapy induced a partial remission and circulating epithelial cells disappeared. When chemotherapy was stopped, blood epithelial cells rose to previous levels. The patient promptly relapsed and, after retreatment with high dose chemotherapy, again became clinically stabilized and blood epithelial cells disappeared. In two patients with recurrence of tumor (Fig. 4 B and C), both blood levels of epithelial cells and tumor burden measured clinically rose; when maintenance chemotherapy was given, both parameters decreased to lower levels. The patient shown in Fig. 4B then relapsed despite continued maintenance chemotherapy, and blood levels of epithelial cells rose. In a patient without evidence of disease on maintenance chemotherapy (Fig. 4D), the blood levels of epithelial cells remained low. These results provide additional evidence that the assay is measuring tumor cells in the blood.
Figure 4.
Changes in blood levels of breast carcinoma cells reflect changes in clinical status. The dark line with darkened squares represents blood levels of epithelial cells and the dotted line with open squares represents the clinical status. The darkened bar on top indicates the length of time for high dose chemotherapy, and the open bar represents the length of time for maintenance chemotherapy. The numbers for clinical status represent the following: I, no evidence of disease; II, stable disease; III, advancing disease; IV, life-threatening disease.
DISCUSSION
Early detection is the hallmark of successful cancer treatment. However, many tumors remain clinically occult until they are far advanced. The reasons for this are varied. For example, the remote anatomical location of the pancreas makes it unlikely that pancreatic carcinomas will be detected before they have invaded neighboring structures. Although the breast is anatomically accessible, breast cancers metastasize very early in their course; consequently, 12–37% of small (<1 cm) mammographically detected breast cancers already have metastasized at diagnosis (19, 20). Even in prostate cancer, in which a prostate-specific antigen can be quantified in the serum, there is a substantial percentage of patients with elevated PSA in which diagnosis remains uncertain. Thus, the urologist must decide whether a biopsy is necessary or, if a biopsy has been performed and is negative, if and when it should be repeated. These examples indicate that there is a need for improved diagnostic techniques for cancer.
In 1869, Ashworth described cells in the blood that resembled those observed in the tumor at autopsy (21). However, it is only in the last 1–2 decades that two techniques were developed, immunohistochemistry and PCR (and reverse transcription PCR), that generally allow detection of one tumor cell in 105–6 blood or bone marrow cells.** The results have changed concepts of the frequency and significance of tumor dissemination. Thus, because hematologic malignancies frequently are associated with particular chromosomal translocations, PCR has been useful in detecting minimal residual disease and in predicting impending relapse in such tumors. Minimal residual disease is associated, in general, with a poor prognosis, but there is considerable evidence that, particularly if transient, it does not preclude a sustained remission of the patient without further treatment (27–30). Both immunocytochemistry and PCR studies of patients with several solid tumors including breast and prostate carcinoma have detected circulating tumor cells in a proportion of these patients with clinically organ-confined disease. Sixteen to 45% of men with “localized” prostate cancer had detectable disease in the peripheral circulation or bone marrow assayed by prostate-specific antigen-specific PCR (31, 32). Analysis by PCR for breast cancer has been hampered by the inability to find a specific target RNA. However, by using a reverse transcription PCR to detect expression of carcinoembryonic antigen in breast and gastrointestinal cancer, 14 of 21 patients had tumor cells detected in the bone marrow (33). In an immunocytochemical study of 135 patients with breast carcinoma, 49 had tumor cells in bone marrow (11).
Both techniques have their limitations. Thus, immunocytochemical assays are unable to quantify tumor burden, and the time-consuming search for a rare cell makes it impractical to phenotype the tumor cells in depth. PCR assays are also difficult to quantify, and there can be false-positive results. In addition, rearrangements of sequences are frequent targets for amplification of RNA sequences by reverse transcription PCR; hence, in these cases, diagnostic tumor tissue usually is needed to confirm the presence of tumor cells.
The assay described here has the following features: (i) 1–2 logs greater sensitivity than the above previously described assays and independent of leukocyte count; (ii) precise enumeration of the number of circulating tumor cells; (iii) use of whole blood; and (iv) suitable for automation. Also, immunophenotyping can be performed on individual tumor cells with regard to organ lineage and, potentially, the presence of activation and invasive markers and detection of oncogenes such as mutated p53.
At this time, we believe the most important test of the usefulness of our assay is to screen for early detection of carcinomas in the general population and, particularly, in high risk patients, e.g., those with BRCA-1 mutations. Will our assay detect early primary tumors or predict them? In this regard, the most pertinent finding to emerge from the present study is that 12 of 14 patients with clinically organ-confined breast cancers and 3 of 3 patients with organ-confined prostate cancers had excess epithelial cells in their blood, and these cells usually had the immunophenotypic and cytomorphologic features consistent with neoplastic cells. Our goal, therefore, is to perform the screening assay entirely by immunomagnetic preparation followed by flow cytometry. This method might entail use of additional channels on the flow cytometer for determination of the organ origin of the carcinoma cells, e.g., prostate-specific membrane antigen+ for prostate, mucin-1+ or progesterone receptor+ for breast. If a cut-off value for a diagnosis of cancer based on blood cells can be obtained and if the positive results are uniformly confirmed by subsequent pathologic analysis, then, in the future, treatment might be initiated earlier when tumor volume is small and, more importantly, the genetically unstable tumor is at an earlier stage in its evolution. These features should increase the likelihood of a clinical cure (34–36).
Although the assay described here appears to be highly sensitive, there are as yet untested requirements that are essential to achieve maximal usefulness. Thus, we have not yet tested the blood of patients with nonmalignant diseases involving mucosa or skin, breast, and prostate. If their blood shows events on flow cytometry, it will be important to determine whether these events can be distinguished from carcinoma cells by present methods, by further alterations in the regimen for flow cytometry, or, if necessary, by appropriate staining and examination of the immunophenotype and cytology of the blood cells obtained by cytospins.
Our preliminary results measuring clinical status and the levels of blood epithelial cells over time are highly suggestive that our assay is measuring circulating tumor cells (Fig. 4). Thus, in all eight patients, there was a general correlation between these two assays. The results also suggest that changes in blood levels of breast carcinoma cells may help in monitoring treatment and determining recurrences. Also, because of the ability to immunophenotype the cells for different molecules, our assay also may be useful in prognostication. Thus, the number and characterization of blood carcinoma cells may allow predictions in individual patients as to whether progressive metastatic growth will develop. The high percentage of blood samples that show carcinoma cells from patients who have no clinical evidence of metastases suggests that the presence of such cells does not necessarily indicate that the circulating tumor cells will survive and grow.
It also would be of interest to test blood on clinically disease-free patients many years after therapy of a breast or prostate carcinoma to determine whether there are circulating tumor cells. There is increasing evidence from clinical observations (37–43), experimental models of dormancy (44–49), and the findings of circulating tumor cells in patients with clinically organ-confined disease as shown in the present study that suggests that many cancers are chronic systemic diseases that are not cured by present day therapy.
Acknowledgments
We thank Drs. Ellen Vitetta and Louis Picker for advice regarding the manuscript, Drs. Joan Reisch and Barbara Foster for help with the statistical analysis, Mr. Thomas Tucker for assistance in sample collection and data processing, and Ms. Cindy Patterson and Sue Chadwick for excellent secretarial assistance. Dr. Emil Racila was supported by National Institutes of Health Fellowship Awards 5-T32-CA66187-02 and T32-CA09082.
ABBREVIATION
- EPCAM
epithelial cell adhesion molecule
Footnotes
References
- 1.Butler T P, Gullino P M. Cancer Res. 1975;35:512–516. [PubMed] [Google Scholar]
- 2.Glaves D, Mayhew E. Cancer Drug Delivery. 1984;1:293–302. doi: 10.1089/cdd.1984.1.293. [DOI] [PubMed] [Google Scholar]
- 3.Liotta L A, Kleinerman J, Saidel G M. Cancer Res. 1974;34:997–1004. [PubMed] [Google Scholar]
- 4.Fidler I J. Eur J Cancer. 1973;9:223–227. doi: 10.1016/s0014-2964(73)80022-2. [DOI] [PubMed] [Google Scholar]
- 5.Ghossein R A, Rosai J. Cancer. 1996;78:10–16. doi: 10.1002/(SICI)1097-0142(19960701)78:1<10::AID-CNCR3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 6.Johnson P W, Burchill S A, Selby P J. Br J Cancer. 1995;72:268–276. doi: 10.1038/bjc.1995.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seiden M, Sklar J L. In: Important Advances in Oncology. DeVita V T, Hellman S, Rosenberg S A, editors. Lippincott-Raven Publishers; 1996. pp. 191–204. [Google Scholar]
- 8.Pelkey T J, Frierson H F, Jr, Bruns D E. Clin Chem. 1996;42:1369–1381. [PubMed] [Google Scholar]
- 9.Pantel K, Riethmuller G. Curr Top Microbiol Immunol. 1996;213:1–18. doi: 10.1007/978-3-642-80071-9_1. [DOI] [PubMed] [Google Scholar]
- 10.Pantel K, Felber E, Schlimok G. J Hematother. 1994;3:315–322. doi: 10.1089/scd.1.1994.3.315. [DOI] [PubMed] [Google Scholar]
- 11.Pantel K, Schlimok G, Braun S, Kutter D, Lindemann F, Schaller G, Funke I, Izbicki J R, Riethmuller G. J Natl Cancer Inst. 1993;85:1419–1424. doi: 10.1093/jnci/85.17.1419. [DOI] [PubMed] [Google Scholar]
- 12.Herlyn D, Herlyn M, Ross A H, Ernst C, Atkinson B, Koprowski H. J Immunol Methods. 1984;73:157–167. doi: 10.1016/0022-1759(84)90041-3. [DOI] [PubMed] [Google Scholar]
- 13.Stahel R A, Gilks W R, Lehmann H P, Schenker T. Int J Cancer. 1994;8:6–26. doi: 10.1002/ijc.2910570704. [DOI] [PubMed] [Google Scholar]
- 14.Liberti P A, Chiarappa J N, Hovsepian A C, Rao C G. In: Fine Particles Science and Technology. Pelizzetti E, editor. Dordrecht, The Netherlands: Kluwer; 1996. pp. 777–790. [Google Scholar]
- 15.Liberti P, Feeley B. In: Cell Separation and Technology. Kompala D S, Todd P, editors. Washington, D.C.: Am. Chem. Soc.; 1991. pp. 268–288. [Google Scholar]
- 16.Hochtlen-Vollmar W, Gruber R, Bodenmuller H, Felber E, Lindemann F, Passlick B, Schlimok G, Pantel K, Riethmuller G. Int J Cancer. 1997;70:396–400. doi: 10.1002/(sici)1097-0215(19970207)70:4<396::aid-ijc4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Pantel K, Schlimok G, Angstwurm M, Weckermann D, Schmaus W, Gath H, Passlick B, Izbicki J R, Riethmuller G. J Hematother. 1994;3:165–173. doi: 10.1089/scd.1.1994.3.165. [DOI] [PubMed] [Google Scholar]
- 18.Ross A A, Cooper B W, Lazarus H M, Mackay W, Moss T J, Ciobanu N, Tallman M S, Kennedy M J, Davidson N E, Sweet D. Blood. 1993;82:2605–2610. [PubMed] [Google Scholar]
- 19.Wilhelm M C, Edge S B, Cole D D, deParedes E, Frierson H F., Jr Ann Surg. 1991;213:600–603. doi: 10.1097/00000658-199106000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chadha M, Chabon A B, Friedmann P, Vikram B. Cancer. 1994;73:350–353. doi: 10.1002/1097-0142(19940115)73:2<350::aid-cncr2820730219>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Ashworth T R. Aust Med J. 1869;14:146. [Google Scholar]
- 22.Gross H J, Verwer B, Houck D, Hoffman R A, Recktenwald D. Proc Natl Acad Sci USA. 1995;92:537–541. doi: 10.1073/pnas.92.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori M, Mimori K, Ueo H, Karimine N, Barnard G F, Sugimachi K, Akiyoshi T. Int J Cancer. 1996;68:739–743. doi: 10.1002/(SICI)1097-0215(19961211)68:6<739::AID-IJC8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Corey E, Arfman E W, Oswin M M, Melchior S W, Tindall D J, Young C Y, Ellis W J, Vesella R L. Urology. 1997;50:184–188. doi: 10.1016/S0090-4295(97)00262-8. [DOI] [PubMed] [Google Scholar]
- 25.Neumaier M, Gerhard M, Wagener C. Gene. 1995;159:43–47. doi: 10.1016/0378-1119(94)00522-t. [DOI] [PubMed] [Google Scholar]
- 26.Matsumura M, Niwa Y, Hikiba Y, Okano K, Kato N, Shiina S, Shiratori Y, Omata M. Biochem Biophys Res Commun. 1995;207:813–818. doi: 10.1006/bbrc.1995.1259. [DOI] [PubMed] [Google Scholar]
- 27.Campana D, Pui C H. Blood. 1995;85:1416–1434. [PubMed] [Google Scholar]
- 28.Nizet Y, Van Daele S, Lewalle P, Vaerman J L, Philippe M, Vermylen C, Cornu G, Ferrant A, Michaux J L, Martiat P. Blood. 1993;85:1618–1625. [PubMed] [Google Scholar]
- 29.Roth M S, Antin J H, Ash R, Terry V H, Gotlieb M, Silver S M, Ginsburg D. Blood. 1992;79:276–282. [PubMed] [Google Scholar]
- 30.Moss T J, Reynolds C P, Sather H N, Romansky S G, Hammond G D, Seeger R C. N Engl J Med. 1991;324:219–226. doi: 10.1056/NEJM199101243240403. [DOI] [PubMed] [Google Scholar]
- 31.Wood D P, Jr, Banks E R, Humphreys S, McRoberts J W, Rangnekar V M. Cancer. 1994;74:2533–2540. doi: 10.1002/1097-0142(19941101)74:9<2533::aid-cncr2820740922>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 32.Ghossein R A, Scher H I, Gerald W L, Kelly W K, Curley T, Amsterdam A, Zhang Z F, Rosai J. J Clin Oncol. 1995;13:1195–1200. doi: 10.1200/JCO.1995.13.5.1195. [DOI] [PubMed] [Google Scholar]
- 33.Gerhard M, Juhl H, Kalthoff H, Schreiber H W, Wagener C, Neumaier M. J Clin Oncol. 1994;12:725–729. doi: 10.1200/JCO.1994.12.4.725. [DOI] [PubMed] [Google Scholar]
- 34.Ries L A G, Kosary C L, Hankey B F, Miller B A, Edwards B K, editors. SEER Cancer Statistics Review, 1973–1994. Betheseda, MD: National Cancer Institute; 1997. NIH Pub. No. 97-2789. [Google Scholar]
- 35.Rimer B K, Schildkraut J. In: Cancer: Principles and Practice of Oncology. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1997. pp. 619–631. [Google Scholar]
- 36.Harris J, Morrow M, Norton L. In: Cancer: Principles and Practice of Oncology. DeVita V T Jr, Hellman S, Rosenberg S A, editors. Philadelphia: Lippincott; 1997. pp. 1557–1616. [Google Scholar]
- 37.Stewart T H M, Raman S. In: Cellular Immune Mechanisms and Tumor Dormancy. Stewart T H M, Wheelock E F, editors. Boca Raton, FL: CRC; 1992. pp. 315–329. [Google Scholar]
- 38.Meltzer A. J Surg Oncol. 1990;43:181–188. doi: 10.1002/jso.2930430312. [DOI] [PubMed] [Google Scholar]
- 39.Berkowitz H, Rosato F, Neiby C P. Am Surg. 1966;32:287–289. [PubMed] [Google Scholar]
- 40.Hadfield G. Br Med J. 1954;2:607–610. doi: 10.1136/bmj.2.4888.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wheelock E F, Weinhold K J, Levich J. Adv Cancer Res. 1981;34:107–140. doi: 10.1016/s0065-230x(08)60240-7. [DOI] [PubMed] [Google Scholar]
- 42.Shaw H M, Beattie C W, McCarthy W H, Milton G W. Arch Surg. 1985;120:1155–1159. doi: 10.1001/archsurg.1985.01390340053010. [DOI] [PubMed] [Google Scholar]
- 43.Callaway M P, Briggs J C. Br J Plast Surg. 1989;42:46–49. doi: 10.1016/s0007-1226(89)90111-2. [DOI] [PubMed] [Google Scholar]
- 44.Uhr J W, Tucker T, May R D, Siu H, Vitetta E S. Cancer Res. 1991;51:5045S–5053S. [PubMed] [Google Scholar]
- 45.Vitetta E S, Tucker T F, Racila E, Huang Y-W, Marches R, Lane N, Scheuermann R, Street N E, Watanabe T, Uhr J W. Blood. 1997;89:4425–4436. [PubMed] [Google Scholar]
- 46.George A J T, Stevenson F K. Int Rev Immunol. 1989;4:271–310. doi: 10.3109/08830188909044783. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson F K, George A J T, Glennie M J. Chem Immunol. 1990;48:126–166. [PubMed] [Google Scholar]
- 48.Wheelock E F, Yang G, Chen L. In: Cellular Immune Mechanisms and Tumor Dormancy. Stewart T H M, Wheelock E F, editors. Boca Raton, FL: CRC; 1992. pp. 53–65. [Google Scholar]
- 49.Slavin S, Ackerstein A, Weiss L, Nagler A, Or R, Naparstek E. In: Cellular Immune Mechanisms and Tumor Dormancy. Stewart T H M, Wheelock E F, editors. Boca Raton, FL: CRC; 1992. pp. 99–107. [Google Scholar]