Abstract
The small HIV-1 accessory protein Vpr (virus protein R) is a multifunctional protein that is present in the serum and cerebrospinal fluid of AIDS patients. We previously showed that Vpr can form cation-selective ion channels across planar lipid bilayers, introducing the possibility that, if incorporated into the membranes of living cells, Vpr might form ion channels and consequently perturb the maintained ionic gradient. In this study, we demonstrate, by a variety of approaches, that Vpr added extracellularly to intact cells does indeed form ion channels. We use confocal laser scanning microscopy to examine the subcellular localization of fluorescently labeled Vpr. Plasmalemma depolarization and damage are examined using the anionic potential-sensitive dye bis(1,3-dibutylbarbituric acid) trimethine oxonol and propidium iodide (PI), respectively, and the effect of Vpr on whole-cell current is demonstrated directly by using the patch-clamp technique. We show that recombinant purified extracellular Vpr associates with the plasmalemma of hippocampal neurons to cause a large inward cation current and depolarization of the plasmalemma, eventually resulting in cell death. Thus, we demonstrate a physiological action of extracellular Vpr and present its mechanistic basis. These findings may have important implications for neuropathologies in AIDS patients who possess significant amounts of Vpr in the cerebrospinal fluid.
HIV-1 has a more complex genome than other retroviruses that encode at least six accessory gene products that play a crucial role in regulating viral replication. One of these is the 96-amino acid virus protein R (Vpr), which is highly conserved in human and simian immunodeficiency viruses (1). Vpr is present in the virion (2, 3) and has been suggested to play a role during the early events of viral replication. Recently, Vpr, along with the matrix protein MA p17, has been demonstrated to be one of the two proteins that are able to facilitate the transport of the viral preintegration complex into the nucleus (4, 5). Incorporation of the viral genome into the nucleus of host cells is essential for viral replication in nondividing cells. Vpr is able to cause a substantial increase in viral replication in primary macrophages but is not essential for replication in T lymphocytes. Other reported functions of Vpr include roles in transactivation (6), induction of cell cycle arrest (refs. 7–11 but see also ref. 12), cell differentiation (13, 14), and apoptosis (15, 16).
Significantly, in addition to its presence in the virion and the nuclei of infected cells, Vpr has been detected in the serum of HIV-positive individuals (17, 18) and in the cerebrospinal fluid (CSF) of AIDS patients with neurological disorders (17). Antibodies recognizing a full-length synthetic Vpr protein have also been detected in serum from HIV-infected patients (19). Although much research effort has focused on elucidating the role of Vpr present in the virion and in the host cell, few studies have addressed the question of possible roles of extracellular Vpr. Levi et al. (17, 20) showed that extracellular Vpr has a profound effect on the control of latency and virus replication, and the C-terminal portion of Vpr has been found to cause mitochondrial dysfunction and apoptosis in CD4+ lymphocytes when present in the extracellular medium (16).
We have reported (21) that purified recombinant Vpr is able to associate with artificial lipid membranes, to incorporate into lipid membranes in a voltage-dependent fashion, and to form cation-selective channels. We proposed that extracellular Vpr, if able to insert into the plasmalemma of living cells, could perturb ion concentration gradients maintained by cells for normal cell function through ion channel formation. In this study we show that extracellular Vpr is indeed able to associate directly with the plasmalemma of cultured rat hippocampal neurons where it causes a large inward current, depolarization of the plasmalemma, and cell death. We show direct electrophysiological evidence of changes in intact cell plasmalemma permeability after exposure to extracellular Vpr, with the characteristics of the observed large cation inward current being consistent with our previous bilayer results (21). Because Vpr is present in the CSF in AIDS patients, the results have significant implications for some of the neuropathologies observed in AIDS patients.
MATERIALS AND METHODS
Expression and Purification of Vpr.
Recombinant Vpr was expressed and purified as described (21). Briefly, it was expressed as a glutathione S-transferase (GST) fusion protein in Escherichia coli from plasmid p2GEX-Vpr. The extracted Vpr-GST fusion protein was purified by affinity chromatography using glutathione-agarose resin (Sigma), and the Vpr portion was cleaved from the fusion protein using thrombin and then further purified by cation-exchange HPLC. Purified Vpr was identified by SDS/PAGE and Western blot analysis using polyclonal anti-Vpr antibodies raised in rabbits (21). Purified Vpr was stored in 20 mM Tris⋅HCl (pH 7.0) containing the zwitterionic detergent 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS, 0.5%), glycerol (20%), and 500 mM NaCl.
Fluorescent Labeling of Vpr.
Pooled fractions of highly purified Vpr were concentrated in CHAPS buffer (20 mM Tris⋅HCl, pH 7.0/0.25% CHAPS/10% glycerol) by using Centricons (Mr 10,000 cut-off, Amicon). Concentrated Vpr (30 g/ml was labeled with the fluorescent dye 5-[4,6-dichlorotriazinyl]aminofluorescein (DTAF or D16, Molecular Probes) whereby 55 μg of Vpr was incubated with DTAF at 0.4 mg/ml in 40 mM borate buffer (pH 9.5) at room temperature for 90 min. Excess DTAF was removed by dialysis against CHAPS buffer and concentration using a Centricon (Centricon 10,000). The concentration of labeled protein was determined by using the Bradford assay (22) with BSA as control. Fluorescent labeling did not alter the ion-channel-forming properties of Vpr (data not shown) in the bilayer assay (21).
Cell Culture.
Rat hippocampal neurons were prepared from neonatal rats as reported (23). Briefly, after removal and trituration of the hippocampi from neonatal rats, dissociated cells were plated on glass coverslips pretreated with poly-(l-lysine) and grown for 5–15 days in a humidified incubator at 5% CO2/95% air in MEM supplemented with fetal bovine serum (10%), serum extender (0.1%), glucose (6%), penicillin (2%), and streptomycin (2%). Rat HTC cells derived from Morris hepatoma 7288C (Flow Laboratories) were propagated in DMEM supplemented with 10% fetal calf serum as described (24). For experiments, cells were grown for 36 h on glass coverslips. Immediately before use, medium was removed and replaced with 2 ml of phenol red-free DMEM/0.02 M Hepes/10% fetal calf serum. Labeled or unlabeled Vpr was only applied to cells in the extracellular medium.
Localization of Fluorescently Labeled Vpr.
Subcellular localization of labeled Vpr after addition to intact cultured rat hippocampal neurons and HTC cells was visualized with a Bio-Rad MRC-600 confocal laser scanning microscope (CLSM). Hippocampal neurons grown on coverslips were exposed to a 3-μl drop of 55% bath solution (140 mM NaCl/5 mM KCl/3 mM CaCl2/2 mM MgCl2/10 mM glucose/10 mM TES, pH 7.3)/20% H2O/8.3% 1 M NaCl containing 2.9 μM labeled Vpr in CHAPS buffer. The solutions were prepipetted into a chamber created by a 3-mm hole in Scotch 3MM double-sided-sticky tape placed on a microscope slide (see ref. 24). A glass coverslip was then placed on top of the coverslip with the neurons and sealed with nail polish. Slides were incubated at 37°C between scans. In experiments with HTC cells, cells grown on coverslips to 50% confluency were exposed to a 5-μl drop of 54% phenol red-free DMEM/20% H2O/6% 1 M NaCl containing 3.5 μM labeled Vpr in CHAPS buffer. The solutions were prepipetted into a 5-mm hole in double-sided-sticky tape as described for neuronal cells with the exception that the coverslip on which the HTC cells were grown was directly sealed with nail polish (24). In control experiments, fluorescently labeled Vpr was replaced by an equivalent volume of CHAPS buffer. Images obtained with the CLSM were quantitatively analyzed using the MacIntosh nih image 1.49 public domain software using the line plot mode (24). Briefly, mean fluorescence was plotted along a line drawn through a cell. Peak values at the membrane were averaged for three to five cells per time point (two measurements per cell) after which values for identical measurements performed for cells in CHAPS buffer alone were subtracted to give the specific membrane fluorescence. These values were then compared with the mean medium fluorescence, again with autofluorescence subtracted, to allow the level of specific membrane accumulation relative to medium Vpr to be calculated.
Cytotoxicity of Vpr.
The cytotoxic effect of Vpr on cultured rat hippocampal neurons and HTC cells was assessed using the CLSM and the membrane-impermeable nucleic-acid-staining fluorescent dye PI (Molecular Probes). Cells were prepared as described above except that unlabeled Vpr (1 or 2.7 μM, final concentration) was used instead of fluorescently labeled Vpr with PI (400 μg/ml in H2O) instead of H2O. For analysis, cells with intense nuclear staining, indicative of cell death, were counted at various time points and expressed as a percentage of the total number of cells.
Plasmalemma Depolarization.
Effects on the membrane potential across the plasmalemma after addition of purified Vpr were monitored with a CLSM in conjunction with the anionic potential-sensitive dye bis(1,3-dibutylbarbituric acid) trimethine oxonol [DiBa-C4(3); Molecular Probes], which has been previously used to examine transmembrane potential changes in various cell types (25–27). Cultured hippocampal neurons were prepared for CLSM as described above for cytotoxicity experiments with the exception that DiBa-C4(3) was added instead of PI to yield a final concentration of 1 μM, and confocal images recorded at several time points up to 15 min at 37°C. As a negative control, cultured hippocampal neurons were exposed to CHAPS buffer alone; a solution of 200 mM KCl was used as a positive control to depolarize the transmembrane potential to 0 mV.
Whole-Cell Patch-Clamp Recording.
Effects of Vpr on the whole cell currents of cultured rat hippocampal neurons and HTC cells were measured by using the patch-clamp technique in the whole cell configuration. Whole-cell currents represent the integrated channel activity over the whole cell. Cultured cells on coverslips were perfused with bath solution (140 mM NaCl/5 mM KCl/3 mM CaCl2/2 mM MgCl2/10 mM glucose/10 mM TES, pH 7.3) at room temperature (23 ± 2°C). Pipettes made from borosilicate glass (Clark Electromedical Instruments, Pangbourne, U.K.) were fire-polished and filled with pipette solution normally containing 150 mM NaCl, 0.5 mM CaCl2, 2 mM MgCl2, 5 mM EGTA, and 10 mM TES, adjusted to pH 7.3. In some experiments, pipette solutions contained either sodium gluconate (150 mM) or choline chloride (122 mM and 28 mM NaCl). Reversal potentials were determined experimentally by altering the holding potential until currents reversed direction and the potential for zero current was recorded. Cells were routinely voltage-clamped at −60 mV. This voltage was chosen (i) because our previous results using the planar lipid bilayer technique (21) indicated that channel activity caused by Vpr was strongly decreased or abolished at positive potentials on the side of the membrane not exposed to Vpr and (ii) to approximate physiological conditions of Vpr present in serum and CSF as closely as possible by holding neurons at potentials close to their resting membrane potential. Whole-cell currents were recorded before and after the addition of purified Vpr by using an Axopatch 200A (Axon Instruments, Foster City, CA). Purified Vpr (0.6 nM ≅ 7 ng/ml) in bath solution was applied directly onto patched cells through gravity-fed drug-delivery tubing. Whole-cell currents were filtered at 5 or 10 kHz, digitized at 44 kHz (Sony), and stored on videotape. For data analysis, currents were replayed through the same system and digitized using an A to D converter interfaced with an IBM-compatible computer. Inward currents are depicted as downward deflections from the zero current level.
RESULTS
Fluorescently Labeled Vpr Accumulates Rapidly at the Plasmalemma.
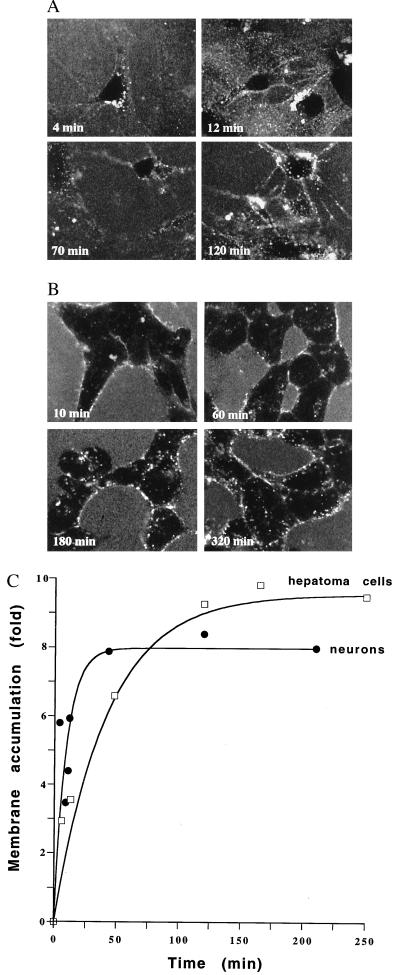
Cultured hippocampal neurons and HTC cells were exposed to fluorescently labeled Vpr, and the subcellular localization was examined at various times with a CLSM. In both cell types, labeled Vpr was predominantly observed at the plasmalemma with no significant amounts of labeled Vpr detected in the cytoplasm or in the nucleus up to 400 min (Fig. 1 and data not shown). Plasmalemmal association of labeled Vpr was observed earlier in neurons than in HTC cells and often appeared inhomogeneous. Quantitative analysis of the fluorescence intensity in the plasmalemma of cells compared with that of the extracellular medium is shown in Fig. 1C, expressed as the membrane accumulation of labeled Vpr relative to the medium. Although the maximal accumulation of (8 ± 0.9)-fold and (9.6 ± 0.8)-fold for neurons and HTC cells, respectively was similar, the rate of accumulation differed significantly, with half-maximal levels of accumulation by neurons and HTC cells, respectively, being achieved 6.5 ± 2.1 min and 29.2 ± 7.8 min after addition of Vpr.
Figure 1.
Visualization of fluorescently labeled Vpr at various times after addition to rat hippocampal neurons (A) and HTC cells (B) using a CLSM. Quantitation of membrane accumulation of fluorescently labeled Vpr compared with medium concentrations over time for hippocampal neurons and HTC cells is shown in C. Each point represents four or five measurements performed with a line plot for each of membrane and medium fluorescence, subsequent to the subtraction of background fluorescence (quantitated in samples lacking fluorescently labeled Vpr) where the SEM for individual measurements was less than 11.6 and 10.9% of the value of the mean for neurons and HTC cells, respectively.
Vpr Causes a Large Cation Current in Neurons.
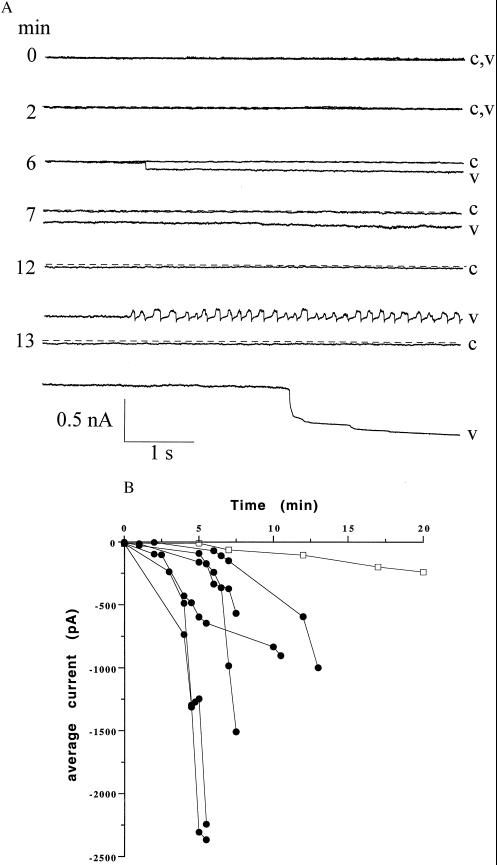
Whole-cell currents of hippocampal neurons voltage-clamped at −60 mV were recorded before and after the extracellular application of Vpr (0.6 nM 7 ng/ml) or CHAPS buffer (control). Current traces at various times from a single cell showing the characteristic response to Vpr are depicted in Fig. 2A. After a delay of 3–8 min, Vpr caused a large inward current (up to −2.4 nA) in all cells (n = 15). The whole-cell inward current either increased gradually or in abrupt jumps (see Fig. 2A, 6 min). In three cells, oscillating currents were observed that appeared to be caused by unclamped action potentials (see Fig. 2A, 12 min). None of the patched cells exposed to Vpr could be held for longer than 15 min, whereas control patched cells exposed to CHAPS buffer were held up to 30 min. Although control cells developed a small inward current over time, no abrupt jumps were observed and the magnitude of inward current was never greater than 15% of the large inward current seen after Vpr exposure. Whole-cell current recordings in HTC cells (n = 5) did not show inward currents of a similar nature up to 30 min after the addition of Vpr (data not shown).
Figure 2.
(A) Typical whole-cell responses from a single cell held at −60 mV after exposure to Vpr for 15 min. The dotted line represents the control whole-cell current before treatment with Vpr. Traces of 6-s recordings in response to CHAPS (c) and Vpr (v) are shown at different times. (B) Whole-cell currents at −60 mV of six individual cells after exposure to Vpr (•) or CHAPS (□) at various times.
The whole-cell currents induced by Vpr in six neurons are shown as current–time graphs in Fig. 2B. The rate of development of the currents and their magnitude were somewhat variable, but in all cases the current was greater than in cells not exposed to Vpr. When the potential was changed, the currents reversed close to the sodium equilibrium potential (about −1 mV with the solutions used). In six cells, the reversal potential was 7 ± 1.6 mV (mean ± SEM). When the pipette solution contained 150 mM sodium gluconate, currents reversed at 1 ± 1.4 mV (n = 8), the lack of effect of the large change in chloride equilibrium potential on the current indicating that there was little if any chloride component. Finally, in eight experiments, 122 mM choline chloride was substituted for 122 mM of the NaCl, and under these conditions, when the sodium equilibrium potential was about +40 mV, the current caused by Vpr reversed at +33 ± 3.2 mV. These results indicate that the inward current caused by Vpr is a sodium current.
Vpr Depolarizes the Plasmalemma of Hippocampal Neurons.
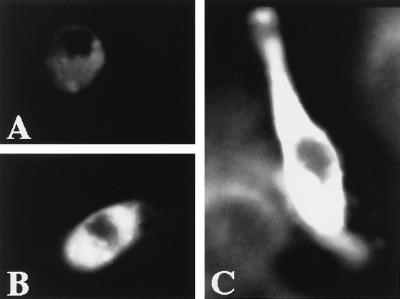
The effect on the transmembrane potential after addition of Vpr to hippocampal neurons was confirmed by using the potential-sensitive dye DiBa-C4(3) in combination with the CLSM, where fluorescence intensity upon binding to the cell membrane at the cytoplasmic side is directly proportional to relative cell membrane depolarization (25, 26). Confocal images of hippocampal neurons exposed to Vpr and DiBa-C4(3) are shown in Fig. 3, the increased fluorescence after exposure to Vpr or high KCl compared with that of CHAPS-treated neurons, indicating a depolarization of the plasma membrane.
Figure 3.
Confocal images of hippocampal neurons in response to the application of CHAPS (A), Vpr (B), and 200 mM KCl (C) with the potential-sensitive dye DiBa-C4(3). Treatments were for 12 min at 37°C.
Vpr Is Cytotoxic to Hippocampal Neurons.
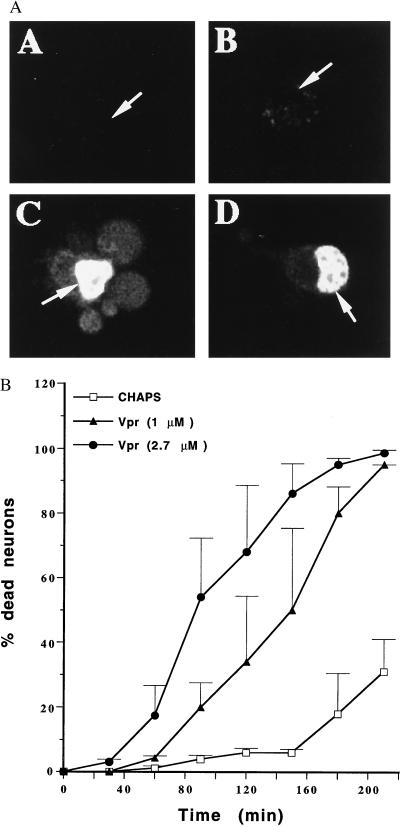
The effects on cell viability after adding Vpr to both hippocampal neurons and HTC cells was compared with that of CHAPS buffer by using the fluorescent dye PI in combination with CLSM. In hippocampal neurons, Vpr resulted in cell death as indicated by bright nuclear staining with PI (Fig. 4A) compared with CHAPS alone (for quantitative data, see Fig. 4B). Dying neurons developed membrane blebs as shown in Fig. 4AC. When Vpr was applied at a higher final concentration (2.7 μM), the rate of cell killing was markedly increased (Fig. 4B). The addition of CHAPS alone did not result in a significantly higher incidence of cell death compared with addition of buffer not containing CHAPS (data not shown). When HTC cells were exposed to Vpr, cell death was only induced at much later times, with the effect indistinguishable from those obtained with CHAPS alone (data not shown).
Figure 4.
(A) Confocal images of hippocampal neurons at various times after exposure to Vpr and the membrane-impermeable nucleic-acid-staining dye PI. Images were taken at 9 (A), 18 (B), 115 (C), and 162 (D) min after exposure to Vpr at 1 (A, B, and D) and 2.7 (C) μM. Arrows indicate the neuronal cell nuclei. (B) Cytotoxic effect of extracellular addition of Vpr expressed as the percentage of dead neurons after treatment with Vpr at the concentrations indicated or CHAPS. Data are averages from nine, five, and four experiments for CHAPS, 2.7 μM Vpr, and 1 μM Vpr, respectively, with SEM indicated by the error bars.
DISCUSSION
This study shows that extracellular Vpr can associate with the plasmalemma of intact cells and cause perturbation of membrane integrity and cell death in hippocampal neurons. This appears to be directly attributable to Vpr’s ability to induce a large inward sodium current, in agreement with predictions based on our planar lipid bilayer experiments (21). Insertion of a cation-selective channel into the plasmalemma would result in a large inward sodium current leading to membrane depolarization and eventually cell death, as observed in this study. The initial membrane depolarization in response to Vpr seen in Fig. 3 presumably is the basis of the action potentials shown in Fig. 2A. These findings are also in agreement with a report (28) demonstrating that the C-terminal portion of Vpr added to the extracellular medium can cause cell membrane permeabilization in yeast cells. Although we cannot exclude the possibility that the inward current observed in our experiments is caused by Vpr modulating other plasmalemmal ion channels, the fact that purified Vpr itself can form cation-selective ion channels in planar lipid bilayers in the complete absence of other proteins (21) supports the idea that the current caused in neurons is flowing through channels formed directly by Vpr itself. That CHAPS is not involved in mediating any of the effects on hippocampal neurons documented in this study is supported by our results using synthetic soluble peptides containing the ion channel functioning domain of Vpr, which have identical effects to full-length Vpr shown herein (unpublished results).
In contrast to a recent report (16) that fluorescein isothiocyanate-labeled peptides containing the C-terminal portion of Vpr added to CD4+ lymphocytes localized in the nucleus, we only observed intracellular localization of full-length Vpr subsequent to cell death in this study. This is consistent with the idea that full-length Vpr only associates with the plasmalemma in intact cells and does not enter cells; in keeping with this, we have been able to show (unpublished data) that labeled Vpr is able to accumulate in the nucleus in a semi-intact cell system where the plasmalemma is perforated (24).
Vpr is present in the CSF of HIV patients with neurological disorders and in the serum of HIV-positive individuals at concentrations of up to 1 ng/ml (17, 18). Importantly, the concentrations of Vpr used in the whole-cell recording experiments are in a comparable range (7 ng/ml), suggesting that Vpr at these concentrations could induce a large inward current leading to depolarization of the plasma membrane. A disruption of the normal resting potential would be particularly detrimental in excitable cells of the central nervous system. In this regard, the observed effect of Vpr on hippocampal neurons in contrast to hepatoma cells may reflect tissue-specific differences in the ability to cope with ion gradient changes and cell membrane depolarization. Our cytotoxicity results also show that although extracellular Vpr can induce cell death in hippocampal neurons when applied at concentrations of 1 μM or higher, it has little effect on hepatoma cells. That Vpr plasmalemmal association occurs faster on hippocampal neurons than on HTC hepatoma cells (see Fig. 1C) may also be indicative of cell-specific effects. Interestingly, HTC cells maintain their resting membrane potential at only −30 to −35 mV (29) compared with −60 to −80 mV in neurons. According to our previous results from bilayer studies (21), this membrane potential would not be sufficient to induce channel activity. This is in agreement with our findings demonstrating the lack of Vpr cytotoxicity for HTC cells despite the observed membrane association. It should be noted that our data for HTC cells (Fig. 1B), although indicating membrane association of Vpr, do not, of course, demonstrate incorporation into the membrane. Differences in resting potential maintained by different cell types may be one of the reasons for cell specificity but other factors such as membrane lipid composition (30) may also be important. Our initial results with C6 glioma cells (unpublished data) support this idea, but definitive conclusions will require the analysis of several other cell types.
The fact that Vpr can incorporate into the plasmalemma of cells to cause large inward sodium currents and plasmalemmal depolarization in vivo implies that extracellular Vpr present in the serum and CSF may play a significant role in AIDS pathology. Although we show herein that addition of full-length Vpr results in an inward current, depolarization of the plasmalemma, and cell death, all of which are probably interdependent, we cannot rule out the possibility that different structural domains of Vpr are responsible for these effects. We are currently testing different domains of Vpr for their cytotoxic and electrophysiological effects. Investigating the cell specificity of the effect of Vpr and understanding the mechanism of its extracellular action may help to develop therapeutic agents that may be useful in treating some of the symptoms in the later stages of AIDS, such as AIDS dementia.
ABBREVIATIONS
- DiBa-C4(3)
bis(1,3-dibutylbarbituric acid) trimethine oxonol
- Vpr
virus protein R
- CSF
cerebrospinal fluid
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- CLSM
confocal laser scanning microscope
- PI
propidium iodide
References
- 1.Tristem M, Marshall C, Karpas A, Hill F. EMBO J. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan X, Matsuda Z, Matsuda M, Essex M, Lee T H. AIDS Res Hum Retroviruses. 1990;6:1265–1271. doi: 10.1089/aid.1990.6.1265. [DOI] [PubMed] [Google Scholar]
- 3.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M A, Gendelman H E, Ratner L, Stevenson M, Emerman M. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen E A, Terwilliger E F, Jalinoos Y, Proulx J, Sodroski J G, Haseltine W A. J Acquired Immune Defic Syndr. 1990;3:11–18. [PubMed] [Google Scholar]
- 7.Jowett J B, Planelles V, Poon B, Shah N P, Chen M L, Chen I S. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Re F, Braaten D, Franke E K, Luban J. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planelles V, Jowett J B, Li Q X, Xie Y, Hahn B, Chen I S. J Virol. 1996;70:2516–2524. doi: 10.1128/jvi.70.4.2516-2524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartz S R, Rogel M E, Emerman M. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emerman M. Curr Biol. 1996;6:1096–1103. doi: 10.1016/s0960-9822(02)00676-0. [DOI] [PubMed] [Google Scholar]
- 13.Levy D N, Fernandes L S, Williams W V, Weiner D B. Cell. 1993;72:541–550. doi: 10.1016/0092-8674(93)90073-y. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Cao J, O’Gorman M R, Yu M, Yogev R. J Virol. 1996;70:5821–5826. doi: 10.1128/jvi.70.9.5821-5826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart S A, Poon B, Jowett J B, Chen I S. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arunagiri C, Macreadie I G, Hewish D, Azad A A. Apoptosis. 1997;2:69–76. doi: 10.1023/a:1026487609215. [DOI] [PubMed] [Google Scholar]
- 17.Levy D N, Refaeli Y, MacGregor R R, Weiner D B. Proc Natl Acad Sci USA. 1994;91:10873–10877. doi: 10.1073/pnas.91.23.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy D N, Refaeli Y, Weiner D B. Curr Top Microbiol Immunol. 1995;193:209–236. doi: 10.1007/978-3-642-78929-8_11. [DOI] [PubMed] [Google Scholar]
- 19.Gras Masse H, Ameisen J C, Boutillon C, Gesquiere J C, Vian S, Neyrinck J L, Drobecq H, Capron A, Tartar A. Int J Pept Protein Res. 1990;36:219–226. doi: 10.1111/j.1399-3011.1990.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 20.Levy D N, Refaeli Y, Weiner D B. J Virol. 1995;69:1243–1252. doi: 10.1128/jvi.69.2.1243-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piller S C, Ewart G D, Premkumar A, Cox G B, Gage P W. Proc Natl Acad Sci USA. 1996;93:111–115. doi: 10.1073/pnas.93.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Premkumar L S, Gage P W, Chung S H. Proc R Soc London B Biol Sci. 1990;242:17–22. doi: 10.1098/rspb.1990.0097. [DOI] [PubMed] [Google Scholar]
- 24.Jans D A, Jans P, Briggs L J, Sutton V, Trapani J A. J Biol Chem. 1996;271:30781–30789. doi: 10.1074/jbc.271.48.30781. [DOI] [PubMed] [Google Scholar]
- 25.Mandler R N, Schaffner A E, Novotny E A, Lange G D, Smith S V, Barker J L. Brain Res. 1990;522:46–54. doi: 10.1016/0006-8993(90)91575-2. [DOI] [PubMed] [Google Scholar]
- 26.Mandler R N, Schaffner A E, Novotny E A, Lange G D, Barker J L. J Neurosci Methods. 1988;22:203–213. doi: 10.1016/0165-0270(88)90041-6. [DOI] [PubMed] [Google Scholar]
- 27.Tanner M K, Wellhausen S R, Klein J B. Cytometry. 1993;14:59–69. doi: 10.1002/cyto.990140111. [DOI] [PubMed] [Google Scholar]
- 28.Macreadie I G, Arunagiri C K, Hewish D R, White J F, Azad A A. Mol Microbiol. 1996;19:1185–1192. doi: 10.1111/j.1365-2958.1996.tb02464.x. [DOI] [PubMed] [Google Scholar]
- 29.Graf J, Henderson R M, Krumpholz B, Boyer J L. J Membr Biol. 1987;95:241–254. doi: 10.1007/BF01869486. [DOI] [PubMed] [Google Scholar]
- 30.Bechinger B. J Membr Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]