Abstract
Human immunodeficiency virus Nef is a myristoylated protein expressed early in infection by HIV. In addition to the well known down-regulation of the cell surface receptors CD4 and MHCI, Nef is able to alter T-cell signaling pathways. The ability to alter the cellular signaling pathways suggests that Nef can associate with signaling proteins. In the present report, we show that Nef can interact with calmodulin, the major intracellular receptor for calcium. Coimmunoprecipitation analyses with lysates from the NIH3T3 cell line constitutively expressing the native HIV-1 Nef protein revealed the presence of a stable Nef-calmodulin complex. When lysates from NIH3T3 cells were incubated with calmodulin-agarose beads in the presence of CaCl2 or EGTA, calcium ion drastically enhanced the interaction between Nef and calmodulin, suggesting that the binding is under the influence of Ca2+ signaling. Glutathione S-transferase-Nef fusion protein bound directly to calmodulin with high affinity. Using synthetic peptides based on the N-terminal sequence of Nef, we determined that within a 20-amino-acid N-terminal basic domain was sufficient for calmodulin binding. Furthermore, the myristoylated peptide bound to calmodulin with higher affinity than nonmyris-toylated form. Thus, the N-terminal myristoylation domain of Nef plays an important role in interacting with calmodulin. This domain is highly conserved in several HIV-1 Nef variants and resembles the N-terminal domain of NAP-22/CAP23, a myristoylated calmodulin-binder. These results for the interaction between HIV Nef and calmodulin in the cells suggested that the Nef might interfere with intracellular Ca2+ signaling through calmodulin-mediated interactions in infected cells.
Keywords: HIV nef, calmodulin, myristoylation, protein-protein interaction
The Nef protein of the human and simian immunodeficiency viruses (HIV-1, HIV-2, and SIV) is a myristoylated protein that is expressed early during the viral infection process (Goldsmith et al. 1995). Nef is critical to the maintenance of a high viral load and for the development of AIDS. Inoculation of Rhesus monkeys with a nef-deletion mutant strain of SIV does not lead to AIDS-like disease, and actually results in long-term immunity against pathogenic SIV (Kestler et al. 1991; Daniel et al. 1992). Furthermore, clinical studies of long-term HIV-infected individuals possessing apparent deletions within the nef gene exhibit no signs of progression to AIDS (Deacon et al. 1995; Kirchoff et al. 1995). The critical function of Nef is not known, but two major in vitro effects on cell function have been observed. One is that Nef induces alterations in cellular signal transduction pathways, and the other is that Nef down-regulates surface expression of the CD4 and MHC class I molecules (Oldridge and Marsh 1998; Peter 1998).
The ability of Nef to alter signal transduction and activation pathways suggests a mechanism that may involve specific molecular interactions between Nef and the cellular signaling proteins. The molecular interactions between Nef and p56Lck kinase and between Nef and other Src families of protein kinases occur through the proline repeat motif in Nef (Saksela et al. 1995; Collette et al. 1996). Furthermore, Nef interacts with c-Raf1 kinase or vacuolar ATPase through the C-terminal conserved acidic sequence (Hodge et al. 1998; Lu et al. 1998). Other proteins have been reported to interact with Nef, including the p21-activated serine/threonine protein kinase (PAK) (Cullen 1996), the MAP kinase (Greenway et al. 1996), protein kinase C (PKC) θ (Smith et al. 1996), and AP2 adaptor protein complex (Piguet et al. 1998).
The structure of the core domain of Nef was determined in solution by NMR methods (Grzesiek et al. 1996, 1997) and X-ray structures (Lee et al. 1996; Arold et al. 1997). These structural studies were performed with recombinant constructs that lack the N-terminal, unstructured domains. The N-terminal region is a myristoylated membrane-targeting domain responsible for both down-regulation of the CD4 receptor and enhancement of viral replication and infectivity (Goldsmith et al. 1995). It has been demonstrated that Nef interacts with CD4 and actin, which are dependent on N-terminal myristoylation of Nef (Harris and Neil 1994; Fackler et al. 1997). Furthermore, several studies have shown that the similarity between the N-terminal domain of Nef and melittin might account for the observed cytotoxicity of the Nef N-terminal peptide (Barnham et al. 1997; Curtain et al. 1998). Thus, the N-terminal domain of Nef is thought to play an important role in the function of this protein through its interactions with membranes and other proteins.
We showed that the myristoyl moiety and the N-terminal domain of some myristoylated proteins are involved in the binding to calmodulin (Takasaki et al. 1999). Brain-specific acidic protein NAP-22/CAP-23, which belongs to the MARCKS family of PKC substrate proteins, binds to calmodulin in a myristoylation-dependent manner (Takasaki et al. 1999; Hayashi et al. 2000). In our studies of the NAP-22/CAP-23 protein-calmodulin interaction, we noticed that the N-terminal sequence of the protein resembles that of Nef protein. This finding led us to examine the interaction between Nef and calmodulin. Calmodulin has been known to act as an intracellular calcium sensor protein. When the intracellular Ca2+ concentration increases, calmodulin can bind up to four Ca2+, changing its conformation and regulating cellular functions such as activation or inhibition of a large number of enzymes, ion channels, and receptors (Crivici and Ikura 1995; James et al. 1995). These Ca2+-dependent interactions of calmodulin with its target proteins have played an important role in intracellular Ca2+ signaling and in various cellular functions including mitogenesis, cell growth, differentiation, and immune response. Thus, the interactions of Nef with calmodulin are likely to be the key to the unknown mechanisms of Nef functions.
We previously showed the in vitro interaction between calmodulin and myristoylated peptide corresponding to the N-terminal region of Nef protein (Hayashi et al. 2002). In the present study, we demonstrate an in vivo and in vitro interaction between intact Nef protein and calmodulin. The N-terminal myristoylation domain of Nef was involved in the interaction with calmodulin. Further, the myristoylated Nef was associated with higher affinity compared to non-myristoylated Nef. Thus, the myristoylation domain of Nef plays an important role in interacting with calmodulin signaling pathways.
Results
Identification of calmodulin as a Nef-interacting protein
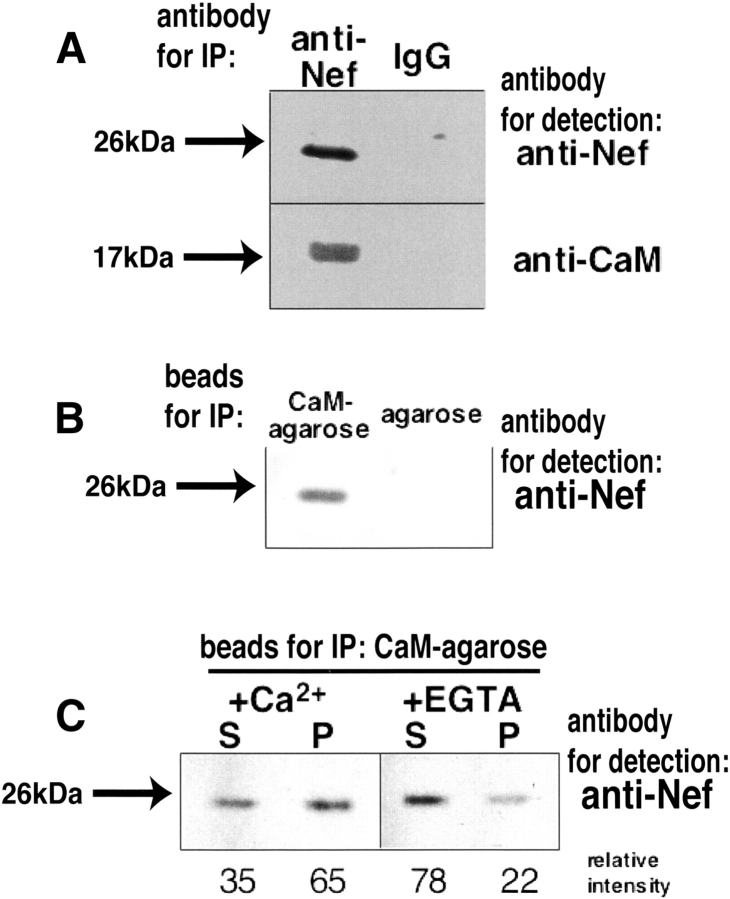
To demonstrate the interaction of Nef with calmodulin in vivo, we performed coimmunoprecipitation analyses using lysates of NIH3T3 cells permanently infected with Nef expression vector (Otake et al. 1994). Cell lysates were incubated with anti-Nef antibody or nonimmunized mouse IgG, followed by incubation with protein A sepharose beads. Immunoprecipitated proteins were resolved on SDS-polyacrylamide gel electrophoresis, and the bound proteins were then detected by Western blotting with the anti-Nef antibody or the anti-calmodulin antibody. As shown in Figure 1A ▶, anti-Nef immunoblot analysis detected the immunoprecipitated Nef that migrated with a molecular mass of ~26 kDa. In contrast, a normal mouse IgG immunoprecipitation did not associate with Nef protein. Immunoprecipitation with anti-Nef antibody followed by Western blotting for calmodulin revealed the presence of calmodulin in anti-Nef immune complexes (Fig. 1A ▶, lower left lane). Immunoprecipitation with nonimmune antisera does not result in the coprecipitation of the proteins. These results demonstrate that Nef interacts with calmodulin in the cells.
Figure 1.
Immunoprecipitation analyses of Nef expressed in NIH3T3 cells. (A) Immunoblot analysis of Nef and calmodulin coimmunoprecipitated from lysates of NIH3T3 cells, which were permanently infected with Nef expression vector. Cell lysates were immunoprecipitated (IP) with the anti-Nef antibody (left lane) and a normal IgG used as a negative control (right lane), and were detected by anti-Nef (upper) and anti-CaM (lower) antibody, respectively. (B) NIH3T3 cells permanently expressing Nef were lysed and incubated with calmodulin-agarose beads (left) or agarose beads without calmodulin (right) in the presence of 2 mM CaCl2. After washing of the agarose beads, the bound proteins were resolved by SDS-PAGE and transferred to membrane. Blots were probed for anti-Nef antibody. (C) Cells were lysed, centrifuged to be separated into soluble and insoluble fraction, and incubated with calmodulin-agarose beads in the presence (left) or absence (right) of CaCl2. S and P represent the soluble fraction and the insoluble fraction, respectively. Blots were probed for anti-Nef antibody. Relative intensities of the bands are also shown under the gel image.
Furthermore, we investigated whether Nef binds to calmodulin in NIH3T3 cell extracts by using a calmodulin agarose assay. Agarose beads with or without calmodulin were incubated with NIH3T3 cell lysates. Precipitated proteins were then analyzed by immunoblotting with the anti-Nef antibody. Figure 1B ▶ shows that Nef was precipitated from lysates of NIH3T3 cells expressing Nef with calmodulin agarose but not with agarose alone, demonstrating the specific interaction between Nef and calmodulin. Furthermore, when lysates from NIH3T3 cells were incubated with calmodulin agarose in the presence of 2 mM CaCl2 or 4 mM EGTA, calcium ion drastically enhanced the interaction between Nef and calmodulin (Fig. 1C ▶).
Nef interacts directly with calmodulin in vitro
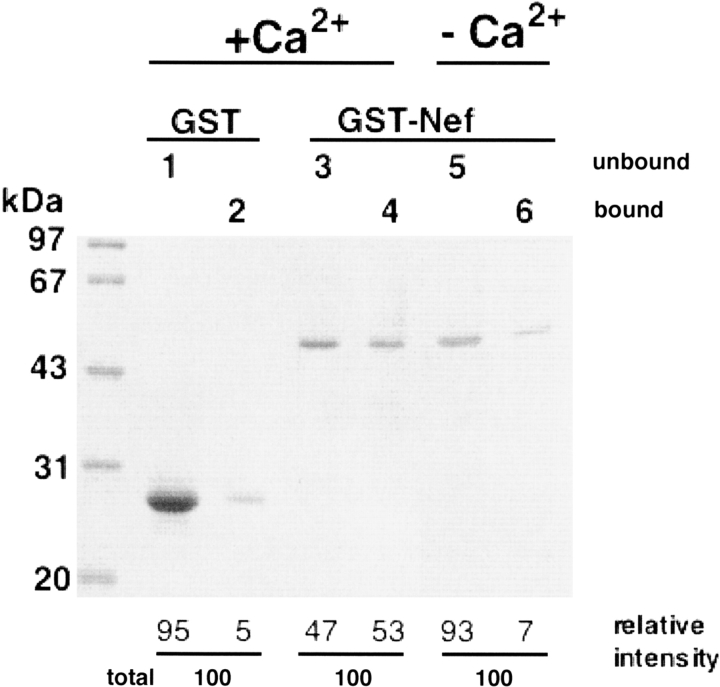
The possibility that the interaction of calmodulin with Nef protein in the NIH3T3 cell lysates required additional cellular factors has not been ruled out. Therefore, to demonstrate that the binding of Nef to calmodulin in vitro is a direct physical interaction between the two proteins, we performed a direct binding assay in which GST fusion Nef protein was reacted with calmodulin agarose beads. GST and GST fusion Nef were mixed with calmodulin agarose beads in the presence or absence of Ca2+. After a short centrifugation, the supernatants and the bound fractions were separated. As shown in Figure 2 ▶, a significant amount of the GST fusion Nef protein bound to the calmodulin agarose, and the amount was significantly reduced in the Ca2+-free buffer.
Figure 2.
Direct interaction between GST-Nef and calmodulin. Aliquots containing GST (lanes 1,2) or GST fusion Nef proteins (lanes 3–6) were incubated with 50 μL of calmodulin-agarose beads in 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 500 μM CaCl2 (lanes 1–4) or 4 mM EGTA (lanes 5,6) for 1 h at 25°C. After a short centrifugation, the supernatants were removed to analyze the unbound fractions (lanes 1,3,5). The protein-bound beads were then extensively washed with the above buffer and processed for SDS-PAGE as the bound fractions (lanes 2,4,6). Relative intensities of the bands are also shown under the gel image.
The ability of Nef to bind to calmodulin was also tested using surface plasmon resonance on a BIAcore instrument. We bound biotinylated calmodulin to streptavidin-coated microsensor chips, which allowed GST fusion Nef to associate with calmodulin and monitored the association and dissociation of GST fusion Nef as a function of time. As shown in the sensorgrams of Figure 3A ▶, GST fusion Nef bound rapidly during the injection phase, reached a steady-state level, then dissociated from the complex. In contrast, no binding signal was observed in the case of GST alone. An addition of buffer containing EGTA reduced the signal to the extent of the baseline (Fig. 3B ▶). Further, we calculated a Kd of 94 nM (kon = 1.49 × 104 M−1s−1, koff = 1.40 × 10−3 M−1s−1) for nonmyristoylated GST fusion Nef-calmodulin interaction, indicating the high-affinity binding. The affinity of CaM/Nef is similar to that of SAP97 (130 nM) (Paarmann et al. 2002), caldesmon (Kd = 1 μM) (Smith et al. 1987) and type 1 inositol 1,4,5-trisphosphate (IP3) receptor (Kd = 700nM) (Yamada et al. 1995). These proteins are representatives of significant calmodulin binders.
Figure 3.
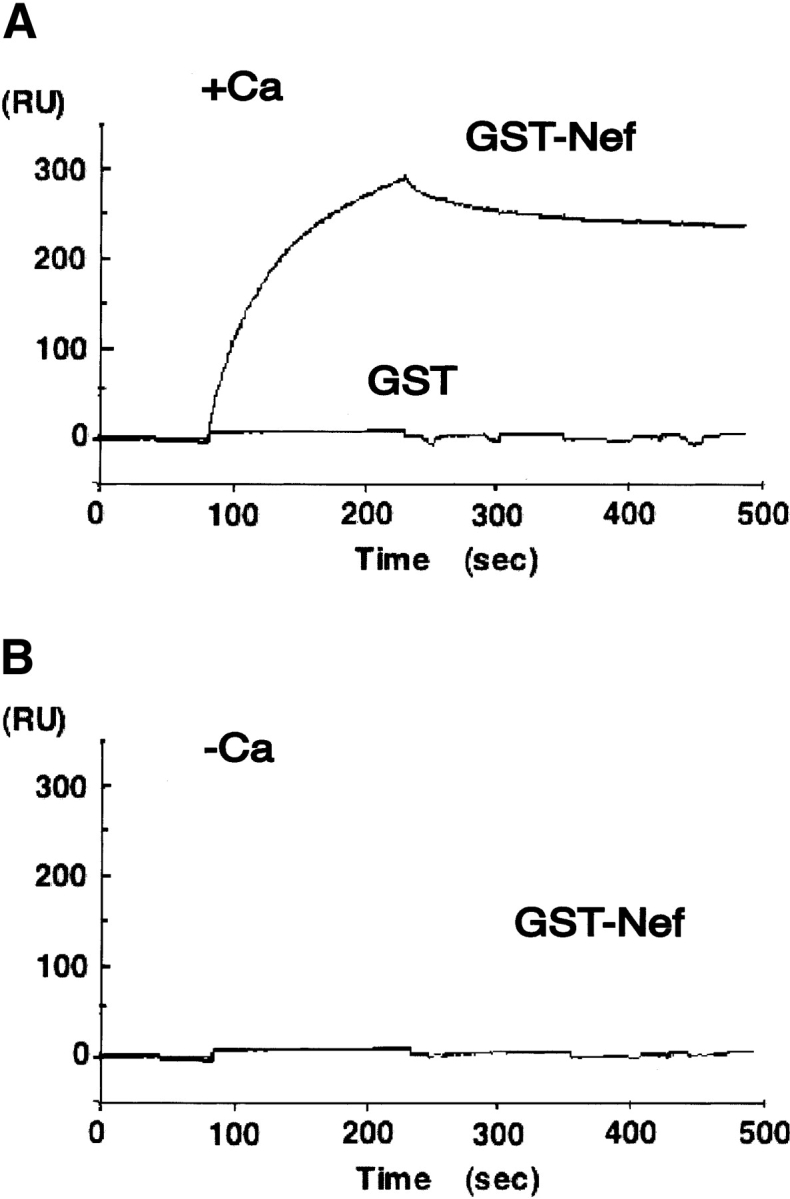
Analyses of binding of Nef to calmodulin using surface plasmon resonance. The calmodulin was trapped on the surface of a sensor chip containing covalently attached streptavidin. For analysis of calmodulin-Nef interaction, solutions of the Nef were injected across chip surfaces containing calmodulin. The running buffer contained 20 mM Tris-HCl, pH 7.4, 100 mM NaCl, 500 μM CaCl2 (A) or 25 mM EGTA (B). GST and GST-Nef were diluted in this buffer to a final concentration of 200 nM prior to injection. The volume of injected sample was 40 μL, and the flow rate was 10 μL/min.
The significance of myristoyl moiety for interaction between CaM and Nef protein
To elucidate the effect of the myristoylation in the interaction between CaM and Nef protein, the ability of myristoylated and nonmyristoylated Nef to bind to calmodulin was tested using surface plasmon resonance on a BIAcore instrument as described in the previous section. For this purpose, intact nonmyristoylated Nef and myristoylated Nef were prepared. The results showed that the myristoylation of Nef protein drastically raises the ability to bind to calmodulin. Analysis of the binding curves from several experimental series revealed two independent dissociation reactions with rate constants (kd) of 5.20 × 10−4 (kd1) and 9.72 × 10−3 (kd2) s−1, respectively. From the association rate constants (ka) (ka1 =.39 × 105 M−1s−1, ka2 = 1.04 × 104 M−1s−1) and the dissociation rate constants, the dissociation constants (KD) of 3.74 × 10−9 (KD1) and 9.36 × 10−7 (KD2) M were calculated. On the other hand, nonmyristoylated Nef showed almost the same KD (176 nM) as that of non-myristoylated GST fusion Nef.
Identification of a calmodulin binding domain of Nef
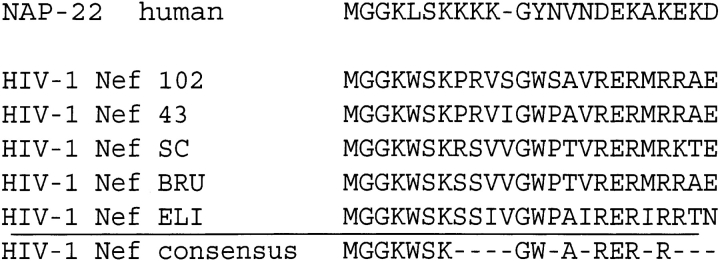
During the course of our studies on the calmodulin binding activity of NAP-22/CAP-23 protein, we noticed sequence similarities between NAP-22/CAP-23 and Nef in the N-terminal myristoylation domain (Takasaki et al. 1999), and we confirmed that a myristoylated peptide corresponding to the N-terminal domain of Nef bound to calmodulin through its myristoyl moiety (Hayashi et al. 2002). Figure 4 ▶ shows the alignment of the N-terminal domain sequences of Nef proteins from different HIV isolates in comparison to that of NAP-22/CAP-23 protein. Since the first nine residues of NAP-22/CAP23 protein (Takasaki et al. 1999) and Nef protein (Hayashi et al. 2002) were sufficient for the binding to calmodulin, and because it was shown that the myristoylated moieties played important roles in the interactions, it was taken for granted that the N-terminal myristoylated domain of intact Nef might be involved directly in the binding to calmodulin.
Figure 4.
Alignment of conserved N-terminal sequences of Nef variants with a corresponding sequence within calmodulin-binding domain of NAP22/CAP23. An HIV-Nef consensus sequence motif is also indicated.
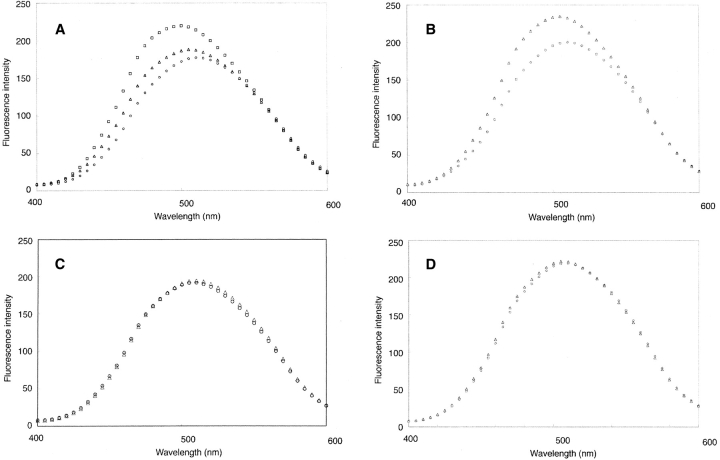
To demonstrate that the N-terminal domain is indeed the calmodulin-binding domain of intact Nef, synthetic myristoylated and nonmyristoylated peptides with different amino acid residues were prepared and tested for their abilities to bind calmodulin. The binding of peptides to calmodulin was carried out by measuring the fluorescence change of dansyl-calmodulin upon binding of the target peptide (Matsubara et al. 1997). The nonmyristoylated peptide (GGKWSKSSVIGWPTVRER) and the myristoylated peptide (myr-GGKWSKSSVVGWPTVRER) were designed on the basis of the sequences of HIV-NL43 and HIV-SC strains. As shown in Figure 5A ▶, the addition of 400 nM myristoylated peptide or nonmyristoylated peptide to 200 nM dansyl-calmodulin in the presence of Ca2+ induced an increase in the intensity and shifts in the peak maxima of the emission spectra, suggesting that both forms bound to calmodulin. However, there was a clear difference in the peak maxima and the level of intensity between the two peptides. Using the fluorescence change, the dissociation constants of both peptides were determined from the titration data to be 8.0 nM and 140.0 nM for the myristoylated form and the nonmyristoylated form, respectively. These results suggested that the calmodulin-binding domain of Nef was within N-terminal 20 amino acid residues, and the myristoylated moiety strongly enhanced the binding.
Figure 5.
Calmodulin binding by N-terminal Nef peptides. (A) The emission spectra of 200 nM dansyl-calmodulin alone (○) in the presence of 400 nM myristoylated Nef peptide (myr-GGKWSKSSVVGWPTVRER) (Δ) and 400 nM nonmyristoylated Nef peptide (GGKWSKSSVIGWPTVRER) (□) are shown. (B) The emission spectra of 200 nM dansyl-calmodulin alone (○) and in the presence of 400 nM short myristoylated Nef peptide (myr-GGKWSKRS) (Δ). (C) The emission spectra of 200 nM dansyl-calmodulin alone (○) and in the presence of 400 nM short nonmyristoylated Nef peptide (GGKWSKRS) (Δ). (D) The emission spectra of 200 nM dansyl-calmodulin alone (○) and in the presence of 400 nM short acetylated Nef peptide (acetyl-GGKWSKRS) (Δ).
Furthermore, to study the role of the N-terminal regions in the myristoylated peptide-calmodulin, a series of the peptides were synthesized, and the binding characteristics to calmodulin were analyzed by fluorescence measurements. Since the N-terminal residues 2–9 of HIV-1 Nef are of a highly conserved nature, the importance of the regions on the binding to calmodulin was also examined. The addition of myristoylated peptide designed on the basis of the sequences of HIV-HV1SC (myr-GGKWSKRS) to dansyl-calmodulin caused an increase in the intensity and a shift of the peak maximum of the emission spectra similar to that observed with the myristoylated peptide with 18 amino acid residues (Fig. 5B ▶). The dissociation constant was determined to be 87.5 nM, which was about 10-fold weaker than that of the longest peptide. On the other hand, no significant change in the fluorescence spectra was observed when the nonmyristoylated peptide (GGKWSKRS) or the acetylated peptide (acetyl-GGKWSKRS) was added to calmodulin (Fig. 5C,D ▶). These results not only indicate that the myristoyl moiety is directly involved in the Nef-calmodulin interaction, but also suggest that the highly conserved regions are also important for the interaction.
Discussion
Several studies have suggested that expression of HIV-1 Nef results in the modulation of intracellular signaling pathways in a variety of cells. The precise molecular bases for these effects are not fully understood, but are likely to be a consequence of the ability of Nef to interact with a variety of signal transduction molecules. Indeed, Nef interacts with a variety of signaling proteins and intracellular kinases, including the Src family kinases, the MAP kinase, protein kinase C, and PAK family kinases (Saksela et al. 1995; Collette et al. 1996; Cullen 1996; Greenway et al. 1996; Smith et al. 1996). In the present study, we demonstrated that Nef binds specifically to calmodulin via its N-terminal myristoylated domain. The Nef-calmodulin interaction occurs in intact cells as demonstrated in Nef-transfected NIH3T3 cells by the coimmunoprecipitation of Nef with calmodulin. We also present evidence that cell-derived Nef coprecipitates with calmodulin agarose in a Ca2+-dependent manner. We identified and characterized the calmodulin-binding domain in Nef using a synthetic peptide. In the fluorescence spectrometry assay, an 18-amino-acid peptide corresponding to the N-terminal myristoylation domain of Nef was found to interact with calmodulin. By using myristoylated synthetic peptide in this region, we confirmed that N-terminal myristoylation could enhance the Nef-calmodulin interaction.
The primary structure of the identified calmodulin-binding domain of Nef shows features similar to those of other calmodulin-binding proteins such as CAP-23/NAP-22 (Fig. 4 ▶). We recently demonstrated that the calmodulin-binding domain of CAP-23/NAP-22 was narrowed down to the myristoyl moiety together with a nine-amino-acid N-terminal basic domain (myristoyl-GGKLSKKKK) (Takasaki et al. 1999). The identified calmodulin-binding domain of Nef contains well conserved hydrophobic and positively charged residues (myristoyl-GGKWSKRS). We speculate that the basic residues contribute to calmodulin binding via electrostatic interaction with acidic residues in calmodulin, whereas the myristoyl group and large hydrophobic amino acids such as tryptophan and leucine seem to play a more important role in calmodulin binding through interactions with the hydrophobic pocket in the globular domains of calmodulin. In the case of CAP-23/NAP-22, myristoylation is more directly involved in the interaction between the protein and calmodulin. Furthermore, the present study shows that myristoylation of Nef is also required for the interaction with calmodulin. Thus, the myristoylation of these calmodulin-binding proteins is not only a mechanism for targeting to membrane fractions, but also plays a direct functional role by mediating protein-protein interactions.
The similarities in sequence between the N-terminal domain of CAP-23/NAP-22 and that of Nef suggest that they may interact with calmodulin in a similar fashion. In this study, different from the case of CAP-23/NAP-22, it was shown that the N-terminal domain of Nef could interact with calmodulin without the myristoyl moiety, but the affinity was found to be about 20-fold weaker than that of myristoylated forms. The myristoylation of Nef has been shown to enhance interaction between Nef and calmodulin, and, furthermore, on the analogy of CAP-23/NAP-22 (Takasaki et al. 1999), the effect is supposed to be reduced by phosphorylation of the neighboring serine residues of Nef. These results indicate that the calmodulin-binding motif located at the N-terminal region of Nef is not sufficient to interact with calmodulin, and the myristoylation directly enhances the interaction in a manner regulated by plural post-translational modifications, myristoylation and phosphorylation.
The N-terminus of Nef is thought to play an important role in the functions of this protein. Goldsmith et al. (1995) demonstrated that deletion of residues 4–7 of Nef resulted in dramatically reduced infectivity. The mutant also had a significantly reduced ability to down-regulate CD4. A similar result by Greenway et al. (1994) showed that Nef caused down-regulation of the surface expression of CD4 and IL2 receptor when transfected into T-lymphocytes, but this did not occur with Nef, which lacked the 19 N-terminal residues. Another report shows that the N-terminus of HIV-1 Nef associated with a protein complex containing Lck and a serine kinase (Baur et al. 1997). Deletion of the fragments 16–22 and/or 11–40 inhibits association of the kinase complex, and significantly reduces the viral infectivity. Thus, the N-terminus of Nef plays an important role in interactions with cellular proteins involved in intracellular signaling and in inhibition of their activities. We also show here that the N-terminus of Nef binds to calmodulin. The surface plasmon resonance analyses of this study and biophysical analyses of previous studies (Hayashi et al. 2000, 2002) independently revealed two states of transitions following the interaction between myristoylated Nef and Ca2+/CaM. Together with the results of the present study and the previous study (Hayashi et al. 2002), it is revealed that when one molecule of myristoylated Nef is bound to Ca2+/CaM, the affinity is very high, although the induced structural change of Ca2+/CaM is very low. On the other hand, when the second molecule of myristoylated Nef binds to Ca2+/CaM, Ca2+/CaM gives rise to drastic structural change despite the lower affinity. Therefore, it is tempting to speculate that at least some of the functional significance of Nef may be mediated by the binding of Nef to calmodulin through the N-terminal myristoylation domain.
We have proposed that calmodulin-binding domains of some proteins are cross-talk points of signal transduction pathways. For instance, calmodulin-binding domains of myristoylated alanine-rich C kinase substrate, GAP-43, CAP-23/NAP-22, endothelial nitric oxide synthase, and src kinase bind to acidic membrane phospholipids (Taniguchi and Manenti 1993; Matsubara 1996; Hayashi et al. 1997, 2000, 2002, 2004; Takasaki et al. 1999). Phosphorylations of the domain by PKC significantly reduce their ability to bind to membrane phospholipids and/or calmodulin. Reversible translocation between the membranes and cytosol in response to PKC-dependent phosphorylation and calmodulin binding plays an important role in the regulation and the function of these proteins. In the case of Nef, N-terminal peptides of Nef possess membrane phospholipid-binding properties (Curtain et al. 1994, 1998). Furthermore, our preliminary results show that the 18 N-terminal amino acid peptide of Nef is phosphorylated by PKC, and the phosphorylation reduces the ability to bind to calmodulin (M. Matsubara and N. Hayashi, unpubl.). Thus, calmodulin, phospholipids, and PKC bind to the same domain, and the bindings are mutually competitive. Therefore, the calmodulin-binding domain of Nef may be involved in the complex regulation and interaction of Nef with other signal transduction pathways, as is also the case with some of the other myristoylated proteins (Hayashi et al. 2002).
Finally, the regulation of Ca2+ signaling in T lymphocytes is a mechanism that modulates the immune response. Therefore, the alteration of Ca2+ homeostasis in Nef-expressing T-cell lines could be of importance in different cellular processes. Ca2+ is also believed to organize and stabilize the calmodulin domain structure in a conformational state that can bind target proteins. The calmodulin concentration in some cells is on the order of micromolar (Klee and Vanaman 1982). Ca2+ release can thus convert these molecules to the Ca2+-bound form. The affinity of Nef for calmodulin is sufficiently high so that under these conditions Nef is free to interact with the cytosolic calmodulin. It is therefore likely that Nef may modulate a diverse array of calmodulin-dependent cell functions. Recent results showed that Nef induces expression of interleukin-10 in a human T-cell line and that induction of interleukin-10 by extracellular Nef involves the Ca2+-calmodulin signal transduction pathway (Brigino et al. 1997). Furthermore, in NIH3T3 cells transfected with Nef, Nef selectively affects the phosphatidylinositol (PI) 3-kinase signaling pathway which is important for cell growth and chemotaxis (Graziani et al. 1996). This observation is of considerable interest, as it was demonstrated that PI 3-kinase activity can be regulated by the binding to calmodulin (Joyal et al. 1997). Therefore, the calmodulin binding by Nef observed in the present study is likely to mediate the functional defects in Nef-infected cells. Further studies are required to unravel the biological significance of calmodulin binding by Nef. The results presented here may provide a new perspective to elucidate the process of HIV-1 infection, and may lead to novel pharmacological strategies against AIDS infection.
Materials and methods
Materials
Calmodulin was purified from bovine brain as described previously (Gopalakrishna and Anderson 1982). Dansyl-calmodulin and calmodulin agarose beads were purchased from Sigma. Other chemicals used were of the highest grade commercially available. The myristoylated peptides of Nef and the nonmyristoylated peptides synthesized using standard Fmoc chemistry were obtained from Research Genetics. The peptides were purified over a reversed-phase column (Vydac218TP, 1.5 × 150 mm) using a linear gradient of H2O-acetonitrile in the presence of 0.1% trifluoroacetic acid. They were judged to be >95% purity by electrospray mass spectrometry (Taniguchi et al. 1994). Peptide concentrations were determined by quantitative amino acid analysis. Anti-Nef monoclonal antibody (A7) prepared as described previously (Fujii et al. 1993) was a kind gift from Y. Fujii (Nagoya City Univ., Nagoya). Anti-calmodulin polyclonal antibody was purchased from Signal Transduction. GST-Nef (HIV-1 NL43) expression plasmid, pGEX-Nef, was also kindly provided by Y. Fujii.
Polyacrylamide gel electrophoresis
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (1970). After staining with Coomassie brilliant blue, the intensity of protein bands was estimated with a Molecular Dynamics personal densitometer SI.
Cell culture and immunoprecipitation assay
The nef-transduced NIH3T3 cells produced as described previously (Otake et al. 1994) were a kind gift from Y. Fujii. The NIH3T3 cells were maintained at 37°C in a humidified 5% CO2 incubator in RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum. For immunoprecipitation experiments, whole-cell lysates were prepared in ice-cold cell lysis buffer (50 mM Tris-HCl, pH 7.5, 0.4 mM EDTA, 1% Nonidet P-40, 0.1% sodium deoxycholate, 2 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL aprotinin). The cell lysates were centrifuged at 15,000 rpm for 15 min to remove insoluble material. The protein concentration of supernatants was determined by Bio-Rad protein assay and adjusted to 1 mg/mL. Equal amounts of protein were precleared by incubation with Protein A Sepharose 4B for 1 h at 4°C. The cleared lysates were incubated with anti-Nef antibody for 1 h at 4°C, protein A Sepharose added, and the mixture was incubated for an additional 1 h. The immune complexes were washed three times with cell lysis buffer. Immunoprecipitated proteins were eluted from the beads by boiling the samples in SDS-PAGE sample buffer. Immunoprecipitated samples were separated by SDS-PAGE (12.5% gels), followed by transfer of the proteins to nitrocellulose membranes. Membranes were blocked by incubation in Tris-buffered saline (10 mM Tris-HCl, pH 7.4, 150 mM NaCl) containing 5% nonfat dry milk for 1 h, followed by 1.5 h incubation in primary anti-Nef antibody (1:1000) or anti-calmodulin antibody (1:1000) diluted in blocking buffer. Membranes were washed extensively in Tris-buffered saline containing 0.05% Tween 20, and then incubated with goat anti-mouse or donkey anti-rabbit horseradish per-oxidase-conjugated secondary antibodies. Membranes were then washed and visualized with the ECL Plus Western blotting detection system and ECL mini-camera (Amersham Pharmacia Bio-tech). For the coprecipitation assay with calmodulin-agarose, cells were lysed in 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 2 mM CaCl2, 0.1 mM PMSF, 10 μg/mL pepstatin, 10 μg/mL leupeptin. Cell lysates were incubated with 50 μL of calmodulin agarose beads or agarose beads with gentle mixing for 1 h at 4°C, and the protein-bound beads were extensively washed with an excess of the above buffer and processed for SDS-PAGE and Western blot analysis as described above.
Protein expression and purification
The GST-Nef expression vector, pGEX-Nef, was used to transform competent Escherichia coli BL21 (DE3) cells. The production and purification of the GST and GST-Nef fusion proteins in E. coli followed the manufacturer’s protocol (Amersham Pharmacia Biotech).
The E. coli strain BL21(DE3)pLysS was transformed with the plasmid pET23d containing the human Nef gene. For non-myr Nef, the cells containing the plasmid were selected with 100 μg/mL ampicillin. A frozen stock of transformed colonies was used to inoculate LB media containing 100 μg/mL ampicillin, and the cells were grown overnight at 37°C. Five hundred mL of LB media containing 100 μg/mL ampicillin was inoculated with the over-night culture. The cells were grown to an OD600 of ~1–1.2, then further grown in the presence of 0.4 mM isopropyl-1-thio-β-galactopylanoside for 5 h. The cells were collected by centrifugation for 20 min at 8000 rpm at 4°C (HITACHI 9-2 rotor) and kept frozen at −20°C. Cells were resuspended and sonicated in 5 volumes of ice-cold buffer (10 mM Tris-HCl, pH 7.5) containing 0.1% Triton X-100, 10 mM dithiothreitol, 1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride). After centrifuging for 25 min at 18,000 rpm at 4°C, the supernatants were collected. The supernatants were then applied to a RESOURCE Q column and eluted with a gradient of 100–600 mM NaCl. The fractions containing the Nef protein were pooled. For myr Nef, the E. coli strain BL21(DE3)pLysS was transformed with the plasmid pBB131NMT, which contained the gene coding for yeast N-myristoyl transferase (NMT) (Duronio et al. 1990). The strain BL21(DE3)pLysS-pBBNMT was then transformed with the plasmid pET23d vector containing the Nef gene. The bacterial culture was performed as described above except that 25 μg/mL kanamycin was also included in the media. Coexpression of Nef and NMT was induced by adding 0.4 mM isopropyl-1-thio-β-galactopylanoside to the log phase culture. The cells were collected by centrifugation, resuspended in 5 volumes of ice-cold buffer (10 mM Tris-HCl, pH 7.5 containing 0.1% Triton X-100, 10 mM dithiothreitol, 1 mM EDTA, 0.2 mM phenylmethylsulfonyl fluoride), and sonicated three times for 5 min with a probe-type sonicator (Branson Sonifier 250). After centrifuging for 25 min at 18,000 rpm at 4°C, the supernatants were collected. The supernatants were then applied to a RESOURCE Q column and eluted with a gradient of 100–600 mM NaCl. The fractions containing the Nef protein were pooled. After adding 7 mM CaCl2 to the supernatant, the solution was loaded on a calmodulin-agarose column equilibrated with 40 mM Tris-HCl buffer (pH 7.5) containing 0.2 M NaCl, 0.1 mM dithiothreitol, and 7 mM CaCl2. The column was washed with 50 mL of the same buffer containing 0.5 M NaCl, 0.1 mM dithiothreitol, 7 mM CaCl2 and then with 50 mL of the buffer containing 0.1 mM dithiothreitol, 0.1 mM CaCl2. Nef was eluted with the same buffer containing 0.6 M NaCl, 1 mM dithiothreitol, and 2 mM EGTA. Fractions containing myr Nef were pooled and then concentrated, substituting the buffer for that containing 20 mM Tris-HCl (pH 7.5) using a Centricon 10 concentrator (Amicon). The purity was confirmed by SDS-PAGE (Fig. 6 ▶). The purified proteins were kept frozen at −80°C until use. Molecular masses of the recombinant non-myr Nef and myr Nef proteins were determined by mass spectrometry to be 23,239 Da and 23,449 Da. The difference, 210 Da, corresponded very well to the mass difference of 210 Da expected for myristoylation.
Figure 6.
Purified recombinant Nef and myristoylated Nef protein. The purity was analyzed by SDS-PAGE. Molecular weight standard proteins (left lane), purified nonmyristoylated Nef protein (center), and purified myristoylated Nef protein (right) are shown.
Mass spectrometry
MALDI-TOF mass spectrometry was done on a Voyager DE Pro (PE Biosystems), and the matrix was 10 mg/mL α-Cyano-4-hydroxycinnamic acid (Sigma) in 0.1% TFA-50% acetonitrile solution. The spectra were displayed and analyzed using GRAMS-MS software, and calibration of the spectra was done using calibration mixture 1 or 2 (PE Biosystems).
Direct binding assay to calmodulin
For the direct binding assay to calmodulin, aliquots containing GST or GST fusion Nef proteins were incubated with 50 μL of calmodulin agarose beads in 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 500 μM CaCl2 or 4 mM EGTA for 1 h at 25°C. The protein-bound beads were then extensively washed with the above buffer and processed for SDS-PAGE and Western blot analysis as before.
Analysis of Nef binding to calmodulin on BIAcore
Direct binding of Nef to calmodulin was also assessed by perfusing solutions of GST-Nef fusion protein over the surface of a BIAcore sensor chip containing calmodulin as described previously (Pronin et al. 1997). Briefly, to immobilize calmodulin, it was biotinylated at lysine residues using NHS-LC-Biotin (Pierce). The calmodulin was desalted on Sephadex G-15 to remove free biotinylation reagent and then trapped on the surface of a sensor chip containing covalently attached streptavidin (sensor chip SA5, BIACORE). This yielded ~700 relative units of calmodulin on the streptavidin chip. For analysis of calmodulin-Nef interaction, solutions of the Nef were injected across chip surfaces containing calmodulin. The running buffer contained 20 mM Tris-HCl, pH 7.4, 100 mM NaCl, and 500 μM CaCl2. GST and GST-Nef were diluted in this buffer to a final concentration of 200 nM prior to injection. The volume of the injected sample was 40 μL, and the flow rate was 10 μL/min. Experiments were performed on a BIAcore2000 instrument, and the data were analyzed using BIAE-valuation 2.1 software (BIACORE).
Fluorescence measurements
Binding of the N-terminal Nef peptides to dansyl-calmodulin was analyzed with a JASCO FP-777 spectrofluorometer in a 1-cm quartz cuvette as described previously (Matsubara et al. 1997, 1998). With the excitation wavelength set at 340 nm, emission spectra of dansyl-calmodulin in the presence or absence of peptides were recorded in 20 mM Tris-HCl, pH 7.4, 100 mM NaCl containing 1 mM CaCl2. Binding of the peptides to calmodulin was monitored by recording the fluorescence emission at 490 nm. Dissociation constants of calmodulin-peptide complexes were determined by a direct fit of the data to the mass (Matsubara et al. 1997).
Acknowledgments
We thank Dr. Y. Fujii for the Nef expression vector, antibody, and Nef-expressing NIH3T3 cells. We also thank Dr. J.I. Gordon for providing the N-myristoyltransferase expression vector. This work was supported in part by grants-in-aid from Fujita Health University, by grants-in-aid for Scientific Research on Priority Areas (C) “Genome Information Science” (14015231, 15014230, and 16014224) to N.H., Scientific Research (C) to N.H., and Young Scientists (B) to M.M. from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
HIV, human immunodeficiency virus
SIV, simian immunodeficiency virus
GST, glutathione S-transferase
MARCKS, myristoylated alanine-rich protein kinase C substrate
PKC, protein kinase C
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.04969605.
References
- Arold, S., Franken, P., Strub, M.-P., Hoh, F., Benichou, S., Benarous, R., and Dumas, C. 1997. The crystal structure of HIV-1 Nef protein bound to the Fyn kinase SH3 domain suggests a role for this complex in altered T cell receptor signaling. Structure 5 1361–1372. [DOI] [PubMed] [Google Scholar]
- Barnham, K.J., Monks, S.A., Hinds, M.G., Azad, A.A., and Norton, R.S. 1997. Solution structure of a polypeptide from the N terminus of the HIV protein Nef. Biochemistry 36 5970–5980. [DOI] [PubMed] [Google Scholar]
- Baur, A., Sass, G., Laffert, B., Willbold, D., Cheng-Mayer, C., and Peterlin, B.M. 1997. The N-terminus of Nef from HIV-1/SIV associates with a protein complex containing Lck and a serine kinase. Immunity 6 283–291. [DOI] [PubMed] [Google Scholar]
- Brigino, E., Haraguchi, S., Koutsonikolis, A., Cianciolo, G.J., Owens, U., Good, R.A., and Day, N.K. 1997. Interleukin 10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway. Proc. Natl. Acad. Sci. 94 3178–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collette, Y., Dutartre, H., Benziane, A., Ramos-Morales, F., Benarous, R., Harris, M., and Olive, D. 1996. Physical and functional interactions of Nef with Lck. J. Biol. Chem. 271 6333–6341. [DOI] [PubMed] [Google Scholar]
- Crivici, A. and Ikura, M. 1995. Molecular and structural basis of target recognition by calmodulin. Annu. Rev. Biophys. Biomol. Struct. 24 85–116. [DOI] [PubMed] [Google Scholar]
- Cullen, B.R. 1996. HIV-1: Is Nef a PAK animal? Curr. Biol. 6 1557–1559. [DOI] [PubMed] [Google Scholar]
- Curtain, C.C., Separovic, F., Rivett, D., Kirkpatrick, A., Waring, A.J., Gordon, L.M., and Azad, A.A. 1994. Fusogenic activity of amino-terminal region of HIV type 1 nef protein. AIDS Res. Hum. Retroviruses 10 1231–1240. [DOI] [PubMed] [Google Scholar]
- Curtain, C.C., Lowe, M.G., Macreadie, I.G., Gentle, I.R., Lawrie, G.A., and Azad, A.A. 1998. Structural requirements for the cytotoxicity of the N-terminal region of HIV type 1 Nef. AIDS Res. Hum. Retroviruses 14 1543–1551. [DOI] [PubMed] [Google Scholar]
- Daniel, M.D., Kirchoff, F., Czajak, S.C., Sehgal, P.K., and Derosiers, R.C. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258 1938–1941. [DOI] [PubMed] [Google Scholar]
- Deacon, N.J., Tsykin, A., Solomon, A., Smith, K., Ludford-Menting, M., Hooker, D.J., McPhee, D.A., Greenway, A.L., Ellett, A., Chatfield, C., et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from blood transfusion donor and recipients. Science 270 988–991. [DOI] [PubMed] [Google Scholar]
- Duronio, R.J., Jackson-Machelski, E., Heuckeroth, R.O., Olins, P.O., Devine, C.S., Yonemoto, W., Slice, L.W., Taylor, S.S., and Gordon, J.I. 1990. Protein N-myristoylation in Escherichia coli: Reconstitution of a eukaryotic protein modification in bacteria. Proc. Natl. Acad. Sci. 87 1506–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fackler, O.T., Kienzle, N., Kremmer, E., Boese, A., Schramm, B., Klimkait, T., Kucherer, C., and Muellur-Lantzsch, N. 1997. Association of human immunodeficiency virus Nef protein with actin is myristoylation dependent and influences its subcellular localization. Eur. J. Biochem. 247 843–851. [DOI] [PubMed] [Google Scholar]
- Fujii, Y., Nishino, Y., Nakaya, T., Tokunaga, K., and Ikuta, K. 1993. Expression of human immunodeficiency virus type 1 Nef antigen on the surface of acutely and persistently infected human T cells. Vaccine 11 1240–1246. [DOI] [PubMed] [Google Scholar]
- Goldsmith, M.A., Warmerdam, M.T., Atchison, R.E., Miller, M.D., and Greene, W.C. 1995. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 69 4112–4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna, R. and Anderson, W.B. 1982. Ca2+-induced hydrophobic site on calmodulin: Application for purification of calmodulin by phenyl-seoharose affinity chromatography. Biochem. Biophys. Res. Commun. 104 830–836. [DOI] [PubMed] [Google Scholar]
- Graziani, A., Galimi, F., Medico, E., Cottone, E., Gramaglia, D., Boccaccio, C., and Comoglio, P.M. 1996. The HIV-1 Nef protein interferes with phospha-tidylinositol 3-kinase activation. J. Biol. Chem. 271 6590–6593. [DOI] [PubMed] [Google Scholar]
- Greenway, A.L., McPhee, D.A., Grgacic, E., Hewish, D., Lucantoni, A., Mac-readie, I., and Azad, A.A. 1994. Nef 27, but not Nef 25 isoform of human immunodeficiency virus-type 1 pNLU.3 down-regulates surface CDU and IL-2R expression in peripheral blood mononuclear cells and transformed T cells. Virology 198 245–256. [DOI] [PubMed] [Google Scholar]
- Greenway, A., Azad, A., Millis, J., and McPhee, D. 1996. Human immunodeficiency virus type 1 Nef binds directly to Lck and mitogen-activated protein kinase, inhibiting kinase activity. J. Virol. 70 6701–6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiek, S., Bax, A., Clore, G.M., Gronenborn, A.M., Hu, J.-S., Kaufman, J., Palmer, I., Stahl, S.J., and Wingfield, P.T. 1996. The solution structure of HIV-1 Nef reveals an unexpected fold and permits delineation of the binding surface for the SH3 domain of Hck tyrosine protein kinase. Nat. Struct. Biol. 3 340–345. [DOI] [PubMed] [Google Scholar]
- Grzesiek, S., Bax, A., Hu, J.-S., Kaufman, J., Palmer, I., Stahl, S.J., Tjandra, N., and Wingfield, P.T. 1997. Refined solution structure and backbone dynamics of HIV-1 Nef. Protein Sci. 6 1248–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, M.P. and Neil, J.C. 1994. Myristoylation-dependent binding of HIV-1 Nef to CD4. J. Mol. Biol. 241 136–142. [DOI] [PubMed] [Google Scholar]
- Hayashi, N. 2002. Multifunctional posttranslational modification: N-myristoylation of proteins regulates protein-protein/protein-lipid interactions and signaling pathway systems in the brain. Res. Signpost 2 33–45. [Google Scholar]
- Hayashi, N., Matsubara, M., Titani, K., and Taniguchi, H. 1997. Circular dichroism and 1H nuclear magnetic resonance studies on the solution and membrane structures of GAP-43 calmodulin-binding domain. J. Biol. Chem. 272 7639–7645. [DOI] [PubMed] [Google Scholar]
- Hayashi, N., Izumi, Y., Titani, K., and Matsushima, N. 2000. The binding of myristoylated N-terminal nonapeptide from neuro-specific protein CAP-23/NAP-22 to calmodulin does not induce the globular structure observed for the calmodulin-nonmyristylated peptide complex. Protein Sci. 9 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, N., Matsubara, M., Jinbo, Y., Titani, K., Izumi, Y., and Matsushima, N. 2002. Nef of HIV-1 interacts directly with calcium-bound calmodulin. Protein Sci. 11 529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, N., Nakagawa, C., Ito, Y., Takasaki, A., Jinbo, Y., Yamakawa, Y., Titani, K., Hashimoto, K., Izumi, Y., and Matsushima, N. 2004. Myristoylation-regulated direct interaction between calcium-bound calmodulin and N-terminal region of pp60v-src. J. Mol. Biol. 338 169–180. [DOI] [PubMed] [Google Scholar]
- Hodge, D.R., Dunn, K.J., Pei, G.K., Chakrabarty, M.K., Heidecker, G., Lautenberger, J.A., and Samuel, K.P. 1998. Binding of c-Raf1 kinase to a conserved acidic sequence within the carboxyl-terminal region of the HIV-1 Nef protein. J. Biol. Chem. 273 15727–15733. [DOI] [PubMed] [Google Scholar]
- James, P., Vorherr, T., and Carafoli, E. 1995. Calmodulin-binding domains: Just two-faced or multi-faceted? Trend Biochem. Sci. 20 38–42. [DOI] [PubMed] [Google Scholar]
- Joyal, J.L., Burks, D.J., Pons, S., Matter, W.F., Vlahos, C.J., White, M.F., and Sacks, D.B. 1997. Calmodulin activates phosphatidylinositol 3-kinase. J. Biol. Chem. 272 28183–28186. [DOI] [PubMed] [Google Scholar]
- Kestler, H.W.I., Ringler, D.J., Mori, K., Panicali, D.L., Sehgal, P.K., Daniel, M.D., and Desrosiers, R.C. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65 651–662. [DOI] [PubMed] [Google Scholar]
- Kirchoff, F., Greenough, T.C., Brettler, D.B., Sullivan, J.F., and Desrosiers, R.C. 1995. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Eng. J. Med. 332 228–232. [DOI] [PubMed] [Google Scholar]
- Klee, C.B. and Vanaman, T.C. 1982. Calmodulin. Adv. Protein Chem. 35 213–321. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Lee, C.-H., Saksela, K., Mirza, U.A., Chait, B.T., and Kuriyan, J. 1996. Crystal structure of the conserved core of HIV-1 Nef complexed with a Src family SH3 domain. Cell 85 931–942. [DOI] [PubMed] [Google Scholar]
- Lu, X., Yu, H., Liu, S.H., Brodsky, F.M., and Peterlin, B.M. 1998. Interactions between HIV1 Nef and vacular ATPase facilitate the internalization of CD4. Immunity 8 647–656. [DOI] [PubMed] [Google Scholar]
- Matsubara, M., Titani, K., and Taniguchi, H. 1996. Interaction of calmodulin-binding domain peptides of nitric oxide synthase with membrane phospholipids: Regulation by protein phosphorylation and Ca2+-calmodulin. Biochemistry 35 14651–14658. [DOI] [PubMed] [Google Scholar]
- Matsubara, M., Hayashi, N., Titani, K., and Taniguchi, H. 1997. Circular dichroism and 1H NMR studies on the structures of peptides derived from the calmodulin-binding domains of inducible and endothelial nitric oxide synthase in solution and in complex with calmodulin. J. Biol. Chem. 272 23050–23056. [DOI] [PubMed] [Google Scholar]
- Matsubara, M., Yamauchi, E., Hayashi, N., and Taniguchi, H. 1998. MARCKS, a major protein kinase C substrate, assumes non-helical conformations both in solution and complex with Ca2+-calmodulin. FEBS Lett. 421 203–207. [DOI] [PubMed] [Google Scholar]
- Oldridge, J. and Marsh, M. 1998. Nef—An adaptor adaptor? Trends Cell Biol. 8 302–305. [DOI] [PubMed] [Google Scholar]
- Otake, K., Fujii, Y., Nakaya, T., Nishino, Y., Zhong, Q., Fujinaga, K., Kameoka, M., Ohki, K., and Ikuta, K. 1994. The carboxy-terminal region of HIV-Nef protein is a cell surface domain that can interact with CD4+ T cells. J. Immunol. 153 5826–5837. [PubMed] [Google Scholar]
- Paarmann, I., Spangenberg, O., Lavie, A., and Konrad, M. 2002. Formation of complexes between Ca2+.calmodulin and the synapse-associated protein SAP97 requires the SH3 domain-guanylate kinase domain-connecting HOOK region. J. Biol. Chem. 277 40832–40838. [DOI] [PubMed] [Google Scholar]
- Peter, F. 1998. HIV Nef: The mother of all evil? Immunity 9 433–437. [DOI] [PubMed] [Google Scholar]
- Piguet, V., Chen, Y.-L., Mangasarian, A., Foti, M., Carpentier, J.-L., and Trono, D. 1998. Mechanism of Nef-induced CD4 endocytosis: Nef connects CD4 with the m chain of adaptor complexes. EMBO J. 17 2472–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin, A.N., Satpaev, D.K., Slepak, V.Z., and Benovic, J. 1997. Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J. Biol. Chem. 272 18273–18280. [DOI] [PubMed] [Google Scholar]
- Saksela, K., Cheng, G., and Baltimore, D. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for downregulation of CD4. EMBO J. 14 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C.W., Pritchard, K., and Marston, S.B. 1987. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J. Biol. Chem. 262 116–122. [PubMed] [Google Scholar]
- Smith, B.L., Krushelnycky, B.W., Mochly-Rosen, D., and Berg, P. 1996. The HIV Nef protein associates with protein kinase C θ. J. Biol. Chem. 271 16753–16757. [DOI] [PubMed] [Google Scholar]
- Takasaki, A., Hayashi, N., Matsubara, M., Yamauchi, E., and Taniguchi, H. 1999. Identification of the calmodulin-binding domain of neuron-specific protein kinase C substrate CAP-23/NAP-22. J. Biol. Chem. 274 11848–11853. [DOI] [PubMed] [Google Scholar]
- Taniguchi, H. and Manenti, S. 1993. Interaction of myristoylated alanine-rich protein kinase C substrate (MARCKS) with membrane phospholipids. J. Biol. Chem. 268 9960–9963. [PubMed] [Google Scholar]
- Taniguchi, H., Manenti, S., Suzuki, M., and Titani, K. 1994. Myristoylated alanine-rich C Kinase Substrate (MARCKS), a major protein kinase C substrate, is an in vivo substrate of proline-directed protein kinase. J. Biol. Chem. 269 18299–18302. [PubMed] [Google Scholar]
- Yamada, M., Miyawaki, A., Saito, K., Nakajima, T., Yamamoto-Hino, M., Ryo, Y., Furuichi, T., and Mikoshiba, K. 1995. The calmodulin-binding domain in the mouse type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 308 (Pt 1) 83–88. [DOI] [PMC free article] [PubMed] [Google Scholar]