Abstract
Background
Increased variability in sexually selected ornaments, a key assumption of evolutionary theory, is thought to be maintained through condition-dependence. Condition-dependent handicap models of sexual selection predict that (a) sexually selected traits show amplified variability compared to equivalent non-sexually selected traits, and since males are usually the sexually selected sex, that (b) males are more variable than females, and (c) sexually dimorphic traits more variable than monomorphic ones. So far these predictions have only been tested for metric traits. Surprisingly, they have not been examined for bright coloration, one of the most prominent sexual traits. This omission stems from computational difficulties: different types of colours are quantified on different scales precluding the use of coefficients of variation.
Methodology/Principal Findings
Based on physiological models of avian colour vision we develop an index to quantify the degree of discriminable colour variation as it can be perceived by conspecifics. A comparison of variability in ornamental and non-ornamental colours in six bird species confirmed (a) that those coloured patches that are sexually selected or act as indicators of quality show increased chromatic variability. However, we found no support for (b) that males generally show higher levels of variability than females, or (c) that sexual dichromatism per se is associated with increased variability.
Conclusions/Significance
We show that it is currently possible to realistically estimate variability of animal colours as perceived by them, something difficult to achieve with other traits. Increased variability of known sexually-selected/quality-indicating colours in the studied species, provides support to the predictions borne from sexual selection theory but the lack of increased overall variability in males or dimorphic colours in general indicates that sexual differences might not always be shaped by similar selective forces.
Introduction
It is usually acknowledged that variation in sexually selected traits is greater than that in comparable naturally selected traits [1]. In fact, the presence of high variance in an extravagant phenotypic trait is often interpreted as evidence for it being sexually selected. On the other hand, sexual ornaments are usually subject to strong, directional selection, which should result in depletion of available (genetic) variability [2], [3]. This apparent discrepancy between theoretical expectation and empirical data, termed the paradox of the lek, has pre-occupied evolutionary biologists for decades [2]–[7]. Recently, a solution has been proposed based on the contention that sexually selected traits show higher condition-dependent expression than non-ornamental traits [6]. The evolution of condition-dependent expression of ornamentation may maintain phenotypic and genetic variability in sexually selected traits as condition itself is expected to have high genetic variance. This variability is unlikely to be depleted by directional selection as variability in condition is probably determined by variation in multiple loci dispersed over the whole genome [6].
Empirical studies that revealed considerable variation in ornamental (sexually selected) traits, often exceeding that found in putatively naturally selected traits, have been instrumental in the development of new theoretical models. However, their conclusions are based on a limited set of traits since comparisons have by and large focused on metric traits such as the size of elongated tail feathers in birds [5], [8]–[10], or eye stalk length in flies [11]. Similar studies on other types of sexual traits are largely missing, an important deficit since patterns of variability may differ between different trait types [8]. Particularly ill studied in this regard is variability in coloration [5], [12], although colours constitute currently some of the best examples of sexually selected traits, especially in birds [13].
Apart from the greater effort and more extensive equipment required to derive objective measurements of coloration (reflectance spectra) compared to metric traits, this omission is most likely largely due to computational difficulties to compare variability in coloration. Unlike metric traits, coefficients of variation are unsuited to estimate variability in coloration because colours are often quantified using arbitrary scales and thus their variance does not scale with the mean [12]. Hence, direct comparisons with putatively naturally selected metric traits (such as tarsus length) are flawed. An alternative would be to compare variability between sexually and naturally selected colours [12]. This has rarely been attempted [but see 14], because different colours are usually described on different scales that are also not directly comparable [see review in 15].
Here we quantify the degree of variation in bright and drab colour patches in six common and well-studied European passerine birds by implementing current models of avian colour vision [16]. Specifically we aim to test the main prediction of the condition-dependent handicap models of sexual selection [11], [17] namely that (a) sexually selected/quality-indicator traits should show amplified variability compared to equivalent non-sexually selected traits. In addition we tested the ensuing prediction that (b) males should be more variable than females, given that they are usually the sexually selected sex. Finally, since the degree of sexual dichromatism is often used as a proxy for sexual selection we also tested the prediction that (c) sexually dimorphic traits should be more variable than monomorphic traits.
Materials and Methods
Study species
Individuals of six passerine birds (blackcap [Sylvia atricapilla], European robin [Erithacus rubecula], blue tit [Cyanistes caeruleus], great tit [Parus major], blackbird [Turdus merula] and greenfinch [Carduelis chloris]) were captured in mist nets in the surroundings of Möggingen (47°75′N, 9°07′E), Germany between March and June 2005 (see Table 1 for sample sizes). Our aim was to estimate the amount of variability in coloration, as perceived by the birds, that would be available for mate choice or rival assessment in a given season and population. We chose to work with live birds instead of museum specimens to avoid introducing other sources of variation that may obscure patterns of variability. In addition to the potential that plumage colours may fade with specimen age, of particular concern are biological sources of variation such as non-systematic differences between years, and differences between collection sites. Nevertheless, museum specimens may constitute valuable sources of data to estimate colour variability especially if it can be shown that patterns of variability broadly agree with those found using wild birds, as in the present study. The species sampled were selected because they provide a diverse array of colours (structural, melanin- and carotenoid-based) and because they are common in the study area, allowing us to obtain the sample sizes that are required to estimate trait variability. The time frame of capture was chosen to include the reproductive season when sexual signalling is presumably intense. All target species are mainly socially monogamous, although low levels of polygyny have been recorded. When unambiguous, birds were sexed by external traits (blackcaps, great tits, blackbirds). Robins, blue tits and greenfinches were sexed using molecular markers [18]–[21]. To identify known sexually and non-sexually selected or quality indicator colours we performed a review of the literature on putative signalling functions of any colour in all study species (Text S1). From this review it became clear that, although these species have been intensively studied, it is not always possible to obtain unambiguous evidence suggesting that a particular colour patch is sexually selected (favoured through agonistic interactions between rivals or through mate choice). Thus, we decided to include also plumage patches where colour expression acts as an indicator of quality or shows condition-dependence. These kinds of traits are usually assumed or hypothesized to convey honest information about the quality of their bearers to potential rivals or mates [22], and thus are likely to be sexually selected and show high variability as well.
Table 1. List of species used in the this study indicating sample size, measured colour patches and their human-perceived colours, probable colour production mechanism, probable signaling function as described in the literature and level of chromatic (ΔSsex) and achromatic (ΔLsex) sexual dimorphism.
| Species | Sample Size | Patch | Human perceived colour | Colour prod. mechanism | Probable signaling function | Sex. dimorph. (jnd) | ||
| males | females | ΔSsex | ΔLsex | |||||
| Robin | 16 | 15 | Back | Brown-grey | Melan. | ? | 0.52 | 0.68 |
| Erithacus rubecula | Breast | Rusty-red | Melan. | Ag.-inter.(?) | 1.67 | 0.17 | ||
| Blackbird | 30 | 10 | Head | Black (males), brown (females) | Melan. | ? | 9.21 | 13.57 |
| Turdus merula | Back | Black (males), brown (females) | Melan. | ? | 5.56 | 8.99 | ||
| Breast | Black (males), brown (females) | Melan. | ? | 8.71 | 17.4 | |||
| Bill | Yellow-orange | Carot. | Q-indic., Ag.-inter., M-choice(?) | 9.44 | 6.8 | |||
| Blackcap | 44 | 22 | Head | Black (males), rusty-red (females) | Melan. | ? | 16.9 | 22.08 |
| Sylvia atricapilla | Back | Brown-grey | Melan. | ? | 1.48 | 0.59 | ||
| Breast | Grey | Melan | ? | 2.74 | 0.88 | |||
| Great tit | 27 | 23 | Head | Black | Struct.+Melan. | Q-indic., M-choice | 5.81 | 3.37 |
| Parus major | Back | Green | Carot.+Melan. | ? | 1.59 | 1.39 | ||
| Breast | Yellow | Carot. | Q-indic. | 0.72 | 3.47 | |||
| Cheek | White | Struct | ? | 0.91 | 1.08 | |||
| Blue tit | 20 | 17 | Head | Blue | Struct. | Q-indic., Ag.-inter., M-choice | 5.25 | 2.0 |
| Cyanistes caeruleus | Back | Grey-green | Carot.+Melan. | ? | 2.68 | 1.53 | ||
| Breast | Yellow | Carot. | Q-indic. | 1.72 | 1.53 | |||
| Cheek | White | Struct. | ? | 1.71 | 1.08 | |||
| Greenfinch | 41 | 20 | Head | Grey-green | Carot.+Melan. | ? | 4.49 | 2.68 |
| Carduelis chloris | Back | Grey-green | Carot.+Melan. | ? | 3.47 | 2.43 | ||
| Rump | Green-yellow (males), green (females) | Carot.+Melan. | ? | 2.26 | 1.54 | |||
| Tail | Yellow | Carot. | Q-indic. | 5.27 | 3.46 | |||
| Breast | Green-yellow (males), brown-green (females) | Carot.+Melan | Q-indic. | 8.10 | 1.87 | |||
Colour production mechanisms (melanin-, carotenoid-based, structural colours and combinations thereof) were collated from the literature when known (see Text S1) or determined based on the shape of reflectance spectra following Doucet et al. [61]. Probable signaling function was categorized as: Q-indic. ( = quality indicator, the expression of colour correlates with aspects of individual quality such as condition, health, parental abilities, etc), Ag.-inter ( = agonistic interaction, expression of the colour determines or influences the outcome of aggressive interactions), and M-choice ( = mate choice, male colour expression determines female preferences, measured by traits such as date of egg-laying, paternity, brood sex ratios, differential allocation patterns, etc.). Bibliographic references in support of the probable signaling function of each coloured patch are given in Text S1.
Reflectance spectrometry
Plumage reflectance of different plumage patches (see Table 1) was measured using an Avaspec 2048 spectrometer connected to a deuterium-halogen light source (Avalight-DHS, Avantes, Eerbek, Netherlands) through a bifurcated fibre optics cable fitted at the end with a plastic cylinder to standardise measuring distance and shield out ambient light. The probe was held perpendicular to the surface of the feathers (or bill in the case of the blackbird) hence illumination and recording angles were both 90°. Reflectance was computed relative to a WS-2 white standard using the program Avasoft 6.2.1. We took a set of five reflectance readings of different predefined and standardized spots in each body part (Table 1). Reflectance values between 300 to 700 nm (in 1 nm steps) were imported into custom made spreadsheets for further analysis. Average reflectance spectra for each species, patch and sex are given in Fig. S1.
Visual modelling
Most diurnal birds present six types of photoreceptors in their retinas, four types of single cones, double cones and rods [23]. While rods are used for vision in low light levels, and double cones (composed of two cells in close electrical and physical contact) are thought to mediate achromatic tasks (luminance or brightness perception), colour vision in diurnal birds depends on the four types of single cones, that are sensitive to very short (VS), short (S), medium (M), and long (L) wavelengths respectively [24]. For each reflectance spectrum we computed cone quantum catches (Qi) for each cone type using the formula:
| (1) |
where λ indicates wavelength, Ri(λ) the sensitivity of the cone type, S(λ) the reflectance spectrum, and I(λ) the spectrum of irradiant light [16]. Vision of passerine birds is chiefly differentiated by the sensitivity maxima of the VS cone, with other cone sensitivities being similar. The species included in the present study all belong to the Passerida, which according to comparative molecular analysis of the opsin gene sequence have U-type eyes with peak sensitivity of the VS cone at 367 nm [25], [26], which has been confirmed through microspectrometry for blue tit and blackbird [27]). Therefore we used generalized spectral cones sensitivities of U-type birds [from Appendix 1 in 26].
Relative (each cone quantum catch divided by the sum of all four) cone quantum catches can be plotted (after mathematical transformation according to [28]) in a three-dimensional tetrahedron where each vertex represents the sole stimulation of a different cone type. Thus, measurements of differently coloured patches are represented by clouds of points in the avian visual space (see Fig. S2). In general the smaller the Euclidean distance between two points in this space, the smaller the difference in visual contrast between the corresponding reflectance spectra, and below a certain threshold distance two spectra will no longer be discriminable. These thresholds are determined by receptor noise, which varies with cone type [16], [29]. Using this model we calculated chromatic discriminability (ΔS) between two points in the tetrahedral space following the equation:
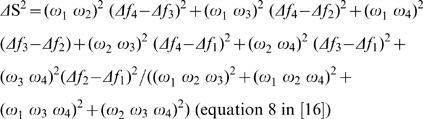 |
(2) |
where
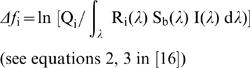 |
(3) |
and Sb(λ) represents the reflectance spectrum of the background (brown bark, see Fig. 1B online appendix), ωi represents receptor noise [16] that was computed using a Weber fraction of 0.05 and cone proportions of 1∶1∶2∶2 (VS:S:M:L; [26]). The Vorobyev-Osorio model we used assumes that colour discriminability depends only on receptor noise and that differences in intensity (i.e. brightness or luminance) are disregarded [16]. This model accurately predicts colour discrimination ability in birds, bees and humans [29] and has been used for example to estimate sexual dichromatism [30] and detectability of birds and fruit to other avian predators and frugivorous birds respectively [31], [32].
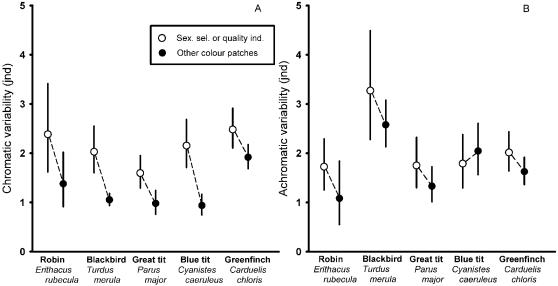
Figure 1. Discriminable chromatic (ΔSvar, A) and achromatic variability (ΔLvar, B) of sexually selected or quality indicator colour patches versus other colour patches in males of five species of European birds.
Depicted are means and 95% confidence intervals (back transformed after Box-Cox transformation prior to analysis).
We also quantified variation in brightness or luminance. Achromatic variation in birds is probably detected by the double cones [33], [34]. We used equations (1) and (3) to compute double cone quantum catches using double cone spectral sensitivity data from Leiothrix lutea provided by Martin Schaefer [35]. The achromatic contrast between two spectra can be computed as:
| (4) |
where ω = 0.05 [36]. For more details on visual modelling see Vorobyev et al. [16] and Siddiqui et al.[36].
The unit for ΔS and ΔL is the jnd (just noticeable difference) and values of >1 can be discriminated by birds, whereas those below this threshold cannot [16]. To estimate the degree of discriminable variation in coloration within each plumage patch for our sample we computed the visual contrast (hereafter ΔSvar or ΔLvar) between each point and a fixed point in space. The chosen point was the joint mean of each cone quantum catch for ΔSvar and the mean double cone quantum catch for ΔLvar, computed separately for each species, sex and plumage patch. Note that this procedure is analogous to a Levene's test for the unequality of variances. Samples with high discriminable variability in coloration should have large mean values of ΔSvar and/or ΔLvar. Thus, this measurement should provide us with a proxy of how much discriminable variability in coloration is available for assessment to potential mates or rivals. The degree of sexual dichromatism for each colour patch was estimated as ΔS or ΔL between the average point of each patch of males and females (as in [30], hereafter ΔSsex or ΔLsex). For a graphical representation of the visual modelling procedure and computation of ΔSvar and ΔSsex see Fig. S3.
The results of the Vorobyev-Osorio model may be influenced by variation in biologically relevant parameters, such as background and type of irradiant light. Neither ΔS nor ΔL values change with background type if we assume that all birds are seen against the same background [data not shown, see also 30]. Variation in irradiant light, on the other hand, may affect ΔS or ΔL, even when all birds are illuminated by the same light [see for example 16]. Thus we repeated all analyses using the following irradiances: forest and woodland shade (measured in the study site, see Fig. S4) and uniform irradiance [as in 30]. Forest shade is typical for the under storey of forests were the light is filtered by green leaves and is rich in intermediate and long wavelengths while woodland shade is found in forest gaps were the direct light from the sun is blocked by the trees, being rich in short wavelengths [37]. These irradiance types thus represent realistic (forest and woodland shade) light environments while their different spectral properties allows us to validate the robustness of the results. Using different irradiances had only small effects on the analyses and the main conclusions of the study are unaffected by the type of illuminant used in the models. Below we present the data using D65 as the sole illuminant but we provide the results for all analyses in Tables S1, S2 and S3.
Statistical analysis
The distribution of ΔSvar and ΔLvar generally did not follow a normal distribution and therefore we Box-Cox [38] transformed the data prior to analysis. To assess differences in ΔSvar and ΔLvar between patches and sexes we used ANOVA including the factors sex, patch and their interaction in the model. If the interaction term was significant we analysed both sexes separately, if not, the interaction term was removed before testing for main effects [39].
Despite the large number of studies on coloration in our target species, published evidence for evolutionary significance of colours is only available for colour patches shown to be sexually selected or indicators of quality (Table 1). In agreement with the general paucity of studies addressing evolutionary significance of drab or cryptic coloration, there appear to be no published studies addressing the signalling function of putatively naturally selected traits in our study species. Therefore, for the purpose of our comparison between sexually and naturally selected colours, we compare ΔSvar and ΔLvar between those patches that have been demonstrated to be important in sexual selection or that are known indicators of individual quality, and those for which no such information is available. This analysis includes only males since there is even less information available for females. See Text S1 for a summary of the evidence for the different colour patches.
To test the hypothesis that the degree of sexual dichromatism in a patch is associated with colour variability we used ordered heterogeneity tests [O-H, 40]. This test is based on the (common) assumption that sexual dichromatism is a valid proxy for the intensity of sexual selection [e.g.41], [42]. The composite statistic rsPc was computed following Rice and Gaines [40], where Pc is the complement of the p value (1-p) obtained for the factor “patch” in the ANOVAs (Table 2). To obtain rsPc, Pc is multiplied by the Spearman's rank correlation coefficient (rs) obtained by correlating ΔSvar or ΔLvar with sexual dichromatism (ΔSsex or ΔLsex respectively) across coloured patches. One-tailed p-values for rsPc were obtained from Fig. 1 in [40]) where k represents the number of different coloured patches measured. O-H tests were performed separately for each species, if the interaction term sex*patch reached significance we computed the tests separatedly for males and females, otherwise the sexes were pooled. O-H tests were not performed for robins as they are redundant given that only two coloured patches were measured.
Table 2. Results of the ANOVAs testing for sex and patch differences in discriminable chromatic (ΔSvar) and achromatic (ΔLvar) variability and corresponding Ordered Heterogeneity tests testing for a positive relationship between levels of variability and sexual dichromatism.
| sex | patch | sex x patch | Ordered heterogeneity tests sexual dichromatism vs. variability | ||
| Robin Erithacus rubecula | ΔSvar | F1,59 = 0.02, p = 0.88 | F1,59 = 11.07, p = 0.0015 | F1,58 = 0.54, p = 0.46 | 1) |
| ΔLvar | F1,59 = 0.39, p = 0.546 | F1,59 = 1.53, p = 0.22 | F1,58 = 0.8, p = 0.389 | 1) | |
| Blackbird Turdus merula | ΔSvar | F1,155 = 2.93, p = 0.088 | F3,155 = 10.08, p<0.0001 | F3,152 = 2.01, p = 0.114 | rsPc = 0.99, k = 4, p<0.001 |
| ΔLvar | F1,155 = 4. 54, p = 0.0347 | F3,155 = 2, p = 0.115 | F3,152 = 0.8, p = 0.524 | rsPc = −0.70, k = 4, p>0.95 | |
| Blackcap Sylvia atricapilla | ΔSvar | F1,194 = 8.78, p = 0.0034 | F2,194 = 3.57, p = 0.026 | F2,192 = 2.81, p = 0.062 | rsPc = 0.48, k = 3, p>0.05 |
| ΔLvar | F1,194 = 2.17, p = 0.142 | F2,194 = 2.69, p = 0.070 | F2,192 = 2.03, p = 0.331 | rsPc = 0.46, k = 3, p>0.1 | |
| Great tit Parus major | ΔSvar | F1,195 = 0.44, p = 0.5 | F3,195 = 24.38, p<0.0001 | F3,192 = 2.56, p = 0.056 | rsPc = −0.39, k = 4, p>0.8 |
| ΔLvar | F1,195 = 1.16, p = 0.281 | F3,195 = 1.22, p = 0.301 | F3,192 = 1.14, p = 0.331 | rsPc = 0, k = 4, p = 0.5 | |
| Blue tit Cyanistes caeruleus | ΔSvar | F1,143 = 0.38, p = 0.53 | F3,143 = 21.38, p<0.0001 | F3,140 = 1.57, p = 0.19 | rsPc = 0.39, k = 4, p>0.1 |
| ΔLvar | F1,143 = 0.22, p = 0.639 | F3,143 = 5.99, p = 0.0007 | F3,140 = 1.68, p = 0.172 | rsPc = 0.19, k = 4, p>0.2 | |
| Greenfinch Carduelis chloris | ΔSvar | --------- | Males: F4,200 = 3.07, p = 0.0173 | F4,295 = 4.75, p = 0.001 | Males: rsPc = 0.59, k = 5, p<0.05 |
| Females: F4,95 = 10.10, p<0.0001 | Females: rsPc = 0.50, k = 5, p>0.05 | ||||
| ΔLvar | --------- | Males: F4,200 = 5.07, p = 0.0007 | F4,295 = 2.85, p = 0.024 | Males: rsPc = 0.099, k = 5, p>0.4 | |
| Females: F4,95 = 4.87, p = 0.0013 | Females: rsPc = 0.49, k = 5, p>0.05 |
Significant terms are depicted in bold.
Ordered heterogeneity tests were not computed for robins as only two patches were measured. In this case chromatic variability (ΔSvar) was higher for the more sexually dichromatic patch (breast) as indicated by Figure 1A, Table 1, and the significant “patch” factor; this was not the case for achromatic variability (ΔLvar) where there was no significant difference in variability between the two patches (Fig. 1G).
Residuals of the final models did not significantly depart from normality except for the ANOVA on ΔLvar for the greenfinch (depicted in Table 2) and the comparison of ΔSvar between sexually selected and putatively non-sexually selected traits in the blackbird. In both cases the Shapiro-Wilk test indicated slight departures from normality (p = 0.048 and 0.044 respectively). Statistical tests were carried out with JMP 5.1.
Results
Chromatic variability: sexually-selected/quality-indicator patches
Variability in coloration was higher for those colour patches for which there is evidence of being sexually selected or indicators of quality when compared with the rest (see Table 1 and Figs. 1 and 2, computed for males only: robin, F1,30 = 4.59, p = 0.0403; blackbird, F1,118 = 31.17, p<0.001; blackcap, no data available; great tit, F1,106 = 9.40, p = 0.0028; blue tit, F1,78 = 27.54, p<0.001; greenfinch, F1,203 = 6.18, p = 0.0137). Patches shown to be sexually selected or quality indicators showed on average 0.87 jnd (range = 0.56 to 1.21 jnd) higher discriminable variability when compared to the rest of the coloured patches in the five studied species (Fig. 1). Results for all illuminants yielded similar results and are presented in Table S1.
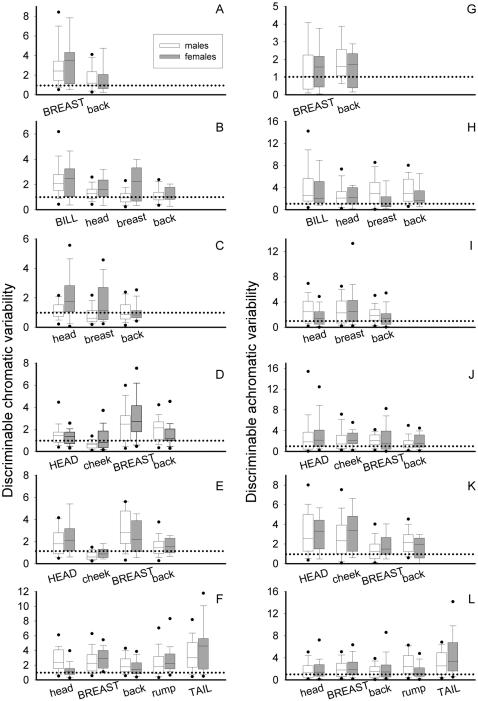
Figure 2. Discriminable chromatic (ΔSvar, left) and achromatic variability (ΔLvar, right) of coloured patches for six species of European birds.
Robin (A, G), blackbird (B, H), blackcap (C, I), great tit, (D, J), blue tit (E, K) and greenfinch (F, L). Depicted are medians, 25th and 75th percentiles (boxes), 10th and 90th percentiles (whiskers) and 5th and 95th percentiles (dots). Coloured patches that have been shown to be sexually selected or indicators of quality are written out in upper case font (see Table 1 for more information). The dotted horizontal line indicates the 1 jnd discriminability threshold.
Chromatic variability: sex differences
In all species there were significant differences in variability between patches (Fig. 2, Table 2) that followed broadly the same pattern in both sexes, with equivalent colour patches being the most variable in males and females (except in the greenfinch, see below). In general there was no evidence that male colours were more variable: colours in males showed similar levels of variability as in females. There was one exception: female colours in the blackcap were more variable than male colours, and there was a similar trend in blackbirds (Table 2). For the greenfinch the sex*patch interaction reached significance. Analysing males and females separately in this species revealed that tail colour showed the highest variability in both sexes but that the order of variability in the other patches was different (Fig. 2). Results for all illuminants yielded similar results and are presented in Table S2.
As the analysis above included all patches, also those with low sexual dichromatism, we repeated the analysis comparing ΔSvar between sexes for the most sexually dichromatic patch in each species (head in blue tits, great tits and blackcaps, bill in blackbirds, tail in greenfinches and breast in robins). Results were unchanged and sex differences were only significant for blackcaps, female (rufous) head colour being more variable than male (black) head colour (Fig. 2; robin[breast]: F1,29 = 0.16, p = 0.689; blackbird[bill]: F1,38 = 0.03, p = 0.862; blackcap[head]: F1,64 = 4.70, p = 0.0339; great tit[head]: F1,48 = 0.28, p = 0.598; blue tit[head]: F1,35 = 0.60, p = 0.441; greenfinch[tail]: F1,59 = 1.29, p = 0.259) . We repeated these tests for each species, sex, patch and illuminant used and the conclusions remained unchanged. These results along with means and 95% confidence intervals are provided in Table S3.
Chromatic variability: sexually dimorphic vs. monomorphic patches
Within-species, coloured patches with higher sexual dichromatism were more variable in the robin, blackbird and male greenfinches but not in blackcaps, great tits, blue tits and female greenfinches, as indicated by the ordered heterogeneity tests (Table 2). Results for all illuminants yielded similar results and are presented in Table S2.
Achromatic variability: sexually-selected/quality-indicator patches
Achromatic variability tended to be higher (average = 0.38 jnd, range = −0.25 to 0.69 jnd) for those coloured patches shown to be sexually selected or indicators of quality compared to the rest in all species except for the blue tit (Table 1 and Figs. 1 and 2) but these differences were not significant (computed for males only; robin: F1,30 = 2.49, p = 0.124; blackbird: F1,118 = 1.7, p = 0.194; blackcap: no data; great tit: F1,106 = 1.94, p = 0.16; blue tit: F1,78 = 0.47, p = 0.492; greenfinch: F1,203 = 2.64, p = 0.105). Results for all illuminants yielded similar results and are presented in Table S1.
Achromatic variability: sex differences
Differences in achromatic variability between patches were much less marked than in ΔSvar (Fig. 2) and only significant for the blue tit and greenfinch (Table 2). In general there were no significant differences in achromatic variability between sexes with the exception of the blackbird, where males seemed more variable than females. The sex*patch interaction was again only significant for the greenfinch. Results for all illuminants yielded similar results and are presented in Table S2.
Comparing variability of males and females for the most sexually dimorphic patch yielded in general similar results as in most cases these differences were not statistically significant, with the exception of the blackcap and blackbird where males were more variable than females (robin[back]: F1,29 = 0.02, p = 0.871; blackbird[bill]: F1,38 = 4, p = 0.052; blackcap[head]: F1,64 = 4.44, p = 0.038; great tit[breast]: F1,48 = 1.01, p = 0.319; blue tit[head]: F1,35 = 0.02, p = 0.884; greenfinch[tail]: F1,59 = 2.85, p = 0.096). We repeated these tests for each species, sex, patch and illuminant used and the conclusions remained unchanged. These results along with means and 95% confidence intervals are provided in Table S3.
Achromatic variability: sexually dimorphic vs. monomorphic patches
Sexually dimorphic patches were not more variable as indicated by the non significant ordered heterogeneity tests (Table 2). Results for all illuminants yielded similar results and are presented in Tables S2.
Discussion
The main findings of the present study can be summarized as follows: we showed that (a) those coloured patches for which there was published information suggesting a sexual signalling or quality-indicator function showed higher levels of variability than the rest (chromatic variability only). Nonetheless, (b) males did not consistently show higher colour variability across species (chromatic and achromatic variability). Finally, (c) the data provided only limited support for the prediction that more sexually dimorphic colour patches are generally more variable than monomorphic colours (chromatic and achromatic variability).
Are sexually-selected/quality-indicator colours more variable?
The ideal test of the predictions of elevated variability in sexually selected colours would be a comparative analysis of variability of all sexually or naturally selected colour patches in a number of species. We developed an index that quantifies variability of different colours on comparable scales, thereby overcoming previous computational difficulties to perform such an analysis. However, information on signaling functions of, or selection pressures on, colour appears only available for patches that look conspicuous to the human eye, which are thus assumed to be important in signalling (Table 1). Similar information on more subtle colours, such as the brown and green colours that are common in many species, is lacking. Therefore we compared variability of those coloured patches known to be sexually selected or indicators of quality with the other measured patches. This comparison demonstrated higher levels of chromatic (but not achromatic) variability in the former, confirming the assumption that sexual selection may be associated with especially variable traits. This result however, should be considered preliminary for two reasons. First, future studies may show that some of the hitherto unstudied coloured patches may also have a function in sexual signaling, and second, we assumed that quality-indicator or condition-dependent colour traits are also sexually selected which may not always be the case. Clearly, more work is needed to be able to confirm that sexually selected colours are more variable than comparable traits, and we hope that the method and results we describe here may stimulate further research in this area.
Interestingly, our results were largely unaffected when using four different types of light environments (see Table S1). This indicates that variability in environmental light conditions, although potentially affecting conspicuousness of birds [e.g. 43], does not greatly affect the degree of discriminable variability between individuals due to colour-constancy, which has been described as the ability of perceiving a given reflectance spectrum as a fixed “colour” under variable illumination [16]. This suggests that a female, for instance, does not gain more or different information by assesing potential mates under different illuminants. The relative insensitivity of chromatic variability to changes in environmental light may be also the reason why chromatic contrast is used for object quality recognition while on the other hand, achromatic contrasts are used for shape recognition and movement detection [33]. This may also explain the lack of consistent differences in achromatic variability between colour patches and between sexually selected and non-sexually selected colours.
Variability and sexual dichromatism
Sexual dichromatism in birds is often thought to be linked to sexual selection intensity [41], [42]. However, although known sexually selected patches showed increased variability, our analysis did not reveal a consistent relationship between variability and sexual dimorphism in coloration. This suggests (based only on patterns of variability) that sexual dichromatism is not always a very precise proxy for sexual selection. Sexual dimorphism in some coloured patches could have arisen due to natural instead of sexual selection (see [44], [45]) or through a combination of both, for instance when habitat differences drive divergence in appearance between the sexes [46]. Additionally, not all sexually dichromatic patches are necessarily quality signals, they may also function to indicate sex, and such signals are likely to be highly optimised and invariant [12]. Alternatively, some naturally selected colours may show genuine high levels of variability. Colours that probably have a camouflage function, for instance the brown-green back plumage in most of the studied species, may show high variability if the background against which they have to blend is highly heterogeneous [12], [47]. Meanwhile, before we can make further progress, we need more information on selection pressures on, and condition-dependence of, dichromatic and monochromatic colours.
Sex differences in variability
We had predicted that males should show higher levels of colour variability than females. The rationale behind this prediction is that sexual signals should show higher levels of condition-dependence (and thus variability) in males than in the corresponding traits in females [17]. Although this prediction is supported by some experimental and correlational studies [11], [48], [49] other researchers have found the opposite pattern (i.e. females being more variable than males, [10], [50]) or no sex difference in variability [9], [51], [52]. Our data seems to mainly add to these last findings since no general increased variability in males was found and, even when including only the most sexually dichromatic patch, males were not generally more variable than females. We suggest that the fact that patterns of variability are broadly similar in males and females might indicate that ornaments may be more often than expected used for mutual mate assessment [53] or be important for status signalling in both sexes, as has been suggested for highly variable morphometric ornaments in females [10]. If these patterns are confirmed in larger datasets it would lend support to the mounting evidence on the importance of female ornamentation [54]. Alternatively, while we show here that females display similar levels of discriminable variability in coloration not all this variability may be equally informative of individual quality. A given amount of variation at the high end of ornament exaggeration (usually males) could provide more information and carry more costs (production costs, detectability to predators) than the same level of variability at the low end (usually females) of ornament exaggeration. This possibility could be assesed by determining the linearity of condition-dependent expression of colours with different levels of exaggeration.
Concluding remarks
While initially riddled with methodological problems [12] studies of colour variability can now be based on physiological models of colour perception. Indeed, currently we can probably quantify better how birds and other animals may perceive variation in coloration than how they perceive size differences (e.g. in tail length), thus providing fresh insights into the longstanding debate on ornament variability. The described method can be used to quantify variability at the intra-individual, intra-specific and inter-specific levels, opening up exciting new research avenues. For example, the highly variable, sexually-selected/quality-indicator colours were often (in 4 out of 5 species) due to the deposition of carotenoids (Table 1). Possibly carotenoid-based colours show intrinsically higher levels of variability, perhaps due to their hypothesized increased condition-dependence [see 55], but also [56]. Likewise, the increased chromatic and the decreased achromatic variability in brown (phaeomelanin- based) plumage of the female blackbird and blackcap compared to the corresponding black (eumelanin-based) plumage in the males could be directly related to the type of pigment used [57]. Whether different mechanisms of colour production have different intrinsic levels of variability, is an intriguing issue that could be pursued further based on a more extensive sampling across bird species and coloured patches. If some traits (for instance carotenoid-based coloration) show systematically higher discriminable variability than others this may explain why they feature more prominently as sexually selected ornaments [58], as only traits with sufficient discriminable variability can effectively be used by rivals or mates for assessment, and this can determine which traits end up being used for signaling [59]. Finally, would patterns of variability differ in species under more intense sexual selection, such as polygynous or lekking species? Intriguingly, some polygynous species have lower levels of variability in tail length than closely-related monogamous species [10], although this result was not confirmed by more comprehensive comparative analyses [50]. Future comparative analyses of colour variability may help to shed light on these issues.
Supporting Information
Differences in chromatic (ΔSvar) and achromatic (ΔLvar) variability between sexually selected or quality indicator colour patches and other colour patches for four different illuminants. Means and 95% confidence intervals have been back-transformed after Box-Cox transformation prior to analysis.
(0.03 MB XLS)
Results of the ANOVAs testing for sex and patch differences in discriminable chromatic (ΔSvar) and achromatic (ΔLvar) variability and Ordered Heterogeneity tests for the four illuminants used.
(0.03 MB XLS)
Indicates level of sexual dimorphism in coloration (ΔSsex and ΔLsex), means and 95% confidence intervals levels of chromatic (ΔSvar) and achromatic variability (ΔLvar) for males and females and associated F-tests for the four used illuminants. Means and 95%CIs have been back-transformed after Box-Cox transformation prior to analysis.
(0.06 MB XLS)
Average reflectance spectra of coloured integumentary patches of six European birds. Open symbols and dashed lines correspond to males and filled symbols and closed lines to females. Vertical error bars represent standard errors.
(6.31 MB TIF)
Graphical representation of coloured integumentary patches of six species of European birds in the avian visual space. In the tetrahedral visual space each vertex represents the theoretical sole stimulation of one cone type (VS: very short, S: short, M: medium, and L: long wavelength sensitive cones). (A) Tetrahedron and all data points plotted to show general scale of the three axes (x, y, z), where higher values of X represent greater stimulation of the L cone and lower stimulation of the M cone, higher Y values represent greater stimulation of the S cone, and higher values of Z greater stimulation of the VS cone. Note that the data points lie in general low along the Z axis due to the use of the D65 illuminant which is relatively poor in UV wavelengths (see Fig. S4). (B) References, open symbols represent males and closed symbols females. (C) Robin. (D) Blackbird. (E) Blackcap. (F) Great tit. (G) Blue tit. (H) Greenfinch.
(5.71 MB TIF)
Graphic representation of the procedures used to compute ΔSvar and ΔSsex. Reflectance spectra of birds (in this example head reflectance of three male and three female blue tits) (A) and background (B) are multiplied by the illuminant (C) and cone sensitivities (D, U-type eyes, from Appendix A in [24]) to obtain light adapted cone quantum catches (E, F) using eqs. 1, 2 in [16]. Cone quantum catches can be plotted (after suitable transformation into x, y, z coordinates, see eqs. A8, A9, A10, A11 in [26]) in the avian visual space, represented here by a tetrahedron (G). Points that lie further apart in this tridimensional space are in general more easily discriminable by the birds, but this depends on receptor noise which differs for the four cone types. To estimate variability for males and females we first computed the discriminability (ΔS) between each point and the sex-specific centroid (i.e. the joint average of the four cone quantum catches, [57], represented here with a square) using eqs. 3, 4, 8 in [16]. Values of ΔS were averaged for males and females separatedly to obtain ΔSvar. Higher values of ΔSvar should thus indicate higher chromatic variability. In this hypothetical example note that males lie further apart in the avian visual space than females and that their ΔSvar is accordingly higher. The chromatic discriminability between male and female centroids provides an estimate of the level of sexual dichromatism (ΔSsex, see [28]).
(5.61 MB TIF)
Irradiance spectra used to compute chromatic and achromatic variability. D65 is the spectrum of standard daylight [16], while green light and woodland shade are irradiance spectra collected in the study area on June and January 2007 respectively. The dotted line represents uniform irradiance as used in some studies [e.g. 28].
(0.51 MB TIF)
Review of evidence of the signaling function of plumage coloration in the six studied species.
(0.13 MB DOC)
Acknowledgments
We are most grateful to Evi Fricke, Juan Masello, Monika Krome, Tanja Vogler, Heidi Schmid, Andreas Schmidt and Uli Querner for help with catching and measuring the birds, to Evi Fricke for managing the library of reflectance spectra and performing the molecular sexing, to Bart Kempenaers, Jim Dale, Martin Schaefer, Tom Tregenza and an anonymous reviewer for providing many insightful comments that improved earlier versions of the manuscript and to Martin Schaefer for input into visual modelling. We thank Wolfgang Fiedler for logistic support at the Vogelwarte Radolfzell.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was funded by the Max Planck Society. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Darwin C. London: John Murray; 1871. The descent of man and selection in relation to sex. [Google Scholar]
- 2.Taylor PD, Williams GC. The Lek Paradox Is Not Resolved. Theor Popul Biol. 1982;22:392–409. [Google Scholar]
- 3.Merilä J, Sheldon BC. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity. 1999;83:103–109. doi: 10.1046/j.1365-2540.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 4.Kirkpatrick M, Ryan MJ. The evolution of mating preferences and the paradox of the lek. Nature. 1991;350:33–38. [Google Scholar]
- 5.Pomiankowski A, Møller AP. A resolution of the lek paradox. Proc R Soc Lond B. 1995;260:21–29. [Google Scholar]
- 6.Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition dependent traits. Proc R Soc Lond B. 1996;263:1415–1421. [Google Scholar]
- 7.Cotton S, Pomiankowski A. Sexual selection: Does condition dependence fail to resolve the ‘lek paradox’? Curr Biol. 2007;17:R335–R337. doi: 10.1016/j.cub.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Alatalo RV, Höglund J, Lundberg A. Patterns of Variation in Tail Ornament Size in Birds. Biol J Linn Soc. 1988;34:363–374. [Google Scholar]
- 9.Fitzpatrick S. Patterns of morphometric variation in birds' tails: length, shape and variability. Biol J Linn Soc. 1997;62:145–162. [Google Scholar]
- 10.Evans MR, Barnard P. Variable Sexual Ornaments In Scarlet-Tufted Malachite Sunbirds (Nectarinia johnstoni) On Mount Kenya. Biol J Linn Soc. 1995;54:371–381. [Google Scholar]
- 11.Cotton S, Fowler K, Pomiankowski A. Condition dependence of sexual ornament size and variation in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Evolution. 2004;58:1038–1046. doi: 10.1111/j.0014-3820.2004.tb00437.x. [DOI] [PubMed] [Google Scholar]
- 12.Dale J. Intraspecific variation in bird coloration. In: Hill GE, McGraw K, editors. Bird Coloration Vol 2 Function and Evolution. Cambridge, Massachusetts: Harvard University Press; 2006. pp. 36–86. [Google Scholar]
- 13.Hill GE, McGraw K, editors. Cambridge, Massachusetts: Harvard University Press; 2006. Bird Coloration. p. 589, 477. [Google Scholar]
- 14.Kemp DJ. Heightened phenotypic variation and age-based fading of ultraviolet butterfly wing coloration. Evol Ecol Res. 2006;8:515–527. [Google Scholar]
- 15.Montgomerie R. Analyzing colors. In: Hill GE, McGraw K, editors. Bird Coloration Vol 1 Mechanisms and Measurements. Massachusetts: Harvard University Press; 2006. pp. 90–147. [Google Scholar]
- 16.Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC. Tetrachromacy, oil droplets and bird plumage colours. J Comp Physiol A. 1998;183:621–633. doi: 10.1007/s003590050286. [DOI] [PubMed] [Google Scholar]
- 17.Cotton S, Fowler K, Pomiankowski A. Do sexual ornaments demonstrate heightened condition-dependent expression as predicted by the handicap hypothesis? Proc R Soc Lond B. 2004;271:771–783. doi: 10.1098/rspb.2004.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffiths R, Double MC, Orr K, Dawson RJG. A DNA test to sex most birds. Mol Ecol. 1998;7:1071–1075. doi: 10.1046/j.1365-294x.1998.00389.x. [DOI] [PubMed] [Google Scholar]
- 19.Ellegren H, Fridolfsson A-K. Male-driven evolution of DNA sequences in birds. Nat Gen. 1997;17:182–184. doi: 10.1038/ng1097-182. [DOI] [PubMed] [Google Scholar]
- 20.Kahn NW, St. John J, Quinn TW. Chromosome-specific intron size differences in the avian CHD gene provide an efficient method for sex identification in birds. Auk. 1998;115:1074–1078. [Google Scholar]
- 21.Quintana F, Somoza G, Uhart M, Cassara C, Gandini P, et al. Sex determination of adult Rock Shags by molecular sexing and morphometric parameters. J Field Ornithol. 2003;74:370–375. [Google Scholar]
- 22.Griffith SC, Pryke SR. Benefits to Females of Assessing Color Displays. In: Hill GE, McGraw K, editors. Bird Coloration Vol 2. Function and Evolution. Cambridge, Massachusetts: Harvard University Press; 2006. pp. 233–279. [Google Scholar]
- 23.Cuthill IC. Color Perception. In: Hill GE, McGraw K, editors. Bird Coloration Vol 1. Mechanisms and Measurements. Cambridge, Massachusetts: Harvard University Press; 2006. pp. 3–40. [Google Scholar]
- 24.Hart NS. The visual ecology of avian photoreceptors. Prog Retin Eye Res. 2001;20:675–703. doi: 10.1016/s1350-9462(01)00009-x. [DOI] [PubMed] [Google Scholar]
- 25.Ödeen A, Håstad O. Complex distribution of avian color vision systems revealed by sequencing the SWS1 opsin from total DNA. Mol Biol Evol. 2003;20:855–861. doi: 10.1093/molbev/msg108. [DOI] [PubMed] [Google Scholar]
- 26.Endler JA, Mielke PW. Comparing entire colour patterns as birds see them. Biol J Linn Soc. 2005;86:405–431. [Google Scholar]
- 27.Hart NS, Partridge JC, Cuthill IC, Bennett ATD. Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J Comp Physiol A. 2000;186:375–387. doi: 10.1007/s003590050437. [DOI] [PubMed] [Google Scholar]
- 28.Kelber A, Vorobyev M, Osorio D. Animal colour vision - behavioural tests and physiological concepts. Biol Rev. 2003;78:81–118. doi: 10.1017/s1464793102005985. [DOI] [PubMed] [Google Scholar]
- 29.Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc Roy Soc Lond B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eaton MD. Human vision fails to distinguish widespread sexual dichromatism among sexually “monochromatic” birds. P Natl Acad Sci USA. 2005;102:10942–10946. doi: 10.1073/pnas.0501891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Håstad O, Victorsson J, Ödeen A. Differences in color vision make passerines less conspicuous in the eyes of their predators. P Natl Acad Sci USA. 2005;102:6391–6394. doi: 10.1073/pnas.0409228102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaefer HM, Levey DJ, Schaefer V, Avery ML. The role of chromatic and achromatic signals for fruit detection by birds. Behav Ecol. 2006;17:784–789. [Google Scholar]
- 33.Osorio D, Miklosi A, Gonda Z. Visual ecology and perception of coloration patterns by domestic chicks. Evol Ecol. 1999;13:673–689. [Google Scholar]
- 34.Campenhausen MV, Kirschfeld K. Spectral sensitivity of the accessory optic system of the pigeon. J Comp Physiol A. 1998;183:1–6. [Google Scholar]
- 35.Maier EJ, Bowmaker JK. Color vision in the passeriform bird, Leiothrix lutea: correlation of visual pigment absorbance and oil droplet transmission with spectral sensitivity. J Comp Physiol A. 1993;172:295–301. [Google Scholar]
- 36.Siddiqi A, Cronin TW, Loew ER, Vorobyev M, Summers K. Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol. 2004;207:2471–2485. doi: 10.1242/jeb.01047. [DOI] [PubMed] [Google Scholar]
- 37.Endler JA. The Color of Light in Forests and Its Implications. Ecol Monogr. 1993;63:1–27. [Google Scholar]
- 38.Sokal RR, Rolf FJ. San Francisco: Freeman; 1981. Biometry. [Google Scholar]
- 39.Grafen A, Hails R. Oxford: Oxford University Press; 2002. Modern Statistics for the Life Sciences. p. 351. [Google Scholar]
- 40.Rice WR, Gaines SD. Extending Nondirectional Heterogeneity Tests to Evaluate Simply Ordered Alternative Hypotheses. P Natl Acad Sci USA. 1994;91:225–226. doi: 10.1073/pnas.91.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn PO, Whittingham LA, Pitcher TE. Mating systems, sperm competition, and the evolution of sexual dimorphism in birds. Evolution. 2001;55:161–175. doi: 10.1111/j.0014-3820.2001.tb01281.x. [DOI] [PubMed] [Google Scholar]
- 42.Owens IPF, Hartley IR. Sexual dimorphism in birds: why are there so many different forms of dimorphism? Proc R Soc Lond B. 1998;265:397–407. [Google Scholar]
- 43.Endler JA, Thery M. Interacting effects of lek placement, display behavior, ambient light, and color patterns in three neotropical forest-dwelling birds. Am Nat. 1996;148:421–452. [Google Scholar]
- 44.Gonzalez-Solis J. Sexual size dimorphism in northern giant petrels: ecological correlates and scaling. Oikos. 2004;105:247–254. [Google Scholar]
- 45.Hunt GR, McLean IG. The ecomorphology of sexual dimorphism in the New-Zealand Rifleman Acanthisitta chloris. Emu. 1993;93:71–78. [Google Scholar]
- 46.Heinsohn R, Legge S, Endler JA. Extreme reversed sexual dichromatism in a bird without sex role reversal. Science. 2005;309:617–619. doi: 10.1126/science.1112774. [DOI] [PubMed] [Google Scholar]
- 47.Merilaita S. Visual background complexity facilitates the evolution of camouflage. Evolution. 2003;57:1248–1254. doi: 10.1111/j.0014-3820.2003.tb00333.x. [DOI] [PubMed] [Google Scholar]
- 48.Møller AP. Sexual selection in the monogamous barn swallow (Hirundo rustica). 1. Determinants of tail ornament size. Evolution. 1991;45:1823–1836. doi: 10.1111/j.1558-5646.1991.tb02690.x. [DOI] [PubMed] [Google Scholar]
- 49.Arden R, Plomin R. Sex differences in variance of intelligence across childhood. Personal Indiv Diff. 2006;41:39–48. [Google Scholar]
- 50.Cuervo JJ, Møller AP. Phenotypic variation and fluctuating asymmetry in sexually dimorphic feather ornaments in relation to sex and mating system. Biol J Linn Soc. 1999;68:505–529. [Google Scholar]
- 51.Komdeur J, Oorebeek M, van Overveld T, Cuthill IC. Mutual ornamentation, age, and reproductive performance in the European starling. Behav Ecol. 2005;16:805–817. [Google Scholar]
- 52.Blanco G, de la Puente J. Multiple elements of the black-billed magpie's tail correlate with variable honest information on quality in different age/sex classes. Anim Behav. 2002;63:217–225. [Google Scholar]
- 53.Kraaijeveld K, Kraaijeveld-Smit FJL, Komdeur J. The evolution of mutual ornamentation. Anim Behav. 2007;74:657–677. [Google Scholar]
- 54.Amundsen T. Why are female birds ornamented? Trends Ecol Evol. 2000;15:149–155. doi: 10.1016/s0169-5347(99)01800-5. [DOI] [PubMed] [Google Scholar]
- 55.Hill GE. Environmental Regulation of Ornamental Coloration. In: Hill GE, McGraw K, editors. Bird Coloration. Vol 1. Mechanisms and Measurements. Cambridge: Harvard University Press; 2006. pp. 507–560. [Google Scholar]
- 56.Griffith SC, Parker TH, Olson VA. Melanin-versus carotenoid-based sexual signals: is the difference really so black and red? Anim Behav. 2006;71:749–763. [Google Scholar]
- 57.Jawor JM, Breitwisch R. Melanin ornaments, honesty, and sexual selection. Auk. 2003;120:249–265. [Google Scholar]
- 58.Gray DA. Carotenoids and sexual dichromatism in North American passerine birds. Am Nat. 1996;148:453–480. [Google Scholar]
- 59.Guilford T, Dawkins MS. Receiver Psychology and the Evolution of Animal Signals. Anim Behav. 1991;42:1–14. [Google Scholar]
- 60.Endler JA, Westcott DA, Madden JR, Robson T. Animal visual systems and the evolution of color patterns: Sensory processing illuminates signal evolution. Evolution. 2005;59:1795–1818. doi: 10.1554/04-669.1. [DOI] [PubMed] [Google Scholar]
- 61.Doucet SM, Mennill DJ, Hill GE. The evolution of signal design in manakin plumage ornaments. Am Nat. 2007;169:S62–S80. doi: 10.1086/510162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differences in chromatic (ΔSvar) and achromatic (ΔLvar) variability between sexually selected or quality indicator colour patches and other colour patches for four different illuminants. Means and 95% confidence intervals have been back-transformed after Box-Cox transformation prior to analysis.
(0.03 MB XLS)
Results of the ANOVAs testing for sex and patch differences in discriminable chromatic (ΔSvar) and achromatic (ΔLvar) variability and Ordered Heterogeneity tests for the four illuminants used.
(0.03 MB XLS)
Indicates level of sexual dimorphism in coloration (ΔSsex and ΔLsex), means and 95% confidence intervals levels of chromatic (ΔSvar) and achromatic variability (ΔLvar) for males and females and associated F-tests for the four used illuminants. Means and 95%CIs have been back-transformed after Box-Cox transformation prior to analysis.
(0.06 MB XLS)
Average reflectance spectra of coloured integumentary patches of six European birds. Open symbols and dashed lines correspond to males and filled symbols and closed lines to females. Vertical error bars represent standard errors.
(6.31 MB TIF)
Graphical representation of coloured integumentary patches of six species of European birds in the avian visual space. In the tetrahedral visual space each vertex represents the theoretical sole stimulation of one cone type (VS: very short, S: short, M: medium, and L: long wavelength sensitive cones). (A) Tetrahedron and all data points plotted to show general scale of the three axes (x, y, z), where higher values of X represent greater stimulation of the L cone and lower stimulation of the M cone, higher Y values represent greater stimulation of the S cone, and higher values of Z greater stimulation of the VS cone. Note that the data points lie in general low along the Z axis due to the use of the D65 illuminant which is relatively poor in UV wavelengths (see Fig. S4). (B) References, open symbols represent males and closed symbols females. (C) Robin. (D) Blackbird. (E) Blackcap. (F) Great tit. (G) Blue tit. (H) Greenfinch.
(5.71 MB TIF)
Graphic representation of the procedures used to compute ΔSvar and ΔSsex. Reflectance spectra of birds (in this example head reflectance of three male and three female blue tits) (A) and background (B) are multiplied by the illuminant (C) and cone sensitivities (D, U-type eyes, from Appendix A in [24]) to obtain light adapted cone quantum catches (E, F) using eqs. 1, 2 in [16]. Cone quantum catches can be plotted (after suitable transformation into x, y, z coordinates, see eqs. A8, A9, A10, A11 in [26]) in the avian visual space, represented here by a tetrahedron (G). Points that lie further apart in this tridimensional space are in general more easily discriminable by the birds, but this depends on receptor noise which differs for the four cone types. To estimate variability for males and females we first computed the discriminability (ΔS) between each point and the sex-specific centroid (i.e. the joint average of the four cone quantum catches, [57], represented here with a square) using eqs. 3, 4, 8 in [16]. Values of ΔS were averaged for males and females separatedly to obtain ΔSvar. Higher values of ΔSvar should thus indicate higher chromatic variability. In this hypothetical example note that males lie further apart in the avian visual space than females and that their ΔSvar is accordingly higher. The chromatic discriminability between male and female centroids provides an estimate of the level of sexual dichromatism (ΔSsex, see [28]).
(5.61 MB TIF)
Irradiance spectra used to compute chromatic and achromatic variability. D65 is the spectrum of standard daylight [16], while green light and woodland shade are irradiance spectra collected in the study area on June and January 2007 respectively. The dotted line represents uniform irradiance as used in some studies [e.g. 28].
(0.51 MB TIF)
Review of evidence of the signaling function of plumage coloration in the six studied species.
(0.13 MB DOC)