Abstract
Galactose is metabolized in humans and other species by the three-enzyme Leloir pathway comprised of galactokinase (GALK), galactose 1-P uridylyltransferase (GALT), and UDP galactose 4'-epimerase (GALE). Impairment of GALT or GALE in humans results in the potentially lethal disorder galactosemia, and loss of either enzyme in yeast results in galactose-dependent growth arrest of cultures despite the availability of an alternate carbon source. In contrast, loss of GALK in humans is not life-threatening, and in yeast has no impact on the growth of cultures challenged with galactose. Further, the growth of both GALT-null and GALE-null yeast challenged with galactose is rescued by loss of GALK, thereby implicating the GALK reaction product, gal-1P, for a role in the galactose-sensitivity of both strains. However, the nature of that relationship has remained unclear. Here we have developed and applied a doxycycline-repressible allele of galactokinase to define the quantitative relationship between galactokinase activity, gal-1P accumulation, and growth arrest of galactose-challenged GALT or GALE-deficient yeast. Our results demonstrate a clear threshold relationship between gal-1P accumulation and galactose-mediated growth arrest in both GALT-null and GALE-null yeast, however, the threshold for the two strains is distinct. Further, we tested the galactose-sensitivity of yeast double-null for GALT and GALE, and found that although loss of GALT barely changed accumulation of gal-1P, it significantly lowered the accumulation of UDP-gal, and also dramatically rescued growth of the GALE-null cells. Together, these data suggest that while gal-1P alone may account for the galactose-sensitivity of GALT-null cells, other factors, likely to include UDP-gal accumulation, must contribute to the galactose-sensitivity of GALE-null cells.
Keywords: galactose, galactosemia, yeast, gal-1P, GALT, GALE, GALK
INTRODUCTION
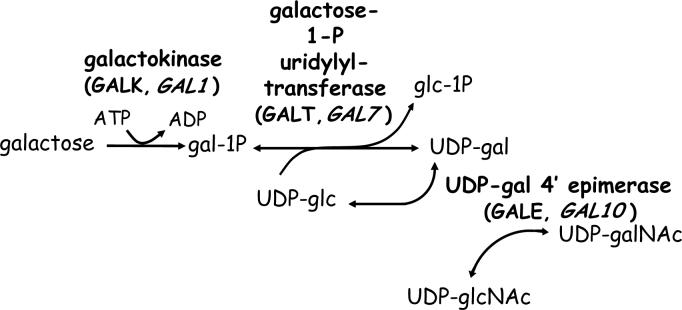
In species ranging from Escherichia coli to mammals galactose is metabolized predominantly through a series of sequential reactions collectively known as the Leloir pathway. The three enzymes that catalyze these reactions are galactokinase (GALK, EC 2.7.1.6) which phosphorylates α-D-galactose (gal) to produce galactose 1-phosphate (gal-1P), galactose-1-phosphate uridylyltransferase (GALT, EC 2.7.7.12) which transfers UMP from UDP-glucose (UDP-glc) to gal-1P, releasing glucose 1-phosphate (glc-1P) and producing UDP-galactose (UDP-gal), and finally, UDP-galactose 4'-epimerase (GALE, EC 5.1.3.2) which interconverts UDP-gal and UDP-glc (Figure 1, [1]). In humans and many other species, GALE also interconverts UDP-N-acetylgalactosamine (UDP-galNAc) and UDP-N-acetylglucosamine (UDP-glcNAc) [2,3]. Enzymes of the Leloir pathway therefore not only mediate the conversion of galactose to glc-1P which can be metabolized for energy, but also control the levels and ratios of four key UDP-sugar substrates required for the biosynthesis of glycoproteins and glycolipids in cells. A deficiency of any one of the Leloir enzymes in humans results in a form of the inherited metabolic disorder, galactosemia [4].
Figure 1. The Leloir pathway of galactose metabolism.
Both the human and yeast gene names are presented for each enzyme (e.g. GALK (human), GAL1 (S. cerevisiae), etc.).
The most common and clinically severe form of galactosemia, classic galactosemia (OMIM 230400), is an autosomal recessive condition that results from profound impairment of GALT [4,5]. Typically asymptomatic at birth, infants with classic galactosemia develop escalating symptoms following exposure to milk, which contains high concentrations of galactose in the form of lactose. Untreated, these infants can succumb to E. coli sepsis within the first days to weeks of life. Although population newborn screening and prompt dietary restriction of galactose relieve or prevent the acute and potentially lethal symptoms of classic galactosemia many, if not most, patients go on to develop serious long-term complications despite early intervention and careful lifelong dietary management [6-9].
Significant impairment of GALE, like GALT, results in potentially lethal sequelae following exposure to dietary galactose. Of note, while many patients with classic galactosemia have little or no detectable GALT activity, even the most severely affected patients with GALE-deficiency demonstrate at least 5% residual activity [10,11]; there are no live born humans who are completely null for GALE. This difference is striking and suggests that although both enzymes function in the same pathway, the quantitative and perhaps also qualitative mechanisms by which they lead to clinical sequelae may be distinct.
Despite decades of study, the underlying bases of pathophysiology in classic GALT-deficiency and GALE-deficiency galactosemia remain unknown [4,12,13]. What we do know is that untreated patients with classic galactosemia experience marked accumulation of gal-1P, and untreated patients with generalized GALE-deficiency experience marked accumulation of both gal-1P and UDP-gal [11,12,14]. Upon dietary restriction of galactose, these metabolic abnormalities subside, although in most treated patients they never fully normalize. Of note, patients with profound impairment of GALK accumulate neither gal-1P nor UDP-gal, and also do not experience any of the potentially lethal sequelae of classic or GALE-deficiency galactosemia. Combined, these data implicate gal-1P for a role in the pathophysiology of disease in both classic GALT and GALE-deficiency galactosemias, and leave open the question of whether UDP-gal or other factors may also play a role.
Studies of galactose; metabolism in yeast have further implicated gal-1P for a role in the toxicity of galactose in GALT and GALE-impaired cells. In particular, both we [15-19] and others [20-23] have demonstrated that both GALT and GALE-deficient yeast arrest their growth in glycerol/ethanol medium upon exposure to even trace levels of galactose, and these cultures also accumulate markedly increased levels of intracellular gal-1P [17]. Further, all of the modifiers identified to date that rescue GALT-null yeast from galactose-mediated growth arrest (i.e., GALK, UGP, IMPase [17,20-23]) appear to function either by preventing the synthesis of gal-1P (e.g., loss of GALK), or by increasing the catabolism of gal-1P (e.g., overexpression of UGP or IMPase). Nonetheless, the quantitative relationship between galactose exposure, gal-1P accumulation, and growth arrest in GALT-null vs. GALE-null yeast has remained unclear. Further, whether the role of gal-1P as a mediator of galactose toxicity in both GALT-null and GALE-null cells is the same or distinct has remained unknown.
Previously, we demonstrated that GALE-null yeast arrest growth upon exposure to galactose at 10-fold lower concentration than do GALT-null yeast [17]. Does this difference imply that GALE-null yeast are hyper-sensitive to gal-1P, or alternatively that a second mechanism of sensitivity, perhaps to another metabolite such as UDP-gal, is super-imposed? To distinguish between these possibilities, we first we generated and applied a doxycycline-repressible allele of GALK that allowed us to titrate the level of gal-1P in GALT-null and GALE-null yeast. This system enabled careful studies of the relationship between gal-1P and growth rate in both strains. Second, we generated and tested a strain of yeast that is both GALT-null and GALE-null. This strain allowed us to uncouple the accumulation of UDP-gal from the accumulation of gal-1P in a galactose-challenged strain. Together, these approaches empowered us to explore the differential roles of gal-1P and UDP-gal as candidate mediators of galactose-sensitivity in GALT-null and GALE-null yeast.
MATERIALS AND METHODS
Yeast Strains
Yeast employed in this work (Table 1) were derived from a haploid strain of S. cerevisiae W303 (MATa: ade 2−1 his 3−11, 15 leu 2−3, 112 ura 3−1 trp1−1 can 1−100 RAD 5+), and propagated using standard techniques [24]. To enable the expression of endogenous yeast GAL genes in both the presence and absence of galactose, we deleted the GAL80 gene from all strains using one-step gene replacement [24,25]. To generate yeast in which the expression of GAL1 encoding galactokinase is regulated by doxycycline, we integrated a doxycycline-repressible promoter [26,27] just upstream of the GAL1 coding sequence. One step gene replacements of GAL7 and GAL10 were accomplished using the procedure described by Alani et al. [25].
Table 1. Yeast strains used in this study.
All yeast strains were derived from the haploid parent W303 and rendered gal80Δ and gal1Δ, gal7Δ, or gal10Δ by one-step gene replacement as described in Materials and Methods. Resulting strains were confirmed by genomic PCR of all relevant loci as well as by enzyme analysis of corresponding gene products.
| Yeast Strain Code (relevant features) | Relevant strain genotype |
|---|---|
| JFy 4793 (wild-type GALK, GALT, GALE) | gal80Δ GAL1 GAL7 GAL10 |
| JFy 4799 (doxycycline-regulated GALK, wild-type GALT, GALE) | gal80Δ dox.GAL1 GAL7 GAL10 |
| JFy 5167 (doxycycline-regulated GALK, GALT-null) | gal80Δ dox.GAL1 gal7Δ GAL10 |
| JFy 4949 (wild-type GALK, GALT-null) | gal80Δ GAL1 gal7Δ GAL10 |
| JFy 3747 (wild-type GALK, GALT-null) | gal80Δ GAL1 gal7Δ GAL10 |
| JFy 4089 (GALK-null and GALT-null) | gal80Δ gal1Δ gal7Δ GAL10 |
| JFy 4958 (doxycycline-regulated GALK, GALE-null) | gal80Δ dox.GAL1 GAL7 gal10Δ |
| JFy 4931 (wild-type GALK, GALE-null) | gal80Δ GAL1 GAL7 gal10Δ |
| JFy 3835 (wild-type GALK, GALE-null) | gal80Δ GAL1 GAL7 gal10Δ |
| JFy 4738 (GALK-null and GALE-null) | gal80Δ gal1Δ GAL7 gal10Δ |
| JFy 5021 (GALT-null and GALE-null) | gal80Δ GAL1 gal7Δ gal10Δ |
Preparation of yeast cultures
All yeast strains were propagated as described previously [19] at 30°C in synthetic medium (SGE) containing 2% glycerol and ethanol respectively. Yeast colonies were inoculated initially into fresh medium and grown for two days to an absorbance (A600) of 3.5 − 4.0. Yeast cultures were then diluted at 106 cells/ml into fresh medium containing 1 μg/ml doxycycline and grown to mid-logarithmic phase (A600 = 0.8 − 1.0) to allow for complete shut down of the dox.GAL1 allele. Thereafter, cells were harvested by centrifugation at 4000 rpm and washed twice in sterile water to remove residual medium drug. Washed cells were then resuspended in fresh medium containing the desired doxycycline concentration, and allowed to grow to an A600 of between 0.8 and 1, at which point the cultures were rediluted to an OD600 of 0.1 in fresh medium containing the same level of doxycycline. These cultures were again allowed to reach an A600 of between 0.8 and 1, at which point they were harvested for analysis.
Preparation of samples for enzyme assays
Five mls of yeast culture at an A600 of between 0.8 and 1.0 were centrifuged at 2500 rpm for 5 minutes, rinsed once in sterile water, and then resuspended in lysis buffer (20mM HEPES pH 8.7, 1mM DTT, 0.3mg Bovine Serum Albumin) supplemented with a cocktail of protease inhibitors (Roche “complete mini” protease inhibitors tablets #11 836 153 001). Acid washed 0.5mm glass beads were added into the cell suspension and the mixture was agitated using a multi-head vortex mixer for 15 minutes at 4°C. Each yeast lysate was passed through a Bio-Spin column (P-30) to remove small metabolites prior to further analysis. Protein concentration was determined using the Bio-Rad protein reagent, as recommended by the manufacturer, using bovine serum albumin as the standard. Enzyme activities were measured as described previously [17].
Analysis of yeast culture growth rates
Yeast cultures grown to an A600 of between 0.8 and 1.0 in doxycycline were diluted to an A600 of 0.1 in fresh medium and allowed to grow one doubling in the presence of the desired concentration of drug prior to analysis of growth rate. Parental and control strains were cultured concurrently. For quantitation of growth rate, yeast cultures were rediluted to A600 of 0.1 into the wells of a 96-well plate in the presence or absence of the desired level of galactose (0, 0.02% or 0.002%) and also the desired level of doxycycline, and incubated with continuous shaking for 36 hours at 30°C on a microplate reader (Bio-Tek, model No. EL808UI) run by KC junior software (Bio-Tek Instrument, Winooski, VT). The microplate reader read culture absorbance of each well at A600 every 30 minutes for the duration of the experiment.
Analysis of metabolites
External galactose and intracellular metabolites were distinguished and quantified by HPLC as described previously [17].
Relationship between gal-1P and cell growth
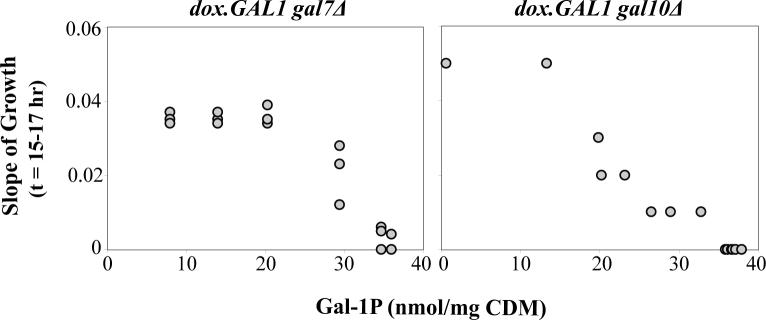
To determine the relationship between galactokinase-dependent changes in cellular gal-1P levels and cell growth in both dox.GAL1gal7Δ and dox.GAL1gal10Δ yeast, the 6-hour internal gal-1P values and the 15-hour to 17-hour slope values from the growth curve data were plotted against each other. Because the galactokinase activity was measured independently in cultures that were sampled for metabolites and cultures that were measured for growth, we calculated a best fit linear equation (y = 11.234x + 38.296, R2 = 0.9217 for dox.GAL1gal7Δ cultures, and y = 5.263x + 14.875, R2 = 0.8183 for dox.GAL1gal10Δ cultures) using log10 transformed galactokinase activity measurements corresponding to the 6-hour internal gal-1P values, and used that equation to calculate gal-1P values that corresponded to the measured galactokinase activities for the growth curve data. Figure 8 presents the relationship between measured 15-hour to 17-hour slope values and corresponding (calculated) gal-1P values.
Figure 8. Growth rates of galactose-challenged dox.GAL1gal7Δ and dox.GAL1gal10Δ cultures as a function of intracellular gal-1P values measured at t=6 hrs.
The values plotted were derived as explained in Materials and Methods using raw data also presented in Figures 6 and 7.
RESULTS
Doxycycline-regulated expression of GALK (GAL1) in yeast
To enable doxycycline-mediated regulation of galactokinase in yeast, we introduced a doxycycline-repressible promoter [26,27] just upstream of the GAL1 translation initiation codon in W303 haploid yeast deleted for GALT (gal7Δ), deleted for GALE (gal10Δ), or wild-type at both loci (GAL7 GAL10, Table 1). To confirm the expression and regulation of GALK in the resultant dox.GAL1gal7Δ, dox.GAL1gal10Δ, and control strains we grew cultures of each in the presence and absence of 1μg/ml doxycycline, prepared lysates, and measured the activities of all three Leloir pathway enzymes in each sample.
Our results (Table 2) demonstrated three important points. First, basal expression of GALK in each of the dox.GAL1 strains cultured in the absence of doxycycline was about 10% of that seen in the wild-type strain. This result was not surprising given our previous experience with the doxycycline-regulated promoter [19], and given the extraordinary strength of the endogenous GAL1 promoter [28]. As described below, this basal level of GALK activity was functionally indistinguishable from wild-type expression in all relevant growth rate experiments. Second, in the presence of 1μg/ml doxycycline, GALK activity in the dox.GAL1 strains was essentially undetectable. In contrast, GALK activity was unaffected by drug in the control strain, and both GALT and GALE enzyme activities were unaffected by drug in all strains tested. Finally, in each strain carrying a deletion of gal7 or gal10, the corresponding enzyme activity was undetectable while activity of the other Leloir enzymes was unaffected. This independence of expression level is an important point given the close proximity of the GAL1, GAL7, and GAL10 genes in the yeast genome, and especially given that GAL1 and GAL10 share a bidirectional promoter [28].
Table 2. Leloir pathway enzyme activity levels in relevant strains cultured in the presence or absence of 1 μg/ml doxycycline.
GALK, GALT, and GALE activities were measured in lysates of wild-type, dox.GAL1, dox.GAL1gal7Δ, and dox.GAL1gal10Δ yeast cultured in the presence verses absence of 1 μg/ml doxycycline. Data are presented as mean ± SEM (n = 3). Means were compared using least significant difference, and all statements of significance are based on P<0.05.
|
Relevant yeast strain genotype |
Doxycycline treatment |
GALK (μmol/mg/min) |
GALT (μmol/mg/min) |
GALE (μmol/mg/min) |
|---|---|---|---|---|
| GAL1 GAL7 | no drug | 9.97 ± 0.61 | 1.71 ± 0.04 | 1.86 ± 0.10 |
|
GAL10 |
1 μg/ml |
11.38 ± 1.26 |
1.92 ± 0.09 |
2.05 ± 0.09 |
| dox.GAL1 | no drug | 1.09 ± 0.12* | 2.21 ± 0.38 | 2.26 ± 0.25 |
|
GAL7 GAL10 |
1 μg/ml |
0.00 ± 0.00 |
2.82 ± 0.19 |
2.48 ± 0.09 |
| dox.GAL1gal7Δ | no drug | 0.60±0.08* | 0.00 ± 0.00 | 2.25 ± 0.17 |
|
GAL10 |
1 μg/ml |
0.06 ± 0.02 |
0.10 ± 0.08 |
1.92 ± 0.70 |
| dox.GAL1 | no drug | 0.70 ± 0.02* | 3.18 ± 0.30 | 0.00 ± 0.00 |
| GAL7 gal10Δ | 1 μg/ml | 0.01 ± 0.00 | 3.55 ± 0.18 | 0.00 ± 0.00 |
Mean value differs significantly from that of the corresponding control value.
To test the impact of the dox.GAL1 allele on growth of gal7Δ and gal10Δ yeast exposed to galactose, we cultured both strains in glycerol/ethanol medium in the presence and absence of galactose. As described in Materials and Methods, we used the minimal levels of galactose previously demonstrated to arrest the growth of gal7Δ and gal10Δ yeast expressing GALK from the wild-type allele (0.02% and 0.002% galactose, respectively) [17]. Wild-type, gal7Δ, gal10Δ, gal1Δgal7Δ, and gal1Δgal10Δ strains were tested in parallel as controls.
As expected, all strains grew indistinguishably in the absence of galactose (Figure 2, panel A). In the presence of 0.02% galactose, the wild-type, dox.GAL1, and gal1Δgal7Δ cultures all grew well, but the gal7Δ and dox.GAL1gal7Δ strains both arrested growth (Figure 2, panel B). Similarly, in the presence of 0.002% galactose wild-type, dox.GAL1, and gal1Δgal10Δ cultures all grew well, but the gal10Δ and dox.GAL1gal10Δ strains both arrested growth (Figure 2, panel C). Together these data reconfirm that loss of GALK relieves galactose-mediated growth arrest of GALT-null or GALE-null yeast [17,20], and demonstrate that despite its compromised basal expression, the dox.GAL1 allele expresses sufficient GALK to halt the growth of dox.GAL1gal7Δ and dox.GAL1gal10Δ strains exposed to trace levels of galactose.
Figure 2. Galactose-sensitivity of GALT-null and GALE-null yeast.
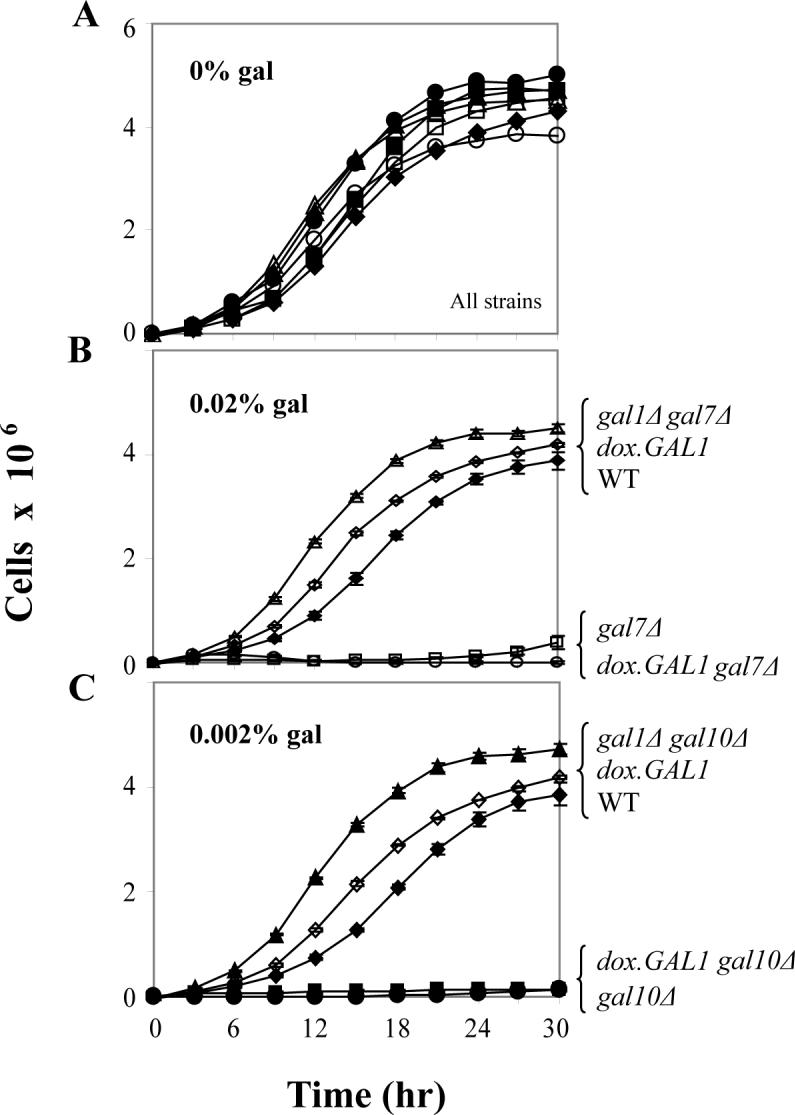
Growth curves of yeast cultured in synthetic medium containing glycerol/ethanol with or without the indicated levels of galactose were performed using 96-well plates as described in Materials and Methods. As we have reported previously [17], GALT-null yeast arrest growth upon challenge with 0.02% galactose, while GALE-null yeast arrest growth upon challenge with 0.002% galactose. Values plotted represent mean ± SEM (n=3).
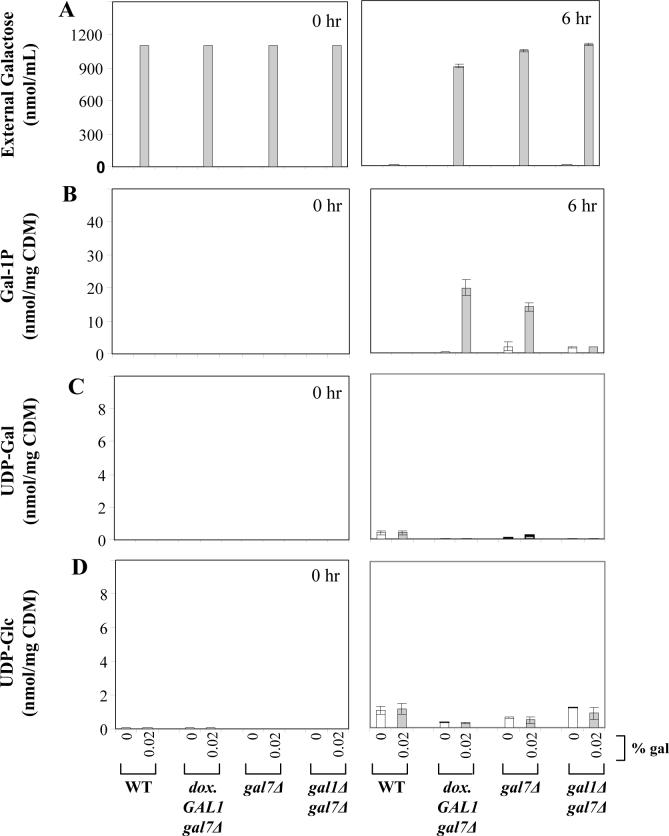
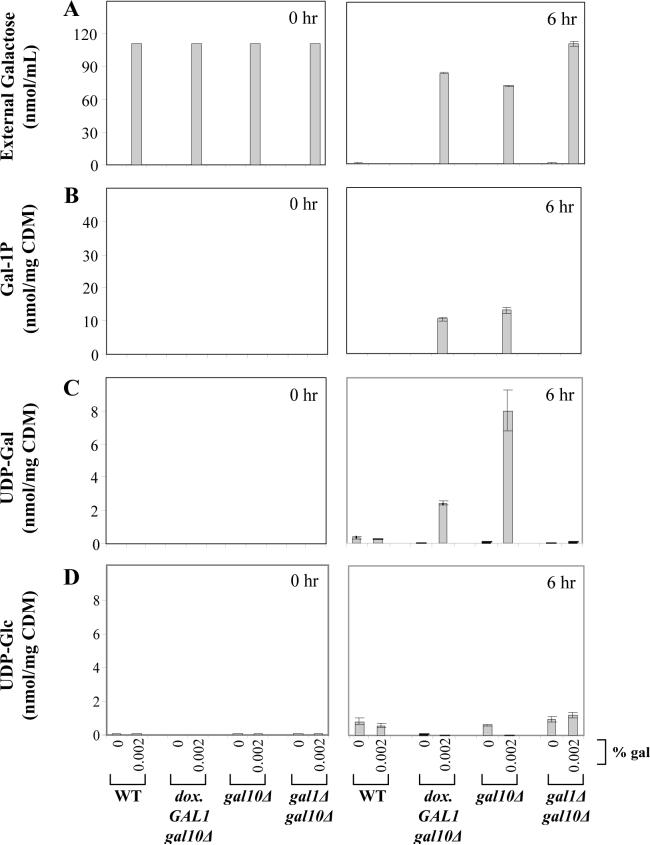
To test the impact of the dox.GAL1 allele on the metabolic profiles of GALT-null or GALE-null yeast cultured in the presence or absence of galactose, we analyzed cultures of both dox.GAL1gal7Δ and dox.GAL1gal10Δ strains in parallel with wild-type, gal7Δ, and gal10Δ controls at t=0 and t=6 hrs following exposure to galactose. We further monitored the ability of each strain to deplete galactose from its surrounding medium. As illustrated in Figure 3 panel A and Figure 4 panel A, only the wild-type cultures were able to deplete galactose fully from their culture medium by 6 hrs. With regard to internal metabolites, as expected, both the gal7Δ and dox.GAL1gal7Δ strains accumulated substantial levels of gal-1P following exposure to 0.02% galactose (Figure 3 panel B); UDP-gal and UDP-glc in both strains remained either undetectable or unaffected by galactose (Figure 3 panels C, D). Also as expected [17], the gal10Δ and dox.GAL1gal10Δ strains accumulated substantial concentrations of both gal-1P and UDP-gal following exposure to 0.002% galactose (Figure 4 panels B, C), and in both of these strains UDP-glc demonstrated a small but notable drop following exposure to galactose (Figure 4 panel D).
Figure 3. Galactose consumption and accumulation of metabolites in GALT-null yeast.
As explained in Materials and Methods, cells were cultured in synthetic medium containing glycerol/ethanol with or without the addition of 0.02% galactose at t=0 hrs. External galactose reflects the level of galactose remaining in the culture medium at the time indicated. As expected, only wild-type cells fully depleted their culture medium of galactose by 6 hrs; the dox.GAL1gal7Δ, gal7Δ, and gal1Δgal7Δ cultures all demonstrated little if any galactose depletion by t=6 hrs. Also as expected, while the dox.GAL1gal7Δ and gal7Δ cells accumulated notable levels of gal-1P following galactose challenge, neither the wild-type nor the gal1Δgal7Δ cells accumulated significant gal-1P. Finally, none of the cells tested demonstrated significant impact of galactose challenge on their UDP-gal or UDP-glc levels. Values plotted represent mean ± SEM (n=3).
Figure 4. Galactose consumption and accumulation of metabolites in GALE-null yeast.
As explained in Materials and Methods, cells were cultured in synthetic medium containing glycerol/ethanol with or without the addition of 0.002% galactose at t=0 hrs. External galactose reflects the level of galactose remaining in the culture medium at the time indicated. As expected, only wild-type cells fully depleted their culture medium of galactose by 6 hrs, and both dox.GAL1gal10Δ and gal10Δ cells accumulated notable levels of gal-1P and UDP-gal and diminished levels of UDP-glc by 6 hrs following galactose challenge. Values plotted represent mean ± SEM (n=3).
Titration of GALK activity with doxycycline in dox.GAL1gal7Δ and dox.GAL1gal10Δ yeast
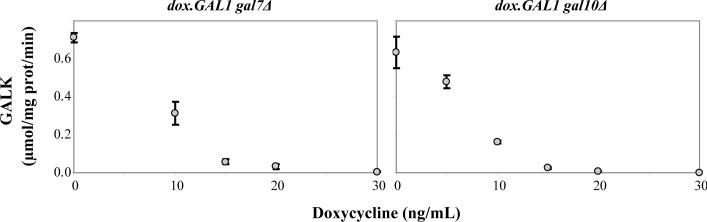
To define the quantitative relationship between doxycycline exposure and GALK activity in the dox.GAL1gal7Δ and dox.GAL1gal10Δ strains we performed titration experiments. As explained in Materials and Methods, GALK was initially repressed to undetectable levels in each strain by exposure to 1μg/ml doxycycline, after which point the cells were washed and diluted into fresh medium containing the desired intermediate level of doxycycline. After approximately 40h, each culture was harvested, processed, and assayed for GALK activity relative to a wild-type control. As illustrated in Figure 5, there was an inverse relationship between the level of doxycycline added to the culture medium and the level of GALK activity detected in the corresponding cell lysate. For both strains, concentrations of doxycycline ≥30ng/ml reduced GALK activity to below the threshold of detection (Figure 5).
Figure 5. Increasing levels of doxycycline result in decreasing expression of GALK in dox.GAL1gal7Δ and dox.GAL1gal10Δ cells.
Values plotted represent mean ± SEM (n=3).
Relationship between GALK activity and galactose-mediated growth arrest in dox.GAL1gal7Δ and dox.GAL1gal10Δ yeast
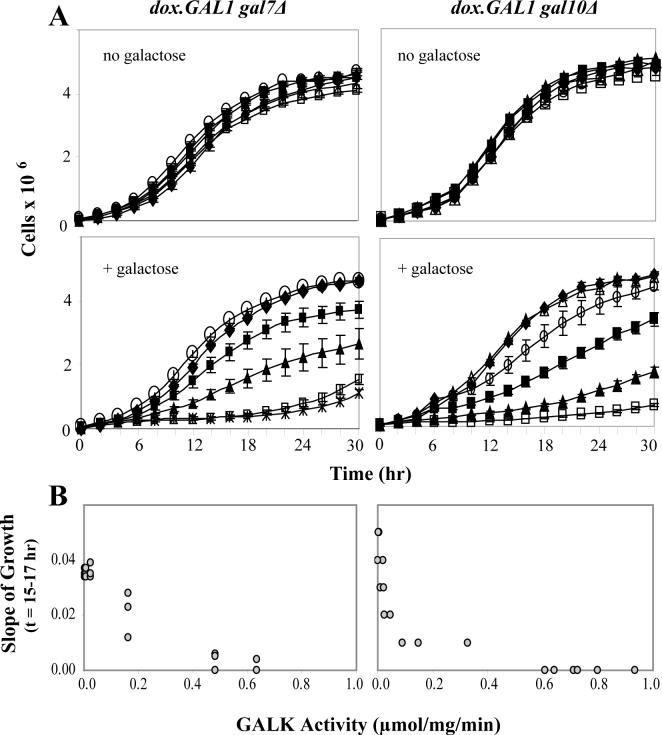
To quantify the relationship between GALK activity and yeast growth in the presence of a fixed concentration of galactose, we monitored the growth rates of the dox.GAL1gal7Δ and dox.GAL1gal10Δ strains under conditions of titrating doxycycline and therefore titrating GALK activity. The levels of galactose applied were the same as those used in Figure 2 (0.02% for dox.GAL1gal7Δ cells and 0.002% for dox.GAL1gal10Δ cells). As a control to rule out the possibility of a direct doxycycline effect on growth, all cultures were also monitored in the absence of galactose. As expected, there was no effect of doxycycline exposure and loss of GALK activity on growth (Figure 6A, top panels). In the presence of galactose (Figure 6A, lower panels), however, we observed a clear and continuous inverse relationship between GALK activity and growth rate of both strains.
Figure 6. Repression of GALK relieves growth-impairment of galactose-challenged dox.GAL1gal7Δ and dox.GAL1gal10Δ cells in a dose-response manner.
(A) Growth curves of cells cultured in the presence or absence of trace galactose (0.02% galactose for dox.GAL1gal7Δ cells and 0.002% galactose dox.GAL1gal10Δ cells). As explained in Materials and Methods, cultures were treated with different concentrations of doxycycline to achieve the intermediate levels of GALK activity plotted. Values plotted represent mean ± SEM (n=3). The different symbols represent different levels of GALK activity in the doxycycline-treated cultures, as follows. For the gal7Δ strain: wild-type (filled diamonds), 0.002 μmol/mg/min (open diamonds), 0.025 μmol/mg/min (filled squares), 0.162 μmol/mg/min (filled triangles), 0.481 μmol/mg/min (cross-hatch), 0.634 μmol/mg/min (open squares). For the gal10Δ strain: wild-type (filled diamonds), 0.005 μmol/mg/min (open diamonds), 0.033 μmol/mg/min (filled squares), 0.058 μmol/mg/min (filled triangles), 0.314 μmol/mg/min (cross-hatch), 0.712 μmol/mg/min (open squares). (B) Data from panels in (A) were re-plotted to illustrate growth rate (slope calculated using 5 data points collected at t=15 hrs, t=15.5 hrs, t=16 hrs, t=16.5 hrs, and t=17 hrs) as a function of GALK activity in the corresponding culture.
To ask whether the quantitative nature of the relationship between GALK activity and growth rate was the same for both the dox.GAL1gal7Δ and dox.GAL1gal10Δ strains, we calculated the slopes of relevant growth curves using the 5 data points collected for each between t=15 and t=17 hrs, and plotted these slopes as a function of GALK activity. As illustrated (Figure 6B), the shapes of these curves were not precisely the same. Indeed, the dox.GAL1gal7Δ strain showed an apparently linear inverse relationship between GALK activity and slope, while the dox.GAL1gal10Δ strain showed an apparently nonlinear, almost logarithmic inverse relationship between GALK activity and slope. Both strains demonstrated complete growth arrest at GALK activity ≥ 0.5 or 0.6 μmol/mg/min.
Relationship between metabolite accumulation and galactose-mediated growth arrest of GALT-null and GALE-null yeast
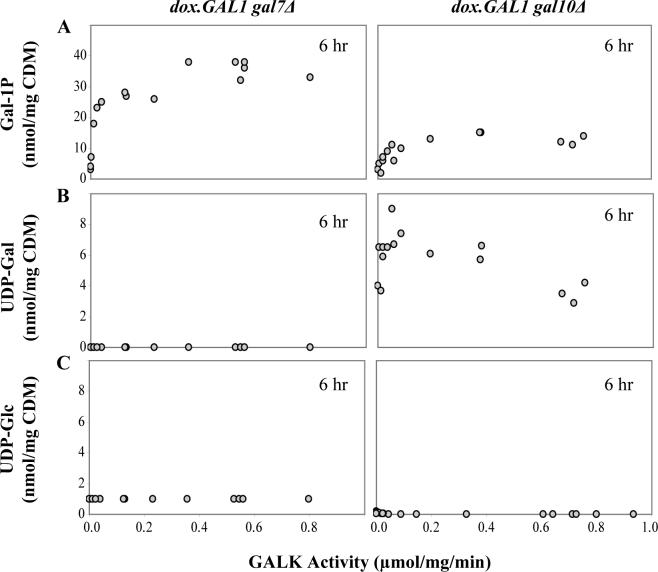
To define the relationship between galactose-mediated growth arrest and actual metabolite accumulation in dox.GAL1gal7Δ and dox.GAL1gal10Δ yeast, we monitored the intracellular levels of gal-1P, UDP-gal, and UDP-glc in cultures of both strains exposed to trace galactose in the presence of titrating levels of doxycycline (Figure 7). All three metabolites were low to undetectable in both strains in the absence of galactose (data not shown). Upon exposure to trace galactose the gal-1P levels in both strains rose precipitously as a function of increasing GALK activity and then leveled off. Although the maximal levels of gal-1P differed by close to a factor of three, the shapes of the curves were comparable, with maximal gal-1P levels achieved in both strains at GALK activities ≥0.2−0.4 μmol/mg/min. The difference in maximal gal-1P values may reflect the fact that the dox.GAL1gal7Δ cultures were exposed to higher concentrations of galactose than were the dox.GAL1gal10Δ cultures (0.02% vs. 0.002%, respectively). As expected, UDP-gal also accumulated in the GALE-null yeast, but not in the GALT-null yeast. Of note, in the GALE-null yeast UDP-gal levels rose even more precipitously as a function of GALK activity than did gal-1P levels, such that maximal UDP-gal was achieved by about 0.05μmol/mg/min GALK. Finally, as expected, UDP-glc levels were depressed in the galactose-challenged dox.GAL1gal10Δ cultures but not in the galactose-challenged dox.GAL1gal7Δ cultures (Figure 7).
Figure 7. Intracellular metabolites in dox.GAL1gal7Δ and dox.GAL1gal10Δ cells measured at t=6 hrs following a galactose challenge.
(A) As expected, both dox.GAL1gal7Δ and dox.GAL1gal10Δ cells accumulated gal-1P in a GALK-dependent manner, such that gal-1P levels rose precipitously as GALK increased from 0 to about 0.2 μmol/mg/min and then leveled off. (B) Also as expected, only the dox.GAL1gal10Δ cells accumulated UDP-gal in a GALK-dependent manner, and the shape of this curve was even more striking; UDP-gal levels rose precipitously as GALK increased from 0 to ∼0.05 μmol/mg/min and then leveled off. (C) Finally, UDP-glc levels were depressed in the galactose-challenged dox.GAL1gal10Δ cultures but not in the galactose-challenged dox.GAL1gal7Δ cultures.
As a further test of the role of gal-1P in galactose-mediated growth arrest of GALT-null and GALE-null yeast, we combined the data from Figures 6B and 7 to view the growth rate of each strain as a function of intracellular gal-1P (Figure 8), rather than as a function of GALK activity (Figure 6B). While both strains showed a clear threshold relationship between gal-1P and inhibition of growth, the shapes of the curves were distinct. Of note, growth of the GALT-null cultures was maximal until gal-1P approached 30 nmol/mg CDM; in contrast, growth rate of the GALE-null cultures was already 50% diminished by gal-1P values of 20 nmol/mg CDM. Both strains were fully growth-arrested when gal-1P values approached ≥35 nmol/mg CDM (Figure 8).
Loss of GALT significantly rescues the growth of galactose-challenged GALE-null yeast
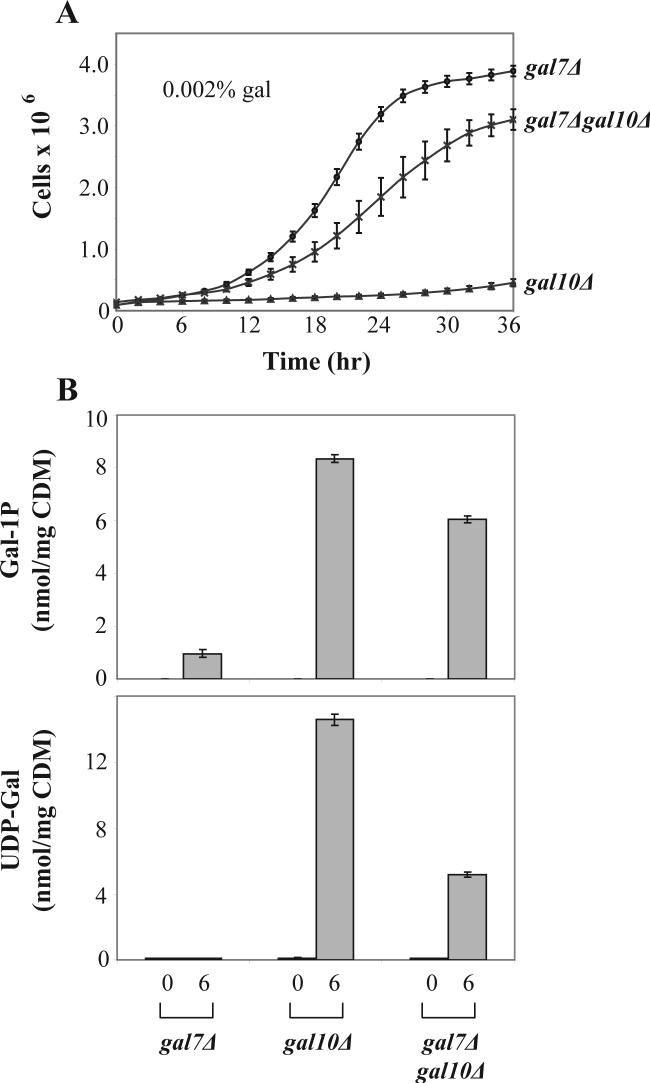
As a final test of the role of gal-1P in mediating the growth arrest of galactose-challenged GALT vs. GALE-null yeast, we created a strain carrying deletions of both GALT and GALE. As expected, in the absence of galactose these cells grew indistinguishably from wild-type, gal7Δ, or gal10Δ cells (data not shown). In the presence of 0.002% galactose, however, which arrests the growth of gal10Δ cells but not gal7Δ cells [17], the gal7Δgal10Δ double deletion cells demonstrated a growth rate only marginally slower than that of their gal7Δ counterparts. Indeed, the slope of the gal7Δgal10Δ growth curve at t=16 hrs was only about 2-fold lower than that of the gal7Δ culture, and more than 12-fold higher than that of the gal10Δ culture (Figure 9, panel A). Loss of GALT therefore was largely protective of the GALE-null cells. Of note, these galactose-challenged, gal7Δgal10Δ double deletion cells demonstrated only a minor dip in their accumulation of gal-1P, but an almost 3-fold drop in UDP-gal accumulation relative to their growth-arrested gal10Δ counterparts. These data clearly contradict the hypothesis that gal-1P alone mediates the galactose-sensitivity of GALE-null cells, and suggest that other factors, likely to include UDP-gal accumulation, must play a role.
Figure 9. Loss of GALT rescues growth of galactose-challenged GALE-null yeast.
(A) Data plotted represent mean ± SEM (n=6). The difference in growth rates, calculated as slopes between t=15 and t=17 hrs, reveals a 2.0-fold decrease between the gal7Δ and gal7Δgal10Δ strains, and a 12.7-fold increase between the gal10Δ and gal7Δgal10Δ strains. (B) Gal-1P levels accumulated in the indicated strains at t=0 and 6 hrs following addition of 0.002% galactose to the culture medium. Values plotted represent mean ± SEM (n=6); both the gal10Δ and gal7Δgal10Δ gal-1P values were statistically significant from those of the gal7Δ strain, as well as statistically significant from one another. (C) UDP-gal levels accumulated in the indicated strains at t=0 and 6 hrs following addition of 0.002% galactose to the culture medium. Values plotted represent mean ± SEM (n=6); both the gal10Δ and gal7Δgal10Δ UDP-gal values were statistically significant from those of the gal7Δ strain, as well as statistically significant from one another.
DISCUSSION
A literature trail extending back more than 40 years implicates gal-1P for a role mediating the toxicity of galactose in eukaryotes deficient in GALT or GALE [15-23]. The nature of that role, however, has remained obscure. Furthermore, whether the role of gal-1P is the same or distinct in GALT vs. GALE-deficient cells has remained unclear. One of the challenges to addressing this point has been that the only way to manipulate gal-1P in living cells was to change the galactose exposure or to eliminate GALK. Changing galactose exposure, by definition, alters variables beyond gal-1P (e.g. galactitol, galactonate). Similarly, eliminating GALK prevents the accumulation of gal-1P in both GALT and GALE-null cells [17], but also prevents the accumulation of UDP-gal and depletion of UDP-glc in GALE-null cells [17]. These data alone therefore cannot resolve the question of whether gal-1P itself plays similar or distinct roles in the galactose-sensitivities of GALT-null and GALE-null yeast.
With the data reported here we have overcome prior limitations to uncouple the level of external galactose exposure from intracellular gal-1P accumulation in yeast. In brief, we have generated and applied a doxycycline-regulated allele of GALK to enable quantitative analysis of the relationship between gal-1P accumulation and growth rate in GALT-null vs. GALE-null yeast. Our results demonstrate that these profiles are markedly distinct. Furthermore, we demonstrate that loss of GALT from galactose-challenged cells already null for GALE barely impacts the accumulation of gal-1P, but significantly rescues growth.
Gal-1P, growth arrest, and galactosemia
Gal-1P is a metabolite that exists only transiently in humans, yeast, and many other eukaryotes. Indeed, the GALT enzyme that normally catabolizes gal-1P demonstrates a mechanism and steady-state condition primed to act quickly and efficiently to eliminate gal-1P[1]; a sizeable fraction of the GALT enzyme detected in human hemolysates or yeast lysates presents as a covalent UMP-bound reaction intermediate, poised to bind and act upon gal-1P [29]. Perhaps reflecting this situation, gal-1P remains undetectable in normal yeast and human cells even under conditions of excess galactose exposure.
Why might accumulation of gal-1P be detrimental? A variety of intriguing hypotheses have been put forward. For example, Gitzelman suggested that gal-1P may inhibit one or more important enzymes that normally metabolize glc-1P, with inhibition constants in the concentration ranges observed in patient hemolysates (>5 mM untreated, ∼0.1 mM treated) [30]. Some of the enzymes reportedly inhibited include glucose-6-phosphatase, phosphoglucomutase, liver glycogen phosphorylase, UDP-glucose pyrophosphorylase, glucose-6-phosphate dehydrogenase, and at high concentrations, UDP-galactose galactosyltransferase (reviewed in [30]). Clearly these are important enzymes. More recently, Bhat also raised the possibility that gal-1P may inhibit the enzyme IMPase, thereby impacting phosphatidylinositol bisphosphate [PI(P)2] dependent signaling in the brain [31].
The work reported here demonstrates two important points with regard to gal-1P and growth arrest in galactose-challenged yeast. First, there is a clear positive correlation between gal-1P accumulation and growth-impairment in yeast. This is true for both GALT-null and GALE-null yeast, and the nature of the relationship appears to involve a threshold of sensitivity for both strains. However, the actual thresholds appear to differ between the strains, such that GALE-null yeast appear markedly more sensitive than do GALT-null yeast (Figure 8). This appearance may be deceptive, however, because at the same time that gal-1P is rising in GALE-null yeast, UDP-gal is also rising and UDP-glc is falling (Figure 7). In fact, UDP-gal rises even more sharply with increased GALK activity than does gal-1P.
The second important point about gal-1P demonstrated here is that gal-1P alone cannot explain the differential growth rates observed in galactose-sensitive yeast. This point is illustrated in Figure 9; gal7Δgal10Δ double deletion yeast accumulated almost comparable levels of gal-1P relative to gal10Δ single-deletion yeast, and yet these double null cells demonstrated significantly rescued growth under conditions of comparable galactose challenge (0.002% galactose). Gal-1P therefore may be sufficient, at high enough concentrations, to halt yeast growth (e.g. for GALT-null cells), but in GALE-null cells a gal-1P threshold alone is unlikely to be the limiting factor.
UDP-glc and UDP-gal
We believe the limiting factor for galactose-sensitivity of GALE-null yeast may be accumulation of UDP-gal and/or depletion of UDP-glc. While reports of abnormalities in the UDP-gal and/or UDP-glc levels or ratios of galactose-challenged patients or mammalian cells with impaired GALT remain controversial [4,12,32-34], it is clear that UDP-gal accumulates dramatically in galactose-challenged patients and mammalian cells with impaired GALE [11,14,35]. Similarly, galactose-challenged GALE-null yeast demonstrate marked accumulation of UDP-gal and also some depletion of UDP-glc [17,19]. Small or large, these variations in UDP-gal and UDP-glc may be key to the pathophysiology of classic and/or epimerase-deficiency galactosemia because they influence the substrate pools required for biosynthesis of important glycoproteins and glycolipids in cells.
Consistent with this hypothesis, we have previously reported that epimerase-deficient Chinese Hamster Ovary (ldlD) cells arrest DNA synthesis upon galactose-challenge, but are rescued from this arrest by uridine treatment which relieves the apparent UDP-glc depletion, although it fails to resolve the abnormal accumulation of gal-1P [35]. In the work reported here we confirm that epimerase-deficient yeast accumulate both UDP-gal and gal-1P in a GALK-dependent manner upon galactose-challenge. We then go on to demonstrate that the galactose-sensitivity of these cells can be partially rescued by a second mutation, loss of GALT, which lowers the accumulation of UDP-gal, but exacerbates rather than lowers the accumulation of gal-1P. These data rule out the possibility that GALE-null yeast are simply hyper-sensitive to gal-1P, and suggest instead that elevated UDP-gal and/or depleted UDP-glc may be the limiting factors defining galactose-sensitivity in these GALE-null cells.
Conclusions and relevance for patients
The results of our studies reported here shed light on the role of gal-1P as a mediator of galactose sensitivity in GALT-null vs. GALE-null yeast. In short, our data resolve an important basic science question by confirming that although gal-1P is key to the galactose-sensitivity of both GALT-null and GALE-null yeast, the mechanism of that sensitivity in the two strains is likely to be distinct. While not directly testing modes of pathophysiology in patients, these data nonetheless raise the important question of what are the appropriate metabolites to monitor in patients with classic vs. epimerase-deficiency galactosemia. At present most patients, whether GALT- or GALE-impaired, are followed with regard to hemolysate gal-1P level and perhaps blood or urinary galactitol. Our data suggest that it might be more appropriate to follow both gal-1P and UDP-gal levels in patients with GALE-deficiency galactosemia.
ACKNOWLEDGMENTS
The authors would like to recognize the many helpful contributions of all members of the Fridovich-Keil lab, and also gratefully acknowledge funding support from the National Institute of Health in the form of NRSA Award DK074297 (to K. Ross) and R01 Awards DK059904 and DK046403 (to J. Fridovich-Keil).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Holden HM, Rayment I, Thoden JB. Structure and function of enzymes of the Leloir pathway for galactose metabolism. J Biol Chem. 2003;278:43885–43888. doi: 10.1074/jbc.R300025200. [DOI] [PubMed] [Google Scholar]
- 2.Piller F, Hanlon MH, Hill RL. Co-purification and characterization of UDP-glucose 4-epimerase and UDP-N-acetylglucosamine 4-epimerase from porcine submaxillary glands. J. Biol. Chem. 1983;258:10774–10778. [PubMed] [Google Scholar]
- 3.Schulz J, Watson A, Sanders R, Ross K, Thoden J, Holden H, Fridovich-Keil J. Determinants of function and substrate specificity in human UDP-galactose 4'-epimerase. J Biol Chem. 2004;279:32796–32803. doi: 10.1074/jbc.M405005200. [DOI] [PubMed] [Google Scholar]
- 4.Tyfield L, Walter J. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C, Beaudet A, Sly W, Valle D, Childs B, Kinzler K, Vogelstein B, editors. McGraw-Hill; New York: 2002. [Google Scholar]
- 5.Zaffanello M, Zamboni G, Schadewaldt P, Borgiani P, Novelli G. Neonatal screening, clinical features and genetic testing for galactosemia. Genet Med. 2005;7:211–212. doi: 10.1097/01.GIM.0000157127.14267.DB. [DOI] [PubMed] [Google Scholar]
- 6.Waggoner DD, Buist NRM, Donnell GN. Long-term Prognosis in Galactosemia: Results of a Survey of 350 Cases. J. Inher. Metab. Dis. 1990;13:802–818. doi: 10.1007/BF01800204. [DOI] [PubMed] [Google Scholar]
- 7.Arn P. Galactosemia. Curr Treat Options Neurol. 2003;5:343–345. doi: 10.1007/s11940-003-0040-x. [DOI] [PubMed] [Google Scholar]
- 8.Antshel K, Epstein I, Waisbren S. Cognitive strengths and weaknesses in children and adolescents homozygous for the galactosemia Q188R mutation: a descriptive study. Neuropsychology. 2004;18:658–664. doi: 10.1037/0894-4105.18.4.658. [DOI] [PubMed] [Google Scholar]
- 9.Ridel K, Leslie N, Gilbert D. An updated review of the long-term neurological effects of galactosemia. Pediatr Neurol. 2005;33:153–161. doi: 10.1016/j.pediatrneurol.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Fridovich-Keil J. Galactosemia: the good, the bad, and the unknown. J Cell Physiol. 2006;209:701–705. doi: 10.1002/jcp.20820. [DOI] [PubMed] [Google Scholar]
- 11.Openo K, Schulz J, Vargas C, Orton C, Epstein M, Schnur R, Scaglia F, Berry G, Gottesman G, Ficicioglu C, Slonim A, Shroer R, Yu C, Rangel V, Kenan J, Lamance K, Fridovich-Keil J. Epimerase-deficiency galactosemia is not a binary condition. Am. J. Hum. Genet. 2006;78:89–102. doi: 10.1086/498985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holton JB, Walter JH, Tyfield LA. In: Metabolic and Molecular Bases of Inherited Disease. 8th Edition Scriver CR, Beaudet AL, Sly SW, Valle D, A., Childs B, Kinzler KW, Vogelstein B, editors. McGraw Hill; 2000. pp. 1553–1587. [Google Scholar]
- 13.Leslie ND. Insights into the pathogenesis of galactosemia. Annu Rev Nutr. 2003;23:59–80. doi: 10.1146/annurev.nutr.23.011702.073135. [DOI] [PubMed] [Google Scholar]
- 14.Walter JH, Roberts REP, Besley GTN, Wraith JE, Cleary MA, Holton JB, MacFaul R. Generalised uridine diphosphate galactose-4-epimerase deficiency. Arch Dis Child. 1999;80:374–376. doi: 10.1136/adc.80.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wohlers T, Fridovich-Keil JL. Studies of the V94M-substituted human UDP-galactose-4-epimerase enzyme associated with generalized epimerase-deficiency galactosemia. J. Inher. Metab. Dis. 2000;23:713–729. doi: 10.1023/a:1005682913784. [DOI] [PubMed] [Google Scholar]
- 16.Riehman K, Crews C, Fridovich-Keil JL. Relationship between genotype, activity, and galactose sensitivity in yeast expressing patient alleles of human galactose-1-phosphate uridylyltransferase. J. Biol. Chem. 2001;276:10634–10640. doi: 10.1074/jbc.M009583200. [DOI] [PubMed] [Google Scholar]
- 17.Ross KL, Davis CN, Fridovich-Keil JL. Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Mol Gen Metab. 2004;83:103–116. doi: 10.1016/j.ymgme.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Wasilenko J, Lucas M, Thoden J, Holden H, Fridovich-Keil J. Functional characterization of the K257R and G319E hGALE alleles found in patients with ostensibly peripheral epimerase deficiency galactosemia. Mol. Gen. Metab. 2005;84:32–38. doi: 10.1016/j.ymgme.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Wasilenko J, Fridovich-Keil J. Relationship between UDP galactose 4'-epimerase activity and galactose sensitivity in yeast. J Biol Chem. 2006;281:8443–8449. doi: 10.1074/jbc.M600778200. [DOI] [PubMed] [Google Scholar]
- 20.Douglas HC, Hawthorne DC. Enzymatic expression and genetic linkage of genes controlling galactose utilization in Saccharomyces. Genetics. 1964;49:837–844. doi: 10.1093/genetics/49.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta DV, Kabir A, Bhat PJ. Expression of human inositol monophosphatase suppresses galactose toxicity in Saccharomyces cerevisiae: possible implications for galactosemia. Bioch et Biophys Acta. 1999;1454:217–226. doi: 10.1016/s0925-4439(99)00037-x. [DOI] [PubMed] [Google Scholar]
- 22.Kabir MA, Khanday FA, Mehta DV, Bhat PJ. Multiple copies of MRG19 suppress transcription of the GAL1 promoter in a GAL80-dependent manner in Saccharomyces cerevisiae. Mol Gen Genet. 2000;262:1113–1122. doi: 10.1007/pl00008654. [DOI] [PubMed] [Google Scholar]
- 23.Lai K, Elsas L. Overexpression of human UDP-glucose pyrophosphorylase rescues galactose-1-phosphate uridyltransferase-deficient yeast. Biochem Biophys Res Commun. 2000;271:392–400. doi: 10.1006/bbrc.2000.2629. [DOI] [PubMed] [Google Scholar]
- 24.Guthrie C, Fink G, editors. Guide to Yeast Genetics and Molecular Biology Vol. 194. Methods in Enzymology. Academic Press, Inc.; San Diego: 1991. [PubMed] [Google Scholar]
- 25.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belli G, Gari E, Aldea M, Herrero E. Functional analysis of yeast essential genes using a promoter-substitution cassette and the tetracycline-regulatable dual expression system. Yeast. 1998;14:1127–1138. doi: 10.1002/(SICI)1097-0061(19980915)14:12<1127::AID-YEA300>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 27.Belli G, Gari E, Piedrafita L, Aldea M, Herrero E. An activator/repressor dual system allows tight tetracycline-regulated gene expression in budding yeast. Nucleic Acids Research. 1998;26:942–947. doi: 10.1093/nar/26.4.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston M. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol Rev. 1987;51:458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson JM, Wells L, Fridovich-Keil JL. Covalent heterogeneity of the human enzyme galactose-1-phosphate uridylyltransferase. J. Biol. Chem. 2000;275:30088–30091. doi: 10.1074/jbc.M005259200. [DOI] [PubMed] [Google Scholar]
- 30.Gitzelmann R. Galactose-1-phosphate in the pathophysiology of galactosemia. Eur J. Pediatrics. 1995;154(Suppl 2):S45–49. doi: 10.1007/BF02143803. [DOI] [PubMed] [Google Scholar]
- 31.Bhat PJ. Galactose-1-phosphate is a regulator of inositol monophosphatase: a fact or a fiction? Med Hypotheses. 2003;60:123–128. doi: 10.1016/s0306-9877(02)00347-x. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y-K, Kaufman FR, Donnell GN, Giudici T, Alfi O, Ng WG. HPLC analysis of uridine diphosphate sugars: decreased concentrations of uridine diphosphate galactose in erythrocytes and cultured skin fibroblasts from classical galactosemia patients. Clinica Chimica Acta. 1995;240:21–33. doi: 10.1016/0009-8981(95)06123-7. [DOI] [PubMed] [Google Scholar]
- 33.Segal S. Defective galactosylation in galactosemia: is low cell UDPgalactose an explanation? Eur J Pediatr. 1995;154:S65–S71. doi: 10.1007/BF02143806. [DOI] [PubMed] [Google Scholar]
- 34.Lai K, Langley S, Khwaja F, Schmitt E, Elsas L. GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology. 2003;13:285–294. doi: 10.1093/glycob/cwg033. [DOI] [PubMed] [Google Scholar]
- 35.Schulz J, Ross K, Malmstrom K, Krieger M, Fridovich-Keil J. Mediators of galactose sensitivity in UDP-galactose 4'-epimerase-impaired mammalian cells. J Biol Chem. 2005;280:13493–13502. doi: 10.1074/jbc.M414045200. [DOI] [PubMed] [Google Scholar]