Abstract
Wnt signaling activates the canonical pathway and induces the accumulation of non-phosphorylated beta-catenin (NPBC) in the nucleus. Although this pathway plays an important role in the maintenance of haematopoietic stem cells as well as in oncogenesis, the significance of nuclear NPBC remains unclear in malignant haematopoiesis. This study examined the expression of nuclear NPBC in bone marrow specimens from 54 and 44 patients with de novo acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS), respectively. On immunohistochemistry with an anti-NPBC antibody, the nuclei were positively stained in 22 and 18 of AML and MDS specimens, respectively. Staining of nuclear NPBC was associated with AML subtypes (M6 and M7), low complete remission (CR) rate, and poor prognosis. Nuclear NPBC was also associated with a high score when using the International Prognostic Scoring System (IPSS) for MDS and with −7/−7q and complex karyotypes. These findings suggest that in situ detection of nuclear NPBC by immunohistochemistry could provide new insights into the pathogenesis and prognosis of AML and MDS.
Keywords: beta-catenin, non-phosphorylated beta-catenin, acute myeloid leukaemia, myelodysplastic syndrome, immunohistochemistry
The Wnt/beta-catenin pathway is involved in the self-renewal and proliferation of haematopoietic stem cells (Reya et al, 2003; Willert et al, 2003). Signaling is initiated by binding of Wnt proteins to transmembrane receptors of the Frizzeled family (Giles et al, 2003). In the absence of Wnt signals, a dedicated complex of proteins that includes the tumor suppressor gene product APC, axin, and glycogen synthase kinase-3-beta (GSK3-beta) phosphorylates specific serine and threonine residues within the N-terminal region of beta-catenin, which leads to the ubiquitination of beta-catenin and its degradation by proteasomes (Conacci-Sorrell et al, 2002; Noort et al, 2002; Staal et al, 2002; Giles et al, 2003). Wnt signals block GSK3beta activity, resulting in the accumulation of non-phosphorylated beta-catenin (NPBC), which is finally translocated to the nucleus (Noort et al, 2002; Staal et al, 2002). Nuclear NPBC interacts with T-cell transcription factor (TCF) and lymphoid enhancer factor (LEF), and it activates target genes such as MYC and CCND1 (He et al, 1998; Tetsu & Maccormick, 1999). Therefore, nuclear NPBC is known to be oncogenic in many solid tumors (Bienz & Clevers, 2000; Polakis, 2000). Mutations of APC, beta-catenin, or axin, which are observed in various tumors, lead to stabilization of NPBC (Morin et al, 1997; Barker & Clevers, 2000).
In the bone marrow (BM), Wnt proteins activate the beta-catenin pathway and the non-obese severe combined immunodeficient (NOD-SCID)-repopulating capacity of normal haematopoietic stem cells. They lead to increased expression of HOXB4 and NOTCH1 implicated in the self-renewal of haematopoietic stem cells (Reya et al, 2003). Up-regulation of the beta-catenin pathway has been suggested in chronic myeloid leukaemia (CML)-derived granulocyte-macrophage progenitor cells (GMPs) and multiple myeloma (MM) cells (Derksen et al, 2004; Jamieson et al, 2004). Furthermore, beta-catenin reportedly plays a significant role in promoting cell proliferation, adhesion, and survival in vitro (Chung et al, 2002). The expression of beta-catenin is also enhanced by oncogenic FLT3 signals and associated with poor prognosis (Tickenbrock et al, 2005; Ysebaert et al, 2006). However, there are some contradictory reports. Studies of conditional knock-out mice with a beta-catenin gene (Ctnnb1) deletion indicated that Ctnnb1 is not indispensable for haematopoiesis (Cobas et al, 2004). Furthermore, an active form of Ctnnb1 compromised haematopoietic stem cell maintenance and blocked differentiation in transgenic mice experiments (Simon et al, 2005). The role of the Wnt/beta-catenin pathway in malignant haematopoiesis therefore needs to be further elucidated.
According to previous studies, the expression of beta-catenin is associated with activation of the Wnt pathway as well as poor prognosis (Tickenbrock et al, 2005; Ysebaert et al, 2006). However, beta-catenin is associated not only with Wnt signaling but also with adherence junctions (Conacci-Sorrell et al, 2002). It is anchored to the cell inner surface membrane via cadherins. In normal bone marrow (BM), the vascular endothelium expresses a significantly higher amount of beta-catenin relative to the level in haematopoietic cells. Accordingly, immunohistochemical detection of nuclear NPBC would enable a better understanding of the role of beta-catenin in leukaemia.
This study investigated the subcellular localization of beta-catenin in BM specimens from acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) patients using two anti-beta-catenin antibodies: one against C-terminal peptides and another against N-terminal non-phosphorylated peptides. The latter antibody detected nuclear NPBC, and positive staining for nuclear NPBC was associated with particular clinical characteristics of AML and MDS.
Materials and methods
Patient samples
For clinical samples, BM clots were obtained during routine diagnostic procedures. Beta-catenin expression was analyzed in BM specimens from patients newly diagnosed at the Nagoya University Hospital between 2000 and 2006 (Table I). The de novo AML patients consisted of 35 men and 19 women with a median age of 53 years (range, 20–81 years), and FLT3 mutations were detected in seven of 22 patients with AML (31·8%). The MDS patients consisted of 28 men and 16 women with a median age of 57 years (range, 22–89 years). BM mononuclear cells were harvested by standard Ficoll/Paque density gradient centrifugation (Amersham Pharmacia Biosciences, Roozendaal, the Netherlands), and were suspended in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 IU/ml of penicillin G and 100 μg/ml of streptomycin.
Table I.
Clinical characteristics of AML and MDS patients according to nuclear NPBC expression.
| Nuclear NPBC+ | Nuclear NPBC- | P-value | |
|---|---|---|---|
| Patients withde novo AML | 22 | 32 | |
| Age (median) | 54 (20–81) | 53 (18–71) | NS |
| Sex/male/female | 18/4 | 17/15 | 0·005 |
| Laboratory data | |||
| WBC (×109/l; median) | 3·5 (0·8–92·5) | 5·3 (0·7–202·1) | NS |
| Hb (g/l; median) | 74 (43–134) | 98 (36–141) | 0·01 |
| PLT (×109/l; median) | 7·6 (0·3–170) | 4·2 (1·1–27·3) | NS |
| PB blasts (%; median) | 24 (0–82) | 39 (0–99) | NS |
| BM blasts (%; median) | 46·2 (20–86·5) | 77·5 (29–98) | 0·02 |
| CR rate | 13/22 (59·1%) | 24/27 (88·9%) | 0·01 |
| Relapse rate | 16/21 (76·2%) | 14/24 (58·3%) | 0·03 |
| Patients with MDS | 18 | 26 | |
| Age | 59 (26–76) | 57 (22–89) | |
| Sex/male/female | 12/6 | 16/10 | 0·05 |
| Laboratory data | |||
| WBC (×109/l; median) | 2·9 (1·2–9·0) | 2·6 (1·6–5·9) | NS |
| Hb (g/l; median) | 75 (46–151) | 85 (47–127) | NS |
| PLT (×109/l; median) | 79 (7–122) | 44 (7–400) | NS |
| BM blasts (%; median) | 3 (0–30) | 5 (0–30) | NS |
| IPSS score* | |||
| Low risk | 0 | 4 | NS |
| Intermediate-1 | 4 | 12 | NS |
| Intermediate-2 | 4 | 4 | NS |
| High risk | 3 | 1 | 0·04 |
NPBC, non-phosphorylated beta-catenin; AML, acute myeloid leukaemia; MDS, myelodysplastic syndrome; WBC, white blood cell count; Hb, hemoglobin concentration; PLT, platelet count; PB, peripheral blood; BM, bone marrow; CR, complete remission; IPSS, International Prognostic Scoring System.
Full data was available in 32 of the 44 patients with MDS.
Antibodies
For immunohistochemical and immunoblot studies, two monoclonal antibodies were used; one was against C-terminal peptides (clone14, IgG1; BD Transduction Laboratories/Life Science Research, Heidelberg, Germany), enabling recognition of pan beta-catenin (PBC), and the other was against N-terminal-peptides (clone 8E4, IgG1; Alexis Biochemicals, Lausanne, Switzerland), enabling recognition of NPBC.
Immunohistochemical staining
Samples were fixed with ice-cold 4% paraformaldehyde for 16–24 h, embedded in paraffin, sectioned transversely (thickness, 3 μm), and processed for immunohistochemistry to determine the localization of beta-catenin. After removal of paraffin with xylene and dehydration with a series of ethanol solutions, the tissue sections were subjected to microwave irradiation (750 W) for 15 min in 0·01 mol/l citrate buffer (pH 6·0). The sections were then placed in an automated immunostainer (Ventana Medical Systems, Tucson, AZ, USA) as described (Xu et al, 2002). For negative controls, primary antibodies were replaced with mouse IgG. The subcellular distribution of beta-catenin (i.e. restriction to the nucleus or presence in the membrane) was assessed without knowledge of the French-America-British (FAB) subtypes, FLT3 mutations or karyotypes. We investigated a single case twice for NPBC expression. The entire section was screened to find the region with the highest immunostaining. The score was determined in each case after counting at least 500 nuclei in 3–5 randomly selected regions. When 20% or more of the BM mononuclear cells were positive for nuclear staining of NPBC, they were classified as nuclear NPBC+. The cut-off value of 20% was determined by the median distribution of the percentage of BM mononuclear cells stained by NPBC. As described in the results, some erythroblasts were positive for NPBC but the number was <20% except in the case of M6 patients. On the other hand, almost all blasts in M7 and other cases tested positive. The discrimination of cell types based on the 20% criterion therefore enabled a clear delineation.
Immunoblotting
Cell lysates from AML cells were extracted as previously described (Ozeki et al, 2004). A total of 1 × 106 cells were directly lysed in sample buffer and then subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis on a 10% gel, and the separated proteins were transferred to a polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA, USA). The membrane was initially incubated at room temperature for 1 h with 5% nonfat milk and 0·1% Tween-20 in Tris-buffered saline and then overnight with mouse monoclonal antibodies at a 1:2000 dilution in the same solution. After washing, the membrane was incubated for 1 h with a 1:5000 dilution of horseradish peroxidase-conjugated mouse antibodies to mouse IgG (MBL, Amersham, Bucks, UK), and immune complexes were then detected with enhanced chemiluminescence (ECL) reagents (Amersham).
Statistical analysis
The χ2 test was used to calculate the difference of frequencies between nuclear NPBC+ and NPBC− groups. The Mann–Whitney U-test was used to compare continuous variables. Kaplan–Meier curves were drawn using statview software (Macintosh; SAS Institute, Cary, NC, USA). P-values <0·05 were considered significant.
Results
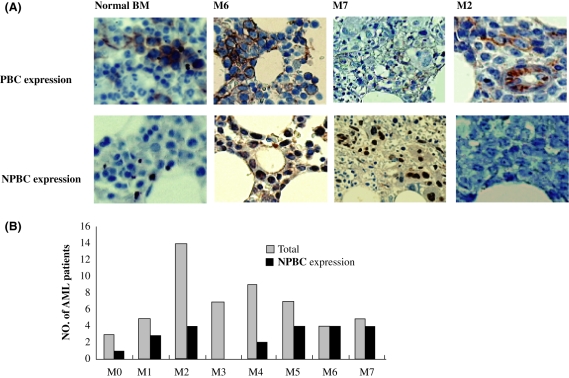
Using an anti-beta-catenin C-terminal peptide antibody, beta-catenin was stained in the membrane and cytoplasm of erythroid cells from normal BM. In de novo AML specimens, significant staining was observed only in M6. This antibody also detected BM vessels whose density was increased in AML specimens as previously reported (Serinsöz et al, 2004; Fig 1A). On the other hand, an anti-N-terminal nonphosphorylated peptide antibody gave no significant staining in the normal BM. In AML specimens, nuclear NPBC was detected in erythroid blasts, megakaryoblasts and some myeloblasts (Fig 1A). In M6 specimens, nuclear NPBC was detected in 30–80% of myelomonocytic cells and nearly 100% of erythroblastic cells (Fig 1A). In M7 specimens, megakaryocytes were also strongly positive for nuclear NPBC (Fig 1A). In total, 20% or more of the BM mononuclear cells were positive for nuclear NPBC in 22 (40·7%) of 54 AML patients (Table I, Fig 1B). There was a strong male predominance of nuclear NPBC+ cases, comprising 81·8% (18/22) in AML patients (Table I). However, the reason for this is unclear. In our cohort study, the karyotypes of female patients correlated to t(8;21)/t(15;17), which did not express nuclear NPBC. Thus the small numbers of studied patients seem to give some bias to the male predominance. A large-scale study is necessary to confirm this association. Nuclear NPBC+ staining was closely associated with AML subtype: it occurred frequently (8/9) in M6 and M7 and rarely (0/7) in M3 (Fig 1B), and nuclear NPBC+ was preferentially detected in erythroid and megakaryoblastic leukaemia compared to other myeloid leukaemias (M6–M7 vs. M0–M5, P < 0·001).
Fig 1.
Specific association of NPBC expression with FAB subtypes of AML specimens. (A) In normal BM clots, PBC is expressed on the membranes of erythroid cells as well as endothelial cells. NPBC was not detected in normal BM clots. Most erythroid cells and endothelial cells showed cell membrane expression of PBC without expression in the nucleus or cytoplasm. NPBC was expressed in leukemia cells and was always restricted to the nucleus, especially in M6 and M7 specimens. Prominent staining of endothelial cells was seen in the vascular tissue in BM derived from an M2 patient with, though NPBC staining was negative in the same specimen. Original magnification ×40. (B) The graph presents data based on nuclear NPBC staining in paraffin sections from 54 patients with AML.
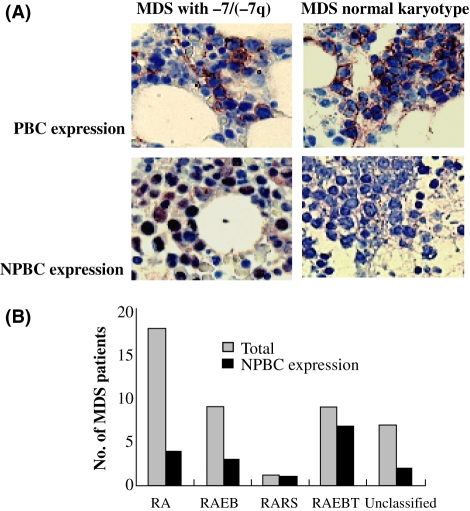
In MDS specimens, erythroid cells and endothelial cells were stained with the anti-beta-catenin C-terminal peptide antibody (Fig 2A). As observed for AML specimens, the cytoplasm and inner membrane were stained by this antibody. The anti-beta-catenin N-terminal nonphosphorylated peptide antibody detected nuclear staining in myeloblasts and erythroblasts that was similar to the pattern seen in AML cases (Fig 2A). Nuclear NPBC was found in 18 (40·9%) of 44 MDS patients, and was related to the FAB classification of MDS (Table I, Fig 2B). Nuclear NPBC+ was preferentially detected in refractory anaemia with excess blasts in transformation (RAEBT) compared to other MDS subtypes [RAEBT versus refractory anaemia (RA)/refractory anaemia with ringed sideroblasts (RARS)/RAEB, P = 0·01].
Fig 2.
Specific association of NPBC expression with FAB subtypes of MDS specimens. (A) Most erythroid cells and endothelial cells showed cell membrane expression of PBC without expression in the nucleus or cytoplasm. NPBC was expressed in erythroid cells and was always restricted to the nucleus, especially in refractory anaemia with excess blasts in transformation (RAEBT) and MDS specimens with −7/−7(q). Original magnification ×40. (B) The graph presents data obtained for nuclear NPBC staining in paraffin sections from 44 patients with MDS. RA, refractory anaemia; RARS, RA with ringed sideroblasts; RAEB, RA with excess blasts.
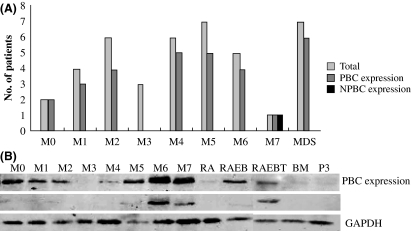
To confirm whether these two antibodies recognized beta-catenin, a total of 41 samples from AML and MDS patients were subjected to immunoblot analysis. The anti-beta-catenin C-terminal peptide antibody detected bands at a molecular weight of 95 kDa, corresponding to beta-catenin, in most samples except for M3 (Fig 3A). The anti-N-terminal nonphosphorylated peptide antibody gave bands of the same size in only a few samples of AML and MDS (Fig 3B). The results of immunoblotting corresponded to those of immunostaining, although the latter was more sensitive than the former.
Fig 3.
Beta-catenin expression of AML cells assessed by immunoblotting. (A) The graph shows data obtained for the expression of NPBC in mononuclear cells from 41 patients with AML, and the data indicate that expression of NPBC is specific to some FAB subtypes, especially to M6 and M7. (B) Representative immunoblots for PBC and NPBC in AML samples. RA, refractory anaemia; RARS, RA with ringed sideroblasts; RAEB, RA with excess blasts; RAEBT, refractory anaemia with excess blasts in transformation.
The above findings suggest that expression of nuclear NPBC could be used to identify some subsets of AML and MDS. Next we studied whether nuclear NPBC was associated with chromosomal abnormalities or genetic alterations. Previous studies suggested that AML-associated translocations, such as t(8;21) and t(15;17), contributed to the activation of gamma-catenin, or that FLT3 mutation might be associated with the stabilization of beta-catenin. In this study, however, nuclear NPBC was never detected in AML with t(8;21) or t(15;17). In AML/MDS with −7/−7q and a complex karyotype, nuclear NPBC was frequently detected (P = 0·007 and P = 0·02, respectively; Table II). Moreover, detection was not related to FLT3 internal tandem duplication (ITD; Table II).
Table II.
Cytogenetic abnormalities and FLT3 mutation according to nuclear NPBC expression.
| Nuclear NPBC+ (N) | Nuclear NPBC− (N) | P-value | |
|---|---|---|---|
| Karyotypes | |||
| t(8;21) | 0 | 3 | NS |
| t(15;17) | 0 | 4 | NS |
| −5/−5q | 4 | 1 | NS |
| −7/−7q | 12 | 6 | 0·007 |
| Complex | 13 | 7 | 0·02 |
| Others | 19 | 2 | NS |
| Normal | 10 | 31 | 0·0003 |
| Unknown | 7 | 1 | 0·02 |
| FLT3 mutation | |||
| Wild type | 3 | 12 | 0·006 |
| ITD | 2 | 5 | NS |
Patients are counted more than once due to the coexistence of more than one cytogenetic abnormality. Complex: patients had three or more cytogenetic abnormalities.
NPBC, non-phosphorylated beta-catenin; ITD, internal tandem repeat; NS, not significant.
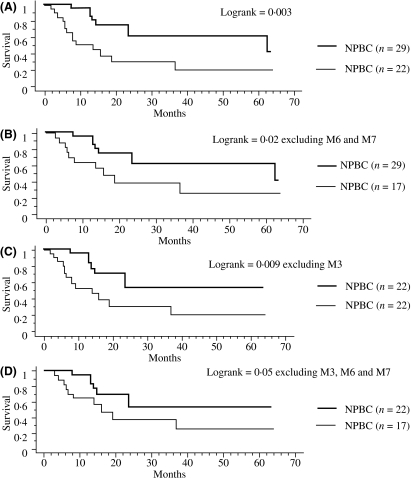
Finally, we studied whether clinical characteristics and outcome were different between nuclear NPBC+ and NPBC− AML patients. NPBC+ AML patients showed significantly lower hemoglobin levels, lower blast percentages in the BM, and lower CR rates (Table I). There were no significant differences between the NPBC+ and NPBC− groups in the MDS patients (Table I). However, nuclear NPBC was associated with a high International Prognostic Scoring System (IPSS) score (Table I). Of note, nuclear NPBC+ AML patients had worse overall survival than NPBC− AML patients (Fig 4A). Even if the M6/M7 subtype and/or M3 subtype was excluded from the analysis, there was still a significant association between nuclear NPBC+ and survival (Fig 4B–D).
Fig 4.
Kaplan–Meier cumulative survival curves were calculated for 51 AML patients (A) and 46 patients excluding subtype M6/M7 (B), 44 patients excluding subtype M3 (C), 39 patients excluding subtype M3/M6/M7 (D), respectively, according to the presence of nuclear NPBC. Comparison of the survival curves using the log-rank test identified nuclear NPBC+ as a prognostic factor.
Discussion
This study used nuclear NPBC as a biomarker for the activated Wnt/beta-catenin signaling pathway. The anti-beta-catenin C-terminal peptide antibody detected total beta-catenin in immunoblots but mainly cytoplasmic and membrane-associated beta-catenin in immunohistological analysis, whereas the anti-beta-catenin N-terminal nonphospholylated peptide antibody detected only nuclear beta-catenin in both analyses (data not shown). Accordingly, it was concluded that the nuclear staining with the latter antibody identified nuclear non-phosphorylated beta-catenin. Nuclear NPBC was detected in 22 (40·7%) of 54 AML patients and 18 (40·9%) of 44 MDS patients. Positive staining of nuclear NPBC was associated with particular AML subtypes (M6 and M7), a low complete remission rate, and poor prognosis. The presence of nuclear NPBC was also associated with a high IPSS score of MDS and with −7/−7q and complex karyotypes.
Previous reports indicated that a significant proportion of AML samples expressed beta-catenin on immunoblot analysis. The expression of beta-catenin is related to CD34 expression, poor prognosis and clonogenic capacity ex vivo (Ysebaert et al, 2006). Furthermore, beta-catenin is expressed in normal CD34+ progenitor cells and the expression level is reduced upon differentiation (Simon et al, 2005). In these studies, however, the total beta-catenin level was analyzed only by immunoblot analysis. Beta-catenin is expressed not only as nuclear NPBC but also as a cadherin-associated protein in the inner cytomembrane (Conacci-Sorrell et al, 2002). The present study found that normal erythroblasts expressed cytoplasmic or membrane-associated beta-catenin but not nuclear NPBC (Fig 1A). Both membrane-associated and nuclear beta-catenin was expressed in malignant erythroblasts in subtype M6 (Fig 1A). These findings suggest that increased nuclear NPBC levels were the result of aberrant signal transduction in the Wnt pathway or an abnormality of beta-catenin itself.
In this study, the expression levels of total and non-phosphorylated beta-catenin were correlated but varied significantly among leukemia samples. In M6 and M7 samples, the expression of beta-catenin was significantly augmented, whereas it was hardly detected in M3 and normal BM samples. These variations suggest the possibility that Wnt/beta-catenin signaling is mediated by multiple factors, such as immaturity, lineage, and oncogenic signals.
We established a correlation between nuclear NPBC+ and poor survival in AML patients. The prognostic value of total beta-catenin expression has been previously studied in AML patients (Ysebaert et al, 2006), but the present study clearly showed for the first time that nuclear NPBC is associated with prognosis. The association was still observed even if M6/M7 and/or M3 patients were excluded from the analysis. Nuclear NPBC might be a new prognostic marker for AML and MDS that can be evaluated by histopathological examination.
Wnts are a family of paracrine and autocrine factors that regulate cell growth and cell fate (Reya et al, 2003). The Wnt autocrine signaling mechanism was initially discovered in human breast and ovarian tumor cell lines as well as in MM primary samples (Bafico et al, 2004; Derksen et al, 2004). Since several Wnt family members have been reported in BM stromal cells, it is possible that leukaemia cells respond to different proteins of the Wnt/beta-catenin pathway secreted by stromal cells in a paracrine fashion (Austin et al, 1997; Van Den Berg et al, 1998; Etheridge et al, 2004). Nuclear NPBC was detected in some cell lines only when they were transplanted in non-obese diabetic/severe combined immunodeficient/gammacnull (NOG) mice (data not shown). Thus, the leukaemia niche may have an important role in nuclear NPBC expression during AML.
MDS is a clonal hematopoietic stem cell disorder characterized by multi-lineage dysplasia and pancytopenia in which further genetic events may be required for the rapid expansion of leukaemic blasts (Heaney & Golde, 1999; Hirai, 2003). Although nuclear NPBC has been studied in many cancers as well as haematological malignancies, it has not been studied in MDS (Morin et al, 1997; Barker & Clevers, 2000; Giles et al, 2003). This is the first study to report the expression of beta-catenin in MDS patients. Here we showed that nuclear NPBC was related to the IPSS score and that secondary AML from MDS showed the highest percentage of nuclear NPBC expression. The Wnt signaling pathway may play an important role in the pathogenesis of the transformation of MDS into AML. Regarding chromosomal abnormalities, −7/−7q and/or complex karyotypes were significantly associated with the presence of nuclear NPBC. According to recent data (Liu et al, 2006), the gene encoding alpha-catenin (CTNNA1) is suppressed by deletion and/or methylation. Both alpha-catenin and beta-catenin bind to the inner membrane of hematopoietic cells, and cadherin binds to actin filaments via the catenin complex. If the expression of alpha-catenin is suppressed in the 5q- genotype or for other reasons, beta-catenin may be abnormally located or activated. In this study, abnormalities of chromosome 5 were associated with the presence of NPBC but this was not statistically significant. The reason why NPBC is associated with chromosome 7 abnormalities remains unclear. A gene encoded by chromosome 7 may be associated with the regulation of the Wnt/beta-catenin pathway. Several possible candidate genes including SFRP4, WNT2, and FZD1 and FZD9 are located at human chromosome 7. There are reports that sFRP4 plays a role in tumor suppression via the Wnt pathway (Hrzenjak et al, 2004; Horvath et al, 2007), although the specific relationship remains unknown. Since many molecules are directly or indirectly associated with the phosphorylation, stabilization and nuclear translocation of beta-catenin, the presence of nuclear NPBC might provide a clue to find a new leukemia-associated signaling mechanism.
In conclusion, in situ detection of nuclear NPBC by immunohistochemistry of paraffin sections from BM specimens could be used to predict the prognosis of AML and MDS. Understanding the mechanisms leading to leukemogenesis in nuclear NPBC+ AML and MDS may lead to new anti-leukemia therapies.
Acknowledgments
This study is partly supported by Grants-in-Aid from National Institute of Biomedical Innovation and from Ministry of Education, Culture, Sports, Science and Technology on the Scientific Research. We thank Kazuko Matsuba for technical assistance, and Mari Otsuka for secretarial assistance.
References
- Austin TW, Solar GP, Ziegler FC, Liem L, Matthews W. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood. 1997;89:3624–3635. [PubMed] [Google Scholar]
- Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Catenins, wnt signaling and cancer. BioEssays. 2000;22:961–965. doi: 10.1002/1521-1878(200011)22:11<961::AID-BIES1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- Chung EJ, Hwang SG, Nguyen PM, Lee S, Kim JS, Kim JW, Henkart PA, Bottaro DP, Soon LL, Bonvini P, Lee SJ, Karp JE, Oh HJ, Rubin JS, Trepel JB. Regulation of leukemic cell adhesion, proliferation and survival by beta-catenin. Blood. 2002;100:982–990. doi: 10.1182/blood.v100.3.982. [DOI] [PubMed] [Google Scholar]
- Cobas M, Wilson A, Ernst B, Mancini SJC, MacDonald HR, Kemler R, Radtke F. Catenin is dispensable for hematopoiesis and lymphopoiesis. Journal of Experimental Medicine. 2004;199:221–229. doi: 10.1084/jem.20031615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conacci-Sorrell M, Zhurinsky J, Ben-ZeÅfev A. The cadherin-catenin adhesion system in signaling and cancer. Journal of Clinical Investigation. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen PWB, Tjin E, Meijer HP, Klok MD, Mac Gillavry HD, Oers MHJV, Lokhorst HM, Bloem AC, Clevers H, Nusse R, Neut RVD, Spaargaren M, Pals ST. Illegitimate WNT signaling promotes proliferation of multiple myeloma cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6122–6127. doi: 10.1073/pnas.0305855101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SL, Spencer GJ, Heath DJ, Genever PG. Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells. 2004;22:849–860. doi: 10.1634/stemcells.22-5-849. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochimica et Biophysica Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Heaney ML, Golde DW. Articles medical progress: myelodysplasia. New England Journal of Medicine. 1999;340:1649–1660. doi: 10.1056/NEJM199905273402107. [DOI] [PubMed] [Google Scholar]
- Hirai H. Molecular mechanisms of myelodysplastic syndrome. Japanese Journal of Clinical Oncology. 2003;33:153–160. doi: 10.1093/jjco/hyg037. [DOI] [PubMed] [Google Scholar]
- Horvath LG, Lelliott JE, Kench JG, Lee CS, Williams ED, Saunders DN, Grygiel JJ, Sutherland RL, Henshall SM. Secreted frizzled-related protein 4 inhibits proliferation and metastatic potential in prostate cancer. Prostate. 2007;67:1081–1090. doi: 10.1002/pros.20607. [DOI] [PubMed] [Google Scholar]
- Hrzenjak A, Tippl M, Kremser ML, Strohmeier B, Guelly C, Neumeister D, Lax S, Moinfar F, Tabrizi AD, Isadi-Moud N, Zatloukal K, Denk H. Inverse correlation of secreted frizzled-related protein 4 and -catenin expression in endometrial stromal sarcomas. The Journal of Pathology. 2004;204:19–27. doi: 10.1002/path.1616. [DOI] [PubMed] [Google Scholar]
- Jamieson CHM, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, Gotlib J, Li K, Manz MG, Keating A, Sawyers CL, Weissman IL. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. New England Journal of Medicine. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- Liu TX, Becker MW, Jelinek J, Wu WS, Deng M, Mikhalkevich N, Hsu K, Bloomfield CD, Stone RM, DeAngelo DJ, Galinsky IA, Issa JP, Clarke MF, Look AT. Chromosome 5q deletion and epigenetic suppression of the gene encoding -catenin (CTNNA1) in myeloid cell transformation. Nature Medicine. 2006;13:78–83. doi: 10.1038/nm1512. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler WK. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Noort MV, Meeldijk J, Zee RVD, Destree O, Clevers H. Wnt signaling controls the phosphorylation status of beta-catenin. Journal of Biological Chemistry. 2002;277:17901–17905. doi: 10.1074/jbc.M111635200. [DOI] [PubMed] [Google Scholar]
- Ozeki K, Kiyoi H, Hirose Y, Iwai M, Ninomiya M, Kodera Y, Miyawaki S, Kuriyama K, Shimazaki C, Akiyama H, Nishimura M, Motoji T, Shinagawa K, Takeshita A, Ueda R, Ohno R, Emi N, Naoe T. Biologic and clinical significance of the FLT3 transcript level in acute myeloid leukemia. Blood. 2004;103:1901–1908. doi: 10.1182/blood-2003-06-1845. [DOI] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes and Development. 2000;14:1837–1851. [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Dome J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signaling in self-renewal of hematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Serinsöz E, Neusch M, Büsche G, Wasielewski RV, Kreipe H, Bock O. Aberrant expression of beta-catenin discriminates acute myeloid leukaemia from acute lymphoblastic leukaemia. British Journal of Haematology. 2004;126:313–319. doi: 10.1111/j.1365-2141.2004.05049.x. [DOI] [PubMed] [Google Scholar]
- Simon M, Grandage VL, Linch DC, Khwaja A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene. 2005;24:2410–2420. doi: 10.1038/sj.onc.1208431. [DOI] [PubMed] [Google Scholar]
- Staal FJT, Noort MV, Strous GJ, Clevers HC. Wnt signals are transmitted through N-terminally dephosphorylated beta-catenin. EMBO Reports. 2002;3:63–68. doi: 10.1093/embo-reports/kvf002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, Maccormick F. Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tickenbrock L, Schwable J, Wiedehage M, Steffen B, Sargin B, Choudhary C, Brandts C, Berdel WE, Tidow CM, Serve H. Flt3 tandem duplication mutations cooperate with Wnt signaling in leukemic signal transduction. Blood. 2005;105:3699–3706. doi: 10.1182/blood-2004-07-2924. [DOI] [PubMed] [Google Scholar]
- Van Den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998;92:3189–3202. [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, Nusse R. Wnt proteins are lipid-modified and can actas stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Xu JL, Lai R, Kinoshita T, Nakashima N, Nagasaka T. Proliferation, apoptosis and intratumoral vascularity in multiple myeloma: correlation with the clinical stage and cytologic grade. Journal of Clinical Pathology. 2002;55:530–534. doi: 10.1136/jcp.55.7.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ysebaert L, Chicanne G, Demur C, Toni FD, Houdellier NP, Ruidavets JB, Mas VMD, Huguet FR, Laurent G, Payrastre B, Manenti S, Sultan CR. Expression of beta-catenin by acute myeloid leukemia cells predicts enhanced clonogenic capacities and poor prognosis. Leukemia. 2006;20:1211–1216. doi: 10.1038/sj.leu.2404239. [DOI] [PubMed] [Google Scholar]