Abstract
This paper presents a new manufacturing method to generate monodisperse microbubble contrast agents with polydispersity index (σ) values of <2% through microfluidic flow-focusing. Micron-sized lipid shell-based perfluorocarbon (PFC) gas microbubbles for use as ultrasound contrast agents were produced using this method. The poly(dimethylsiloxane) (PDMS)-based devices feature expanding nozzle geometry with a 7 μm orifice width, and are robust enough for consistent production of microbubbles with runtimes lasting several hours. With high-speed imaging, we characterized relationships between channel geometry, liquid flow rate Q, and gas pressure P in controlling bubble sizes. By a simple optimization of the channel geometry and Q and P, bubbles with a mean diameter of <5 μm can be obtained, ideal for various ultrasonic imaging applications. This method demonstrates the potential of microfluidics as an efficient means for custom-designing ultrasound contrast agents with precise size distributions, different gas compositions and new shell materials for stabilization, and for future targeted imaging and therapeutic applications.
Introduction
Ultrasound contrast agents are encapsulated microbubbles with diameters of the order of 1 to 10 μm. The use of contrast agents in ultrasonic imaging has been shown to improve the accuracy of detecting functional abnormalities, and provides the potential for early detection and characterization of disease.1,2 Owing to the density and compressibility of their gas core, these stabilized microbubbles are substantially more echogenic than the interfaces between different types of tissue, and therefore improve the sensitivity and specificity of 2-D and 3-D ultrasound imaging by increasing the reflection of sound waves. They have a proven clinical utility, particularly as a diagnostic tool in cardiology,3 radiology,4 and oncology.5
The size of a microbubble contrast agent affects its ability to cross the pulmonary microcirculation as well as the degree of its reflectivity of ultrasound. They must be below 7 μm in diameter to safely pass through the microvessels of the lungs without causing obstruction, but the ultrasound scattering efficiency of a microbubble is a function of the sixth power of its radius, meaning that smaller microbubbles also have poor reflectivity.6 Thus the optimum microbubble size is between 2 and 5 μm in diameter.
In-vivo contrast agents have enabled only small increases in the ultrasound signal at target sites.7,8 Although methods to improve sensitivity are currently underway, such as delivering a higher payload of contrast agents to the target site and adding site-specific adhesion molecules to the shell, little has been done towards optimization of the size of contrast agents. Conventional methods used to produce lipid-encapsulated microbubble suspensions rely on simple agitation to entrain a portion of the bulk gas phase into the bulk aqueous phase. The random nature of this homogenization process results in a highly polydisperse size distribution.9 The current leading FDA-approved ultrasound contrast agent DEFINITY® (Bristol-Myers Squibb Medical Imaging) has microspheres that average in size from 1.1 to 3.3 μm, but the maximum bubble diameter can be as large as 20 μm. Since the resonance frequency of a microbubble depends on its diameter, an aliquot of current contrast agents has a wide range of resonance frequencies. Limitations in bandwidth of ultrasound transducers reduce the sensitivity of the imaging system to a small portion of the contrast agent population.
Microfluidic systems are ideal for biomedical (bio-MEMS) applications due to their small size and batch manufacturability, providing a versatile platform for rapidly performing complex syntheses, measurements, and analysis.10-17 The potential exists for the use of microfluidics for highly specialized applications in large markets. For example, the ability to form micron-sized bubbles or drops is important in many pharmaceutical and food processing applications, from processes to actual products.18 Microfluidic techniques have been shown to generate highly controlled droplet dispersions19-24 and microbubbles.24-27
Several groups have recently utilized hydrodynamic flow-focusing methods for the mass-generation of microbubbles, but to date no group has demonstrated microbubble production in the diameter range required for use as ultrasound contrast agents, or the feasibility of producing lipid shell-based perfluorocarbon gas microbubbles. In this work, we demonstrate a practical microfluidic manufacturing technique for the generation of monodisperse microbubbles with parameters optimized for use as ultrasonic contrast agents that does not rely on the randomness of mechanical agitation.
Background
Microbubble production methods
Conventional mechanical agitation techniques produce sufficient force to introduce gas from the ambient environment into the liquid solution, resulting in the formation of stabilized microbubbles. The rate of reciprocation and motion is important in determining the amount and size of the microbubbles formed.28 A popular mechanical shaker used by hospitals to create a leading ultrasound contrast agent has a shaking frequency of approximately 4500 oscillations per minute.
The flows of fluids in microfluidic systems are usually characterized by low Reynolds numbers (laminar flow), dominated by viscous stresses and pressure gradients. To create a condition within passive microfluidic devices to generate microbubbles, flow-focusing is utilized to force a central stream of gas and two side sheath flows of a liquid mixture through a narrow orifice into a second chamber held at ambient pressure. The focusing effect of the surrounding flow of liquid creates a microjet which breaks at the orifice into microbubbles.
Expanding the nozzle geometry generates monodisperse microbubbles, focusing the bubble break-off location to one single point located at the orifice (Fig. 1). The narrowest point incurs the highest shear force, and the subsequent nozzle expansion generates a velocity gradient in the flow direction that allows the head of the gas thread to break continuously at the orifice, which provides uniform control of bubble sizes.29
Fig. 1.

3-D rendering of the flow-focusing expansion chamber. The widths of the liquid and gas inlet channels (Wl and Wg) are 50 and 35 μm respectively. The orifice width is 7 μm. Channel height h is 25 μm.
For liquids with moderate to small surface tension and an aqueous viscosity range, Garstecki et al.30 summarized a scaling law relation [eqn (1)] for the diameter of the bubbles db produced by fluidic systems having the relation (Qg/Ql < 1), where Qg and Ql are the gas and liquid flow rates, and D is the orifice diameter:
| (1) |
In microfluidic flow-focusing systems, the bubble size primarily scales with the liquid and gas flow rates, with the continuous-phase surface tension having a negligible effect.30
Ideal contrast agents
Microbubble contrast agents with longer survival times are composed of higher molecular weight gases and more rigid shell materials.28,31-34
Gas composition is a major factor in determining the length of time a microbubble lasts in the circulation. The diffusivity of a gas is described by the Ostwald partition coefficient L = Cwater/Cgas which is equal to the ratio of the amount concentrations C in the liquid and in the gas. Nitrogen has a high Ostwald coefficient (L = 14 480 at 35 °C) and therefore a higher water solubility compared with a PFC gas such as n-C3F8 (L = 530 at 35 °C). An adapted model by Kabalnov et al.35 [eqn (2)] describes the rate of bubble shrinkage by the dissolution of gas in the bloodstream, where D is the gas diffusivity in water, L is the Ostwald partition coefficient, Patm is atmospheric pressure, P* is an excess pressure term impacted by the blood pressure and gas metabolism, γ is the interfacial tension, r is the bubble radius, and t is time:
| (2) |
The Laplace pressure ΔP = 2γ/r is the main mechanism responsible for the disappearance of a bubble. The use of high molecular weight gases such as perfluorocarbons reduces diffusion out of the microbubble core and enhances bubble stability and circulation lifetime by counterbalancing the Laplace and blood pressures.31
A stabilized shell for the microbubble is ideally composed of multiple components serving different functions (Fig. 2).34 An amphiphilic biocompatible phospholipid shell in the form of a monolayer is ideal, where the water-insoluble hydrocarbon tails are in contact with the gas core, leaving the charged phosphate head groups to interact with a polar aqueous environment. The primary lipid shell component, a saturated diacyl phosphatidyl choline such as DPPC, is effectively neutral and lowers the interfacial tension, adding rigidity and reducing gas escape. A negatively charged shell component such as DPPA is added to enhance stabilization by repulsive forces, preventing direct contact between microbubbles. The third component, an emulsifier such as a poly(ethylene glycol) (PEG) or PEGylated material bound to the lipid membrane of the microbubble, prevents coalescence and provides a physical barrier to various enzymatic agents, adsorption of blood plasma proteins, and prevents phagocytosis by macrophages of the immune system.36
Fig. 2.

Stabilized microbubble concept. The PFC gas core resists the combined Laplace and blood pressure forces.
Materials and methods
Design of the microfluidic channels
Critical channel widths were incorporated in the flow-focusing device [Fig. 3(a)] such as a narrow 7 μm orifice [Fig. 3(b)] and closely spaced filtering channels [Fig. 3(c)] to prevent clogging due to PDMS debris accumulation. Reduction in the widths of the liquid and gas inlet channels to 50 and 35 μm respectively enabled the use of lower liquid flow rates and gas delivery pressures to generate <5 μm microbubbles.
Fig. 3.
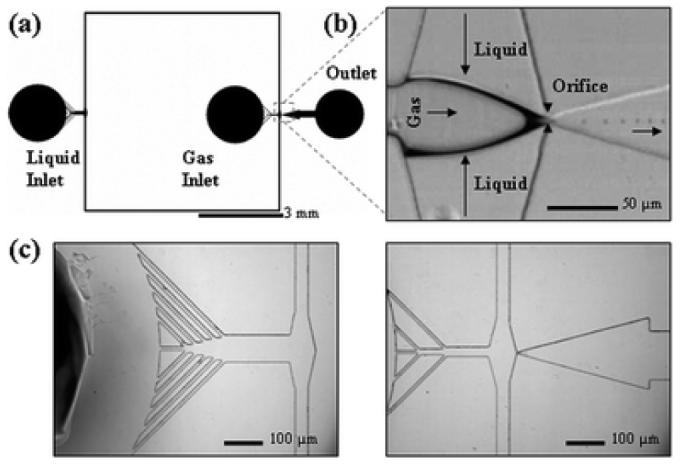
(a) Schematic view of the microfluidic flow-focusing device. All channels have a rectangular cross section and a height of 25 μm. The widths of the liquid and gas inlet channels are {50, 75, 100 μm} and {35, 50 μm} respectively. The devices feature an expanding nozzle with a range of orifice widths {7, 10, 15, 20, 25 μm}. The outlet channel connects to an open reservoir for bubble collection. (b) Main functional area with a 7 μm orifice and 3 μm microbubble generation. The arrows indicate direction of flow. (c) Magnified images of liquid inlet (left) and gas inlet (right) filtering channels.
The gas inlet distance from the orifice region was decreased to reduce gas diffusion. To minimize bubble contact, the outlet reservoir immediately follows the expansion chamber. The punched outlet reservoir hole is of the same size as the punched inlet holes to reduce large pressure variations that will affect bubble stability.
Chemicals
Microbubbles were prepared using specific lipid shell and gas core components similar to those described for formulation of commercially manufactured ultrasound contrast agents (Table 1). In one recipe, the lipids DPPC (1,2-dipalmitoyl-sn-glycero-3-phosphocholine, Avanti Polar Lipids), DPPA (1,2-dipalmitoyl-sn-glycero-3-phosphate) and poly(ethylene glycol) (PEG) lipid conjugate DPPE-PEG5000 (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxypoly(ethylene glycol)-5000], Avanti Polar Lipids) were combined at an 81 : 8 : 10 ratio of molar percentages, dissolved in chloroform (CHCl3) and exposed to nitrogen under vacuum to create a homogenous mixture. The fluorescent probe DiI-C18 (1,1′-dilinoleyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate, Molecular Probes) was added at 1 mol% for fluorescence microscopy studies. Water, purified using a Millipore system and mixed with NaCl (sodium chloride, Fisher) to give a 4 mg mL−1 saline solution, was added to the vial containing the lipid mixture, sonicated at room temperature for 20 min, and combined with a 10% aqueous glycerol/propylene glycol (GPW) mixture. In another recipe, the lipid DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine, Northern Lipids) and surfactant emulsifier PEG-40 stearate (polyoxyethylene 40 stearate – Myrj 52, Sigma-Aldrich) were combined at a 9 : 1 molar ratio and prepared in a similar manner as described previously. Ultra pure DI water containing 2% Tween 20 (polyoxyethylene 20 sorbitan monolaurate, Sigma-Aldrich) surfactant was used for initial microbubble size experiments.
Table 1.
Lipid microbubble compositions
| Component | Abbreviation | Name | Molecular weight | Category |
|---|---|---|---|---|
| Shell | DPPC | 1,2-Dipalmitoyl-sn-glycero-3-phosphocholine | 734 | Phospholipid |
| Shell | DPPA | 1,2-Dipalmitoyl-sn-glycero-3-phosphate | 671 | Phospholipid |
| Shell | DSPC | 1,2-Distearoyl-sn-glycero-3-phosphocholine | 790 | Phospholipid |
| Shell | PEG-40 stearate | Polyoxyethylene 40 stearate (Myrj 52) | 2704 | Surfactant emulsifier |
| Shell | DPPE-PEG5000 | 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[methoxypoly(ethylene glycol)-5000] | 5745 | Lipopolymer emulsifier |
| Shell | DiI-C18 | 1,1′-Dilinoleyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate | 934 | Lipophilic membrane stain |
| Gas core | N2 | Nitrogen | 28 | Osmotic agent |
| Gas core | C4F8 | Octafluorocyclobutane | 200 | Osmotic agent |
Microfabrication and assembling
Fabrication of the poly(dimethylsiloxane) (PDMS)-based microfluidic flow-focusing device followed standard soft lithography techniques.10 First, a 3 inch silicon wafer was spin-coated with a 25 μm layer of a UV-curable epoxy (SU8-25, MicroChem) and exposed to UV-light through a high resolution 20 000 dpi photomask containing the channel pattern and developed. The wafer was used to cast a replica in PDMS (Sylgard 184, Dow Corning) consisting of a 10 : 1 prepolymer and curing agent ratio and bonded to clean soda lime glass (Corning) after oxygen plasma treatment.
Nitrogen (N2, Airgas) or octofluorocyclobutane (C4F8, Specialty Chemical Products) is supplied from a pressurized tank via flexible Tygon tubing and delivered into the gas inlet of the microfluidic chamber using a homemade micro flow meter consisting of a high-accuracy filled pressure gauge (Cole-Parmer EW-68022-02, Cole-Parmer Instrument Company) coupled by a three-way pressure gauge tee to a micro-metering needle valve assembly (Upchurch P-445, Upchurch Scientific). The continuous liquid phase mixture is pumped at a constant flow rate using a digitally controlled syringe pump (Pico Plus, Harvard Apparatus).
Analysis and imaging of the lipid microbubbles
An inverted Nikon microscope and high-speed camera (Fastcam PCI-10K, Photron Ltd.) is used to capture still images and record movies of the microbubbles. A file viewer (PFV, Photron Ltd.) and image analysis program (ImageJ, NIH) are used for data processing and measurements.
For bubbles in contact with the top and bottom PDMS walls, the volume was approximated as Vb = πdb2h/4, where db is the channel wall width and channel height h is 25 μm. The polydispersity index σ =δ/davg× 100% was calculated from the average bubble size davg and standard deviation δ, determined by measuring the sizes of at least 100 microbubbles from recorded images.
For imaging of the stained lipid membranes, the microbubbles in the form of a foam were collected using a glass pipette from the outlet reservoir. Aliquots were transferred onto glass slides and secured with plastic cover slips to reduce fluid flow. The sample was positioned on the stage of an upright fluorescence microscope (Nikon Eclipse E800) and illuminated by a mercury lamp using the optical filters for illumination with green light, λ = 500–550 nm (TRITC filter). Detection of fluorescence occurred at λ = 569 nm due to DiI labeling and bubbles were imaged at 10× and 40× by a color CCD camera (MicroFire 2 MP, Olympus America).
Results and discussion
Visualization
Channel geometry in addition to the liquid and gas flow rates are used for precise control of the bubble sizes. The bubble volume Vb depends on the ratio of the gas pressure P and liquid flow rate Q (Fig. 4). Vb increases with P for a fixed Q. An increase in Q results in a decrease in Vb.
Fig. 4.

Sequence of high-speed images showing the relationship between N2 gas flow rate and bubble size upon application of several different liquid flow rates (0.5–2.0 μL s−1). The widths of the liquid and gas inlet channels are 50 and 35 μm respectively, and the orifice size is 7 μm.
An equilibrium point is reached when the liquid and gas phase form an interface of equal pressure upstream of the orifice. At this point, no bubbles are produced since there is no pressure drop along the longitudinal axis of the device and the tip of the gas stream does not enter the orifice. Higher Q values increase the equilibrium point with P, and decreases the working bubble generation range when varying P. There is a critical regime near the equilibrium point where the system generates bubbles of different sizes.
Several device geometries at various Q and P are able to produce micron-sized bubbles. Depending on the flow rates, it is possible to create microbubbles which are substantially smaller in diameter than the diameter of the exit orifice.
We observed several channel-related effects on bubble size. PDMS is a gas permeable material with a permeability to nitrogen of 245 × 10−10 cm3(STP) cm cm−2 s−1 cmHg−1, and reducing the distance between the gas inlet and orifice results in a lower P necessary to produce the same Vb for a fixed Q. Increasing the gas channel width Wg to match the liquid channel width Wl decreases Vb at the same Q and P, and increases the uniformity of bubbles produced.
Production rates as high as one bubble per microsecond, or 6 × 107 bubbles per minute, have been measured, compared with commercial production of ca. 1010 bubbles per mL during a 45 s agitation of one vial of DEFINITY® in a VialMix activation device.
Stabilization and analysis of the lipid microbubbles
The choice of shell material greatly affects the microbubble dissolution time (Fig. 5). Upon generation, the microbubbles quickly adjust to the downstream conditions and we observe some degree of dissolution at the outlet reservoir when using multiple components for the microbubble shells.
Fig. 5.

Relationships between microbubble shell materials and dissolution time using the same PFC gas and flow conditions.
While monodisperse inside the generation chamber, purely Tween 20-coated microbubbles exist only a few minutes after generation [Fig. 5(a)] due to rapid bubble collapse from the Laplace pressure. A shell composed entirely of PEG-40 stearate or DPPE-PEG5000 helps preserve microbubble monodispersity [Fig. 5(b,c)]. The polydispersity index σ of these microbubbles was calculated to be <2% after generation, but they slowly expand by air influx and Ostwald ripening.
There was no observed change in the size of lipid-coated microbubbles from minutes [Fig. 5(d,e)] to hours after generation. Although highly size-stable for more than two weeks, the polydispersity of these lipid-coated microbubbles can be >50% when using high liquid flow rates and gas pressures (Q > 1.0 μL s−1, P > 10 psi) to increase production. Increasing Q and P decreases the distance between exiting bubbles, and these contact interactions cause them to coalesce due to the lower shell resistance in a high flow velocity environment as in the expansion chamber. In addition, DPPC and DSPC lipids exist as liposomal particles in the aqueous continuous phase, and their opening up and spreading as a monolayer at the gas–liquid interface upstream of the orifice – a dynamic adsorption process – is effected at high rates of flow. Using lower Q and P (Q < 1.0 μL s−1, P < 5 psi) results in highly monodisperse and stable 5 μm lipid-coated microbubbles, at the expense of production rate (Fig. 6). Fluorescence microscopy confirms the existence of the coatings. The constant brightness along the membrane wall suggests a constant membrane thickness. In contrast, the polydispersity of DEFINITY®, the benchmark for our study, is >50% when prepared with the standard mechanical agitation techniques.
Fig. 6.

Fluorescence microscopy image of ca. 5 μm lipid/PFC microbubbles. Microbubbles of varying sizes can be formed under controlled conditions.
Studies on optimizing parameters for long-term stability (>one month) after production are currently underway. In theory, a N2/PFC gas mixture helps to maintain bubble size by setting an osmotic equilibrium with water-soluble gases, enabling the PFC gas to resist the Laplace and outer fluid pressures. The issue of creating long-lasting lipid shell-based PFC gas microbubbles in microfluidic systems poses interesting studies concerning the impact of device geometry and scale, flow parameters, and the synergy between shell, internal components, and the surrounding medium on the stabilization of the microbubbles.
Conclusions
In summary, we have demonstrated a microfluidic flow-focusing system for manufacturing monodisperse microbubble contrast agents in the size range desired for ultrasonic imaging. The microfluidic device is fully compatible with the pharmaceutical ingredients in existing contrast agents and can serve as a low-cost alternative for current manufacturing systems. Eliminating the size disparity issue is the most noteworthy aspect of our device. With a monodisperse contrast agent population, we expect similar microbubble radial oscillations from pulses of ultrasound, and thus a smaller variation in the received echoes. This we hope translates into a better ultrasound image. Acoustic testing of these agents is outside the scope of the current paper, but will be demonstrated in future work.
A significant benefit of microfluidic systems is the ability to integrate multiple assays or steps (i.e. synthesis, purification, analysis, and diagnostics) on a single device. Given the straightforwardness of the basic flow-focusing design, this approach is easy to implement for creating a multiplexed microbubble generator that can achieve the level of contrast agent required for imaging.
Our successful production of lipid shell-based PFC gas microbubbles demonstrates the potential of microfluidics as an efficient means for custom-designing contrast agents with different gas composition and new shell materials for stabilization. A nice prospect is the building of functionalized ‘smart’ microbubbles for targeted imaging and therapeutic applications such as localized drug delivery.
Acknowledgements
The authors would like to thank Lisen Wang for microfabrication assistance, and funding from UC Discovery Grant BIO-ELE04-10462 and by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant # 1 R21 EB005325-01.
References
- 1.Dayton PA, Ferrara KW. J. Magn. Reson. Imaging. 2002;16:362. doi: 10.1002/jmri.10173. [DOI] [PubMed] [Google Scholar]
- 2.Lanza GM, Wickline SA. Prog. Cardiovasc. Dis. 2001;44:13. doi: 10.1053/pcad.2001.26440. [DOI] [PubMed] [Google Scholar]
- 3.Wei K, Kaul S. Curr. Opin. Cardiol. 1997;12:539. doi: 10.1097/00001573-199711000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Forsberg F, Liu JB, Merton DA, Rawool NM, Goldberg BB. J. Ultrasound Med. 1995;14:949. doi: 10.7863/jum.1995.14.12.949. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg BB, Raichlen JS, Forsberg F. Ultrasound Contrast Agents. Martin Dunitz Ltd.; London: 2001. [Google Scholar]
- 6.Palma L. Dalla, Bertolotto M. Eur. Radiol. 1999;9(Suppl 3):S338. doi: 10.1007/pl00014069. [DOI] [PubMed] [Google Scholar]
- 7.Schumann PA, Christiansen JP, Quigley RM, McCreery TP, Sweitzer RH, Unger EC, Lindner JR, Matsunaga TO. Invest. Radiol. 2002;37:587. doi: 10.1097/00004424-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Leong-Poi H, Christiansen J, Klibanov AL, Kaul S, Lindner JR. Circulation. 2003;107:455. doi: 10.1161/01.cir.0000044916.05919.8b. [DOI] [PubMed] [Google Scholar]
- 9.Hunter RJ. Foundations of colloid science. Oxford University Press; Oxford: 1986. [Google Scholar]
- 10.Whitesides GM, Ostuni E, Takayama S, Jiang XY, Ingber DE. Annu. Rev. Biomed. Eng. 2001;3:335. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 11.Hansen CL, Skordalakes E, Berger JM, Quake SR. Proc. Natl. Acad. Sci. U. S. A. 2002;99:16531. doi: 10.1073/pnas.262485199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramsey JM, Jacobson SC, Knapp MR. Nat. Med. 1995;1:1093. doi: 10.1038/nm1095-1093. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Wu H, Mao C, Whitesides GM. Anal. Chem. 2002;74:1772. doi: 10.1021/ac0109422. [DOI] [PubMed] [Google Scholar]
- 14.Zheng B, Roach LS, Ismagilov RF. J. Am. Chem. Soc. 2003;125:11170. doi: 10.1021/ja037166v. [DOI] [PubMed] [Google Scholar]
- 15.Tan YC, Fisher JS, Lee AI, Cristini V, Lee AP. Lab Chip. 2004;4:292. doi: 10.1039/b403280m. [DOI] [PubMed] [Google Scholar]
- 16.Chan EM, Alivisatos AP, Mathies RA. J. Am. Chem. Soc. 2005;127:13854. doi: 10.1021/ja051381p. [DOI] [PubMed] [Google Scholar]
- 17.Hung LH, Choi KM, Tseng WY, Tan YC, Shea KJ, Lee AP. Lab Chip. 2006;6:1. doi: 10.1039/b513908b. [DOI] [PubMed] [Google Scholar]
- 18.Stone HA, Stroock AD, Ajdari A. Annu. Rev. Fluid Mech. 2004;36:381. [Google Scholar]
- 19.Thorsen T, Roberts RW, Arnold FH, Quake SR. Phys. Rev. Lett. 2001;86:4163. doi: 10.1103/PhysRevLett.86.4163. [DOI] [PubMed] [Google Scholar]
- 20.Nisisako T, Torii T, Higuchi T. Lab Chip. 2002;2:24. doi: 10.1039/b108740c. [DOI] [PubMed] [Google Scholar]
- 21.Anna SL, Bontoux N, Stone HA. Appl. Phys. Lett. 2003;82:364. [Google Scholar]
- 22.Xu Q, Nakajima M. Appl. Phys. Lett. 2004;85:3726. [Google Scholar]
- 23.Tan YC, Hettiarachchi K, Siu M, Pan YR, Lee AP. J. Am. Chem. Soc. 2006;128:5656. doi: 10.1021/ja056641h. [DOI] [PubMed] [Google Scholar]
- 24.Garstecki P, Fuerstman MJ, Stone HA, Whitesides GM. Lab Chip. 2006;6:437. doi: 10.1039/b510841a. [DOI] [PubMed] [Google Scholar]
- 25.Ganan-Calvo AM, Gordillo JM. Phys. Rev. Lett. 2001;87:274501. doi: 10.1103/PhysRevLett.87.274501. [DOI] [PubMed] [Google Scholar]
- 26.Garstecki P, Gitlin I, DiLuzio W, Whitesides GM, Kumacheva E, Stone HA. Appl. Phys. Lett. 2004;85:2649. [Google Scholar]
- 27.Gordillo JM, Cheng ZD, Ganan-Calvo AM, Marquez M, Weitz DA. Phys. Fluids. 2004;16:2828. [Google Scholar]
- 28.Unger EC, Matsunaga TO, Yellowhair D. US Patent and Trademark Office; Washington, DC: 1998. [Google Scholar]
- 29.Tan YC, Cristini V, Lee AP. Sens. Actuators, B. 2006;114:350. [Google Scholar]
- 30.Garstecki P, Ganan-Calvo AM, Whitesides GM. Bull. Pol. Acad. Sci.: Tech. Sci. 2005;53:361. [Google Scholar]
- 31.Schutt EG, Klein DH, Mattrey RM, Riess JG. Angew. Chem., Int. Ed. 2003;42:3218. doi: 10.1002/anie.200200550. [DOI] [PubMed] [Google Scholar]
- 32.Borden MA, Martinez GV, Ricker J, Tsvetkova N, Longo M, Gillies RJ, Dayton PA, Ferrara KW. Langmuir. 2006;22:4291. doi: 10.1021/la052841v. [DOI] [PubMed] [Google Scholar]
- 33.Talu E, Lozano MM, Powell RL, Dayton PA, Longo ML. Langmuir. 2006;22:9487. doi: 10.1021/la062095+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borden MA, Pu G, Runner GJ, Longo ML. Colloids Surf., B. 2004;35:209. doi: 10.1016/j.colsurfb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Kabalnov A, Klein D, Pelura T, Schutt E, Weers J. Ultrasound Med. Biol. 1998;24:739. doi: 10.1016/s0301-5629(98)00034-9. [DOI] [PubMed] [Google Scholar]
- 36.Scott MD, Murad KL. Curr. Pharm. Des. 1998;4:423. [PubMed] [Google Scholar]


