Abstract
Although a 22-kDa human growth hormone (hGH) is the predicted protein product of the hGH-N gene, a pleiotropic collection of uncharacterized molecular weight and charge isoforms is also produced. Using chromatography and preparative SDS-PAGE under reducing conditions we isolated an unusually stable mercaptoethanol-resistant (MER) 45-kDa hGH. A 5-h incubation at 100°C in the presence of 2-mercaptoethanol was required to convert approximately 90% of MER-45-kDa hGH into a 22-kDa hGH. Other reductants were not as effective in splitting MER-45-kDa hGH. After fracturing MER-45-kDa hGH, the 22-kDa hGH fragments would spontaneously reassociate if the reductant was removed; however, alkylation of cysteine residues prevented their reassociation. Identical amino acid sequences for the first six N-terminal residues were obtained for MER-45-kDa hGH and its 22-kDa hGH cleavage product. Structural identity of MER-45-kDa hGH and 22-kDa hGH was demonstrated by MALDI-TOF mass spectrometry of tryptic digests. MER-45-kDa hGH did not break up upon incubation with EDTA and EGTA. The significance of this work to our understanding of the structure of hGH isoforms is that it demonstrates that MER-45-kDa hGH is not a single chain polypeptide but is instead a homodimer of 22-kDa hGH monomers. The MER-45-kDa hGH dimer is held together by interchain disulfide bonds and not by divalent metal cation bridges. Additionally, MER-45-kDa hGH’s interchain disulfide links are exceptionally resistant to reducing agents and thus confer extreme stability to the homodimer.
Keywords: human growth hormone, isoform, dimer, mass spectrometry, preparative electrophoresis, purification, protein structure
Pituitary GH is an important regulator of growth, metabolism, and development in humans and animals (Strobl and Thomas 1994; Ho et al. 1996; Okada and Kopchick 2001; Renaville et al. 2002; Waters and Kaye 2002). Growth hormones have long been known to stimulate body weight gain (Evans and Simpson 1931), increase the growth rate of animals (Brumby 1959; Machlin 1972), influence the metabolism of proteins, nucleic acids, lipids, and carbohydrates (Altszuler 1974; Cheek and Hill 1974; Goodman and Schwartz 1974; Kostyo and Nutting 1974), enhance the body’s ratio of protein to fat (Brumby 1959; Machlin 1972; Muir et al. 1983; Chung et al. 1985), and influence the partitioning of nutrients to various organs (Bauman et al. 1982; Bauman 1999).
Pituitary hGH is a heterogeneous mixture of structural isoforms that make up nearly 10% of the dry weight of the pituitary gland (Singh et al. 1974; Lewis et al. 1980; Charrier and Martal 1988; Chen et al. 1989; Chene et al. 1989; Baumann 1991, 1999; Lewis 1992; Lewis et al. 1994, 2000; Boguszewski 2003). Human GH is synthesized by two genes, the GH-N (for normal) gene expressed by the pituitary gland and the GH-V (for variant) gene expressed by the placenta (Chen et al. 1989; Baumann 1991; Barrera-Saldana 1998). In addition to different gene loci, other mechanisms have been shown to contribute to hGH pleiotrophy and include alternative mRNA splicing, posttranslational modifications, post-secretory events, and metabolic conversions (Charrier and Martal 1988; Chene et al. 1989; Baumann 1991, 1999; Lewis et al. 1994, 2000). The main 22-kDa isoform of hGH consists of 191 amino acid residues, and it has two intramolecular disulfide bridges (Cys 53–Cys 165 and Cys 182–Cys 189). Other hGH isoforms include glycosylated hGHs with molecular weights of 24 kDa and 12 kDa (Diaz et al. 1993; Haro et al. 1996), a 20-kDa hGH, deamidated hGHs, phosphorylated hGHs, a 35-kDa hGH, oligomeric hGHs, and cleaved hGHs with molecular weights of 17 kDa and 5 kDa (Singh et al. 1974; Lewis et al. 1980, 1994, 2000; Baumann 1991, 1999; Lewis 1992).
Although the storage form of hGH is not clearly established, bovine GHs are stored in the secretory granules of somatotrophs in the adenohypophysis as intermolecular disulfide-linked oligomers (Jacobs and Lee 1975; Lorenson and Jacobs 1982; Lorenson et al. 1983). These oligomers express low immunoactivity but can be reduced in vitro with 2-mercaptoethanol or dithiothreitol to immunologically active monomeric forms. Dimeric and larger forms of GH are also found in serum and tissue homogenates, but the cellular location or mechanism of interconversion are still unclear (Lorenson and Jacobs 1982; Lewis et al. 1987, 2000; Baumann 1991, 1999; Nindl et al. 2003).
Oligomeric GHs exist as isoforms of various molecular weights. Several reports describe the characterization of 45-kDa hGH oligomers (Lewis et al. 1977, 2000; Brostedt and Roos 1989). However, the 45-kDa hGH preparations are a mixture of at least four different isoforms that include (1) aggregated 22-kDa hGH monomers that are dissociable with urea, (2) interchain disulfide dimers that are dissociable with 2-mercaptoethanol, (3) interchain disulfide dimers that have been cleaved in the large dusulfide loop, and (4) mercaptoethanol-resistant (MER) 45-kDa hGHs that do not dissociate into monomers by reduction of interchain disulfide bonds with 2-mercaptoethanol. Specifically, Lewis et al. (1977) reported that MER-45-kDa hGH did not dissociate into 22-kDa hGH when treated for 5 min at 100°C in either 1% mercaptoethanol or 1% dithiothreitol. Although Lewis and coworkers described the existence of MER-45-kDa hGH, its structure was not determined. However, they reported that the NH2- and COOH-terminal residues were both phenylalanine, which are the predicted residues for 22-kDa hGH. Hence, the MER-45-kDa hGH could be (1) a hGH dimer held together by unusually stable disulfide bridges, (2) a hGH dimer held together with metal bridges, or (3) a single-chain polypeptide. The purpose of this investigation was to ascertain the structure of the MER-45-kDa hGH. The hypothesis that the MER-45-kDa hGH is an unusually stable interchain disulfide dimer of 22-kDa hGH was tested in this work. In this communication we report the isolation and structural analysis of the MER-45-kDa hGH. The work serves to fill a gap in our knowledge of the structure of hGH molecular isoforms.
Recent reports have addressed the biological significance of the MER-45-kDa hGH. Although the binding constants were not reported, initial work showed that MER-45-kDa hGH binds to both GH and PRL receptors with high affinities based on ED50 values for the displacement of radiolabeled bovine GH from GH receptors of bovine liver membranes and displacement of radiolabeled ovine PRL from PRL receptors of lactating rabbit mammary gland membranes (Grigorian et al. 2002). The MER-45-kDa hGH was reported to bind to hGH receptors of IM9 lymphocytes and to stimulate the proliferation of Nb2 lymphoma cells (Grigorian et al. 2003). The MER-45-kDa hGH was also shown to have anti-proliferative effects in MCF-7, MDA-MB-231, and T47D human breast cancer cell lines (Muñoz et al. 2004).
Results
Isolation of MER-45-kDa hGH by preparative electrophoresis and electroelution
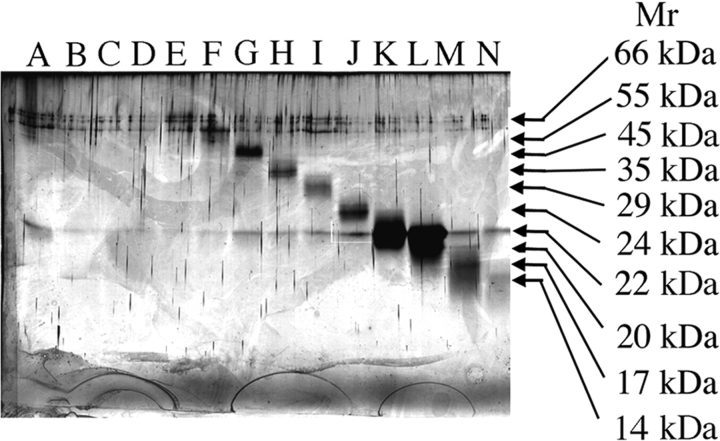
Molecular weight isoforms of hGH were isolated from each other by preparative SDS-PAGE under reducing conditions and recovered by electroelution. An analytical SDS polyacrylamide gel, run under reducing conditions, of the fractions obtained by preparative SDS-PAGE is shown in Figure 1 ▶. The sample in lane G comprises an aliquot of the fraction corresponding to the isolated MER-45-kDa hGH. The preparative SDS-PAGE procedure also produced samples rich in 35-kDa hGH (lane H), 24-kDa hGH (lane I), 22-kDa hGH (lanes K, L) and low molecular weight hGH isoforms (lanes M, N). We have previously demonstrated (Haro et al. 1998) by Western blot analysis that these are hGH isoforms. Higher molecular weight bands seen in the zone 50–66 kDa in all lanes of the gel are attributed to keratin contamination of commercially acquired 2-mercaptoethanol (Ochs 1983; Shapiro 1987; Paul-Pletzer and Parness 2001), which is a component of the SDS-PAGE sample buffer.
Figure 1.
Isolated fractions of hGH molecular weight isoforms obtained by preparative SDS-PAGE under reducing conditions. Lanes A–N of the analytical 13.5% SDS polyacrylamide gel show the purity of electroeluted hGH fractions obtained from a preparative SDS polyacrylamide gel run in the presence of 2-mercaptoethanol. Lane G represents the MER-45-kDa hGH used in subsequent studies.
Transformation of MER-45-kDa hGH into 22-kDa hGH subunits with 2-mercaptoethanol
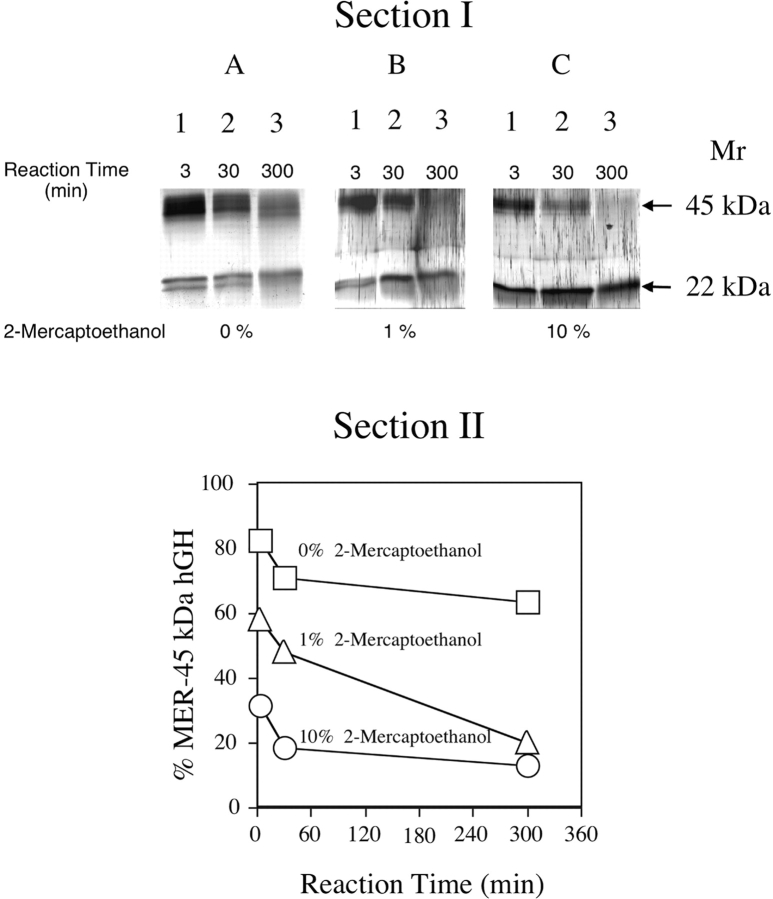
MER-45-kDa hGH was obtained by preparative SDS-PAGE under reducing conditions; however, we analyzed it to ascertain whether or not it is a disulfide-linked hGH dimer held together by unusually stable disulfide bridges. Therefore, the effects of reducing agents on changing MER-45-kDa hGH to an apparent molecular weight of 22 kDa was investigated. The SDS polyacrylamide gels run under reducing conditions, shown in Figure 2 ▶, Section I, contain MER-45-kDa hGH samples that were incubated at 100°C in the absence or presence of reducing agent (1% v/v or 10% v/v 2-mercaptoethanol) for 3 min, 30 min, and 300 min (5 h). Treatment of MER-45-kDa hGH with reducing agent produced protein bands in the gels with apparent molecular weights of 45 kDa and 22 kDa. Amounts of MER-45-kDa hGH and 22-kDa hGH after the end of the reaction varied and were dependent on the incubation conditions. The conversion of MER-45-kDa hGH into 22-kDa hGH in the absence of 2-mercaptoethanol (Fig. 2A ▶) was slower than in the presence of 2-mercaptoethanol at 1% (Fig. 2B ▶) or 10% (Fig. 2C ▶). Quantities of hGH isoforms determined by scanning densitometry were used to plot the percent of total hGH that comprised MER-45-kDa hGH as a function of time as shown in Figure 2 ▶, Section II. The kinetics of the reaction show that after 300 min, the percent of MER-45-kDa hGH remaining in the sample was 67%, 20%, and 10% when incubated with 2-mercaptoethanol at 0%, 1%, and 10%, respectively. Thus, the formation of 22-kDa hGH from MER-45-kDa hGH was slower in the absence of 2-mercaptoethanol than in its presence. Figure 2 ▶ shows that there is a progressive decrease of protein in the absence of 2-mercaptoethanol with increased incubation times. This is most likely due to adherence of protein in the sample to the reaction vessel over time. This decrease in protein seen in the gels may seem more pronounced than would be indicated in the graph of Figure 2 ▶ due to expression of the data as a percentage of total protein that is ascribed to MER-45-kDa hGH. The band spreading seen in Figure 2 ▶ as well as the doublet 22-kDa band may be the result of hGH micro-heterogeneity caused by glycosylation (Haro et al. 1996), phosphorylation (Lewis 1992), or C-terminal proteolytic enzymatic cleavage (Chene et al. 1989).
Figure 2.
Kinetics of the conversion of MER-45-kDa hGH into 22-kDa hGH in the absence and presence of 2-mercaptoethanol as a function of time. (Section I) Analytical SDS-PAGE pattern of the dissociation of the MER-45-kDa hGH at 100°C in the absence of 2-mercaptoethanol (A), in the presence of 1% 2-mercaptoethanol (B), and in the presence of 10% 2-mercaptoethanol (C). Samples in lanes 1–3 were incubated for 3 min, 30 min, and 300 min (5 h), respectively. (Section II) Plot of the densitometric analysis of the gel in Section I showing the conversion of MER-45-kDa hGH into 22-kDa hGH in the absence and presence of 2-mercaptoethanol at 100°C as a function of time. Concentrations of 2-mercaptoethanol in the incubation mixture were 0% (□), 1% (▵), and 10% (○).
Transformation of MER-45-kDa hGH into 22-kDa hGH subunits with other reducing agents
Conversion of MER-45-kDa hGH into 22-kDa hGH in the presence of other reducing agents was also investigated (Table 1). Overall, the amount of MER-45-kDa hGH decreased and concomitantly the amount of 22-kDa hGH increased as a function of time in the presence of reducing agents. An increase in the reaction temperature from 25°C to 60°C and 95°C increased the amount of 22-kDa hGH produced from 6% to 20% and 76%, respectively, when incubated in the presence of 10 mM TCEP-HCL for 5 h. Similarly, as the reaction temperature increased from 25°C to 60°C and 95°C, the percentage of 22-kDa hGH increased from 5% to 27% and 50%, respectively, when incubated in the presence of 10 mM glutathione for 5 h. Increasing the concentrations of reductants from 10 mM to 100 mM produced a slight or insignificant increase in percent of 22-kDa hGH formed after a 5-h incubation at 95°C, as indicated below. With respect to TCEP-HCL, the increase in production of 22-kDa hGH was from 76% to 83%. No difference was observed in the case of glutathione. Incubation of MER-45-kDa hGH with 100 mM DTT for 5 h at 95°C converted 55% of the sample to 22-kDa hGH.
Table 1.
The percentage of total protein converted to 22-kDa hGH from MER-45-kDa hGH samples after a 5-h incubation in either TCEP-HCl, glutathione, or DTT at 25°C, 60°C or 95°C
| Temperature | |||
| Reductant | 25°C % 22-kDa hGH | 60°C % 22-kDa hGH | 95°C % 22-kDa hGH |
| TCEP-HCl (10 mM) | 6.0% | 20.0% | 76.0% |
| Glutathione (10 mM) | 5.0% | 27.0% | 50.0% |
| DTT (100 mM) | — | — | 55.0% |
Reassociation of dissociated MER-45-kDa hGH subunits
Reducing MER-45-kDa hGH with 1.4 M 2-mercaptoethanol generated 22-kDa hGH proteins and the tendency of dissociated MER-45-kDa hGH subunits to reassociate was examined. Incubation of MER-45-kDa hGH in 1.4 M 2-mercaptoethanol for 5 h at 100°C produced dissociated 22-kDa hGH subunits as shown in Figure 3 ▶, Section I, lane 1. After reducing the concentration of reductant to 20 mM by dialysis the 22-kDa hGH subunits reassociated to form MER-45-kDa hGH (lane 2). Densitometric analysis indicated that MER-45-kDa hGH in the sample accounted for approximately 63% of the total hGH. After removing the reductant by dialysis, MER-45-kDa hGH in the sample increased to approximately 94% of total hGH as shown in lane 3.
Figure 3.
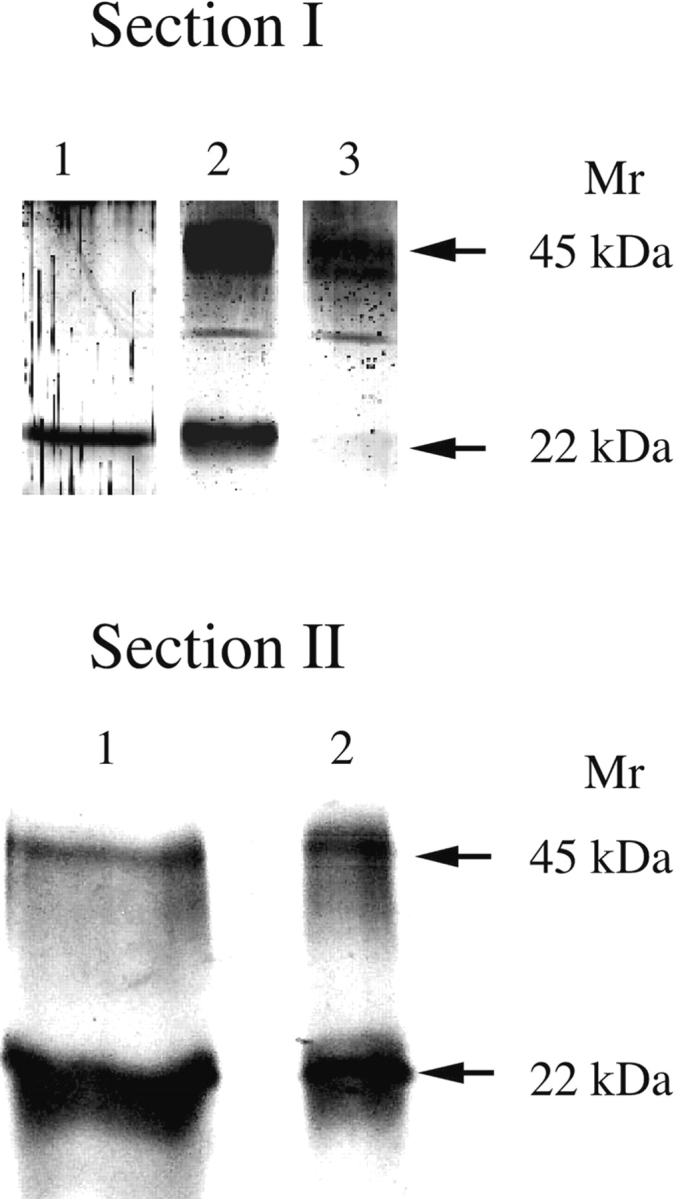
Formation of MER-45-kDa hGH by reassociation of 22-kDa hGH subunits and prevention of their reassociation by modification with iodoacetamide. An analytical 13.5% SDS polyacrylamide gel in the absence of reductants was used to separate samples and to assess the degree of 22-kDa hGH subunit association. (Section I) MER-45-kDa hGH was dissociated into 22-kDa hGH subunits by incubation in 10% mercaptoethanol at 100°C for 5 h (lane 1). The dissociated 22-kDa hGH samples were then dialyzed against 5 mM ammonium bicarbonate buffer containing 20 mM 2-mercaptoethanol (lane 2) or with buffer in absence of reductant (lane 3). (Section II) MER-45-kDa hGH was dissociated into 22-kDa hGH subunits by incubation in 10% mercaptoethanol at 100°C for 5 h, and 22-kDa hGH subunits were treated with 7 M iodoacetamide for 15 min at room temperature (lane 1). The dissociated and modified 22-kDa hGH samples were then dialyzed against 10 mM Tris-HCl buffer (lane 2).
Although 22-kDa hGH subunits generated by reduction of MER-45-kDa hGH reassociated to form dimers, modification of subunit cysteines with iodoacetamide prevented their dimerization. Incubation of MER-45-kDa hGH in 10% 2-mercaptoethanol at 100°C for 5 h generated dissociated 22-kDa hGH subunits whose cysteines were modified with iodoacetamide as shown in Figure 3 ▶, Section II, lane 1. However, after removing the reductant by dialysis, the cysteine-modified 22-kDa hGH subunits did not dimerize as shown in Figure 3 ▶, Section II, lane 2, indicating that MER-45-kDa hGH is an interchain disulfide dimer.
Influence of divalent metal cation chelators on dissociation of MER-45-kDa hGH
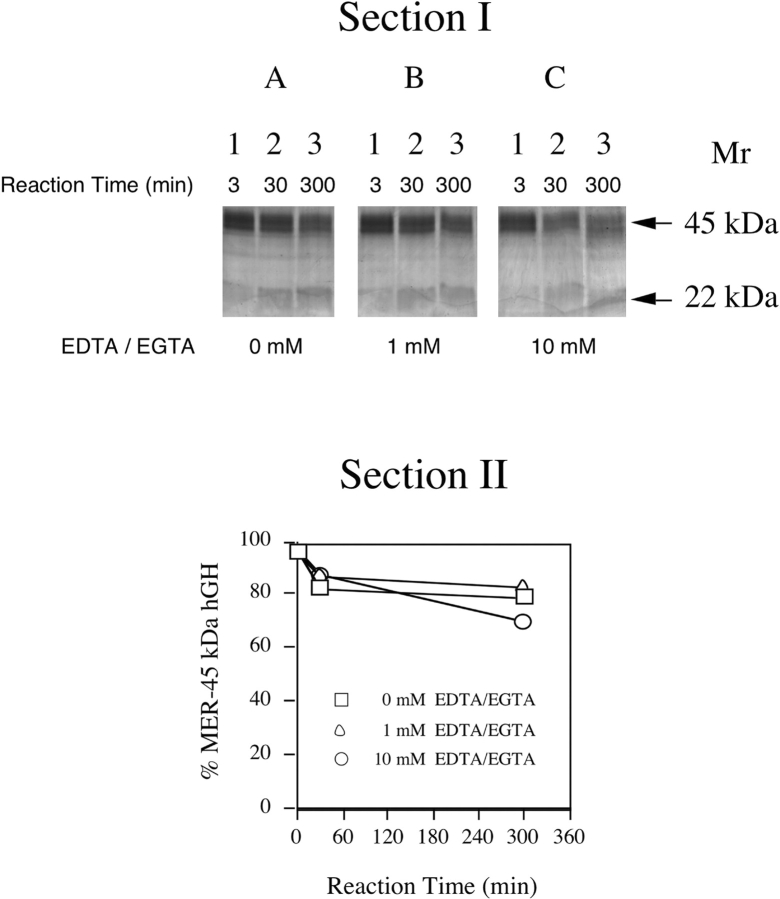
The abilities of divalent cation metal chelators to convert the MER-45-kDa hGH into a 22-kDa hGH were investigated. The SDS-polyacrylamide gels run under nonreducing conditions shown in Figure 4 ▶, Section I, contain MER-45-kDa hGH samples that were incubated at 100°C in the absence (Fig. 4A ▶) or presence of EDTA and EGTA for 3 min, 30 min, and 300 min (5 h) at 1 mM (Fig. 4B ▶) or 10 mM (Fig. 4C ▶). The kinetics of the conversion of MER-45-kDa hGH into 22-kDa hGH in the absence and presence of EDTA and EGTA is shown in a plot of the densitometric analysis of analytical SDS-polyacrylamide gels in Figure 4 ▶, Section II. The conversion of MER-45-kDa hGH into 22-kDa hGH in the absence of divalent metal cation chelators (□) was not dramatically different than the conversion in the presence of EDTA and EGTA at either 1 mM (▵) or 10 mM (○), as indicated by approximately superimposed conversion rate curves. Therefore, the presence of divalent metal cation chelators did not have a prominent effect upon the conversion of the MER-45-kDa hGH into 22-kDa hGH compared to their absence.
Figure 4.
Kinetics of the conversion of MER-45-kDa hGH into 22-kDa hGH in the absence and presence of divalent metal cation chelators under nonreducing conditions. (Section I) Analytical SDS-PAGE pattern of the dissociation of the MER-45-kDa hGH at 95°C in the absence of EDTA/EGTA (A), in the presence of 1 mM EDTA/EGTA (B), in the presence of 10 mM EDTA/EGTA (C). Samples in lanes 1–3 were incubated for 3 min, 30 min, and 300 min (5 h), respectively. (Section II) Plot of the densitometric analysis of the gel in Section I showing the conversion of MER-45-kDa hGH into 22-kDa hGH in the absence and presence of EDTA/EGTA at 95°C as a function of time. Concentrations of EDTA and EGTA in the incubation mixture were 0 mM (□), 1 mM (▵), and 10 mM (○).
N-terminal amino acid sequence analysis of MER-45-kDa hGH, 22-kDa hGH, and the dissociated 22-kDa hGH subunits produced through the reduction of MER-45-kDa hGH
N-terminal amino acid sequence analyses of MER-45-kDa hGH and of its 22-kDa hGH conversion product were carried out to ascertain if the 22-kDa hGH conversion product is the result of the dissociation of a disulfide dimer or the result of cleavage of a single chain polypeptide. MER-45-kDa hGH and 22-kDa hGH were obtained by preparative electrophoresis, transferred to a Problott membrane, and subjected to automated Edman degradation. Incubation of MER-45-kDa hGH with 10% 2-mercaptoethanol at 95°C for 5 h produced the 22-kDa hGH break-up product, referred to as 22-kDa hGH*, which was then isolated by preparative electrophoresis and subjected to N-terminal amino acid sequence analysis. The N-terminal amino acid sequence of the first six amino acid residues of MER-45-kDa hGH, 22-kDa hGH, and the break-up product of MER-45-kDa hGH were all Phe-Pro-Thr-Ile-Pro-Leu. Identical sequences of MER-45-kDa hGH and of the MER-45-kDa hGH break-up product imply that 22-kDa hGH* is not produced by cleavage of a single polypeptide and consequently that MER-45-kDa hGH is a dimer of 22-kDa hGH.
MALDI-TOF/MS of tryptic peptides obtained from digests of 22-kDa hGH and MER-45-kDa hGH
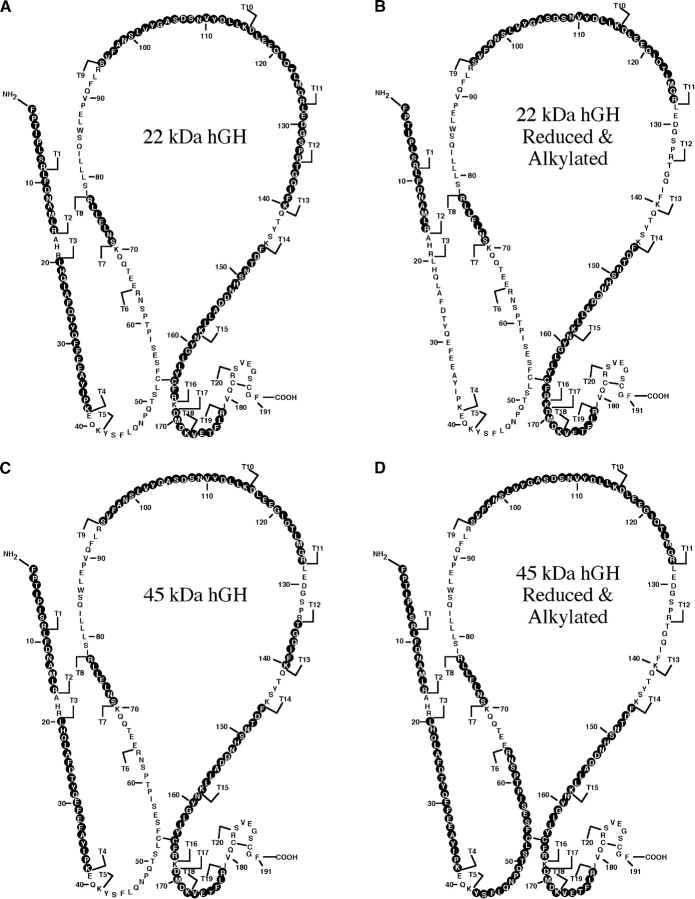
To assess the structural similarities of 22-kDa hGH and MER-45-kDa hGH we compared the mass maps of their tryptic digests. Peptide masses obtained by MALDI-TOF/MS of in-gel tryptic digests of 22-kDa hGH, MER-45 kDa hGH or reduced and alkylated preparations of each hormone are summarized in Table 2. Based on the sequence of hGH, the known specificity of trypsin, and the theoretical mass of each tryptic peptide, the in-gel-generated tryptic peptides could be matched to a location in the predicted structure of hGH. The data in Table 2 show that a number of peptides with almost equal masses were generated from tryptic digests of (1) 22-kDa hGH, (2) reduced and alkylated 22-kDa hGH, (3) MER-45-kDa hGH, and (4) reduced and alkylated MER-45-kDa hGH. Only two peptides were found solely in the unmodified 22-kDa hGH digest (T12 and T19). The mass of a cysteine-containing peptide detected by MALDI-TOF increased after the modification of peptide digests with iodoacetamide (T16 vs. T16c). Upon oxidation of methionine residues, the mass of the methionine-containing peptides also increased (T2 vs. T2b; T18-T19d vs. T18-T19b,d; T11 vs. T11b). Peptide T15-T16-T17d was found only in the 22-kDa hGH digest (reduced and alkylated) while peptide T6c was found only in the MER-45-kDa hGH digest (reduced and alkylated). The recovered tryptic peptides of the MER-45-kDa hGH and 22-kDa hGH were identical to each other or to that of the published sequence for hGH (Chen et al. 1989). In Figure 5 ▶, the recovered tryptic peptides of the MER-45-kDa hGH and 22-kDa hGH are superposed on the predicted amino acid sequence of hGH. Black circles with white lettering represent amino acid residues of recovered tryptic peptides. Although not all of the tryptic peptides are recovered in this procedure, the method enabled us to obtain intense spectra for many peptides with an extensive sequence coverage of each protein. There were 13 peptides recovered for the 22-kDa hGH and 12 peptides recovered for the MER-45-kDa hGH. Eleven of the recovered tryptic peptides (T1, T2, T4, T8, T10, T11, T13, T15, T16, T18, and T19) were identical in both 22-kDa hGH and MER-45-kDa hGH. Tryptic peptides T3, T5, T7, T9, T12, T14, T20, and T21 were not recovered in either 22-kDa hGH or MER-45-kDa hGH. Obtaining 11 identical peptides from the 13 tryptic peptides of 22-kDa hGH and 12 tryptic peptides of MER-45-kDa hGH, with the remaining peptides corresponding to predicted tryptic peptides of hGH, is a strong indication that MER-45-kDa hGH is a homodimer of 22-kDa hGH.
Table 2.
Peptide masses obtained by MALDI-TOF/MS of in-gel tryptic digests of 22-kDa hGH, 45-kDa hGH, or their reduced and alkylated derivatives
| Expected mass of hGH tryptic peptide | Numeric order of tryptic peptide (T)in hGH sequence | Tryptic peptide’s range of residues in hGH | Amino acid sequence of hGH tryptic peptide | Observed mass of 22-kDa hGH tryptic peptide | Observed massa of 22-kDa hGH tryptic peptide | Observed mass of MER-45-kDa hGH tryptic peptide | Observed massa of MER-45-kDa hGH tryptic peptide |
| 693.39 | T13 | 135–140 | TGQIFK | 693.48 | — | 693.40 | — |
| 764.43 | T19 | 173–178 | VETFLR | 764.52 | — | — | — |
| 773.38 | T12 | 128–134 | LEDGSPR | 773.46 | — | — | — |
| 844.49 | T8 | 71–77 | SNLELLR | 842.61 | 844.58 | 844.50 | 844.51 |
| 930.54 | T1 | 1–8 | FPTIPLSR | 930.65 | 930.62 | 930.54 | 930.54 |
| 979.50 | T2 | 9–16 | LFDNAMLR | 979.62 | 979.59 | 979.50 | 979.51 |
| 995.50 | T2b | 9–16 | LFDNAMLR | 995.61 | 995.59 | 995.50 | 995.50 |
| 1148.56 | T16 | 159–167 | NYGLLYCFR | 1148.55 | — | 1148.55 | — |
| 1205.58 | T16c | 159–167 | NYGLLYCFR | — | 1205.69 | — | 1205.61 |
| 1253.62 | T18–T19d | 169–178 | DMDKVETFLR | 1253.78 | 1253.73 | 1253.60 | 1253.62 |
| 1269.61 | T18–T19b,d | 169–178 | DMDKVETFLR | — | 1269.73 | 1269.57 | 1269.64 |
| 1361.67 | T11 | 116–127 | DLEEGIQTLMGR | 1361.87 | 1361.81 | 1361.66 | 1361.70 |
| 1377.67 | T11b | 116–127 | DLEEGIQTLMGR | 1377.85 | 1377.80 | 1377.63 | 1377.68 |
| 1489.69 | T15 | 146–158 | FDTNSHNDDALLK | 1489.89 | 1489.83 | 1489.69 | 1489.73 |
| 2262.13 | T10 | 95–115 | SVFANSLVYGASDSNVYDLLK | 2262.33 | 2262.36 | 2262.96 | 2262.18 |
| 2342.13 | T4 | 20–38 | LHQLAFDTYQEFEEAYIPK | 2342.37 | — | 2341.98 | 2342.09 |
| 2673.26 | T6c | 42–64 | YSFLQNPTSLCFSESIPTPSNR | — | — | — | 2673.23 |
| 2676.25 | T15–T16–T17d | 146–168 | FDTNSHNDDALLKNYGLLYCFRK | — | 2676.40 | — | — |
a Reduced and alkylated protein.
b Oxidized methionine.
c Alkylated peptide.
d Incomplete cleavage.
Figure 5.
Sections of the hGH amino acid sequence that match the masses of tryptic peptides obtained from 22-kDa hGH, 45-kDa hGH, or their reduced and alkylated preparations. Peptide mass maps were produced by MALDI-TOF analysis of in-gel tryptic digestion of 22-kDa hGH, 45-kDa hGH, or their reduced and alkylated preparations. Mass information was matched to the masses of predicted hGH tryptic peptides. Matched tryptic peptides are shown for 22-kDa hGH (A), 22-kDa hGH reduced and alkylated (B), MER-45-kDa hGH (C), and MER-45-kDa hGH reduced and alkylated (D). Black circles with white lettering represent amino acid residues in the tryptic digests matched to tryptic peptides of hGH. Tryptic peptides (T1–T21) of hGH are labeled in order starting from the amino terminus.
Discussion
In the present work, preparative electrophoresis in combination with electroelution enabled us to obtain fractions containing MER-45-kDa hGH in several hours. The purification scheme is a great improvement over our preliminary work (Hernandez et al. 1998). The previous purification scheme required weak anion-exchange chromatography on a TSK-DEAE anion-exchange column followed by Sephacryl-100 molecular sieve chromatographic fractionation and produced MER-45-kDa hGH fractions containing several other hGH isoforms including a large amount 22-kDa hGH. In addition, the partial isolation of MER-45-kDa hGH required several days to complete. The new isolation procedure for the preparation of MER-45-kDa hGH by preparative electrophoresis in combination with electroelution facilitates the isolation of hGHs ranging in weight from 14 to 45 kDa. We have previously demonstrated (Haro et al. 1998) by SDS-PAGE and by Western blot analysis that the proteins in our starting material contain immunoreactive hGH isoforms of molecular masses of 17, 20, 22, 24, 29, 36, and 45 kDa.
Our studies with the isolated MER-45-kDa hGH demonstrate that it can be transformed into a 22-kDa hGH. There is an accelerated transformation of MER-45-kDa hGH into 22-kDa hGH in the presence of 2-mercaptoethanol compared to its absence. Additionally, the quantity of MER-45-kDa hGH that changes into 22-kDa hGH increases with (1) increasing concentrations of 2-mercaptoethanol, (2) increasing temperature of incubation in 2-mercaptoethanol, and (3) increasing reaction time in 2-mercaptoethanol. The other reducing agents (TCEP-HCL, glutathione, DTT) also catalyzed the conversion of MER-45-kDa hGH into 22-kDa hGH with similar dependencies on time and temperature. However, those reductants were not used at high concentrations (1.4 M) comparable to that of mercaptoethanol because at those concentrations normal banding patterns of proteins in the SDS polyacrylamide gels are distorted (data not shown). Although the mechanism is not clear, the high concentrations of reductants TCEP-HCl and glutathione in the sample buffer interfered with the electrophoretic migration of proteins in the gel to produce highly distorted protein migration banding patterns, similar to distorted bands that occur if there is too much salt in the sample or if the sample pH is incorrect (Hames 1998). Consequently, an analysis of the proportions of MER-45-kDa hGH and 22-kDa hGH could not be carried out for these reductants at high concentrations. The accelerated metamorphosis of MER-45-kDa hGH into 22-kDa hGH in the presence of reducing agents suggests that MER-45-kDa hGH is a disulfide dimer of 22-kDa hGH monomers.
The results of the reassociation of dissociated MER-45-kDa hGH subunits clearly show that the change of MER-45-kDa hGH to a 22-kDa hGH isoform is a reversible process. Lowering the concentration of 2-mercaptoethanol from 1.4 M to 20 mM promoted 63% of the dissociated monomers to reassociate and reform interchain disulfide linked MER-45-kDa hGH. Complete removal of 2-mercaptoethanol increased to 94% the amount of the dissociated monomers that connected to reform the interchain disulfide linked MER-45-kDa hGH. The reassociated subunits are not associated via hydrophobic interactions because the presence of SDS in the polyacrylamide gels would have disrupted such subunit interactions. Brostedt and Roos (1989) have demonstrated that hydrophobically associated 45-kDa hGH dimers are split into monomers when separated by SDS-PAGE. The reassociation of subunits in the absence or diminished concentrations of reductant and maintenance of their associated state during SDS-PAGE indicate that the subunits are associated through interchain disulfide linkages.
In the absence of 2-mercaptoethanol partial conversion of MER-45-kDa hGH into 22-kDa hGH subunits was apparent in the analytical SDS polyacrylamide gels. This conversion may be attributed to thermal cleavage of disulfide bridges catalyzed by extended incubation at elevated temperatures. In a study of a dimeric isoform of recombinant bovine GH, mild heat (37°C) cleaved one disulfide bond (Thamann 1998). More intense heating (75°C) caused the cleavage of two cystines. Similarly, heating of MER-45-kDa hGH at 95°C–100°C under nonreducing conditions would have promoted cleavage of disulfide bridges and partial generation of 22-kDa hGH subunits.
Our amino acid sequence data infer that the breakup of MER-45-kDa hGH is the result of the dissociation of subunits and not due to the cleavage of a single 45-kDa poly-peptide chain. Cleavage of a single polypeptide chain would have produced two or more peptides with different N-terminal amino acid sequences in the Edman degradation analysis. However, the Edman degradation analysis produced a single polypeptide sequence corresponding to the N-terminal sequence of hGH. These data prompt us to conclude that the breakup of MER-45-kDa hGH in the presence of 2-mercaptoethanol is the result of the dissociation of 22-kDa hGH subunits.
If MER-45-kDa hGH and 22-kDa hGH are identical proteins, then their tryptic peptide mass maps will be the same. The database matching of MALDI-TOF mass spectra of tryptic peptides produced from MER-45-kDa hGH provided information about peptide identities that infers that MER-45-kDa hGH is a dimer of 22-kDa hGH monomers. In a hypothetical circumstance, a tryptic peptide map of a 22-kDa hGH dimer would produce tryptic peptides identical to those of the monomer; however, a single-chain 45-kDa protein would produce a tryptic peptide map different than that of 22-kDa hGH. When searched against the published databases, the peptide mass map produced by MALDI-TOF analysis of in-gel tryptic digestion of MER-45-kDa hGH matched the mass information that links the identity of the protein to hGH. Additionally, the theoretical masses of tryptic peptides produced from in-gel digestion of 22-kDa hGH matched the predicted mass values for hGH peptides (Table 2; Fig. 6). Tryptic digestion therefore produces fragments from both MER-45-kDa hGH and 22-kDa hGH identical to the predicted monomeric hGH sequence. Tryptic peptides not derived from hGH were not apparent. The 11 identical peptides recovered from the tryptic digests of the MER-45-kDa hGH and 22-kDa hGH demonstrate identity of the two proteins. These data further support the notion that 45 kDa hGH is a dimer of 22-kDa hGH monomers.
Isolation of a 22-kDa hGH/20-kDa hGH heterodimer and a 20-kDa hGH/20-kDa hGH homodimer have been reported (Chapman et al. 1981; Brostedt and Roos 1988, 1989); however our data do not support the presence of these dimers in the MER-45-kDa hGH sample. First, there was no evidence of a 20-kDa hGH band in the SDS-PAGE gels upon reduction of MER-45-kDa hGH. Second, since the 20-kDa hGH is a hGH that lacks amino acid residues 32–46, a tryptic digest of 20-kDa hGH would produce a tryptic peptide 4 corresponding to amino acid residues 20–31 + 47–64 of 22-kDa hGH comprising the sequence (LHQLAFDTYQEFN-PQTSLCFSESIPTPSNR) with a monoisotopic mass of 3526.65 (when reduced and alkylated with acetamide). This peptide has been reported as a peptide generated by tryptic cleavage of 20-kDa hGH (Hearn et al. 1983). In our experiments, the tryptic peptides of MER-45-kDa hGH contained a peptide with a mass identical to tryptic peptide 4 of 22-kDa hGH (residues 20–38; Table 2) but the mass spectra did not display a mass corresponding to a tryptic peptide 4 of 20-kDa hGH. Neither was there a tryptic peptide in the unreduced MER-45-kDa hGH corresponding to an unreduced tryptic peptide 4 of 20-kDa hGH linked to tryptic peptide 14 of 20-kDa hGH (NYGLLYCFR) with a predicted monoisotopic mass of 4615.17. Differences in the presence or absence of peptide masses obtained among the protein samples is the consequence of the variable recoveries of individual tryptic peptides from the different polyacrylamide gel pieces containing tryptic digests of 22-kDa hGH or MER-45-kDa hGH.
Therefore, our data do not provide evidence for the presence of either a 22-kDa hGH/20-kDa hGH heterodimer or a 20-kDa hGH/20-kDa hGH homodimer.
The anatomical connectivity of intrachain and interchain disulfide bonds of monomeric and dimeric isoforms of GH is complex and has been examined in natural and recombinant bovine GHs (Graf et al. 1975; Mao 1990; Tou et al. 1993). Graf et al. determined that the conditions leading to the cleavage of both intrachain disulfide bridges in human GH caused the reduction of only one of the intrachain disulfide bonds in the structure of the bovine GH, which is located at the COOH-terminal region (Graf et al. 1975). Mao has provided a discussion of the molecular topology and dimerization of recombinant bovine GH (Mao 1990). Experiments were carried out on dimers of recombinant bovine somatotropin and some possible structural models for the intrachain and interchain linkages in disulfide-linked dimers of recombinant bovine somatotropin were proposed. These models include (1) parallel and anti-parallel bGH polypeptide chains with symmetric interchain disulfide bonds, (2) catenane dimers of bGH, and (3) dissymetric disulfide-linked dimers of bGH. Structural characterizations of two refold dimers generated during the isolation of recombinant bovine somatotropin from inclusion bodies were carried out (Violand et al. 1989; Tou et al. 1993). By means of peptide mapping using enzymatic cleavage and selective DTT reduction these scientists demonstrated that one of these refold dimers was an anti-parallel cross-linked dimer. Another refold dimer was a concatenane dimer in which two monomers were held together by the interlocking of two large disulfide loops. Similar structural models of disulfide-linked dimers of hGH are theoretically possible. Therefore, a topology of disulfide linkages that are dissymetric or that result in formation of catenane structures may be responsible for the extraordinary stability of the MER-45-kDa hGH disulfide dimer.
According to Cunningham et al. (1991), zinc ions (Zn2+) induce the dimerization of hGH. We conducted studies using divalent metal cation chelators to accelerate the conversion of MER-45-kDa hGH into 22-kDa hGH by dissociation of postulated hGH dimeric complexes held together by divalent metal cations. The divalent metal cation chelators did not accelerate the conversion of MER-45-kDa hGH into 22-kDa hGH. These data suggest that MER-45-kDa hGH is not a hGH dimer wherein the monomers are held together by divalent metal cations.
Although prolonged exposure of proteins to oxygen-containing buffers leads to oxidation and disulfide bond formation, it is unlikely that MER-45-kDa hGH is formed artificially during the initial purification procedures from human pituitary extracts. In this respect a study performed by Lewis et al. (1977) is useful. Under experimental conditions similar to those used in our purification they demonstrated that a 45 kDa hGH homodimer does not form during a prolonged exposure of monomeric hGH to oxygen-containing buffers in the absence of reducing agents. They also demonstrated that the MER-45-kDa hGH was present in extracts of fresh pituitary glands by SDS-PAGE. Other investigators have shown that disulfide-linked oligomers of bovine GH are present in the secretory granules of somatotrophs (Jacobs and Lee 1975; Lorenson and Jacobs 1982; Lorenson et al. 1983). Human pitituitary adenomas secrete MER-45-kDa hGH in culture (Talamantes et al. 1981) and MER-45-kDa hGH is present in the circulation (Baumann 1999; Nindl et al. 2003). These studies indicate that MER-45-kDa hGH dimers are not the result of purification conditions but rather that they are naturally occurring hGH isoforms that are constituents of the pituitary gland and circulatory system. Estimates of the percentage of total pituitary hGH that is MER-45-kDa hGH have been reported as 5% (Lewis et al. 1987) and 2% (Baumann 1991, 1999). Estimates of the isoform in serum along with other oligomers that were thought to be non-disulfide linked, prior to our findings, were estimated to be approximately 1% of the circulating isoforms in serum (Baumann 1991).
In summary, our data are consistent with the view that the MER-45-kDa hGH variant is not a single-chain polypeptide. Instead, the data support our hypothesis that MER-45-kDa hGH is a dimer of 22-kDa hGH subunits. The MER-45-kDa hGH dimer is held together by interchain disulfide bonds but not by divalent metal cation bridges. Moreover, the molecular topological linkages responsible for preserving the integrity of the MER-45-kDa hGH dimer are not easily broken. The impact of this work is that it expands our knowledge of the structure and biochemical properties of one of the molecular weight isoforms of hGH.
Materials and methods
Source of hGH
Partially purified hGH from human pituitary extracts was prepared as previously described (Haro et al. 1996). Human pituitaries of unknown age, sex, disease state, and so on were provided as a batch by the National Hormone and Pituitary Program. Fresh frozen human pituitary glands (850) were homogenized (5°C) in 5 mL of saline per gram of tissue then centrifuged for 45 min at 20,000g (5°C). The supernatant was frozen, thawed, recentrifuged, and filtered through paper. The filtrate was made 2 M in (NH4)2SO4, stirred overnight (5°C), and the precipitate collected by centrifugation at 10,000g for 30 min (5°C). The precipitate from the pituitary extract was suspended in 50 mM NaHCO3 and chromatographed on a Sephadex G-100 column (10 × 100 cm) in 10 mM NH4HCO3 (5°C). Pooled fractions from the Sephadex G-100 column containing MER-45-kDa hGH as determined by analytical SDS-PAGE (Laemmli 1970) was used as starting material for preparative electrophoresis.
Chemicals
Acrylamide, N,N′-methylene-bis-acrylamide, SDS, TEMED, ammonium persulfate, EPPS, CAPS, EGTA, 2-mercaptoethanol, DTT, glutathione (reduced form), iodoacetamide, 2,5-dihydroxy-benzoic acid, and Coomassie R-250 were purchased from Sigma. EDTA was purchased from Fisher Scientific. 2-Amino-2-(hydroxymethyl)-1,3-propanediol, isopropyl alcohol, methanol, acetic acid, and glycerol were purchased from EM Science. TCEP-HCl was obtained from Pierce.
Preparative electrophoresis and electroelution
Preparative electrophoresis of the partially purified hGH under reducing conditions using the Laemmli SDS-PAGE system (4% T, 2.7% CBIS stacking gel; 10% T, 2.7% CBIS running gel) (Laemmli 1970) was carried out in the Mini-PROTEAN II Electrophoresis Cell (Bio-Rad; gel size 7 × 8 cm, gel thickness 1.0 mm) at 200 V for 55–60 min to obtain the hGH fraction containing MER-45-kDa hGH. Electroelution of the separated proteins from the gel was carried out using the Mini Whole Gel Eluter (Bio-Rad) with a current of 100 mA for 30 min using a 32 mM Tris/30 mM EPPS electroelution buffer at pH 8.1.
Analytical SDS-PAGE and silver staining
Analytical SDS-PAGE analysis of protein samples under reducing and nonreducing conditions was carried out using discontinuous polyacrylamide gels (4% T, 2.7% CBIS stacking gel; 13.5% T, 2.7% CBIS separating gel) according to the method of Laemmli (1970) using a Mini-PROTEAN II Electrophoresis Cell. The gels were silver-stained according to Ansorge (1985).
Exposure of MER-45-kDa hGH to various disrupters of oligomeric structure
Fractions containing MER-45-kDa hGH were obtained by preparative electrophoresis under reducing conditions. MER-45-kDa hGH samples were then treated with Laemmli (1970) sample buffer (0.125 M [w/v] Tris-HCl at pH 6.8, 4% [w/v] SDS; 20% [v/v] glycerol) at a ratio of 1:1 (v/v). In parallel experiments the MER-45-kDa hGH samples were treated with Laemmli sample buffer containing either 2-mercaptoethanol at 1% (v/v) or 10% (v/v), TCEP-HCl at 10 mM or 100 mM, glutathione at 10 mM or 100 mM, or DTT at 100 mM. Other MER-45-kDa hGH samples were incubated with Laemmli sample buffer in the absence or presence of EDTA and EGTA at either 1 mM or 10 mM without 2-mercaptoethanol. MER-45-kDa hGH samples were incubated for various times (3 min, 30 min, 300 min) at various temperatures (25°C, 60°C, 95°C, and 100°C). Upon completion of the incubations, protein components were separated by analytical SDS-PAGE (Laemmli 1970) and then silver stained (Ansorge 1985).
Quantitative evaluation of protein bands in SDS polyacrylamide gels
Stained SDS polyacrylamide gels were scanned with an imaging densitometer (Bio-Rad, Model GS-700). The Molecular Analyst program (Bio-Rad, version 2.1) was used to calculate the amount of protein in each band, expressed as volume = (density × area), using the densitometry data.
Reassociation of MER-45-kDa hGH subunits
MER-45-kDa hGH was dissociated into 22-kDa protein subunits by incubation in the presence of 10% (1.4 M) mercaptoethanol at 100°C for 5 h. Dissociated protein subunits were then examined for their abilities to reassociate by dramatically reducing the concentration of reductant from 1.4 M to 20 mM or to 0 M. Dialysis of dissociated protein subunits was carried out in Slide-A-Lyzer Dialysis Cassettes (Pierce; MWCO 10,000, capacity 0.1–0.5 mL) against 10,000 vol of 5 mM ammonium bicarbonate (pH 7.6) in the presence and absence of 20 mM 2-mercaptoethanol at 4°C for 48 h with three changes of the dialysis buffer. After, dialysis samples were analyzed for the presence of monomers and dimers by analytical SDS-PAGE in the absence of 2-mercaptoethanol.
To modify SH-groups in 22-kDa protein subunits derived from MER-45-kDa hGH the method of Creighton (1980) with a modification described by Lewis et al. (1977) was employed: 10% mercaptoethanol was used instead of DTT. The protein sample was incubated in a buffer containing 10 mM Tris-HCl and 1 mM EDTA (pH 8.0) in the presence of 10% mercaptoethanol at 100°C for 5 h. Thereafter iodoacetamide (7 M, with a 10-fold molar excess above the quantity of mercaptoethanol, in 0.25 M Tris-HCl at pH 8.0) was added to the incubated protein. The protein sample was incubated with the modifying agent for 15 min at room temperature. Thereafter the dialysis of dissociated and modified protein subunits against 10 mM Tris-HCl (pH 7.6) was carried out as described above. After, dialysis samples were analyzed for the presence of monomers and dimers by analytical SDS-PAGE in the absence of 2-mercaptoethanol.
N-terminal amino acid sequence analysis of MER-45-kDa hGH, 22-kDa hGH, and the dissociated 22-kDa hGH subunits produced through the reduction of MER-45-kDa hGH
Samples containing 22-kDa hGH and MER-45-kDa hGH for N-terminal amino acid sequence analysis were separated using discontinuous polyacrylamide gels (4% T, 2.7% CBIS stacking gel; 13.5% T, 2.7% CBIS running gel) according to the method of Laemmli (1970) using the Mini-PROTEAN II Electrophoresis Cell. The proteins were then transferred onto a ProBlott membrane (Applied Biosystems) using the electroblotting buffer (10 mM CAPS, 10% methanol at pH 11) in the Mini-PROTEAN II Electrophoresis Cell at 50 V for 30 min at room temperature. The protein bands were detected by Coomassie blue staining (0.1% Coomassie blue R-250 in 40% methanol/5% acetic acid) and excised.
Dissociated 22-kDa protein subunits derived from MER-45-kDa hGH were obtained for amino acid sequence analysis by incubation of MER-45-kDa hGH with 10% 2-mercaptoethanol at 95°C for 5 h with subsequent preparative electrophoresis. The sample containing dissociated 22-kDa protein subunits was dialyzed in a Slide-A-Lyzer Dialysis Cassette (Pierce; MWCO 10,000, capacity 0.1–0.5 mL) against 2000 vol of 5 mM NH4HCO3 (pH 7.6) at 4°C for 48 h with three changes of the dialysis buffer before N-terminal amino acid sequence analysis.
N-terminal amino acid sequences of protein samples were determined by automated Edman degradation (Hunkapiller et al. 1983) using the P.E. Biosystems 491 CLC Procise Sequencer (Applied Biosystems).
MALDI-TOF/MS of tryptic peptides obtained from digests of 22-kDa hGH and MER-45-kDa hGH
22-kDa hGH and MER-45-kDa hGH samples were prepared by a combination of chromatography and SDS-PAGE. Starting material containing hGH isoforms was separated by anion-exchange chromatography on a DEAE-TSK Toyopearl SP-650 column in the presence of EDTA and EGTA as previously described (Haro et al. 1998). Fractions rich in both MER-45-kDa hGH and 22-kDa hGH were solubilized in Laemmli sample buffer and heated for 3 min at 95°C. The samples (approximately 10 μg of protein per lane) were separated on discontinuous SDS polyacrylamide gels (4% T, 2.7% CBIS stacking gel; 13.5% T, 2.7% CBIS running gel) according to the method of Laemmli (1970) under reducing conditions and visualized with Coomassie blue staining (0.1% Coomassie blue R-250 in 40% methanol/5% acetic acid). Gel slices containing MER-45-kDa hGH and 22-kDa hGH were excised and proteins digested with trypsin (Shevchenko et al. 1996). The in-gel digestion was performed with 5–10 ng/μL sequencing grade-modified trypsin (Promega) in 40 mM ammonium bicarbonate and incubated overnight at 37°C. To reduce the MER-45-kDa hGH and 22-kDa hGH samples, DTT (100 mM) was used. Iodoacetamide (500 mM) was used for the alkylation of MER-45-kDa hGH and 22-kDa hGH. The resulting digests were analyzed by MALDI-TOF/MS using an Applied Biosystems Voyager-DE STR operated in reflector mode using delayed extraction. Samples were applied to the target and embedded in a UV-absorbing matrix consisting of a saturated solution of 2,5-dihydroxybenzoic acid in 50% acetonitrile/0.1% trifluoroacetic acid. The peptide mass maps produced by MALDI-TOF/MS were searched against the published databases by means of the MS-Fit module in Protein Prospector (Clauser et al. 1999) to provide information about the identity of the protein(s) in each band.
Acknowledgments
We thank Dr. U.J. Lewis for providing the starting material for our research, the National Pituitary Program for providing the pituitaries, Steve Mouton of the Institutional Protein Core Facility of the University of Texas Health Science Center at San Antonio for conducting the N-terminal amino acid sequencing of our samples, and Dr. Susan T. Weintraub and Chris Carroll of the Institutional Mass Spectrometry Laboratory of the University of Texas Health Science Center at San Antonio for conducting the mass spectrometry. This work was supported by grants GM08194 and GM60655 of the NIH.
Abbreviations
SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis
MER-45-kDa hGH, mercaptoethanol-resistant 45-kDa human growth hormone
TEMED, N, N, N′, N′-tetramethylethylenediamine
EPPS, N-[2-hydroxyethyl]piperazine-N′-[3-propanesulfonic acid]
CAPS, 3-[cyclohexylamino]-1-propanesulfonic acid
EGTA, ethylene glycolbis(β-aminoethylether)-N, N, N′, N′-tetraacetic acid
EDTA, ethylenedi-aminetetraacetic acid
DTT, dithiothreitol
TCEP-HCl, tris(2-carboxyethyl)phosphine hydrochloride
hGH, human growth hormone
MALDI-TOF/MS, matrix-assisted laser desorption/ionization time-of-flight/mass spectrometry
Article published online ahead of print. Article and publication date are at http://www.proteinscience.org/cgi/doi/10.1110/ps.041048805.
References
- Altszuler, N. 1974. Actions of growth hormone on carbohydrate metabolism. In Handbook of physiology, Section 7: Endocrinology, Vol. 4, Part 2 (eds. E. Knobil and W.H. Sawyer), pp. 233–252. American Physiological Society, Washington, DC.
- Ansorge, W. 1985. Fast and sensitive detection of protein and DNA bands by treatment with potassium permanganate. J. Biochem. Biophys. Methods 11 13–20. [DOI] [PubMed] [Google Scholar]
- Barrera-Saldana, H.A. 1998. Growth hormone and placental lactogen: Biology, medicine and biotechnology. Gene 211 11–18. [DOI] [PubMed] [Google Scholar]
- Bauman, D.E. 1999. Bovine somatotropin and lactation: From basic science to commercial application. Domest. Anim. Endocrinol. 17 101–116. [DOI] [PubMed] [Google Scholar]
- Bauman, D.E., Eisemann, J.H., and Currie, W.B. 1982. Hormonal effects on partitioning of nutrients for tissue growth: Role of growth hormone and prolactin. Fed. Proc. 41 2538–2544. [PubMed] [Google Scholar]
- Baumann, G. 1991. Growth hormone heterogeneity: Genes, isohormones, variants, and binding proteins. Endocrin. Rev. 12 424–449. [DOI] [PubMed] [Google Scholar]
- ———. 1999. Growth hormone heterogeneity in human pituitary and plasma. Horm. Res. 51 (Suppl. 1): 2–6. [DOI] [PubMed] [Google Scholar]
- Boguszewski, C.L. 2003. Molecular heterogeneity of human GH: From basic research to clinical implications. J. Endocrinol. Invest. 26 274–288. [DOI] [PubMed] [Google Scholar]
- Brostedt, P. and Roos, P. 1988. Isolation of four isomers of the 20,000 dalton variant of human pituitary growth hormone. Prep. Biochem. 18 277–291. [DOI] [PubMed] [Google Scholar]
- ———. 1989. Isolation of dimeric forms of human pituitary growth hormone. Prep. Biochem. 19 217–229. [DOI] [PubMed] [Google Scholar]
- Brumby, P.J. 1959. The influence of growth hormone on growth in young cattle. N.Z.J. Agric. Res. 2 683–689. [Google Scholar]
- Chapman, G.E., Rogers, K.M., Brittain, T., Bradshaw, R.A., Bates, O.J., Turner, C., Cary, P.D., and Crane-Robinson, C. 1981. The 20,000 molecular weight variant of human growth hormone. Preparation and some physical and chemical properties. J. Biol. Chem. 256 2395–2401. [PubMed] [Google Scholar]
- Charrier, J. and Martal, J. 1988. Growth hormones. 1. Polymorphism. (Minireview). Reprod. Nutr. Dev. 28 857–887. [DOI] [PubMed] [Google Scholar]
- Cheek, D.B. and Hill, D.E. 1974. Effect of growth hormone on cell and somatic growth. In Handbook of physiology, Section 7, Vol. 4 (eds. R.O. Greep and E.B. Astwood), pp. 159–185. American Physiological Society, Washington, DC.
- Chen, E.Y., Liao, Y.-C., Smith, D.H., Barrera-Saldana, H.A., Gelinas, R.E., and Seeburg, P.H. 1989. The human growth hormone gene locus: Nucleotide sequence, biology, and evolution. Genomics 4 479–497. [DOI] [PubMed] [Google Scholar]
- Chene, N., Martal, J., de la Llosa, P., and Charrier, J. 1989. Growth hormones. II. Structure-function relationships. Reprod. Nutr. Dev. 29 1–25. [DOI] [PubMed] [Google Scholar]
- Chung, C.S., Etherton, T.D., and Wiggins, J.P. 1985. Stimulation of swine growth by porcine growth hormone. J. Animal. Sci. 60 118–130. [DOI] [PubMed] [Google Scholar]
- Clauser, K.R., Baker, P.R., and Burlingame, A.L. 1999. Role of accurate mass measurement (+/− 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal. Chem. 71 2871–2882. [DOI] [PubMed] [Google Scholar]
- Creighton, T.E. 1980. Counting integral numbers of amino acid residues per polypeptide chain. Nature 284 487–488. [DOI] [PubMed] [Google Scholar]
- Cunningham, B.C., Mulkerrin, M.G., and Wells, J.A. 1991. Dimerization of human growth hormone by zinc. Science 253 545–548. [DOI] [PubMed] [Google Scholar]
- Diaz, M.J., Dominguez, F., Haro, L.S., Ling, N., and Devesa, J. 1993. A 12-kilodalton N-glycosylated growth hormone-related peptide is present in human pituitary extracts. J. Clin. Endocrin. Metab. 77 134–138. [DOI] [PubMed] [Google Scholar]
- Evans, H.M. and Simpson, M.E. 1931. Hormones of the anterior hypophysis. Am. J. Physiol. 98 511–546. [Google Scholar]
- Goodman, H.M. and Schwartz, J. 1974. Growth hormone and lipid metabolism. In Handbook of physiology, Section 7: Endocrinology, Vol. 4, Part 2 (eds. E. Knobil and W.H. Sawyer), pp. 211–231. American Physiological Society, Washington, DC.
- Graf, L., Li, C.H., and Bewley, T.A. 1975. Selective reduction and alkylation of the COOH-terminal disulfide bridge in bovine growth hormone. Int. J. Peptide Protein Res. 7 467–473. [DOI] [PubMed] [Google Scholar]
- Grigorian, A.L., Bustamante, J.J., Aguilar, R.M., Martinez, A.O., and Haro, L.S. 2002. 45 kDa Mercaptoethanol-resistant human growth hormone binds to somatogenic and lactogenic receptors. Program of the 84th Annual Meeting of the Endocrine Society, p. 555. San Francisco, CA.
- Grigorian, A.L., Bustamante, J.J., Aguilar, R.M., Muñoz, J., Martinez, A.O., and Haro, L.S. 2003. Characterization of 45 kDa mercaptoethanol-resistant human growth hormone by radioimmunoassay and bioassays. Program of the 85th Annual Meeting of the Endocrine Society, p. 381. Philadelphia, PA.
- Hames, B.D. 1998. Gel electrophoresis of proteins: A practical approach, 3rd ed. pp. 34–35. Oxford University Press, Oxford, UK.
- Haro, L.S., Lewis, U.J., Garcia, M., Bustamante, J., Martinez, A.O., and Ling, N.C. 1996. Glycosylated human growth hormone (hGH): A novel 24 kDa hGH-N variant. Biochem. Biophys. Res. Commun. 228 549–556. [DOI] [PubMed] [Google Scholar]
- Haro, L.S., Cubriel, A., Bustamante, J., Flores, R., and Martinez, A.O. 1998. Divalent metal cation chelators enhance chromatographic separation of structurally similar macromolecules: Separation of human growth hormone isoforms. J. Chromat. B 720 39–47. [DOI] [PubMed] [Google Scholar]
- Hearn, M.T.W., Grego, B., and Chapman, G.E. 1983. Separation and assignment of the tryptic peptides of human growth hormone (hGH) and the 20K Dalton variant by reversed phase high performance liquid chromatography. J. Liquid Chrom. 6 215–228. [Google Scholar]
- Hernandez, P., Bustamante, J., and Haro, L.S. 1998. Analysis of a novel growth hormone variant from human pituitaries. 1998 Annual Meeting of the American Society for Biochemistry and Molecular Biology, Washington, DC. FASEB J. 12 A1363. [Google Scholar]
- Ho, K.K., O’Sullivan, A.J., and Hoffman, D.M. 1996. Metabolic actions of growth hormone in man. Endocr. J. 43 S57–S63. [DOI] [PubMed] [Google Scholar]
- Hunkapiller, M.W., Hewick, R.M., Dreyer, W.J., and Hood, L.E. 1983. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 91 399–413. [DOI] [PubMed] [Google Scholar]
- Jacobs, L.S. and Lee, Y.-C. 1975. Polymeric growth hormone and prolactin in secretory granules: Sulfhydryl bonding masks immunoreactive sites. Program of the 57th Annual Meeting of the Endocrine Society, p. 83. New York.
- Kostyo, J.J. and Nutting, D.F. 1974. Growth hormone and protein metabolism. In Handbook of physiology, Section 7: Endocrinology, Vol. 4, Part 2 (eds. E. Knobil and W.H. Sawyer), pp. 187–210. American Physiological Society, Washington, DC.
- Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Lewis, U.J. 1992. Growth hormone: What is it and what does it do? Trends Endocrinol. Metab. 3 117–121. [DOI] [PubMed] [Google Scholar]
- Lewis, U.J., Peterson, S.M., Bonewald, L.F., Seavey, B.K., and VanderLaan, W.P. 1977. An interchain disulfide dimer of human growth hormone. J. Biol. Chem. 252 3697–3702. [PubMed] [Google Scholar]
- Lewis, U.J., Singh, R.N., Tutwiler, G.F., Sigel, M.B., VanderLaan, E.F., and VanderLaan, W.P. 1980. Human growth hormone: A complex of proteins. Recent Prog. Horm. Res. 36 477–508. [DOI] [PubMed] [Google Scholar]
- Lewis, U.J., Singh, R.N.P., Lewis, L.J., and Abadi, N. 1987. Detection of multiple forms of human growth hormone. In Acromegaly: A century of scientific and clinical progress (eds. R.J. Robbins and S. Melmed), pp. 27–34. Plenum, New York.
- Lewis, U.J., Sinha, Y.N., and Haro, L.S. 1994. Variant forms and fragments of human growth hormone in serum. Acta. Paediatr. Suppl. 399 29–31. [DOI] [PubMed] [Google Scholar]
- Lewis, U.J., Sinha, Y.N., and Lewis, G.P. 2000. Structure and properties of members of the hGH family: A review. Endocrin. J. 47 S1–S8. [DOI] [PubMed] [Google Scholar]
- Lorenson, M.Y. and Jacobs, L.S. 1982. Thiol regulation of protein, growth hormone, and prolactin release from isolated adenohypophysial secretory granules. Endocrinology 110 1164–1172. [DOI] [PubMed] [Google Scholar]
- Lorenson, M.Y., Robson, D.L., and Jacobs, L.S. 1983. Detectability of pituitary PRL and GH by immunoassay is increased by thiols and suppressed by divalent cations. Endocrinology 112 1880–1882. [DOI] [PubMed] [Google Scholar]
- Machlin, L.J. 1972. The effect of porcine growth hormone on growth and carcass composition of the pig. J. Anim. Sci. 35 794–800. [DOI] [PubMed] [Google Scholar]
- Mao, B. 1990. Molecular topology and dimerization of recombinant bovine somatotropin. Biopolymers 30 645–647. [DOI] [PubMed] [Google Scholar]
- Muir, L.A., Wien, S., Dugvette, P.F., Rickes, E.L., and Cordes, E.H. 1983. Effects of endogenous growth hormone and diethylstilbesterol on growth and carcass composition of growing lambs. J. Anim. Sci. 56 1315–1323. [DOI] [PubMed] [Google Scholar]
- Muñoz, J., Esquivel, J., Bustamante, J.J., Martinez, A.O., and Haro, L.S. 2004. Anti-proliferative effects of a 2-mercaptoethanol resistant 45 kDa human growth hormone variant in MCF-7, MDA-MB-231 and T47D human breast cancer cell lines. Program of the 86th Annual Meeting of the Endocrine Society, p. 375. New Orleans, LA.
- Nindl, B.C., Kraemer, W.J., Marx, J.O., Tuckow, A.P., and Hymer, W.C. 2003. Growth hormone molecular heterogeneity and exercise. Exerc. Sport Sci. Rev. 31 161–166. [DOI] [PubMed] [Google Scholar]
- Ochs, D. 1983. Protein contaminants of sodium dodecyl sulfate-polyacrylamide gels. Anal. Biochem. 135 470–474. [DOI] [PubMed] [Google Scholar]
- Okada, S. and Kopchick, J.J. 2001. Biological effects of growth hormone and its antagonist. Trends Mol. Med. 7 126–132. [DOI] [PubMed] [Google Scholar]
- Paul-Pletzer, K. and Parness, J. 2001. Elimination of keratin contaminant from 2-mercaptoethanol. Anal. Biochem. 289 98–99. [DOI] [PubMed] [Google Scholar]
- Shapiro, S.Z. 1987. Elimination of the detection of an artifactual 65 kDa keratin band from immunoblots. J. Immunol. Methods 102 143–146. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. 1996. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68 850–858. [DOI] [PubMed] [Google Scholar]
- Singh, R.N.P., Seavey, B.K., and Lewis, U.J. 1974. Heterogeneity of human growth hormone. Endocrine Res. Commun. 1 449–464. [DOI] [PubMed] [Google Scholar]
- Strobl, J.S. and Thomas, M.J. 1994. Human growth hormone. Pharmac. Rev. 46 1–34. [PubMed] [Google Scholar]
- Talamantes, F., Lopez, J., Lewis, U.J., and Wilson, C.B. 1981. Multiple forms of growth hormone: Detection in medium from cultured pituitary adenoma explants. Acta Endocrinol. 98 8–13. [DOI] [PubMed] [Google Scholar]
- Thamann, T.J. 1998. Raman spectroscopic studies of a dimeric form of recombinant bovine growth hormone. Anal. Biochem. 265 202–207. [DOI] [PubMed] [Google Scholar]
- Tou, J.S., Violand, B.N., Schlittler, M.R., and Jennings, M.G. 1993. Structural characterization of the two refold dimers of recombinant bovine somatotropin (bST). J. Protein Chem. 12 237–245. [DOI] [PubMed] [Google Scholar]
- Violand, B.N., Takano, M., Curran, D.F., and Bentle, L.A. 1989. A novel concatenated dimer of recombinant bovine somatotropin. J. Protein Chem. 8 619–628. [DOI] [PubMed] [Google Scholar]
- Waters, M.J. and Kaye, P.L. 2002. The role of growth hormone in fetal development. Growth Horm. IGF Res. 12 137–146. [DOI] [PubMed] [Google Scholar]