Abstract
A peroxidase is present in the chorion of Aedes aegypti eggs and catalyzes chorion protein cross-linking during chorion hardening, which is critical for egg survival in the environment. The unique chorion peroxidase (CPO) is a glycoprotein. This study deals with the N-glycosylation site, structures, and profile of CPO-associated oligosaccharides using mass spectrometric techniques and enzymatic digestion. CPO was isolated from chorion by solubilization and several chromatographic methods. Mono-saccharide composition was analyzed by HPLC with fluorescent detection. Our data revealed that carbohydrate (D-mannose, N-acetyl D-glucosamine, D-arabinose, N-acetyl D-galactosamine, and L-fucose) accounted for 2.24% of the CPO molecular weight. A single N-glycosylation site (Asn328-Cys- Thr) was identified by tryptic peptide mapping and de novo sequencing of native and PNGase A-deglycosylated CPO using matrix-assisted laser/desorption/ionization time-of-flight mass spectrometry (MALDI/TOF/MS) and liquid chromatography/tandem mass spectrometry (LC/MS/MS). The Asn328 was proven to be a major fully glycosylated site. Potential tryptic glycopeptides and profile were first assessed by MALDI/TOF/MS and then by precursor ion scanning during LC/MS/MS. The structures of N-linked oligosaccharides were elucidated from the MS/MS spectra of glycopeptides and exoglycosidase sequencing of PNGase A-released oligosaccharides. These CPO-associated oligosaccharides had dominant Man3GlcNAc2 and Man3 (Fuc) GlcNAc2 and high mannose-type structures (Man4–8GlcNAc2). The truncated structures, Man2GlcNAc2 and Man2 (Fuc) GlcNAc2, were also identified. Comparison of CPO activity and Stokes radius between native and deglycosylated CPO suggests that the N-linked oligosaccharides influence the enzyme activity by stabilizing its folded state.
Keywords: Aedes aegypti, chorion peroxidase, glycosylation
The chorion of insect eggs is a proteinacious structure and undergoes a hardening process either before or right after oviposition, which leads to the formation of a highly protective chorion or eggshell (Margaritis 1985a). It has been determined that peroxidase-catalyzed chorion protein cross-linking is one of the major mechanisms contributing to the formation of rigid and insoluble chorion in Drosophila (Petri et al. 1976; Mindrinos et al. 1980; Margaritis 1985b; Keramaris et al. 1996; Trougakos and Margaritis 1998) and Aedes aegypti (Li et al. 1996; Han et al. 2000). During chorion hardening, the specific CPO catalyzes protein crosslinking through dityrosine and trityrosine formation (Li et al. 1996). In insects, embryonation of eggs takes place in the environment. This requires several days or even weeks, depending upon the temperature. During embryonation, the hardened chorion protects the developing embryo against potential environmental adversities, especially desiccation during dry weather conditions. Therefore, peroxidase-mediated chorion protein crosslinking is a crucial biochemical event contributing to the survival of insects in the environment.
CPO is undoubtedly an important enzyme in insects, but there have been limited studies of the enzyme because it is extremely difficult to isolate enough pure CPO from the chorion for structural and functional characterization. Our previous study dealing with the biochemical characterization of an A. aegypti CPO revealed that the enzyme has extremely high specific activity to tyrosine and is strongly resistant to SDS, organic solvents, and heat (Han et al. 2000). Recently, we isolated the cDNA of A. aegypti CPO based on partial sequence data derived from LC/MS/MS of partially purified CPO (Li et al. 2004). Our data suggest that A. aegypti CPO is a structurally unique peroxidase and undergoes extensive posttranslational modifications, including proteolytic processing and glycosylation (Han et al. 2000; Li et al. 2004). These post-translation modifications may be closely related to its unique biochemical properties.
There are two potential N-glycosylation sites (Asn328 and Asn430) and several potential O-glycosylation sites in mature CPO (Li et al. 2004). In mammals and plants, it has been recognized that the carbohydrate moiety may modulate the folding and biochemical properties of the glycoproteins, as well as mediate their conformation, activity, antigenicity, location, and stability (Rademacher et al. 1988; van Huystee and McManus 1998; Varki et al. 1999). N-glycosylation is one of the two types of protein glycosylation and has drawn more attention. In N-glycosylation, oligosaccharide is attached to an asparagine residue (R-CONH2) of the domain Asn-X-Ser/Thr, where X can be any amino acids except for proline. Although it has been suggested that the N-glycosylation pathway in insect cells is similar to that observed in mammalian cells, there has been a paucity of data on the structures of protein-associated oligosaccharides in insects (Williams et al. 1991; Varki et al. 1999; Seppo and Tiemeyer 2000; Stephens et al. 2004). Glycosylation of some plant or mammalian peroxidases has been reported (Kurosaka et al. 1991; van Huystee et al. 1992; van Huystee and McManus 1998), but we have not found related data in any insect peroxidases. To characterize the glycosylation of CPO, we isolated the enzyme from A. aegypti chorion (Li et al. 2004) and analyzed its monosaccharide composition, glycosylation site, and the structures of its oligosaccharides using mass spectrometric techniques combined with enzymatic digestion. We also investigated the effect of glycosylation on CPO activity. This study provides helpful information for understanding CPO function and localization in mosquito eggs and should also serve as a useful reference for the structural characterization of other insect glycoproteins.
Results
CPO purification
CPO was purified by DEAE-Sepharose, phenyl- Sepharose, Superdex, and Mono-Q columns. Finally, a single peak with peroxidase activity was observed in the chromatogram (detection at 280 nm) during the Mono-Q step (Fig. 1 ▶). A single protein band was also shown on SDS-PAGE gel (Fig. 1 ▶, inset). A total of 40 μg (0.2 μg/μL) of CPO was obtained from 8000 ovary pairs and the purified enzyme displayed a specific activity of 4.6 ± 0.78 μmol/min/mg under the described assay conditions.
Figure 1.
Chromatogram of CPO on Mono Q column and purity of CPO based on SDS-PAGE, after purification with DEAE-Sepharose, phenyl-Sepharose, and Superdex columns.
CPO monosaccharide composition
Analysis of acid hydrolysates using HPLC with fluorescence detection determined the presence of D-mannose, N-acetyl D-glucosamine, D-arabinose, N-acetyl D-galactosamine, and L-fucose in CPO (Fig. 2 ▶). Among them, D-mannose and N-acetyl D-glucosamine were the major monosaccharides. D-arabinose, N-acetyl D-galactosamine, and L-fucose were the minor ones (Table 1). D-galactose, D-arabinose, N-glycolyl neuraminic acid, and N-acetyl neuraminic acid were not detected. Because approximately equal amounts of glucose and xylose were observed in the chromatograms of sample and blank PVDF control (data not shown), they were considered to come from PVDF membrane (Anumula and Du 1999). In addition, no sialic acid peaks were observed in the chromatogram of the hydrolysate (not shown). These results indicate that N-linked oligosaccharides are dominant in CPO glycoyslation. Based on the amounts of individual monosaccharides detected, carbohydrates constitute ~2.24% of the total molecular weight in CPO.
Figure 2.
Typical chromatogram of 2-anthranilic acid-labeled monosaccharides in CPO TFA hydrolysate. (A)Monosaccharide standards (see Table 1 for abbreviations); (B) monosaccharides from CPO. Glucose and xylose were considered to come from PVDF membrane, because essentially equal amounts of glucose and xylose were observed in the chromatogram of a blank PVDF control (data not shown). A summary of monosaccharide composition is in Table 1.
Table 1.
Monosaccharide composition of chorion peroxidase (CPO)
| Monosaccharides | Percentage in total carbohydrates (w/w)a | Residues per molecule of CPOb |
| N-Acetyl D-glucosamine (GlcNAc) | 30.8 ± 1.24 | 2.2 |
| N-Acetyl D-galactosamine (GalNAc) | 2.29 ± 0.12 | 0.2 |
| D-Galactose (Gal) | NDc | — |
| D-Mannose (Man) | 63.0 ± 3.21 | 5.5 |
| D-Glucose (Glc) | ND | — |
| D-Arabinose (Ara) | 2.72 ± 0.82 | 0.3 |
| D-Xylose (Xyl) | ND | — |
| L-Fucose (Fuc) | 1.29 ± 0.56 | 0.1 |
| N-Glycolyl neuraminic acid (Neu5Gc) | ND | — |
| N-Acetyl neuraminic acid (Neu5Ac) | ND | — |
N-glycosylation sites
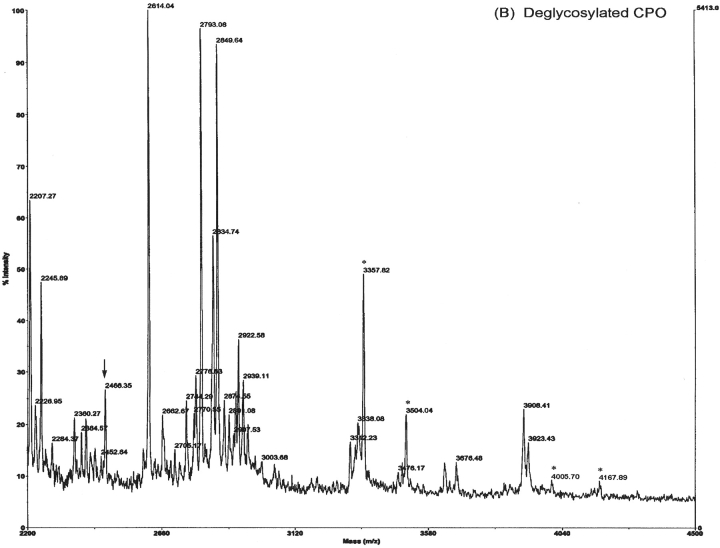
There were two potential N-glycosylation sites (Asn328- Cys-Thr and Asn430-Arg-Thr) in the amino acid sequence of the mature CPO (Li et al. 2004). A number of peptides with m/z ranging from 3195.40 to 4167.49 were observed in the MALDI/MS spectrum of native CPO tryptic peptides (Fig. 3A ▶), but these peaks disappeared in the MALDI/MS spectrum of deglycosylated CPO tryptic peptides with concomitant detection of a cluster of new peaks with m/z 2423.29, 2437.76, 2451.95, and 2466.14 (Fig. 3B ▶), suggesting their potential glycopeptide identity. When the same deglycosylated tryptic CPO sample was analyzed by LC/MS/MS, the MS/MS spectra of the precursor ions corresponding to m/z 2423.29, 2437.76, 2451.95, and 2466.14 clearly indicated that they were derived from the same peptide fragment (318HGQA IECCTPDCTAPLFGPHR338) that contained a potential glycosylation site (Asn328-Cys-Thr, where Asn328 was converted to Asp after PNGase deglycosylation). The differences in mass units of these precursor ions were due to in vitro modification of its three cysteine residues. The ion with m/z 2423.29 contained three carboxyamidomethyl cysteine residues (average mass 160.19 per residue), which were formed during iodoacetamide alkylation after SDS-PAGE. M/z 2437.76 contained two carboxyamidomethyl cysteine residues and one propionamide cysteine residue (average mass 174.22 per residue, formed through reaction with free acrylamide during SDS-PAGE). M/z 2451.95 contained one carboxyamidomethyl cysteine residue and two propionamide cysteine residues. M/z 2466.14 had three propionamide cysteines. Based on MS/MS spectra, the propionamide cysteine residues or carboxyamidomethyl cysteine residues and their corresponding positions in the peptide backbone can be determined based on adjacent ions. Figure 4 ▶ shows the structural elucidation of the precursor ion m/z 2466 based on its MS/MS spectrum. The N-glycosylation site was reconfirmed by analysis of native CPO tryptic peptides during LC/ESI/MS/MS analysis (see Figs. 7 ▶, 8 ▶, below).
Figure 3.
MALDI/MS spectra of tryptic peptides of CPO. (A) Native CPO: The enlarged mass range shows putative glycopeptides with m/z 3195.40, 3357.36, 3503.40, 3518.65, 3681.39, 3843.70, 4005.76, and 4167.49, indicated with asterisk. The arrow shows the position of the deglycosylated peptides. (B) PNGase A-deglycosylated CPO: Peaks indicated with an arrow are putative deglycosylated peptide after modification with acrylamide and/or iodoacetamide (m/z 2423.29, 2437.76, 2451.95, and 2466.14). The structure of m/z 2466.14 is illustrated in Figure 4 ▶.
Figure 4.
ESI/MS/MS spectrum and de novo sequence of a deglycosylated CPO peptide of m/z 823.13 with 3+ charge status and an observed molecular mass of 2465.88. There is an N-glycosylation site (Asn*-Cys-Thr, where Asn was converted to Asp after PNGase A deglycosylation) in the fragment. All cysteine residues are acrylamide adduct in this structure.
Figure 7.
ESI/MS/MS spectrum and structure of a CPO glycopeptide m/z 1120.09, with 3+ charge status and an observed molecular mass of 3356.30.
Figure 8.
ESI/MS/MS spectrum and structure of a CPO glycopeptide m/z 1168.43, with 3+ charge status and an observed molecular mass of 3502.74.
Because no peaks were observed from m/z 2423 to m/z 2466 in the MALDI/MS spectrum of native CPO tryptic peptides, it is apparent that the Asn328-Cys-Thr site of CPO molecules is fully glycosylated. Although mature CPO contains another potential N-glycosylation site (Asn430-Arg-Thr), there is no evidence of its glycosylation.
N-glycan profiles and sequences
Glycopeptides were screened by precursor ion scanning using LC/ESI/MS/MS and by the presence of diagnostic ions of m/z 163 (Hex) and m/z 204 (HexNAc) (Hex=hexose) in their corresponding MS/MS spectra. In the TIC and reconstructed chromatograms, all glycopeptides with N-linked oligosaccharides were eluted within 16.5–18.8 min under the described elution conditions (Fig. 5 ▶). Figure 6 ▶ shows the profile of these N-glycopeptides. Analysis of the MS/MS spectra of these detected precursor ions clearly indicated that they were glycopeptides. The high intensive peaks of m/z 163.20 (Hex), m/z 204.03 (HexNAc), and m/z 366.04 (HexHexNAc) were recorded in their MS/MS spectra. The absence of the m/z 292 (sialic acid) in these MS/MS spectra indicated that sialic acids were not present in the glycopeptides, which is consistent with the results from monosaccharide determination (Table 1). These spectra also showed characteristics of both peptide and oligosaccharide structures. Significant Y serial ions with peptide glycoside, such as the [M-162]+ and [M- 203]+, were observed in higher mass ranges. These results, combined with monosaccharide analysis and exoglycosidase digestion, indicated that these oligosaccharides had a high mannose-type structure. In some spectra, m/z 147.01 (fucose) was observed, with the coexistence of Y and [Y- 146]+ ions, indicating that fucose is attached to asparagine-linked GlcNAc. Typical MS/MS spectra and their de novo sequences are illustrated in Figure 7 ▶ and Figure 8 ▶. A summary of the putative structures and profile of oligosaccharides is listed in Table 2. These oligosaccharides are highmannose type, including the dominant Man3GlcNAc2 and Man3 (Fuc)GlcNAc2 cores, truncated structure Man2- GlcNAc2 and Man2(Fuc)GlcNAc2, and Man4–8(Fuc)Glc- NAc2.
Figure 5.
TIC of tryptic peptides of CPO (bottom) and reconstructed ion chromatograms of glycopeptides, obtained by reverse phase-capillary LC/ESI/MS/MS (QTOF). All N-glycopeptides are eluted within 16.5–18.8 min.
Figure 6.
ESI/MS spectrum of elutants within 16.5–18.8 min, showing N-glycopeptide (oligosaccharide) profile of CPO.
Table 2.
N-oligosaccharide structures and profile
Exoglycosidase digestion of PNGase A-released oligosaccharides provided additional support for their structures. Due to limitation in the amount of oligosaccharides, only the most abundant ions, m/z 933.64 and m/z 1079.68 (Na+ adductive ions), were detected in the oligosaccharide pool (Fig. 9A ▶). The m/z 1079.68 disappeared in the aliquot of oligosaccharides after digestion with α-fucosidase with concomitant increase in the intensity of m/z 933.64 (Fig. 9B ▶), indicating the release of a fucose residue from the N-glycan core [Man3Fuc GlcNAc2 + Na]. The m/z 933 ion remained, and no new peaks were observed in the aliquot of oligosaccharides digested with both α-fucosidase and β-acetyl hexosaminidase (Fig. 9C ▶). Them/z 933 ion disappeared, and a new peak of m/z 609.48 [ManGlcNAc2 + Na] was detected in the aliquot of oligosaccharide digested with α-fucosidase, β-acetyl hexosaminidase, and α-mannosidase (Fig. 9D ▶). These results confirmed the carbohydrate structures of the glycopeptide m/z 3357.36 and m/z 3503.40 deduced based on their corresponding MS/MS spectra in Figure 7 ▶ and Figure 8 ▶.
Figure 9.
MALDI/MS spectrum of the products of exoglycosidase digests of PNGase A-released oligosaccharides from CPO. All molecular weights represent the average mass of the respective [M + Na]+. (A) Oligosaccharide pools of PNGase A-released glycans; (B) after digestion with Bovine epididymis α-fucosidase; (C) after digestion with both α-fucosidase and Jack bean β acetyl hexosaminidase; and (D) after digestion with α-fucosidase, β-acetyl hexosaminidase, and Jack bean α-mannosidase.
Effect of glycans on CPO activity
Deglycosylation of CPO by PNGase under native conditions and subsequent activity assay of the deglycosylated enzyme in comparison with the activity of the enzyme incubated in the same buffer without PNGase indicated that N-glycans influence CPO activity. Figure 10 ▶ illustrates the substrate saturation curves of the control CPO (10A) and deglycosylated CPO (10B). Based on Lineweaver-Burk Plot, the Km of the deglycosylated CPO to guaiacol (2.6 ± 0.6 mM) was slightly smaller than that of the control sample (3.3 ± 0.8 mM), but its Vmax (0.4 ± 0.04 nmol/min) was much lower than that of the control sample (0.7 ± 0.10 nmol/min).
Figure 10.

Substrate saturation curves for control (A) and PNGase A-deglycosylated CPO (B). The reaction solution (0.5 mL) consisted of 0.2 μg of control or deglycosylated CPO, 1 mM H2O2, and 0.5–10 mM guaiacol in 50 mM sodium phosphate buffer (pH 7.0). Km and Vmax were determined using Lineweaver-Burk Plot. Mean ± s.d., n=3.
Peptide mapping of the control and PNGase A-treated CPO showed that although some glycopeptides were observed in PNGase A-treated CPO, most of its glycopeptide peaks were greatly diminished as compared with those of untreated sample (Fig. 11A,B ▶). Moreover, a new peak of m/z 2466.35 (deglycopeptide) was observed in the treated sample. These results provide the physical evidence for CPO deglycosylation by PNGase A. The profiles of other peptides of CPO and deglycosylated CPO were essentially the same.
Figure 11.
MALDI/MS spectra of tryptic products of control (A) and PNGase A-treated CPO (B). The asterisks and arrow indicate glycopeptide peaks and deglycopeptide peak, respectively. Compared with the tryptic peptides of control CPO (Fig. 10A ▶), most of the glycopeptide peaks were greatly diminished in PNGase-treated CPO sample with the detection of a new peak of m/z 2466.35 (deglycopeptide) at the same time (Fig. 10B ▶).
The elution time of CPO and deglycosylated CPO and their calculated Stokes radius are shown in Figure 12 ▶. The Stokes radius was 25.8 A¢ª , 26.0 A¢ª , and 26.3 A¢ª for CPO without incubation, CPO incubated without PNGase, and deglycosylated CPO, respectively. Although the Stokes radius of the deglycosylated CPO was not greatly different from that of controls, the trend was evident. These data indicate that long-term incubation (50 mM citrate-phosphate buffer, pH 5.0, 24 h at 37°C) tends to relax CPO native structure, and the presence of N-oligosaccharide structures stabilizes its folded state. However, incubation condition or deglycosylation showed no apparent effect on the thermal stability or proteolytic resistance of CPO (data not shown). In addition, the Stokes radius (25.8 A¢ª ) of CPO (MW 68,000) was much smaller than that (30.5 A¢ª ) of ovalbumin (MW 43,000), suggesting that CPO has a tightly packed structure.
Figure 12.
Stokes radius of CPO and PNGase-deglycosylated CPO based on gel filtration chromatography. CPO1, without any treatment; CPO2, incubation without PNGase A; and de CPO, PNGase-deglycosylated CPO. Standards: 1, thyroglobulin; 2, γ-globulin; 3, catalase; 4, bovine albumin; 5, ovalbumin; 6, myoglobin; 7, vitamin B12. LC conditions: Superose FPLC column (300 × 10 mm, Pharmacia), 50 mM phosphate buffer (pH 7.0), containing 0.15 M NaCl, flow rate at 0.4 mL/min, detection at 280 nm. All values are means of four repeats.
Discussion
CPO-mediated chorion protein crosslinking is an important biochemical event in insects (Chapman 1985). Dityrosine or trityrosine crosslinking among chorion proteins is formed during CPO-mediated reactions, leaving the nascent developing embryo protected by a tough, impermeable barrier (chorion). CPO is synthesized by follicle cells and then secreted and assembled with other proteins into an intact chorion. In mosquitoes, CPO-mediated chorion protein crosslinking occurs in the environment, which is an important part of the overall chorion hardening process and which must be achieved in a short period (1–2 h) after oviposition. Therefore, the reaction conditions for CPO are somewhat different than those in vivo and the enzyme has evolved to play an essential role in the mosquito eggs. The environmental pressure and selection may explain why the enzyme has a high specific activity to tyrosine and is highly resistant to denaturing conditions (Han et al. 2000).
Characterization of CPO glycosylation is essential toward a comprehensive understanding of the unique function or behavior of the enzyme during chorion formation and hardening. However, determination of the CPO glycosylation site and oligosaccharide structures has been a great challenge. CPO activity is strictly limited within chorion and can be easily detected in situ in developing or mature eggs (Li et al. 2004), but it is extremely difficult to isolate enough pure enzyme for its structure or carbohydrate moiety characterization. To isolate CPO, ovaries containing mature eggs have to be individually dissected from female mosquitoes under a dissecting scope, which is extremely time consuming. Moreover, the chorion proteins are <10% of the total egg proteins, and CPO is <1% of the total chorion proteins. After protein solubilization, a series of purification steps are necessary to obtain CPO with electrophoretic purity. A total of 40 μg of pure CPO was obtained from 8000 ovaries. Based on initial results, it seemed impractical to completely work out the primary structures of CPO oligosaccharides with the released oligosaccharides by mass spectrometry or enzymatic digestion, due to limitations in the amount of sample and relatively low MALDI signal of sugars. Therefore, glycopeptides were selected as the analytical targets and capillary LC/ESI/MS/MS (Q-TOF) with a nanospray source in combination with exoglycosidase sequencing was used for oligosaccharide structural elucidation.
Monosaccharide composition was determined first. This result was useful to obtain an overall perception in terms of oligosaccharide types and structures. This is particularly true when mass spectrometric techniques are used to elucidate their structures, because hexoses or hexosamines have the same molecular mass as a class. Monosaccharide composition analysis revealed that CPO-associated carbohydrate has high levels (total 93.8%) of D-mannose and N-acetyl D-glucosamine. These results clearly indicate that N-glycosylation is dominant in CPO glycosylation. In addition to N-acetyl D-galactosamine and L-fucose, arabinose that is rare in animals was detected. In Table 1, the relative amount of fucose is lower than that determined in Table 2. It is likely that some fucose was destroyed or absorbed on apparatus during hydrolysis and determination.
There are four potential N-glycosylation sites in the deduced amino acid sequence of CPO: Asn95, Asn189, Asn328, and Asn430 (Li et al. 2004). Our previous study showed that the first 210 amino acid residues in CPO were proteolytically digested from its mature protein, so that Asn328 and Asn430 were the remaining potential N-glycosylation sites in mature CPO. Asn328-Cys-Thr was identified as the sole N-glycosylation motif by comparing the MALDI/MS spectra of tryptic fragments of CPO before and after deglycosylation. It was further confirmed by de novo sequencing of the target peptide (318HGQAIECCTPDCTAPLFGPHR338). The disappearance of a group of peptide ions following deglycosylation and the concomitant formation of a new cluster of peptide ions in comparison with those of native CPO tryptic fragments helped tremendously in subsequent screening of glycopeptide precursor ions during LC/MS/MS. The N-glycopeptides were all from the same peptide backbone, so that the sum of all N-glycopeptides (with different mass units due to heterogeneity of its oligosaccharides) is equivalent to the content of any other tryptic peptides. In addition, the varying derivatization of the three cysteine residues further decreased the relative concentration of the individual glycopeptides, so their signal intensities were low during LC/MS/MS. Consequently, without the comparison of tryptic peptide mapping between the native CPO tryptic fragments and deglycosylated tryptic fragments, these low-intensity peaks corresponding to glycopeptides could have easily been overlooked during LC/MS/MS analysis.
It has been suggested that the protein N-glycosylation pathway in insect cells is similar to that observed in mammalian cells (Varki et al. 1999). In mammalian cells, a dolichol-linked precursor oligosaccharide (Glc3 Man9GlcNac2) is first transferred to a newly synthesized protein. The oligosaccharide is then further processed in the endoplasmic reticulum and Golgi, involving trimming by exoglycosidases and adding new sugar residues by glycosyltransferases. The trimming and extension reactions can yield a complex or hybrid type of oligosaccharides. These enzymes are also sensitive to the environment within the cells, such as cell types and physiological status. Hence glycoproteins usually exist as complex mixtures of glycoforms. Unlike mammalian cells, most insect cells have extremely low levels of glycosyltransferase activities and high active exoglycosidases (β-hexosaminidase), and the processing pathway in insect cells is usually completed with the final structure of Man3GlcNAc2. The results of this study show that, in addition to the dominant Man3 GlcNAc2 and Man3 (Fuc) GlcNAc2, significant amounts of high mannose-type structures (Man4–8GlcNAc2) and truncated structures (Man2GlcNAc2, Man2FucGlcNAc2) are also present. In addition, it is possible that the single fucose residue presenting in core is α1,6-linked fucose (Fabini et al. 2001). Similar high mannose-type structures have been found in membrane preparation of Drosophila melanogaster (Williams et al. 1991). In CPO, there was no Xyl β (1,2)-Man β (1,4), Fuc α(1,3)-Glc Nac β (1,3), or hybrid structure that is predominantly present in some plant peroxidase (Kurosaka et al. 1991; Van Huystee and McManus 1998). Based on monosaccharide analysis, the N-glycosylation site, on average, is occupied by a Man 5–Man 6 structure, but based on the MS/MS spectra of glycopeptides, it is apparent that the average structure is a Man 3–Man 4 glycan, which might be due to the presence of O-linked mannose in CPO.
Removal of carbohydrate from a protein often affects its folding, kinetics, antigenicity, or resistance (Moura et al. 1991; Giraud et al. 1992; Tams and Welinder 1995, 1998; Duarte-Vazquez et al. 2003). It is evident that the presence of N-glycans has a positive impact on CPO activity. PNGase-deglycosylated CPO has a lower Vmax, and slightly larger Stokes radius than those with native CPO, suggesting that N-glycans may influence CPO activity by stabilizing its native structure (including the conformation of its catalytic domain), but its contribution to CPO stability is limited. In addition, CPO is synthesized in follicle cells and transferred to eggshell. These sugar chains may also have a function of localization or assembling during eggshell development. CPO has unusual stability (high resistance to SDS and organic solvent); therefore, there must be other dominant factors contributing to its physical stability. However, CPO is sensitive to reducing agents, such as DTT (Han et al. 2000). Mature CPO has 14 conserved cysteine residues. Its ability to resist denaturation by SDS but proneness to strong reducing agents suggest that formation of multidisulfide bonds might play a crucial role in CPO stability.
In summary, we have identified an N-glycosylation site and elucidated its N-oligosaccharide structures and profile in CPO. Our data suggest that CPO-associated oligosaccharides have a positive impact on the enzyme activity by stabilizing its folded state. They also may play a role in CPO folding or transportation to the chorion layer after its synthesis in follicle cells. Data regarding the glycosylation site and glycan primary structures provide some basis for further study of the biological significance of oligosaccharides in CPO.
Materials and methods
Materials
PNGase A and monosaccharide standards, Dowex AG3X4A (OH−), Dowex AG50X12 (H+), DHB and α-CN, TFA, 2- aminobenzoic acid, sodium cyanoborohydride, o-phenylenediamine, and sodium meta-periodate were purchased from Sigma. DHB and α-CN were recrystallized from ethanol before use. Glucose homopolymer standards, Bovine epididymis α-fucosidase, Jack bean β-acetyl hexosaminidase, and Jack bean α-mannosidase were from ProZyme. Modified trypsin was from Promega. PVDF membrane was from Amersham. ZipTip C18 was from Millipore. Spectrapor dialysis membrane tubing (12,000 and 1000 MW cutoff) was from Spectrum Laboratory. Fresh Mini-Q water was used to prepare all buffers. Other laboratory chemicals were purchased from Sigma or Fisher.
CPO purification and activity assay
Chorion isolation and protein solubilization were based on a previously described method (Li et al. 2004). All operations were done at 0°C–4°C. Six thousand matured ovary pairs were broken by sonication in 1% triton X100, 1 mM PMSF, and 5 mM EDTA-Na2. The chorion sediments were isolated and then homogenized in 100 mM sodium phosphate buffer (pH 7.0), containing 1% CHAPS, 2 M urea, 1 mM PMSF, and 5 mM EDTA-Na2, to released CPO. Solubilized CPO was purified successively on a DEAE-Sepharose column with a linear gradient of 10–500 mM NaCl in 10 mM sodium phosphate buffer (pH 7.0), a phenyl-Sepharose column with a gradient of 0%–10% glycerin in the phosphate buffer, a Superdex gel filtration column with the phosphate buffer containing 0.15 M NaCl, and finally a Mono-Q column with a gradient of 0– 300 mM NaCl in the phosphate buffer, respectively. Purity of the isolated CPO was analyzed by SDS-PAGE.
Protein concentration was determined at 280 nm with a U2001 spectrophotometer (Hitachi). The typical reaction mixture consisted of 5 mM guaiacol, 1 mM hydrogen peroxide, and varying amounts of CPO in 0.5 mL of 50 mM phosphate buffer (pH 7.0) (Bergmeyer 1974). CPO activity was determined spectrophometrically based on absorbance increases at 436 nm (ɛ =25,000) at 25°C.
Monosaccharide analysis
Monosaccharide determination was based on methods described by Weitzhandler et al. (1993) and Anumula (1994, 1995). SDS-PAGE was performed with 12% polyacrylamide gel and 0.1%SDS (Laemmli 1970). CPO was applied to individual wells of the polyacrylamide gel with 2 μg each. After electrophoresis, CPO was transferred to PVDF membrane. For neutral and amino monosaccharide analysis, CPO on PVDF membrane was hydrolyzed in 20% TFA for 6 h at 100°C. The released monosaccharides were derivatized with aminobenzoic acid and sodium cyanoborohydride (Weitzhandler et al. 1993; Anumula 1994). Determination of the derivatized monosaccharides was achieved with a LaChrom D7000 HPLC system with fluorescent detector. HPLC conditions: C18 column, 5 μm particle, 4.6 × 150 mm; fluorescence detection at Ex 360 nm and Em 425 nm; mobile phase A, 0.2% (v/v) 1-butylamine, 0.5% (v/v) phosphoric acid, and 1.0% (v/v) tetrahydrogenfuran in water; mobile phase B, 50% acetonitrile in mobile phase A; gradient profile, 5%B from 0 to 10 min, 5%–12% B from 11 to 35 min, and 12%–100%B from 36 to 40 min, 1 mL/min of flow rate. For sialic acid analysis, the CPO on PVDF was first hydrolyzed with a 0.25 M sodium bisulfate for 20 min at 80°C and then derivatized with o-phenylenediamine for 40 min at 80°C (Anumula 1995). HPLC conditions: C18 column, 5 μm particle, 4.6 × 150 mm; fluorescence detection at Ex 230 nm/Em 425 nm; gradient profile, 10% B from 0 to 20 min and 10%–100% B from 21 to 25 min. Monosaccharide standards and blank were subjected to the same hydrolysis and derivatization processes and analyzed by HPLC under identical conditions.
In-gel digestion with trypsin
CPO was electrophoresed on SDS polyacrylamide gel, stained with Coomassie blue, and destained with 40% methanol containing 7% acetic acid. CPO band was cut from the gel and transferred into a 0.6-mL siliconized microcentrifuge tube (Fisher). After DTT reduction and iodoacetamide alkylation, CPO was digested with trypsin in 50 mM Tris-HCl (pH 8.0) for 16 h at 37°C. Tryptic peptides were extracted from the gel using 50% acetonitrile in water plus sonication. After evaporation in a Speedvac, peptides were redissolved in 0.1% TFA for subsequent analysis using MALDI/TOF/MS or LC/MS/MS.
Enzymatic deglycosylation
In enzymatic deglycosylation, a denaturing protocol was used (Kuster et al. 1997). CPO (6 μg) was first digested with trypsin. The tryptic peptides were incubated with PNGase A (24 μ units) in 5 μL of 50 mM citrate-phosphate buffer (pH 5.0) for 24 h at 37°C. The released oligosaccharides were separated from peptides by withdrawing samples through ZipTip C18. Glycans in aqueous solution were desalted by drop dialysis against water using Spectrapor tubing (1000 MW cut off) and then stored for enzymatic sequencing. Deglycosylated peptides were eluted from ZipTip C18 by 60% acetonitrile in 0.1% TFA and subsequently analyzed using MALDI/TOF/MS or LC/MS/MS.
Oligosaccharide sequencing by exoglycosidase digestion
PNGase A-released oligosaccharides from 10 μg of CPO were dissolved in 8 μL of 20 mM sodium acetate (pH 5.5) and divided into four aliquots. The samples were digested by incubating with 1 μL of each of following exoglycosidase arrays: (1) acetate buffer (control), (2) Bovine epididymis α-fucosidase (1 unit/mL), (3) α-fucosidase and Jack bean β-acetyl hexosaminidase (30 unit/mL), and (4) α-fucosidase, β-acetyl hexosaminidase and Jack bean α-mannosidase (100 unit/mL) (Sutton et al. 1994; Kuster et al. 1997). Each reaction mixture was adjusted to 5 μL with acetate buffer and incubated overnight at 37°C. Products were purified by loading into a microcolumn packed with 5 μL of Dowex AG3 (OH−) (bottom), 5 μL of Dowex AG50 (H+) (center), and 5 μL of C18 (top) and eluted with 100 μL of water. After concentration and drop-dialysis against water, the samples were applied to MALDI/TOF/MS. N-linked oligosaccharide structures were deduced based on mass profiles and shifts in mass spectra before and after digestion with the applied enzyme arrays.
MALDI/TOF/MS for peptide mapping
Mass spectra were acquired on a Voyager-DE STR (Applied Biosystems) with a delayed extraction ion source. Sugar sample was finally dissolved in water and mixed with freshly prepared DHB matrix (10 mg of DHB in 1 mL of 10 mM NaCl-10% ethanol) on a target plate. Oligosaccharides were observed as [M+Na]+ in positive ion mode. Peptides or proteins were dissolved in 0.1% TFA and mixed with α-CN matrix (saturated solution in 50% acetonitrile) or DHB (10 mg/mL in ethanol), respectively, and observed as [M+H]+ in positive ion mode. Putative glycopeptides were evaluated by MALDI/TOF/MS peptide profiles before and after deglycosylation, with the aid of the deduced CPO sequence and Peptide Cutter (http://us.expasy.org/tools/peptidecutter).
Capillary LC/ESI/MS/MS for glycopeptide sequencing
The capillary LC/ESI/MS/MS system consisted of a CapLC XE fitted with a NanoEase 75-μm C18 column, an OPTI-PAK C18 Trap column, and a Q-TOF micro mass spectrometer with a nanospray source (Waters Micromass). Peptide separation was achieved by gradient elution with mobile phase A (5% acetonitrile in 0.1% formic acid) and mobile phase B (90% acetonitrile in 0.1% formic acid). The following gradient profile was applied: 5% B from 0 to 5 min, 5%–40% B from 6 to 40 min, and 40%–90% B from 40 to 65 min. In MS analysis, precursor ion scanning was used to search for glycopeptides. The m/z 204 and m/z 366 in their corresponding MS/MS spectra were used as marker ions. The TIC chromatogram and reconstructed ion chromatograms were used to display the overall and glycopeptide elution profiles, respectively. In MS/MS analysis, glycopeptides were extracted into the collision cell for dissociation, and the structures of the glycopeptides were elucidated based on their MS/MS spectra.
Glycosylation and activity
To determine the effect of N-oligosaccharides on CPO activity, 10 μg of native CPO was deglycosylated by incubation with 10 μunit/μ LPNGase A in 20 μL of 50 mM citrate-phosphate buffer (pH 5.0) for 24 h at 37°C. CPO sample, incubated in the same buffer in the absence of PNGAse A, served as a control. The effect of oligosaccharides on CPO kinetic parameters (Km and Vmax) was determined using Lineweaver-Burke model (Sigma Plot). To verify deglycosylation and possible peptide mapping change, a portion of the treated CPO was applied to SDS-PAGE, digested by trypsin, and analyzed by MALDI/TOF/MS.
The effect of oligosaccharides on CPO physical stability was assessed by possible changes in the hydrodynamic volumes (Stokes radius) of native and deglycosylated CPO (after incubation with PNGase A for 24 h at 37°C) (Light and Higaki 1987; Al-Obeidi and Light 1988). The Stokes radius was determined by gel filtration based on those described in the literature (Light and Higaki 1987; Rogers et al. 1997). For thermal stability, 2 μg of native or deglycosylated CPO was added to 50 μL of 50 mM sodium phosphate buffer (pH 7.0) and incubated at 55°C for designed time periods. Samples were then immediately cooled on ice and their residual activity was assayed. For proteolytic resistance, 2 μg of native or deglycosylated CPO was incubated with 0.05 μg/μL trypsin in 50 mM Tris-HCl buffer (pH 8.0) at 37°C. Aliquots were removed at different time periods and the remaining activity was determined.
Acknowledgments
This work is supported by NIH grants AI 37789 and C06 RR 16515-01.
Abbreviations
CPO, chorion peroxidase
α-CN, α-cyano-4-hydoxycinnamic acid
DTT, dithiothreitol
DHB, 2,5-dihydroxylbenzoic acid
LC/ESI/MS/MS, liquid chromatography/electrospray ionization/tandem mass spectrometry
MALDI/TOF/MS, matrix-assisted laser/desorption/ionization time-of-flight mass spectrometry
PMSF, phenyl methyl sulforyl fluoride
PVDF, polyvinylidene difluoride
SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis
TIC, total ion current
TFA, trifluoroacetic acid
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.051419105.
References
- Al-Obeidi, A.M and Light, A. 1988. Size-exclusion high performance liquid chromatography of native trypsinogen, the denatured protein, and partially refolded molecules. Further evidence that non-native disulfide bonds are dominant in refolding the completely reduced protein. J. Biol. Chem. 263 8642–8645. [PubMed] [Google Scholar]
- Anumula, K.R. 1994. Quantitative determination of monosaccharides in glycoproteins by high performance liquid chromatography with highly sensitive fluorescence detection. Anal. Biochem. 220 275–283. [DOI] [PubMed] [Google Scholar]
- ———. 1995. Rapid quantitative determination of sialic acids in glycoproteins by high-performance liquid chromatography with a sensitive fluorescence detection. Anal. Biochem. 230 24–30. [DOI] [PubMed] [Google Scholar]
- Anumula, K.R. and Du, P. 1999. Characterization of carbohydrates using highly fluorescent 2-aminobenzoic acid tag following gel electrophoresis of glycoproteins. Anal. Biochem. 275 236–242. [DOI] [PubMed] [Google Scholar]
- Bergmeyer, H.U. 1974. Methods of enzymatic analysis, 2nd ed. Academic Press, New York.
- Chapman, R.F. 1985. The insects: Structure and function, 4th ed. Cambridge University Press, Cambridge, UK.
- Duarte-Vazquez, M.A., Garcia-Almendarez, B.E., Rojo-Dominguez, A., Whitaker, J.R., Arroyave-Hernandez, C., and Regalado, C. 2003. Monosaccharide composition and properties of a deglycosylated turnip peroxidase isozyme. Phytochemistry 62 5–11. [DOI] [PubMed] [Google Scholar]
- Fabini, G., Freilinger, A., Altmann, F., and Wilson, I.B.H. 2001. Identification of core α1,3-fucosylated glycans and cloning of the requisite fucosyltransferase cDNA from Drosophila melanogaster: Potential basis of the neural anti-horseradish peroxidase epitope. J. Biol. Chem. 176 28058–28067. [DOI] [PubMed] [Google Scholar]
- Giraud, A., Franc, J.L., Long, Y., and Ruf, J. 1992. Effects of deglycosylation of human thyroperoxidase on its enzymatic activity and immunoreactivity. J. Endocrinol. 132 317–323. [DOI] [PubMed] [Google Scholar]
- Han, Q., Li, G., and Li, J. 2000. Purification and characterization of chorion peroxidase from Aedes aegypti eggs. Arch. Biochem. Biophys. 378 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keramaris, K.E., Margaritis, L.H., Zografou, E.N., and Tsiropoulos, G.J. 1996. Egg laying suppression in Drosophila melanogaster (Diptera: Drosophilidae) and Dacus (Bactrocera) oleae (Diptera: Tephritidae) by phloroglucinol, a peroxidase inhibitor. Bull. Entomol. Res. 86 369–375. [Google Scholar]
- Kurosaka, A., Yano, A., Itoh, N., Kuroda, Y., Nakagawa, T., and Kawasaki, T. 1991. The structure of a neural specific carbohydrate epitope of horseradish peroxidase recognized by anti-horseradish peroxidase antiserum. J. Biol. Chem. 266 4168–4172. [PubMed] [Google Scholar]
- Kuster, B., Wheeler, S.F., Hunter, A.P., Dwek, R.A., and Harvey, D.J. 1997. Sequencing of N-linked oligosaccharides directly from protein gels: In-gel deglycosylation followed by matrix-assisted laser desorption/ionization mass spectrometry and normal-phase high-performance liquid chromatography. Anal. Biochem. 250 82–101. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Li, J., Hodgeman, B.A., and Christensen, B.M. 1996. Involvement of peroxidase in chorion hardening in Aedes aegypti. Insect Biochem. Mol. Biol. 26 309–317. [DOI] [PubMed] [Google Scholar]
- Li, J.S., Kim, S.R., and Li, J. 2004. Molecular characterization of a novel peroxidase involved in Aedes aegypti chorion protein cross-linking. Insect Biochem. Mol. Biol. 34 1195–1203. [DOI] [PubMed] [Google Scholar]
- Light, A. and Higaki, J.N. 1987. Detection of intermediate species in the refolding of bovine trypsinogen. Biochemistry 26 5556–5564. [DOI] [PubMed] [Google Scholar]
- Margaritis, L.H. 1985a. Structure and physiology of the eggshell. In Comprehensive insect physiology, biochemistry and pharmacology (eds. G.A. Kerkut and L.I. Gilbert), Vol. 1, pp.154–230. Pergamon Press, Oxford, UK.
- ———. 1985b. The eggshell of Drosophila melanogaster. 3. Covalent crosslinking of the chorion proteins involves endogenous hydrogen peroxide. Tissue Cell 17 553–559. [DOI] [PubMed] [Google Scholar]
- Mindrinos, M.N., Petri, W. H., Galanopoulos, V.K., Lombard, M.F., and Margaritis, L.H. 1980. Cross linking of the Drosophila melanogaster chorion involves a peroxidase. Rouxs Arch. Dev. Biol. 189 87–196. [DOI] [PubMed] [Google Scholar]
- Moura, E.G., Pazos-Moura, C.C., Yokoyama, N., Dorris, M.L., and Taurog, A. 1991. Enzymatic deglycosylation of porcine thyroid peroxidase: Effects on catalytic activity and immunoreactivity. Acta Endocrinol. (Copenh.) 124 107–114. [DOI] [PubMed] [Google Scholar]
- Petri, W.H., Wyman, A.R., and Kafatos, F.C. 1976. Specific protein synthesis in cellular differentiation: The eggshell proteins of Drosophila melanogaster and their program of synthesis. Dev. Biol. 49 85–99. [DOI] [PubMed] [Google Scholar]
- Rademacher, T.W., Parekh, R.B., and Dwek, R.A. 1988. Glycobiology. Annu. Rev. Biochem. 57 785–838. [DOI] [PubMed] [Google Scholar]
- Rogers, K.S., Rodwell, V.W., and Geiger, P. 1997. Active form of Pseudomonas mevalonii 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochem. Mol. Med. 61 114–120. [DOI] [PubMed] [Google Scholar]
- Seppo, A. and Tiemeyer, M. 2000. Function and structure of Drosophila glycans. Glycobiology 10 751–760. [DOI] [PubMed] [Google Scholar]
- Stephens, E., Sugars, J., Maslen, S.L., Williams, D.H., Packman, L.C., and Ellar, D.J. 2004. The N-linked oligosaccharides of aminopeptidase N from Manduca sexta. Eur. J. Biochem. 271 4241–4258. [DOI] [PubMed] [Google Scholar]
- Sutton, C.W., O’Neill, J.A., and Cottrell, J.S. 1994. Site-specific characterization of glycoprotein carbohydrates by exoglycosidase digestion and laser desorption mass spectrometry. Anal. Biochem. 218 34–36. [DOI] [PubMed] [Google Scholar]
- Tams, J.W. and Welinder, K.G. 1995. Mild chemical deglycosylation of horseradish peroxidase yields a fully active, homogeneous enzyme. Anal. Biochem. 228 48–55. [DOI] [PubMed] [Google Scholar]
- ———. 1998. Glycosylation and thermodynamic versus kinetic stability of horseradish peroxidase. FEBS Lett. 421 234–236. [DOI] [PubMed] [Google Scholar]
- Trougakos, I.P. and Margaritis, L.H. 1998. Immunolocalization of the temporally “early” secreted major structural chorion proteins, Dvs38 and Dvs36, in the eggshell layers and regions of Drosophila virilis. J. Struct. Biol. 23 111–123. [DOI] [PubMed] [Google Scholar]
- Van Huystee, R.B. and McManus, M.T. 1998. Glycans of higher plant peroxidases: Recent observations and future speculations. Glycoconj. J. 15 101–106. [DOI] [PubMed] [Google Scholar]
- Van Huystee, R.B., Sesto, P.A., and O’Donnell, J.P. 1992. Number and size of oligosaccharides linked to peanut peroxidase. Plant Physiol. Biochem. 30 147–152. [Google Scholar]
- Varki, V., Cummings, R., Esko, J., Freeze, H., Hart, G., and Marth, J. 1999. Essentials of glycobiology, pp. 31–39, 285–304. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [PubMed]
- Weitzhandler, M., Kadlecek, D., Avdalovic, N., Forte, J.G., Chow, D., and Townsend, R.R. 1993. Monosaccharide and oligosaccharide analysis of proteins transferred to polyvinylidene fluoride membranes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Biol. Chem. 268 5121–5130. [PubMed] [Google Scholar]
- Williams, P.J., Wormald, M.R., Dwek, R.A., Rademacher, T.W., Parker, G.F., and Roberts, D.R. 1991. Characterization of oligosaccharides from Drosophila melanogaster glycoproteins. Biochim. Biophys. Acta 1075 146–153. [DOI] [PubMed] [Google Scholar]