Abstract
After female mosquitoes ingest blood from vertebrate hosts, exopeptidases and endopeptidases are required for digesting blood proteins in the midgut into amino acids, which female mosquitoes use to build yolk proteins. These proteases are not always present in the midgut, and their diverse expression patterns suggest that production of these enzymes is highly regulated in order to meet specific physiological demands at various stages. Here we report identification of a serine-type protease, JHA15, in the yellow fever mosquito Aedes aegypti. This protein shares high sequence homology with chymotrypsins, and indeed exhibits specific chymotrypsin enzymatic activity. The JHA15 gene is expressed primarily in the midgut of adult female mosquitoes. Our results indicate that its transcription is activated by juvenile hormone in the newly emerged female adults. Although its mRNA profile is similar to that of the early trypsin gene, we found that JHA15 proteins were readily detected in the midgut epithelium cells of both non-blood-fed and blood-fed mosquitoes. Analysis of polysomal RNA further substantiated that synthesis of JHA15 occurs before and shortly after blood feeding. Knocking down expression of JHA15 resulted in no evident phenotypic changes, implying that functional redundancy exists among those proteolytic enzymes.
Keywords: Mosquito, Juvenile hormone, Serine Protease, Midgut, Blood Feeding
1. Introduction
Both male and female mosquitoes can feed on sugar sources such as plant nectar and honey dew, but female adults of many mosquito species require at least one blood meal to complete each gonotrophic cycle and successfully lay eggs (Clements, 1992; Attardo et al., 2005). The imbibed blood is digested in the mosquito gut, and amino acids, lipids, and other nutrients are used for egg yolk protein biosynthesis and egg development (Raikhel and Dhadialla, 1992). While the insect steroid hormone 20-hydroxyecdysone controls egg maturation after blood feeding, preparation of newly emerged adult female mosquitoes to become competent for egg production is regulated by juvenile hormone (JH)(Hagedorn, 1994; Raikhel et al., 2005).
JH III is the only JH analogue identified from larval and adult Aedes aegypti (Baker et al., 1983). JH is secreted by a pair of endocrine glands behind the brain called the corpora allata. Secretion of JH begins autonomously soon after emergence of the adult. JH levels increase during the first two days and appear to decline gradually in the absence of a blood meal (Shapiro et al., 1986). Blood feeding stimulates an increase in JH esterase activity and inhibition of JH production. JH esterase specifically degrades JH, causing a rapid decrease in hemolymph JH titers (Shapiro et al., 1986).
After eclosion, young female adults embark on a 2−3 days post-emergence development, taking place simultaneously in many tissues (Klowden, 1997). Many aspects of this development are under the control of JH (Hagedorn, 1994). Surgical removal of corpora allata (allatectomy) from newly emerged adult mosquitoes blocks these developmental changes. For example, a batch of synchronously developing follicles separates from the germarium shortly after eclosion. The follicles, called primary follicles, grow to approximately 100 μm in length and by 48−72 h enter a morphologically quiescent “resting stage” in which they remain until the female feeds on blood (Hagedorn et al., 1977). When Ae. aegypti females are allatectomized within one hour of emergence, their primary ovarian follicles show little or no growth and do not develop to the previtellogenic resting stage. Full previtellogenic development in these allatectomized mosquitoes can be rescued by implantation of corpora allata or by topical application of either JH or its analogue, methoprene (Gwadz and Spielman, 1973; Raikhel and Lea, 1991).
After eclosion, the trophocyte cells in the fat body undergo a proliferation of ribosomes (Raikhel and Lea, 1990), and an increase in ploidy (Dittmann et al., 1989). The fat body is then remodeled into an efficient protein factory and becomes responsive to 20E (Flanagan and Hagedorn, 1977; Ma et al., 1988). Female Ae. aegypti mosquitoes that have been allatectomized at emergence fail to synthesize yolk protein precursors in response to 20E injection unless the corpora allata has been implanted (Flanagan and Hagedorn, 1977). At about the same time, JH stimulates the ovary to assemble the necessary machinery for protein uptake, and renders the follicles competent to respond to ovary ecdysteroidogenic hormone after a blood meal (Shapiro and Hagedorn, 1982). In the midgut, aggregation of rough endoplasmic reticulum forms after emergence and disappears after blood feeding. Rossignol et al (1982) demonstrated that the aggregation was blocked if JH secretion was prevented by allatectomy at emergence.
Despite the essential roles of JH in the post-emergence development, the molecular mode of action of JH has remained very much a mystery. The JH response appears to implicate regulation of gene expression. In the midgut, JH stimulates accumulation of the early trypsin (AaET) mRNA after adult emergence (Noriega et al., 1997). Abdominal ligation immediately after adult eclosion, isolating the midgut from the corpora allata (the source of JH), abrogates transcription of AaET. When these ligated abdomens are treated with methoprene, the expression of AaET is restored (Noriega et al., 1997). In a separate experiment, injection of recombinant juvenile hormone esterase (JHE) to the newly-emerged females causes an increase of JHE activity in the hemolymph and dampens the normal post-emergence transcription of AaET (Edgar et al., 2000). It appears that both transcriptional and post-transcriptional control mechanisms are involved in regulation of gene expression by JH. The competence factor βFTZ-F1 is a key transcription factor in the 20E response in adult female mosquitoes (Zhu et al., 2006). Translation of βFTZ-F1 mRNA, not its transcription, is closely correlated to endogenous JH titers and can be stimulated in the fat body of newly emerged mosquito by in vitro JH treatment (Zhu et al., 2003).
Using suppression subtractive hybridization to identify JH target genes, we isolated a chymotrypsin-like protease gene (JHA15) in the Aedes aegypti mosquito. Transcription of JHA15 is considerably activated after eclosion by JH in the midgut of adult female mosquitoes, and the transcriptional profile of JHA15 is similar to that of AaET. While the early trypsin protein is produced only after a blood meal, the JHA15 protein is detected in the midgut even before blood feeding, revealing the diverse gene expression patterns in the post-emergence development.
2. Materials and methods
2.1. Mosquitoes
The Aedes aegypti mosquito strain UGAL/Rockefeller was reared at 27°C and 80% relative humidity with a photoperiod cycle of 16 h light/8 h dark (Hays and Raikhel, 1990). Larvae were fed a standard diet (Lea, 1964), and adults were fed on a 10% sucrose solution continuously by wick. Abdominal ligations were performed as described by Hagedorn et al (1977) to remove the influence of juvenile hormone from the corpora allata. Juvenile hormone III and methoprene (Sigma) was dissolved in acetone and topically applied to the abdomen of newly emerged mosquito (Noriega et al., 1997).
2.2. Suppression subtractive hybridization
Abdomen ligations were performed on female mosquitoes within 30 minutes of adult emergence. Abdomens were then topically applied with either 200 ng of JH III (in 0.5 μl of acetone) or acetone only, and incubated in a humidified chamber. After 6 hours, the abdomens were collected and mRNA was extracted using the Oligotex mRNA mini kit (Qiagen). Starting with mRNA of 100 ng each from the JH-treated and acetone-treated samples, we carried out cDNA synthesis using the SMART PCR synthesis kit (Clontech). Suppression subtractive hybridization was then performed using the PCR-Select cDNA subtraction kit (Clontech) to create forward- and reverse-subtracted DNA pools. PCR products generated by the forward subtraction (cDNA enhanced by the JH treatment) were cloned into the T/A cloning vector pCR2.1 (Invitrogen). Transformation of E. coli strain TOP10F′ (Invitrogen) formed a subtracted cDNA library of about 2,000 colonies on agar plates (LB + Ampicillin + Xgal), 70% of which were white.
Differential screening of the above mentioned cDNA library was performed using the PCR-Select Differential Screening kit (Clontech) according to the manufacturer's instructions. 96 clones of the forward-subtracted library were randomly picked for PCR colony screening with primers flanking the TA cloning site (T7 and M13R). The 96 PCR amplified inserts were dot blotted onto two replicate nylon membranes. Each filter was then incubated with either forward-subtracted or reverse-subtracted cDNA probes, and visualized by autoradiography.
2.3. RNAi, reverse transcription and real-time PCR
Synthesis of double-stranded RNAs (dsRNAs) and microinjection were performed as described previously (Zhu et al., 2003). AaET and luciferase dsRNAs were produced using procedures described by Lu et al. (2006). The JHA15 template for synthesis of dsRNA was generated by a PCR reaction using primers JHA15 DSF (5'-TACGTAATACGACTCACTATAGGGGTTCTCATCGTTACATCCAAAGTTC-3') and JHA15 DSR (5'-TACGTAATACGACTCACTATAGGGGGCTTTTGAAATCACTATCTCTCA-3'). RNA extraction, reverse transcription, and semi-quantitative PCR analyses were carried out as previously described (Chen et al., 2004). PCR primers for RT-PCR were listed as follows: JHA15 forward primer (5'-TTATCGCCGAAAAGTGGATTCTAA-3'); JHA15 reverse primer (5'-CACCCGGAATGAATTATTTGGTTA-3'); Early trypsin forward primer (5'-AATACAGATCCTGCGGCCTA-3'); Early trypsin reverse primer (5'-CCATTATACTGCGGGTGAGG-3'); rpS7 forward primer (5'-GAGATCGAGTTCAACAGCAAGAAG-3'); rpS7 reverse primer (5'-TCTTGTACACTGACGTGAAGGTGTC-3'). Conditions of the PCR amplification are as follows: 94°C (2 min), then 28 cycles at 94°C (30 s)/56°C (30 s)/72°C (30 s), and a final extension at 72°C for 10 min.
Real-time PCR was performed using the iCycler iQ system (Bio-Rad), as previously reported (Zhu et al., 2003). Reactions were performed in 96-well plates with a QuantiTect SYBR PCR kit (Qiagen). JHA15 forward primer (5'-TGCAATTGGTTCTTGTTTCG-3'); JHA15 reverse primer (5'-CCGCACTTGAACTCATCCTT-3'); Early trypsin forward primer (5'-AATACAGATCCTGCGGCCTA-3'); Early trypsin reverse primer (5'-CCATTATACTGCGGGTGAGG-3'); Late trypsin forward primer (5'-GTTGAACTCTCTCAGGGACGTG-3'); Late trypsin reverse primer (5'-GGCCGTCATTAGTTGTATCCTC-3'); rpS7 forward primer (5'-CCCGGAGCCCTACCTATAAACTAT-3'); rpS7 reverse primer (5'-GCAGCACAAAGATGATTTATGCAC-3'). Real-time PCR was performed in triplicate and normalized to the rpS7 mRNA for each sample.
2.4. In situ hybridization and immunostaining
Dissected midguts were fixed for 1 hour at 4 C with 4% formaldehyde in phosphate-buffered saline (PBS) and dehydrated by successive washes in 20%, 40%, 60%, 80% and 100% methanol and kept in ethanol overnight at −20°C. The samples were re-hydrated in PBS by successive washes in 80%, 60%, 40%, 20% ethanol. Permeabilization was carried out by incubating the samples with proteinase K (50 μg/ml) for 10 min followed by fixation for 30 min in PBS containing 4% formaldehyde. Sense and antisense probes were generated with the PCR DIG Probe Synthesis Kit (Roche Applied Science). Hybridization and detection were carried out according to the Nonradioactive In Situ Hybridization Application Manual (Roche Applied Science).
The entire coding region of JHA15 was cloned into the bacterial expression vector pRSET-A (Invitrogen). The His-tagged fusion protein was expressed in the E. coli strain BL21(DE3)pLysS after IPTG induction, and purified using ProBond nickel-chelating resin (Invitrogen). The protein sample was then sent to Cocalico Biologicals, Inc (Reamstown, PA) to raise antibodies in rats. Antisera were partially purified by affinity chromatography using the ImmunoPure IgG purification kit (Pierce, Rockford, IL). The immunostaining procedure was performed as described previously (Zhu et al., 2006).
2.5. Enzyme activity assay
Chymotrypsin activity of the His-tagged recombinant JHA15 protein was assayed in both putative zymogen and activated forms as describe by Shen et al.(2000). Synthetic substrate N-succinyl-Ala-Ala-Pro-Phe-p-nitroanilide (AAPF) (Sigma) was used for the chromogenic reactions. For enzyme activation, 50 nanograms of recombinant JHA15 proteins were treated with 5 ng trypsin (Sigma) for 10 minutes at room temperature. The activated recombinant JHA15 was then incubated for 30 minutes at 25°C with 100 μl of 5 μM substrate in 10 mM Tris HCl (pH 8.0). The amount of the hydrolytic product, nitroaniline, was determined by the change of absorbance at 410 nm using a Beckman spectrophotometer (Beckman, Fullerton, CA). When the chymotrypsin inhibitor chymostatin was used, residual activity was determined after a 15-min preincubation of the enzyme with 25 μM of the inhibitor.
2.6. Polysomal RNA preparation
50 each non-blood-fed female mosquitoes (4−5 days after emergence) and blood-fed mosquitoes (1 h after a blood meal) were flash frozen in liquid nitrogen immediately after harvesting and stored at −80°C prior to RNA extraction. Frozen whole-body samples were ground using a mortar and pestle, homogenized in 1 ml lysis buffer [140 mM KCl, 1.5 mM MgCl2, 20mM Tris-HCl, pH 8, 1% Triton X-100, 1% sodium deoxycholate, 0.2 U/ml RNase inhibitor, 1 mg/ml heparin and 1 mM dithiothreitol], and transferred to an Eppendorf tube. After 10 min incubation on ice with occasional vortexing, the extracts were centrifuged for 10 min at 12,000 g to remove cell debris and nuclei. The supernatant was loaded directly onto a 20−60% linear sucrose gradient containing 20 mM Tris-HCl (pH 8), 140 mM KCl, 1.5 mM MgCl2 and 0.1 mg/ml cycloheximide, and centrifuged at 120,000 g for 180 min at 4°C in an SW50.1 rotor. Fractions were collected from the top of each gradient with continuous monitoring of the absorbance at 254 nm and treated directly with proteinase K. After phenol-chloroform extraction and isopropanol precipitation, RNA was re-suspended in water and then re-purified with RNeasy kit (Qiagen). RT-PCR was performed on equivalent volumes of total RNA extracted from each fraction.
3. Results
3.1. Cloning of JHA15, a serine protease gene in female mosquitoes
In order to identify mosquito genes that are governed by JH III during post-emergence development, we exploited the suppression subtractive hybridization technique, which offers enrichment of rare differentially expressed sequences. Abdomen ligations were performed on the newly emerged female mosquitoes, before the endogenous JH titers began to elevate significantly. Those mosquitoes were then treated with topical application of JH III or acetone (solvent control). Using RNA extracted from those samples, we generated a subtracted cDNA library enriched for Aedes transcripts that are up-regulated by JH III in the newly-emerged female mosquitoes.
Screening of 96 randomly selected colonies from this library resulted in two cDNA clones whose transcriptions were significantly up-regulated by the JH treatment (Figure 1). One clone corresponded to the early trypsin gene (AaET), a well characterized JH target gene in the midgut (Noriega et al., 1996; Noriega et al., 1997). The other one, JHA15, putatively encodes a serine-type endopeptidase. Full-length coding region sequence of the JHA15 gene was obtained after rapid amplification of cDNA ends (RACE), and the entire sequence has been deposited in the GenBank database (Accession No. AY957559). This gene is identical to AAEL001703 in the recently published Aedes aegypti genome annotation, and is located on the supercontig1.39 (2,531,926−2,532,952).
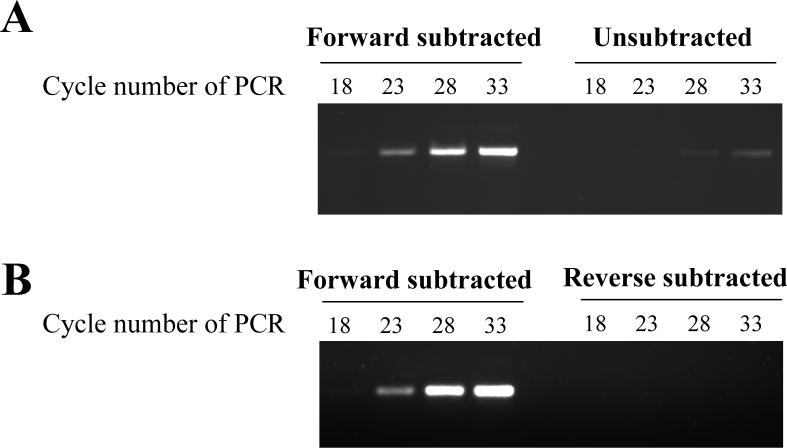
Figure 1.
Enrichment of JHA15 cDNAs by suppression subtractive hybridization. For forward subtraction, tester cDNA was prepared from mosquito abdomens treated with JH III and driver cDNA was from mosquito abdomens treated with acetone. PCR-Select cDNA subtraction was carried out as described in the Materials and Methods. PCR amplification of JHA15 cDNA was performed on subtracted and unsubtracted (JH III-stimulated) cDNA (A), and forward-subtracted and reverse-subtracted cDNA (B).
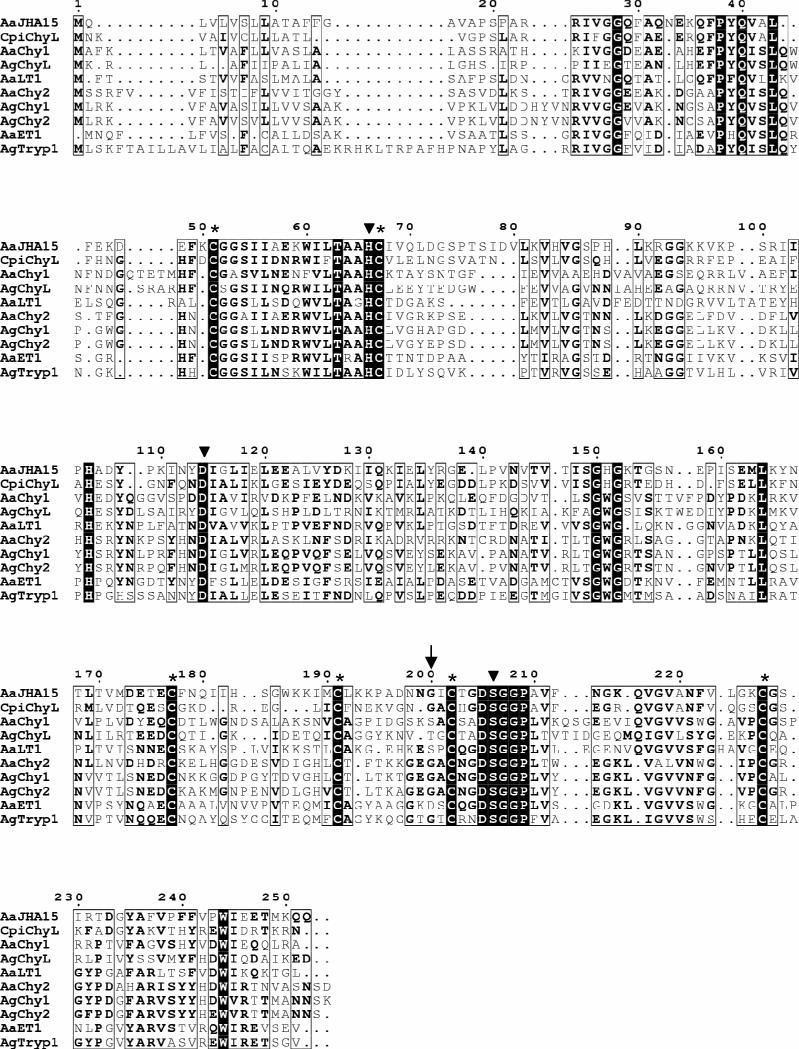
The JHA15 cDNA, 946 bp in length, encodes a putative 252-aa polypeptide. Sequence analysis predicts a 19-aa signal peptide with a cleavage site on the carboxyl side of Pro19 and a proprotein cleavage site on the carboxyl side of Arg24, leaving an active protein of 228 aa (Figure 2). The deduced amino acid sequence of the mature enzyme shares 68.9% and 64.5% amino acid similarity with Ae. aegypti early trypsin (GenBank access number 2211307A) and chymotrypsin (AAB01218), respectively. The catalytic triad of serine proteases His66(H), Asp114(D), and Ser206(S) is conserved in this putative protein. Protein structure analysis predicts a hydrophobic substrate binding pocket, characteristic of chymotrypsins. Three conserved cysteine bridges, common to all known invertebrate chymotrypsinogens (Jiang et al., 1997), are also found in analogous positions in this chymotrypsin-like protease.
Figure 2.
Multiple sequence alignment of the deduced Aedes aegypti JHA15 with other insect serine proteases. The sequences listed were retrieved from GenBank and the alignment was performed using MultAlin (http://bioinfo.genopoletoulouse.prd.fr/multalin/multalin.html). Solid triangles (▼) mark the active site residues of the catalytic triad (His/Asp/Ser), and the Gly residue characteristic of chymotrypsin-like serine proteases is indicated by an arrow (↓). Six conserved cysteines corresponding to the sites of the predicted disulfide bridges are denoted by asterisks (*). AaJHA15, Ae. aegypti chymotrypsin-like serine protease, AAX56968; CpiChyL, Culex pipiens chymotrypsin-like serine protease, AY958427; AaChy1, Ae. aegypti chymotrypsin 1, AAB01218; AgChyL, Anopheles gambiae chymotrypsin-like serine protease, AAC02700; AaLT1, Ae. aegypti late trypsin, AAA29356; AaChy2, Ae. aegypti chymotrypsin II-like protein, AAF43707; AgChy1, An. gambiae chymotrypsin-like protease ANCHYM1, CAA83568; AgChy2, An. gambiae chymotrypsin-like protease ANCHYM2, CAA83567; AaET1, Ae. aegypti early trypsin, 2211307A; AgTryp1, An. gambiae trypsin 1, CAA80513.
3.2. Chymotrypsin enzymatic activity of recombinant JHA15
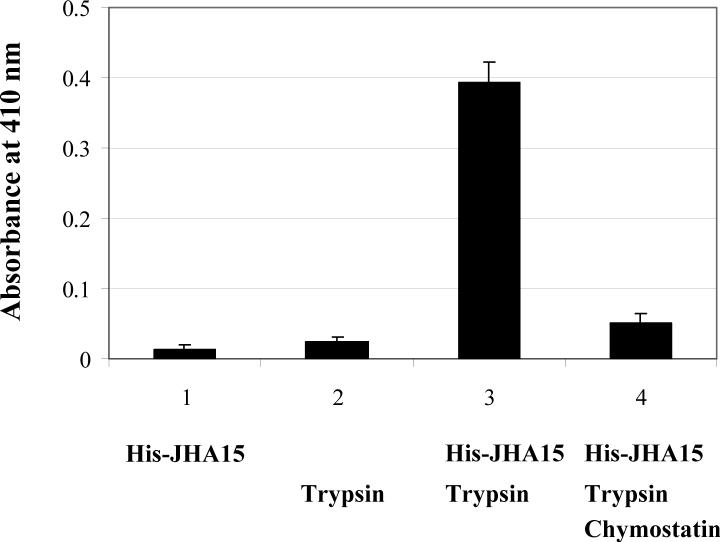
To verify its endopeptidase activity, the recombinant His-tagged JHA15 protein was overexpressed in E. coli and purified by Ni-NTA affinity chromatography. Consistent with our expectations for a zymogen, the full length fusion protein did not cleave a synthetic chymotrypsin substrate AAPF (N-succinyl-Ala-Ala-Pro-Phe-nitroanilide) (Figure 3). After activation by trypsin treatment, the mature JHA15 clearly demonstrated its chymotrypsin-like activity. As a control, trypsin by itself did not hydrolyze the substrate. The catalytic properties of JHA15 were substantially inhibited by chymostatin, a specific inhibitor of α-, β-, γ- and δ-chymotrypsin.
Figure 3.
Activity assay of recombinant AaJHA15. Purified His-JHA15 fusion protein was tested for chymotrypsin activity using synthetic substrate AAPF (N-succinyl-Ala-Ala-Pro-Phe-nitroanilide). The experiment was performed as described in Materials and Methods. JHA15 was activated by trypsin treatment, and the enzyme activity is shown as the increase in the absorption at 410 nm. A blank control was used to correct the spontaneous hydrolysis of the substrate. Chymostatin is a strong inhibitor of many proteinases, including chymotrypsin, chymotrypsin-like serine proteinases, chymases, and lysosomal cysteine proteinases. Values are means ± SEM.
3.3. Juvenile hormone-regulated JHA15 transcription in the midgut of newly-emerged female mosquitoes
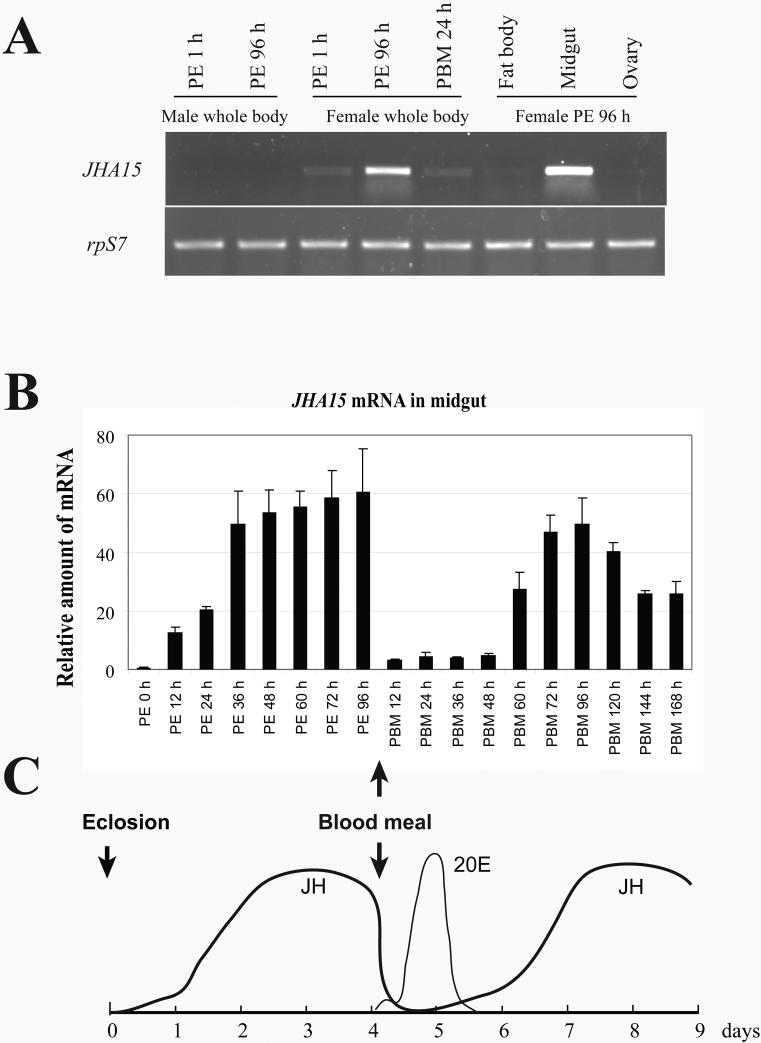
RT-PCR analysis of the JHA15 mRNA in the adult mosquitoes revealed that its expression is female-mosquito-specific, and is limited primarily to the midgut (Figure 4A). Further characterization with real time RT-PCR indicated that the JHA15 transcripts are scarce at eclosion in the midgut of adult females and start to increase approximately 12 h post eclosion (PE) (Figure 4B). They reach a plateau by 36 h PE and remain at a nearly constant level until a blood meal is taken. Shortly after blood feeding, JHA15 mRNA levels decrease and remain at relatively low levels until 48 h post blood meal (PBM), then begin to increase again. By 72 h PBM, the amount of JHA15 mRNA is comparable to that before blood ingestion. These results were confirmed by subsequent in situ hybridization study of JHA15 mRNA in the midgut (Figure 5), revealing a transcriptional profile similar to that reported for AaET (Noriega et al., 1996).
Figure 4.
mRNA expression profile of JHA15 in adult female Aedes aegypti. (A) Female-specific expression of JHA15 in adult mosquitoes. Total RNAs were isolated from whole body and dissected tissues of adult mosquitoes at different stages. JHA15 transcripts were measured using semi quantitative RT-PCR. The ribosomal protein S7 gene was used as control. PE, post-eclosion; PBM, post-blood meal. (B) Expression of JHA15 in the midgut. JHA15 transcripts were measured using real-time RT-PCR and normalized to S7 expression. Arbitrary units are plotted against developmental time. Representative data (mean±SEM) from three independent experiments are shown. (C) Titers of juvenile hormone III (JH III) and 20-hydroxyecdysone (20E) in the female adult Aedes aegypti. The figure is modified from Shapiro et al. (1986) and Hagedorn et al. (1975).
Figure 5.
Detection of JHA15 mRNA in the midgut of female mosquitoes. In situ hybridization was performed on whole-mounts of midgut tissues using a DIG labeled antisense mRNA probe for JHA15. Sense strand RNA probes were used as the negative controls, and no evident signals were detected in the control panels shown in the bottom.
The expression profile of JHA15 is closely correlated with the change in JH titers during vitellogenic cycles (Fig. 4C), suggesting that the transcription of JHA15 in previtellogenic midgut is under the control of JH III. We repeated the abdomen ligation experiments and measured changes of the JHA15 transcripts in response to the topical JH application. Transcription of JHA15 was induced within 2 h after JH treatment, and reached its peak at about 6 h, while acetone could not invoke such effects (Figure 6). In the same experiment, AaET mRNA increased gradually after exposure to exogenous JH, and the maximal mRNA levels were found between 16 and 24 h.
Figure 6.
JH enhances the JHA15 mRNA levels in the newly emerged female adults. (A) Effects of topical application of JH on the JHA15 expression. Abdominal ligations were performed on female mosquitoes within 30 minutes of adult emergence. These abdomens were then treated with 200 ng of JH III dissolved in 0.5 μl of acetone. mRNA levels of AaET and JHA15 were analyzed using semi quantitative RT-PCR. rpS7 mRNA was measured as internal control. The RT-PCR products were stained with ethidium bromide. (B) Transcription of JHA15 in response to increasing doses of JH and methoprene. Females mosquitoes were ligated within 30 minutes of adult emergence, and the abdomens were topically treated with the indicated amounts of JH III or Methoprene. The experimental doses of JH and methoprene were chosen based on a previous experiment performed by Noriega et al. (1997). The samples were collected at 6 h after hormone application, and were subjected to real time RT-PCR analysis. Representative data (mean±SEM) from at least three independent experiments are shown.
We then went on testing effects of different doses of JH on JHA15 expression. The activation of JHA15 in response to JH was dose-dependent. Methoprene, a juvenile hormone analogue, was more potent in stimulating JHA15 transcription (Figure 6).
3.4. Synthesis of the JHA15 protein in the blood-fed and non-blood-fed female mosquitoes
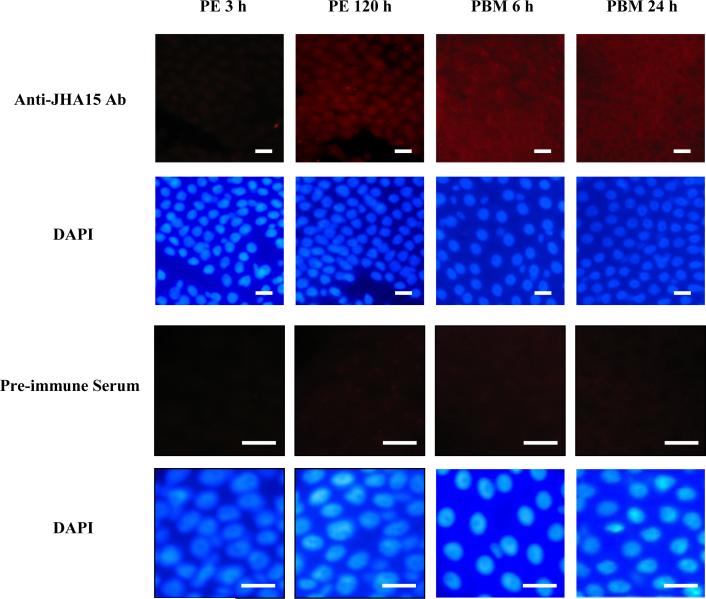
Although transcription of AaET is stimulated by JH in the non-blood-fed female mosquitoes, accumulation of the early trypsin protein is detected only after blood ingestion (Noriega et al., 1996; Noriega et al., 1997). The similar mRNA profiles of AaET and JHA15 prompted us to investigate whether translation of JHA15 mRNA is repressed in the mosquito prior to blood feeding. Immunohistochemical staining with polyclonal antibodies against JHA15 indicated that this chymotrypsin-like protease was not detectable in the midgut of newly emerged mosquitoes, but was found in the mature female mosquitoes both before and after a blood meal (Figure 7).
Figure 7.
Immunostaining of the JHA15 proteins in the midgut. The midgut epithelium cells were stained with polyclonal antibodies against JHA15 (red), and with DAPI to visualize nuclei (blue). For negative controls, preimmune serum was used instead of the primary antibody. White scale bars represent 10 μm.
A reliable measure of translational status of cellular mRNAs is the degree of their associations with ribosomes (Tzamarias et al., 1989; Arava et al., 2003). mRNAs in polyribosomes (or polysomes) are usually undergoing active translation, whereas translationally inactive mRNAs are often defined as those sequestered in non-polysomal complexes (ribonucleoprotein particles and monoribosome). To compare the translational states of the AaET and JHA15 mRNA, we used sucrose gradient sedimentation to isolate polysomal and nonpolysomal complexes from female mosquitoes before and after blood feeding, and measured relative levels of the AaET and JHA15 mRNAs in polysomes. As shown in Figure 8, while almost all the AaET mRNAs were in the nonpolysomal complexes in non-blood-fed mosquitoes, the proportion of the AaET mRNA molecules in polysomes was significantly increased in midgut cells at 1 h PBM, in agreement with the stimulated AaET protein synthesis discovered by Noriega et al (1996) using western blot analysis. Consistent with our aforementioned immunostaining results, we found that the JHA15 mRNA was associated with polysomes both before and after blood feeding, suggesting that production of the JHA15 protein does not rely on blood feeding.
Figure 8.
Polysome distribution of the JHA15 mRNA in Aedes aegypti before and after blood feeding. Whole body extracts from non-blood-fed female mosquitoes (PE 5d) or blood-fed mosquitoes (PBM 1h) were sedimented by centrifugation in a 20−60% sucrose gradient, and 0.35-ml fractions were collected. (A) Absorbance profiles at 254 nm (top) are shown together with analysis of rRNA. RNA was extracted from each fraction and half of the RNA was used in electrophoresis on 1% agarose/formaldehyde gel followed by ethidium bromide staining. The positions of the 40S, 60S, 80S, and polysomal peaks are indicated. (B) The remaining purified RNA was used for RT-PCR analysis of the AaET and JHA15 genes. This experiment was performed three times with similar results.
3.5. Knockdown of JHA15 expression and effect on blood meal digestion
To assess the role of JHA15 in the midgut, we utilized double-stranded RNA (dsRNA)-mediated interference to inhibit JHA15 expression. Injection of JHA15 dsRNA into the newly emerged mosquitoes resulted in over 80% reduction of JHA15 transcript abundance at 5 days post eclosion, compared to the dsAaET-injected and uninjected controls (Figure 9). No clear phenotype was associated with silencing of the JHA15 gene. Blood depletion, yolk protein synthesis, oviposition and egg viability were not markedly affected in JHA15 RNAi animals (data not shown). Moreover, simultaneous knockdown of both AaET and JHA15 did not alter expression of the late trypsin gene (AaLT) in the midgut after blood ingestion.
Figure 9.
Effect of injection of JHA15 dsRNA on expression of the late trypsin gene. Newly emerged female mosquitoes were injected with dsRNA corresponding to either AaET or JHA15, or both. Firefly luciferase dsRNA was used as an injection control as described by Lu et al. (2006). The injected mosquitoes were allowed a period of 5 days for recovery and were then fed blood. (A) Mosquitoes were collected at the indicated time points after a blood meal. The extracted RNAs were analyzed by real time RT-PCR and normalized to the rpS7 mRNA for each sample. WT, uninjected Ae. aegypti Rockefeller/UGAL strain; Luc, injected with double-stranded RNA complementary to the firefly luciferase gene; AaET, injected with AaET dsRNA; JHA15, injected with JHA15 dsRNA; AaET+JHA15, injected with AaET and JHA15 dsRNA. AaLT, the Ae. aegypti late trypsin gene. (B) Female mosquitoes were collected at 5 days after RNA injection (before blood feeding) and the JHA15 proteins in the midgut were examined by Western blot analysis. The lower panel shows levels of β-actin as a loading control.
4. Discussion
Transcriptome analyses of midgut of Aedes aegypti and Anopheles gambiae mosquitoes have revealed transcripts of a spectrum of proteolytic enzymes both before and after blood feeding (Sanders et al., 2003; Abraham et al., 2004; Dana et al., 2005; Marinotti et al., 2006; Warr et al., 2007). Some of these proteases exhibit diverse expression patterns in the midgut of adult female mosquitoes. For instance, genes encoding late trypsin (Barillas-Mury et al., 1991), carboxypeptidase A (Edwards et al., 2000) and some chymotrypsins (Jiang et al., 1997) are expressed only after blood ingestion, while many other genes expressed in both blood-fed and non-blood-fed mosquitoes. Among the latter group is the early trypsin gene (AaET), whose expression is transcriptionally activated in the newly emerged mosquitoes by JH and translationally stimulated by an increase in the concentration of amino acids in the midgut cells after ingestion of a blood meal (Noriega and Wells, 1999).
In searching for genes controlled by JH during post-emergence development, we identified a chymotrypsin-like serine protease gene, JHA15. The close correlation between its transcriptional profile and the changes in JH titers, along with the results of the abdominal ligation experiment, suggests that this gene is transcriptionally regulated by JH in the midgut of adult female mosquitoes. JHA15 and AaET are both regulated at transcriptional level by JH, with very similar transcription patterns. It is intriguing that post-transcriptional regulation of those two genes is quite divergent, resulting in different protein profiles in the midgut.
Noriega et al.(1999) have demonstrated that an increase in the concentration of amino acids in the midgut is sufficient to induce translation of AaET mRNA, although the molecular mechanism behind this phenomenon remains unknown. Coiled membranous whorls composed of rough endoplasmic reticulum (RER) have been speculated to play a role in inhibiting translation of some mRNAs in sugar-fed mosquitoes (Devenport et al., 2006). The RER assembles into whorls soon after emergence in midgut epithelial cells of the non-blood-fed female Aedes aegypti mosquitoes, and spreads out around the cell cytoplasm after the blood meal (Bertram and Bird, 1961). It will be very interesting to test whether AaET and JHA15 mRNAs are sequestered in the whorls, and whether these associations contribute to selective translation of individual mRNA species in the midgut epithelium cells.
Blood feeding induces a biphasic increase in midgut trypsin activities in adult Aedes aegypti females (Noriega and Wells, 1999). During the first few hours following a blood meal, relatively small amounts of early trypsin protein are produced, presumably as a result of translational regulation of AaET mRNA in response to an initial digestion of blood proteins. Between 8 and 36 hours after blood feeding, large amounts of late trypsin are manufactured accounting for the majority of the endoproteolytic cleavage of the protein components of blood. Late trypsin expression has been postulated to depend on the early trypsin activity based on a previous study using soybean trypsin inhibitor (Barillas-Mury et al., 1995). This connection, however, is disputed by a more recent study, in which late trypsin expression was not affected either by blocking early trypsin activity with different trypsin inhibitors, or by reducing early trypsin expression via dsRNA-mediated RNAi (Lu et al., 2006). The different expression patterns of JHA15 and AaET imply that these two genes have disparate functions in the adult female mosquitoes. However, injection of dsRNA corresponding to either gene does not lead to obvious changes in phenotype. Even reduction of both JHA15 and AaET expression has no significant effects on blood depletion, transcription of the late trypsin gene, peritrophic matrix formation in the midgut, and vitellogenin synthesis in the fat body. Numbers and viability of eggs laid by each individual are also very similar between the controls and AaET/JHA15 double knockdown mosquitoes. Our preliminary study shows that more than 30 serine-type endopeptidase genes are transcriptionally upregulated in the adult female Aedes mosquitoes during the first two days after eclosion (Zhu et al., unpublished observation). It is not currently clear whether all these genes are expressed in the midgut epithelium cells, and if they are subjected to translational repression in the sugar-fed mosquitoes. Nonetheless, this abovementioned observation suggests a possible scenario in which some of the proteases may have both distinct and overlapping functions, and compensation of one member by another may mask the function of individual proteolytic enzymes. Translation of AaET mRNA depends on a boost in the amino acid concentrations in the midgut, and the translation takes place within 1 h post blood feeding, suggesting that some digestive enzymes responsible for initial breaking down of blood proteins exist in the midgut prior to blood ingestion. JHA15 is likely among this group of exopeptidases and endoproteases.
Numerous past studies have demonstrated that JH plays an essential role during mosquito post-emergence development (Klowden, 1997). Although the physiological effects have been well documented, the mode of JH action during these 2−3 days window remains largely unknown. In order to elucidate molecular basis of this stage-specific JH response, we used subtractive hybridization to isolate mosquito genes whose transcription are controlled by JH in the young adult females. The pilot screen yielded two cDNA clones corresponding to AaET and JHA15, two protease genes that are primarily expressed in the midgut. Wu et al.(2006) have reported that methoprene affects midgut remodeling in Aedes aegypti during metamorphosis by interfering with expression of the components of the 20-hydroxyecdysone signaling pathway. Our earlier study has shown that mRNA levels of the broad complex gene (broad) are elevated in the fat body of 2-day-old females after in vitro exposure to JH III (Chen et al., 2004). Whether similar mechanisms are exploited by mosquitoes for JH actions during post-emergence development will be addressed in further investigation. Nevertheless, discovering two JH-inducible genes in the newly emerged female mosquitoes demonstrates that regulation of gene expression is clearly involved in the JH actions. Recent publication of Aedes aegypti genome annotation enables us to carry out global analysis of gene expression in response to the elevated JH titers. This approach should facilitate identification of JH target genes in the mosquito, and lead to molecular dissection of the JH signaling.
Acknowledgements
The authors thank Mr. Jeff Busch for critical reading of the manuscript. This work was supported by National Institutes of Health Grant AI036959 (to A.S.R.) and financial support from the Virginia Tech Institute for Biomedical & Public Health Sciences (to J.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham EG, Islam S, Srinivasan P, Ghosh AK, Valenzuela JG, Ribeiro JMC, Kafatos FC, Dimopoulos G, Jacobs-Lorena M. Analysis of the Plasmodium and Anopheles transcriptional repertoire during ookinete development and midgut invasion. J. Biol. Chem. 2004;279:5573–5580. doi: 10.1074/jbc.M307582200. [DOI] [PubMed] [Google Scholar]
- Arava Y, Wang YL, Storey JD, Liu CL, Brown PO, Herschlag D. Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U S A. 2003;100:3889–3894. doi: 10.1073/pnas.0635171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardo GM, Hansen IA, Raikhel AS. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem. Mol. Biol. 2005;35:661–675. doi: 10.1016/j.ibmb.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Baker FC, Hagedorn HH, Schooley DA, Wheelock G. Mosquito juvenile hormone: Identification and bioassay activity. J. Insect Physiol. 1983;29:465–470. [Google Scholar]
- Barillas-Mury C, Graf R, Hagedorn HH, Wells MA. cDNA and deduced amino acid sequence of a blood meal-induced trypsin from the mosquito, Aedes aegypti. Insect Biochem. 1991;21:825–831. [Google Scholar]
- Barillas-Mury CV, Noriega FG, Wells MA. Early trypsin activity is part of the signal-transduction system that activates transcription of the late trypsin gene in the midgut of the mosquito, Aedes aegypti. Insect Biochem. Mol. Biol. 1995;25:241–246. doi: 10.1016/0965-1748(94)00061-l. [DOI] [PubMed] [Google Scholar]
- Bertram DS, Bird RG. Studies on mosquito-borne viruses in their vectors. I. The normal fine structure of the midgut epithelium of the adult female Aedes aegypti (L.) and the functional significance of its modification following a blood meal. . Trans. R. Soc. Trop. Med. Hyg. 1961;55:404–423. doi: 10.1016/0035-9203(61)90085-2. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhu J, Sun G, Raikhel AS. The early gene Broad is involved in the ecdysteroid hierarchy governing vitellogenesis of the mosquito Aedes aegypti. J. Mol. Endocrinol. 2004;33:743–761. doi: 10.1677/jme.1.01531. [DOI] [PubMed] [Google Scholar]
- Clements AN. The biology of mosquitoes, Volume 1. Development, Nutrition, and Reproduction. Chapman & Hall; New York: 1992. [Google Scholar]
- Dana AN, Hong YS, Kern MK, Hillenmeyer ME, Harker BW, Lobo NF, Hogan JR, Romans P, Collins FH. Gene expression patterns associated with blood-feeding in the malaria mosquito Anopheles gambiae. BMC Genomics. 2005;6:5. doi: 10.1186/1471-2164-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenport M, Alvarenga PH, Shao L, Fujioka H, Bianconi ML, Oliveira PL, Jacobs-Lorena M. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as a heme-binding protein. Biochemistry. 2006;45:9540–9549. doi: 10.1021/bi0605991. [DOI] [PubMed] [Google Scholar]
- Dittmann F, Kogan PH, Hagedorn HH. Ploidy levels and DNA-synthesis in fat body cells of the adult mosquito, Aedes aegypti. Arch. Insect Biochem. Physiol. 1989;12:133–143. [Google Scholar]
- Edgar KA, Noriega FG, Bonning BC, Wells MA. Recombinant juvenile hormone esterase, an effective tool for modifying juvenile hormone-dependent expression of the early trypsin gene in mosquitoes. Insect Mol. Biol. 2000;9:27–31. doi: 10.1046/j.1365-2583.2000.00154.x. [DOI] [PubMed] [Google Scholar]
- Edwards MJ, Moskalyk LA, Donelly-Doman M, Vlaskova M, Noriega FG, Walker VK, Jacobs-Lorena M. Characterization of a carboxypeptidase A gene from the mosquito, Aedes aegypti. Insect Mol. Biol. 2000;9:33–38. doi: 10.1046/j.1365-2583.2000.00159.x. [DOI] [PubMed] [Google Scholar]
- Flanagan TR, Hagedorn HH. Vitellogenin synthesis in the mosquito: The role of juvenile hormone in the development of responsiveness to ecdysone. Physiol. Entomol. 1977;2:173–178. [Google Scholar]
- Gwadz RW, Spielman A. Corpus allatum control of ovarian development in Aedes aegypti. J. Insect Physiol. 1973;19:1441–1448. doi: 10.1016/0022-1910(73)90174-1. [DOI] [PubMed] [Google Scholar]
- Hagedorn HH. The endocrinology of the adult female mosquito. Adv. Dis.Vect. Res. 1994;10:109–148. [Google Scholar]
- Hagedorn HH, O'Connor JD, Fuchs MS, Sage B, Schlaeger DA, Bohm MK. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc. Natl. Acad. Sci. U S A. 1975;72:3255–3259. doi: 10.1073/pnas.72.8.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn HH, Turner S, Hagedorn EA, Pontecorvo D, Greenbaum P, Pfeiffer D, Wheelock G, Flanagan TR. Postemergence growth of the ovarian follicles of Aedes aegypti. J. Insect Physiol. 1977;23:203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- Hays AR, Raikhel AS. A novel protein produced by the vitellogenic fat body and accumulated in mosquito oocytes. Roux's Arch. Dev. Biol. 1990;199:114–121. doi: 10.1007/BF02029559. [DOI] [PubMed] [Google Scholar]
- Jiang QJ, Hall M, Noriega FG, Wells M. cDNA cloning and pattern of expression of an adult, female-specific chymotrypsin from Aedes aegypti midgut. Insect Biochem. Mol. Biol. 1997;27:283–289. doi: 10.1016/s0965-1748(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Klowden MJ. Endocrine aspects of mosquito reproduction. Arch. Insect Biochem. Physiol. 1997;35:491–512. [Google Scholar]
- Lea AO. Studies on the dietary and endocrine regulation of autogenous reproduction in Aedes taeniorhynchus (Wied.). J. Med. Entomol. 1964;39:40–44. doi: 10.1093/jmedent/1.1.40. [DOI] [PubMed] [Google Scholar]
- Lu SJ, Pennington JE, Stonehouse AR, Mobula MM, Wells MA. Reevaluation of the role of early trypsin activity in the transcriptional activation of the late trypsin gene in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 2006;36:336–343. doi: 10.1016/j.ibmb.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Ma M, Zhang JZ, Gong H, Gwadz R. Permissive action of juvenile hormone on vitellogenin production by the mosquito, Aedes aegypti. J. Insect Physiol. 1988;34:593–596. [Google Scholar]
- Marinotti O, Calvo E, Nguyen QK, Dissanayake S, Ribeiro JMC, James AA. Genome-wide analysis of gene expression in adult Anopheles gambiae. Insect Mol. Biol. 2006;15:1–12. doi: 10.1111/j.1365-2583.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Colonna AE, Wells MA. Increase in the size of the amino acid pool is sufficient to activate translation of early trypsin mRNA in Aedes aegypti midgut. Insect Biochem. .Mol. Biol. 1999;29:243–247. doi: 10.1016/s0965-1748(98)00132-5. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Pennington JE, Barillas-Mury C, Wang XY, Wells MA. Aedes aegypti midgut early trypsin is post-transcriptionally regulated by blood feeding. Insect Mol. Biol. 1996;5:25–29. doi: 10.1111/j.1365-2583.1996.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Shah DK, Wells MA. Juvenile hormone controls early trypsin gene transcription in the midgut of Aedes aegypti. Insect Mol. Biol. 1997;6:63–66. doi: 10.1046/j.1365-2583.1997.00154.x. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Wells MA. A molecular view of trypsin synthesis in the midgut of Aedes aegypti. J. Insect Physiol. 1999;45:613–620. doi: 10.1016/s0022-1910(99)00052-9. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Juvenile hormone controls previtellogenic proliferation of ribosomal RNA in the mosquito fat body. Gen. Comp. Endocrinol. 1990;77:423–434. doi: 10.1016/0016-6480(90)90233-c. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Lea AO. Control of follicular epithelium development and vitelline envelope formation in the mosquito; role of juvenile hormone and 20-hydroxyecdysone. Tissue Cell. 1991;23:577–591. doi: 10.1016/0040-8166(91)90015-l. [DOI] [PubMed] [Google Scholar]
- Raikhel AS, Brown MR, Belles X. Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive molecular insect science. Vol. 3. Elsevier; Boston: 2005. pp. 433–491. [Google Scholar]
- Rossignol PA, Spielman A, Jacobs MS. Rough endoplasmic reticulum in midgut cells of mosquitos (Diptera: Culicidae): aggregation stimulated by juvenile hormone. J. Med. Entomol. 1982;19:719–721. [Google Scholar]
- Sanders HR, Evans AM, Ross LS, Gill SS. Blood meal induces global changes in midgut gene expression in the disease vector, Aedes aegypti. Insect Biochem. Mol. Biol. 2003;33:1105–1122. doi: 10.1016/s0965-1748(03)00124-3. [DOI] [PubMed] [Google Scholar]
- Shapiro AB, Wheelock GD, Hagedorn HH, Baker FC, Tsai LW, Schooley DA. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J. Insect Physiol. 1986;32:867–877. [Google Scholar]
- Shapiro JP, Hagedorn HH. Juvenile hormone and the development of ovarian responsiveness to a brain hormone in the mosquito, Aedes aegypti. Gen. Comp. Endocrinol. 1982;46:176–183. doi: 10.1016/0016-6480(82)90199-x. [DOI] [PubMed] [Google Scholar]
- Shen Z, Edwards MJ, Jacobs-Lorena M. A gut-specific serine protease from the malaria vector Anopheles gambiae is downregulated after blood ingestion. Insect Mol. Biol. 2000;9:223–229. doi: 10.1046/j.1365-2583.2000.00188.x. [DOI] [PubMed] [Google Scholar]
- Tzamarias D, Roussou I, Thireos G. Coupling of GCN4 mRNA translational activation with decreased rates of polypeptide chain initiation. Cell. 1989;57:947–954. doi: 10.1016/0092-8674(89)90333-4. [DOI] [PubMed] [Google Scholar]
- Warr E, Aguilar R, Dong YM, Mahairaki V, Dimopoulos G. Spatial and sex-specific dissection of the Anopheles gambiae midgut transcriptome. BMC Genomics. 2007;8:37. doi: 10.1186/1471-2164-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Parthasarathy R, Bai H, Palli SR. Mechanisms of midgut remodeling: juvenile hormone analog methoprene blocks midgut metamorphosis by modulating ecdysone action. Mech. Dev. 2006;123:530–547. doi: 10.1016/j.mod.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Zhu J, Chen L, Raikhel AS. Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. U S A. 2003;100:13338–13343. doi: 10.1073/pnas.2234416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JS, Chen L, Sun GQ, Raikhel AS. The competence factor beta Ftz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol. Cell. Biol. 2006;26:9402–9412. doi: 10.1128/MCB.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]